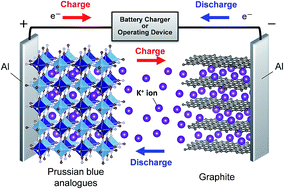
Recently, research on novel and low cost batteries has been widely conducted to realize large-scale energy storage systems. However, few of the battery systems have delivered performance equal to that of Li-ion batteries. Herein, we propose non-aqueous K-ion batteries by developing hexacyanoferrate(II) compounds (so-called Prussian blue analogues), K1.75Mn[FeII(CN)6]0.93·0.16H2O and K1.64Fe[FeII(CN)6]0.89·0.15H2O, as affordable positive electrode materials. In particular, K1.75Mn[FeII(CN)6]0.93·0.16H2O prepared by a simple precipitation process delivers a high capacity of 141 mA h g−1 at 3.8 V as the average operating potential, resulting in a comparable energy density to that of LiCoO2, with excellent cyclability and rate performance in K half-cells. Operando X-ray diffraction measurements reveal that the excellent electrochemical performance of this material is attributed to its open and flexible framework, which can realize fully reversible K+ extraction/insertion and a structural change from monoclinic to tetragonal via cubic phases. For the first time, we demonstrate an inexpensive high-voltage K-ion full cell with a K1.75Mn[Fe(CN)6]0.93·0.16H2O/graphite configuration to prove its feasibility as a new promising battery system for an environmentally friendly future.