Open Access Article
Open Access ArticleImpact of structure and homo-coupling of the central donor unit of small molecule organic semiconductors on solar cell performance†
Pieter
Verstappen
a,
Ilaria
Cardinaletti
b,
Tim
Vangerven
b,
Wouter
Vanormelingen
a,
Frederik
Verstraeten
a,
Laurence
Lutsen
ac,
Dirk
Vanderzande
ac,
Jean
Manca
d and
Wouter
Maes
*ac
aDesign & Synthesis of Organic Semiconductors (DSOS), Institute for Materials Research (IMO-IMOMEC), Hasselt University, Universitaire Campus, Agoralaan – Building D, B-3590 Diepenbeek, Belgium. E-mail: wouter.maes@uhasselt.be; Tel: +32 11268312
bMaterials Physics Division, Institute for Materials Research (IMO-IMOMEC), Hasselt University, Universitaire Campus, Wetenschapspark 1, B-3590 Diepenbeek, Belgium
cIMEC, IMOMEC, Universitaire Campus, Wetenschapspark 1, B-3590 Diepenbeek, Belgium
dX-LaB, Hasselt University, Universitaire Campus, Agoralaan – Building D, B-3590 Diepenbeek, Belgium
First published on 24th March 2016
Abstract
Currently, both low bandgap conjugated polymers and small molecule analogues are employed as electron donor components in state of the art bulk heterojunction organic photovoltaics, providing similar record efficiencies (∼10%). However, to evaluate molecular structure-device performance relations and (in particular) the effect of material purity, small molecule chromophores can be considered to be more versatile probes. In the present study, we have synthesized three small molecule donor materials with a varying central electron-rich building block, inspired by the well-known high-performance small molecule p-DTS(FBTTh2)2. The influence of this structural modification on the physicochemical material properties, electro-optical characteristics and solar cell performance is analysed. Most importantly, it is shown that the presence of homo-coupled side products generated during Stille cross-coupling reactions – which can be very hard to remove, even for small molecule semiconductors – is detrimental to solar cell performance, with a noticeable effect on the open-circuit voltage.
Introduction
Organic solar cells have attracted a huge amount of attention as a promising “future-proof” energy production technology because of their additional appealing features (compared to standard Si photovoltaics) such as attractive look (different colours and transparency), flexible and light-weight character and the possibility to produce large area devices via simple and cheap printing processes.1 In state of the art bulk heterojunction organic photovoltaics (BHJ OPV), the photoactive layer consists of two finely intermixed materials, acting as the electron donor and acceptor.2 Although noteworthy efforts have been conducted to identify viable alternatives for (methano)fullerene n-type materials,3 phenyl-C61-butyric acid methyl ester (PC61BM) and phenyl-C71-butyric acid methyl ester (PC71BM) are still most often employed. On the other hand, the donor material has undergone significant evolution over time. During the early days of OPV, almost all studies focused on poly(p-phenylene vinylene) (PPV) and poly(3-hexylthiophene) (P3HT) as workhorse conjugated polymers, generating power conversion efficiencies (PCE's) of approximately 3 and 5%, respectively.4 In recent years, the focus has shifted to push–pull type copolymers. These polymeric semiconductors are composed of electron rich (donor, D) and electron poor (acceptor, A) (heterocyclic) moieties, copolymerized in an alternating fashion by Pd-catalyzed polycondensation reactions. By employing this strategy, intramolecular charge transfer occurs, lowering the bandgap and allowing to harvest significantly more light in comparison to the homopolymers, leading to current reports of solar cell efficiencies approaching and even exceeding 10%.5The advent of the push–pull concept has delivered a toolbox to material chemists not only to effectively lower the bandgap, but also to tune the frontier molecular orbital energy levels rather independently.1b,6 Nowadays, sufficiently low HOMO–LUMO gaps can already be achieved with relatively short chain “small” molecules. Furthermore, such mall molecules offer some specific advantages compared to their polymeric counterparts, i.e. less batch-to-batch variation because of their uniform and defined molecular structures and easier purification (although polymer materials may be advantageous in terms of large scale solution processing).1i,7 Consequently, the interest in small molecule organic solar cells has risen rapidly and PCE's in the proximity of 10% have also been reported recently.8 Among others, Bazan et al. developed a D–A–D–A–D strategy based on fluorinated benzothiadiazole (BT) and dithienosilole (DTS), affording the well-known small molecule p-DTS(FBTTh2)2, yielding PCE's exceeding 8%.8b,9 On the other hand, Chen and co-workers employed a D–A–D concept to achieve OPV devices with similar efficiencies.8c,10
Nowadays, it is generally accepted that small changes to the chemical structure of the light-harvesting chromophores can lead to strong variations in final solar cell performance. For example, it was reported that substitution of alkoxy side chains by alkylthio groups effectively improves solar cell efficiency, leading to devices with almost 10% PCE.8c It has also been shown that side chain length and position are of major importance for the development of high-performance small molecule organic solar cells.11 However, the impact of such structural changes is very hard to predict. Additional investigations of structure-device relations are hence certainly still required to make further advances in the field and to evolve from a trial and error to an intelligent design approach. On the other hand, the effect of minor amounts of (organic) impurities on device performance has been underexposed so far and needs further attention, even though it is widely known that purity is of utmost importance in organic electronics. It was for instance shown that the presence of low molar mass materials in polymers can greatly affect both the initial performance and the lifetime of the resulting polymer solar cells.12 Moreover, although small molecules are often regarded as “easy to purify”, Heeger and co-workers already stated that the presence of trace amounts of impurities, which can be hard to remove, significantly influences the solar cell properties, illustrating the need for a better understanding of their effect on the device parameters.13
In this work, the central donor unit in the well-known “Bazan” small molecule (p-DTS(FBTTh2)2)8b,9 was varied and its impact on the physicochemical properties of the resulting materials and the final solar cell characteristics was studied. Three different donor units were chosen, based on their previous use in low bandgap OPV copolymers, i.e. 4H-cyclopenta[2,1-b:3,4-b′]dithiophene (CPDT),1b,14N-acyl-substituted dithieno[3,2-b:2′,3′-d]pyrrole (DTP)15 and thieno[3,2-b]thiophene (TT).16 Incorporation of the CPDT and DTP components allows to study the effect of the bridging atom of these fused bithiophene donor moieties, whereas a more crystalline small molecule is targeted by the introduction of the TT moiety. To guarantee sufficient solubility of the full small molecule series, some additional side chains were introduced on the thiophene units as compared to p-DTS(FBTTh2)2.8b,9 The novel small molecules were fully characterized and their solar cell properties were determined, along with studies on the film morphology and charge carrier mobility of the donor materials and their blends with PC71BM. A noteworthy and strong effect of molecular purity was observed for the DTP-based material, for which homo-coupling of the central donor unit occurred to some extent in the final Stille cross-coupling reaction, with a remarkable detrimental effect on OPV performance.
Results and discussion
Material synthesis and characterization
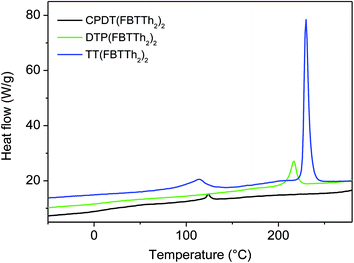
All three small molecules were prepared following the same synthetic pathway, outlined in Scheme 1. 3,5′-Dihexyl-2,2′-bithiophene (1) was synthesized according to a literature procedure and subsequently monostannylated.17 Stille cross-coupling of precursor 2 with 4,7-dibromo-5-fluorobenzo[c][1,2,5]thiadiazole yielded molecule 3. The targeted small molecules were then obtained via Stille cross-coupling of precursor 3 with the bis(trimethylstannyl) derivatives of the appropriate donor molecules. CPDT(FBTTh2)2 and DTP(FBTTh2)2 showed excellent solubility in common organic solvents (e.g. THF, chloroform and toluene). TT(FBTTh2)2, on the other hand, only showed a reasonable solubility in CS2 at room temperature or in chlorinated solvents (e.g. 1,1,2,2-tetrachloroethane and chlorobenzene) at elevated temperatures. Since material purity is of major importance for the fabrication of electronic devices, CPDT(FBTTh2)2 and DTP(FBTTh2)2 were purified by flash column chromatography (on silica) and recycling preparative size exclusion chromatography (prep-SEC). Due to its limited solubility, TT(FBTTh2)2 was purified through Soxhlet extraction with methanol, acetone, n-hexane and chloroform. 1H NMR analysis indicated high purity of the CPDT(FBTTh2)2 and TT(FBTTh2)2 small molecule semiconductors (see ESI†). For DTP(FBTTh2)2, some (minor) impurities could be observed (vide infra).The thermal properties of the small molecule series were investigated by rapid heat-cool calorimetry (RHC) (Fig. 1, Table 1). RHC was chosen above regular differential scanning calorimetry (DSC) because of its increased sensitivity to thermal transitions as a result of the fast scanning rates and the low sample amounts required.18 From the obtained results, it is clear that variation of the central donor unit has a major impact on both the melting temperature (Tm) and melting enthalpy (ΔHm). As anticipated, the small molecule with the highest degree of crystallinity was acquired by employing thieno[3,2-b]thiophene as the central (substituent-free) donor unit.16 This material showed two melting temperatures, one at a relatively low temperature (120 °C) and a second at higher temperature (230 °C), with the latter exhibiting a large ΔHm (48.8 J g−1), indicating highly crystalline character. On the other hand, relatively low ΔHm values were observed for both CPDT(FBTTh2)2 and DTP(FBTTh2)2, demonstrating a more amorphous nature. For CPDT(FBTTh2)2, the low melting enthalpy was accompanied by a low melting temperature.
| T m a (°C) | ΔHma (J g−1) | λ max b (nm) solution | λ max (nm) film | E OPg c (eV) | HOMOd (eV) | LUMOd (eV) | E ECg e (eV) | |
|---|---|---|---|---|---|---|---|---|
| a Determined by RHC. b In chloroform. c Optical HOMO–LUMO gap, determined by the onset of the solid-state UV-Vis spectrum. d Determined by CV from the onsets of oxidation and reduction. e Electrochemical HOMO–LUMO gap. f Determined for the non-purified sample. | ||||||||
| CPDT(FBTTh2)2 | 124 | 2.6 | 607 | 677 | 1.64 | −5.43 | −3.41 | 2.02 |
| DTP(FBTTh2)2 f | 217 | 11.0 | 581 | 681 | 1.52 | −5.35 | −3.43 | 1.92 |
| TT(FBTTh2)2 | 120/230 | 11.6/48.8 | 553 | 575 | 1.72 | −5.63 | −3.38 | 2.25 |
Normalized UV-Vis absorption spectra of the three small molecules in solution (CHCl3) and thin film are shown in Fig. 2. The UV-Vis spectra in chloroform nicely demonstrate that the introduction of more electron rich donor units results in a bathochromic shift. TT(FBTTh2)2 showed maximum absorption around 550 nm (λmax). When the central donor unit was changed to N-acyl-DTP, a red-shift of 30 nm was acquired, and an even higher λmax was obtained when the electron rich CPDT unit was incorporated (Table 1). In thin film, the absorption profiles were broadened and red-shifted. It was also observed that the absorption profiles of CPDT(FBTTh2)2 and DTP(FBTTh2)2 were more strongly red-shifted in comparison to TT(FBTTh2)2, despite the higher degree of crystallinity of the latter, as perceived from RHC. From the UV-Vis spectra in thin film, the optical HOMO–LUMO gaps were determined (Table 1). The smallest optical gap was observed for DTP(FBTTh2)2 (1.52 eV). Modifying the central donor moiety to CPDT slightly increased the optical gap, which further raised by implementation of TT.
 | ||
| Fig. 2 Normalized UV-Vis absorption spectra for the small molecule series in chloroform solution (top) and in thin film (bottom) (non-purified DTP(FBTTh2)2 was used here). | ||
The HOMO and LUMO energy levels of the small molecules were estimated via cyclic voltammetry (CV) from the onset of the oxidation and reduction peaks, respectively (Table 1, Fig. S1†). While similar LUMO energy levels were observed for the three small molecules, the HOMO energy levels were (non-surprisingly) significantly influenced by the variation of the central donor unit. Incorporation of TT seemed to yield small molecules with deep HOMO levels, while introduction of the electron rich DTP unit resulted in the highest HOMO level and the smallest electrochemical HOMO–LUMO gap (1.92 eV).
Solar cell analysis
The small molecule electron donor materials were then used to fabricate standard architecture photovoltaic cells, in combination with PC61BM or PC71BM (Table 2, S1–S3†). The devices were prepared by spin-coating the active layer blends on a PEDOT:PSS (poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)) hole transport layer, deposited on an ITO (indium tin oxide) transparent electrode. Non-surprisingly, the best processing conditions and optimal donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor ratio were found to be different for each donor material, and they are reported in Table 2, together with the average output parameters determining the optimal performance. The current density (J)–voltage (V) curves of the best performing solar cells are shown in Fig. 3. The use of chlorobenzene (CB) as active layer casting solvent allowed to reach the optimal performance for the solar cells of all three small molecules in blends with PC71BM. Due to the low solubility imposed by the TT moiety, TT(FBTTh2)2 had to be deposited from a high temperature CB solution. The use of 1,8-diiodooctane (DIO) allowed for an additional increase of current for the TT(FBTTh2)2:PC71BM device, reaching a best efficiency of 3.0%. For all compounds, the EQE spectra of the optimal solar cell devices (Fig. S2†) showed a clear contribution of both the donor material and the fullerene derivative.
acceptor ratio were found to be different for each donor material, and they are reported in Table 2, together with the average output parameters determining the optimal performance. The current density (J)–voltage (V) curves of the best performing solar cells are shown in Fig. 3. The use of chlorobenzene (CB) as active layer casting solvent allowed to reach the optimal performance for the solar cells of all three small molecules in blends with PC71BM. Due to the low solubility imposed by the TT moiety, TT(FBTTh2)2 had to be deposited from a high temperature CB solution. The use of 1,8-diiodooctane (DIO) allowed for an additional increase of current for the TT(FBTTh2)2:PC71BM device, reaching a best efficiency of 3.0%. For all compounds, the EQE spectra of the optimal solar cell devices (Fig. S2†) showed a clear contribution of both the donor material and the fullerene derivative.
| Donor | Acceptor | D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio A ratio |
Solventa | J sc (mA cm−2) | V oc (V) | FF (%) | PCEb (%) |
|---|---|---|---|---|---|---|---|
| a CB = chlorobenzene, DIO = 1,8-diiodooctane. b Average values over at least 3 devices. The best device performance is shown in parentheses. c Before removal of the homo-coupled species. d Processed at 85 °C. | |||||||
| CPDT(FBTTh2)2 | PC71BM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
CB | 8.39 | 0.85 | 39.7 | 2.83 (3.10) |
| DTP(FBTTh2)2 c | PC71BM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CB | 6.75 | 0.50 | 39.6 | 1.34 (1.37) |
| DTP(FBTTh2)2-pure | PC71BM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CB | 8.37 | 0.67 | 44.1 | 2.56 (2.76) |
| TT(FBTTh2)2 d | PC71BM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CB + 0.2% DIO | 9.13 | 0.79 | 36.4 | 2.63 (2.96) |
Despite extensive optimization of the processing parameters, the performances of all small molecules remained moderate. While for CPDT(FBTTh2)2 and TT(FBTTh2)2 reasonable results were obtained (up to 3% PCE), the solar cell performances of DTP(FBTTh2)2 (PCE around 1%) were very poor. The parameter that contributes most to limiting the final efficiency of the devices presented in this study is the FF, which can possibly be related to the occurrence of recombination processes or to an offset of the hole mobility in the donor material and the electron mobility in the acceptor phase (1 × 10−3 cm2 V−1 s−1 for PC71BM19).20 To evaluate charge transport, the hole mobility was assessed for the neat small molecule donor materials. Field-effect transistors (FETs) were prepared in the bottom gate bottom contacts configuration by depositing the small molecules from a CB solution on a SiO2 layer, thermally grown on highly n-doped Si. Gold source and drain contacts were pre-patterned on the substrate, on top of a titanium adhesion layer. Although the observed high threshold voltages (Fig. S3†) suggest the possibility of occurring bias stress,21 the estimated mobilities do not deviate too far from the values reported for other commonly employed organic semiconductors (∼10−4 cm2 V−1 s−1 for P3HT22). Comparable hole mobilities were extracted for CPDT(FBTTh2)2 and TT(FBTTh2)2, 4.5 × 10−4 and 3.5 × 10−4 cm2 V−1 s−1, respectively. Unfortunately, no mobility value could be extracted for DTP(FBTTh2)2, due to difficulties in the deposition on the SiO2 substrate.
To examine the photoactive layer blend morphology on the nanoscale, atomic force microscopy (AFM) images were acquired in PeakForce Tapping™ mode. In all cases, the best performing films appeared to be fully intermixed, with little to no evidence of phase separation nor crystallization (Fig. 4).
 | ||
| Fig. 4 AFM scans of the active layer blends (resulting in best solar cell performances) based on (a) CPDT(FBTTh2)2, (b) DTP(FBTTh2)2 and (c) TT(FBTTh2)2. | ||
Homo-coupling defects
Besides the modest FF of all solar cell devices prepared from the novel small molecules, a noteworthy observation is also the low Voc value obtained for the optimal device based on DTP(FBTTh2)2 (0.50 V, compared to values of 0.79–0.85 V for the other materials), especially since such an inferior value is not expected based on the HOMO energy levels as derived from CV (Table 1). Detailed analysis of the 1H NMR spectrum of DTP(FBTTh2)2 (see ESI†) revealed the presence of ‘small’ impurities, which might be causing the Voc drop. Despite the fact that this small molecule was purified by the same two-step procedure as CPDT(FBTTh2)2 (1× flash column chromatography and 1× prep-SEC), some side product(s) seemed to remain in this sample. To determine the chemical structure of the impurities, MALDI-TOF mass spectrometry analysis was performed (see ESI†). The MALDI spectrum clearly identified the major impurity to be a structure with an additional DTP unit, pointing to the occurrence of homo-coupling of the organotin species during the final Stille cross-coupling reaction (Scheme 1), which leads to a small molecule with two central adjacent DTP units (DTP(FBTTh2)2-homo, see Fig. 5).23 It has to be emphasized that MALDI appears to be an extremely sensitive technique for the detection of molecular (homo-coupling) impurities, whereas 1H NMR analysis only allowed to state that the amount of the side product(s) was below 10%.It has recently been reported that the presence of homo-coupling in low bandgap copolymers has a detrimental effect on solar cell performance, and especially on Voc.24 Unfortunately, it can be very hard to identify homo-coupling defects in polymer structures (in a direct way) and its importance hence remains to be elucidated in more detail. To investigate if the low Voc value for the DTP-based small molecule can be correlated to the occurrence of homo-coupling, additional tedious purification was performed by prep-SEC. Separation of both products was not straightforward due to pronounced tailing on the preparative SEC column (Fig. S4†). The material had to be injected and collected multiple times before the small molecule could be obtained in pure form (denoted as DTP(FBTTh2)2-pure). Obviously, the use of such an elaborate purification procedure is not desirable in a commercial setting, clearly demonstrating the need for optimized synthetic protocols affording defect-free materials.
In previous reports, the presence of a low energy tail in the UV-Vis absorption spectrum has been associated to the presence of homo-coupling in low bandgap copolymers.24 However, despite the red-shifted absorption spectrum of DTP(FBTTh2)2-homo, the UV-Vis absorption profiles of DTP(FBTTh2)2 and DTP(FBTTh2)2-pure are nearly identical (Fig. S5†), suggesting that UV-Vis spectroscopy is not the most appropriate technique to decide about the presence of homo-coupled impurities and clearly demonstrating the need to analyse materials by multiple techniques to discover and identify (minor) impurities, which is of course much easier for small molecules.
Subsequently, the solar cell performance of DTP(FBTTh2)2-pure was evaluated and the results were compared to the non-purified sample (Table 2, Fig. 3). As anticipated, complete removal of the homo-coupled side product resulted in an enhancement of the Voc of almost 0.2 V to a value of 0.67 V, still below the values for the other two materials but in much better agreement with the trend in HOMO energy levels. Furthermore, besides the Voc, also the Jsc and FF benefited from the removal of DTP(FBTTh2)2-homo, leading to a final average solar cell efficiency of 2.56%. The purification of the DTP-based small molecule also enabled to deposit it on SiO2 and to extract the hole mobility from FETs. This mobility turned out to be 2.0 × 10−5 cm2 V−1 s−1, hence somewhat below the other two small molecules.
To acquire more insight in the nature of the Voc changes, Fourier transform photocurrent spectroscopy (FTPS) studies were performed. It has been widely shown that mixing of an organic semiconductor with a fullerene gives rise to interfacial charge transfer (CT) states, which determine the Voc of organic solar cells.25 To probe the absorption of the CT state, FTPS spectra were acquired for the optimized devices based on the three small molecules. The results are shown in Fig. 6, together with the fits of eqn (1) to the CT band.26
 | (1) |
 | ||
| Fig. 6 FTPS spectra of the CT region for optimized devices, with fits to eqn (1) indicated by the dashed lines. The fitting parameters can be found in Table S4.† | ||
As expected, the DTP(FBTTh2)2:PC71BM device exhibits the lowest ECT value (= 1.22 eV, Table S4†) and the CPDT(FBTTh2)2:PC71BM device the highest (= 1.43 eV), corresponding to the observed trend in Voc. A linear relationship between ECT and Voc has been observed in the past for many different polymer and small molecule OPV systems, where the difference between ECT and qVoc (ΔE) is typically 0.60 ± 0.07 eV, with q being the elementary charge.27 These energy losses (ΔE) could originate from radiative and non-radiative mechanisms.26 The best performing (∼3% PCE) CPDT(FBTTh2)2 and TT(FBTTh2)2 based devices yield values of 0.58 and 0.54 eV for ΔE, respectively, agreeing with the often observed trend in ΔE. On the other hand, a remarkably high energy loss of 0.70 eV was observed for the solar cell devices based on non-purified DTP(FBTTh2)2. After complete removal of DTP(FBTTh2)2-homo, this energy loss was significantly reduced to 0.55 eV, as the Voc considerably increased and the ECT values for both materials remained similar (Fig. 6 and Table S4†). Burke et al. recently developed a model to understand Voc losses.28 They mention that properties such as the number of CT states, CT lifetime and energetic interfacial disorder can strongly influence Voc. The present results lead to suggest that homo-couplings might have a strong effect on the energetic interfacial disorder, leading to increased Voc losses. Further studies to confirm this hypothesis are still required, however.
Conclusions
Generally, as compared to conjugated polymers, small molecule chromophores have the advantage of a more straightforward and reproducible synthesis, combined with the possibility of purifying these materials more effectively by classical organic synthesis purification methodologies (e.g. recrystallization). However, in this study, we have shown that purification of small molecule organic semiconductors is not as straightforward as often projected. Notwithstanding the use of recycling preparative size exclusion chromatography in combination with standard column chromatography, the presence of (minor amounts of) side products could be demonstrated by MALDI-TOF MS analysis. These impurities could be related to the use of the Stille cross-coupling reaction, as they were identified as homo-coupled products. The presence of the homo-coupled species was shown to have a detrimental effect on final solar cell performance. Furthermore, there seems to be a large influence of homo-coupling on the ΔE = ECT − Voc relationship, as the presence of such side products results in a large deviation from the empirical ΔE = 0.6 eV relation. Therefore, considerable care is required to avoid the formation of homo-coupled side products, something that is currently undervalued in the OPV field.23,24 In this respect, small molecules are obviously interesting model systems to optimize Stille reactions standardly applied for low bandgap copolymers. On the other hand, the presence of homo-coupled impurities might also have a (strong) effect on OPV device stability,12 which is currently under investigation within our group.Furthermore, it was shown that variation of the central donor unit greatly affects the physicochemical properties of the studied small molecule materials. The crystalline character was modified to a large extent. Despite the large difference in crystallinity, the materials showed comparable solar cell performances (up to ∼3% PCE). The considerably lower efficiencies as compared to p-DTS(FBTTh2)2 might be related to the significantly reduced hole mobility for the small molecules reported in this manuscript (with regards to the high mobility, up to 0.14 cm2 V−1 s−1, of p-DTS(FBTTh2)2 (ref. 9)). Finally, it has been confirmed once more that establishing general design rules for small molecule solar cells is not trivial, as optimization of a certain parameter very often leads to (unexpected) negative effects.
Experimental section
Materials and instruments
Preparative (recycling) size exclusion chromatography was performed on a JAI LC-9110 NEXT system equipped with JAIGEL 1H and 2H columns (eluent CHCl3, flow rate 3.5 mL min−1). NMR spectra were recorded in CDCl3, unless stated otherwise, and chemical shifts (δ, in ppm) were determined relative to the residual CHCl3 (7.26 ppm) absorption or the 13C resonance shift of CDCl3 (77.16 ppm). High resolution electrospray ionization mass spectrometry (ESI-MS) was performed using an LTQ Orbitrap Velos Pro mass spectrometer equipped with an atmospheric pressure ionization source operating in the nebulizer assisted electrospray mode. The instrument was calibrated in the m/z range 220–2000 using a standard solution containing caffeine, MRFA and Ultramark 1621. MALDI-TOF mass spectra were recorded on a Bruker Daltonics Ultraflex II Tof/Tof. 1 μL of the matrix solution (16 mg mL−1 DTCB (trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile) in CHCl3) was spotted onto an MTP Anchorchip 600/384 MALDI plate. The spot was allowed to dry and 1 μL of the analyte solution (0.5 mg mL−1 in CHCl3) was spotted on top of the matrix. Reported masses are those corresponding to the first peaks of the isotopic patterns. UV-Vis measurements were performed on a VARIAN Cary 500 UV-Vis-NIR spectrophotometer at a scan rate of 600 nm min−1. The films for the UV-Vis measurements were prepared by drop casting a solution of the small molecule in chloroform on a quartz substrate. The solid-state UV-Vis spectra were used to estimate the optical HOMO–LUMO gaps (from the wavelength at the intersection of the tangent line drawn at the low energy side of the absorption spectrum with the x-axis: Eg (eV) = 1240/(wavelength in nm)). Rapid heat-cool calorimetry (RHC) experiments were performed on a prototype RHC of TA Instruments, equipped with liquid nitrogen cooling and specifically designed for operation at high scanning rates.18 RHC measurements were performed at 250 or 500 K min−1 in aluminum crucibles, using helium (6 mL min−1) as a purge gas. Electrochemical measurements (cyclic voltammetry) were performed with an Eco Chemie Autolab PGSTAT 30 potentiostat/galvanostat using a three-electrode microcell with a platinum working electrode, a platinum counter electrode and a Ag/AgNO3 reference electrode (silver wire dipped in a solution of 0.01 M AgNO3 and 0.1 M NBu4PF6 in anhydrous acetonitrile). The reference electrode was calibrated against ferrocene/ferrocenium as an external standard. Samples were prepared by dip coating the platinum working electrode in the respective small molecule solutions (also used for the solid-state UV-Vis measurements). The CV measurements were done on the resulting films with 0.1 M NBu4PF6 in anhydrous acetonitrile as electrolyte solution. To prevent air from entering the system, the experiments were carried out under a curtain of argon. Cyclic voltammograms were recorded at a scan rate of 100 mV s−1. For the conversion of V to eV, the onset potentials of the first oxidation/reduction peaks were used and referenced to ferrocene/ferrocenium, which has an ionization potential of −4.98 eV vs. vacuum. This correction factor is based on a value of 0.31 eV for Fc/Fc+vs. SCE29a and a value of 4.68 eV for SCE vs. vacuum:29b . The reported values (Table 1, Fig. S1†) are the means of the first four redox cycles.
. The reported values (Table 1, Fig. S1†) are the means of the first four redox cycles.
Material synthesis
Unless stated otherwise, all reagents and chemicals were obtained from commercial sources and used without further purification. Solvents were dried by a solvent purification system (MBraun, MB-SPS-800) equipped with alumina columns. Precursors 3,5′-dihexyl-2,2′-bithiophene (1),17 4,7-dibromo-5-fluorobenzo[c][1,2,5]thiadiazole,30 2,6-bis(trimethylstannyl)-4-(2′-ethylhexyl)-4-octyl-4H-cyclopenta[2,1-b:3,4-b′]dithiophene,30 and 2,6-bis(trimethylstannyl)-N-(2′-ethylhexanoyl)dithieno[3,2-b:2′,3′-d]pyrrole15 were prepared according to literature procedures. 2,5-Bis(trimethylstannyl)thieno[3,2-b]thiophene was purchased from Sigma-Aldrich.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane, 70
dichloromethane, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) and recycling prep-SEC yielded the pure product as an orange solid (1.10 g, 64%). 1H NMR (400 MHz, CDCl3): δ 7.96 (s, 1H), 7.65 (d, J = 10.1 Hz, 1H), 7.05 (d, J = 3.6 Hz, 1H), 6.77 (d, J = 3.6, 1H), 2.82 (q, J = 7.8 Hz, 4H), 1.71 (quint, J = 7.3 Hz, 4H), 1.48–1.23 (m, 12H), 0.96–0.82 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 160.9 (d, 1JC–F = 251.2 Hz, 1H), 154.4 (d, 3JC–F = 7.4 Hz, 1H), 149.1 (1H), 147.3 (1H), 140.2 (1H), 135.0 (1H), 134.1 (1H), 133.0 (1H), 132.3 (1H), 127.5 (d, 3JC–F = 10.3 Hz, 1H), 126.3 (1H), 124.8 (1H), 115.5 (d, 2JC–F = 30.9 Hz, 1H), 95.8 (d, 2JC–F = 24.8 Hz, 1H), 31.8 (1H), 31.7 (2H), 30.7 (1H), 30.3 (1H), 29.6 (1H), 29.4 (1H), 29.0 (1H), 22.8 (1H), 22.7 (1H), 14.3 (2H).
30) and recycling prep-SEC yielded the pure product as an orange solid (1.10 g, 64%). 1H NMR (400 MHz, CDCl3): δ 7.96 (s, 1H), 7.65 (d, J = 10.1 Hz, 1H), 7.05 (d, J = 3.6 Hz, 1H), 6.77 (d, J = 3.6, 1H), 2.82 (q, J = 7.8 Hz, 4H), 1.71 (quint, J = 7.3 Hz, 4H), 1.48–1.23 (m, 12H), 0.96–0.82 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 160.9 (d, 1JC–F = 251.2 Hz, 1H), 154.4 (d, 3JC–F = 7.4 Hz, 1H), 149.1 (1H), 147.3 (1H), 140.2 (1H), 135.0 (1H), 134.1 (1H), 133.0 (1H), 132.3 (1H), 127.5 (d, 3JC–F = 10.3 Hz, 1H), 126.3 (1H), 124.8 (1H), 115.5 (d, 2JC–F = 30.9 Hz, 1H), 95.8 (d, 2JC–F = 24.8 Hz, 1H), 31.8 (1H), 31.7 (2H), 30.7 (1H), 30.3 (1H), 29.6 (1H), 29.4 (1H), 29.0 (1H), 22.8 (1H), 22.7 (1H), 14.3 (2H).
General synthesis protocol. Precursor 3 (200 mg, 0.354 mmol), 2,6-bis(trimethylstannyl)-4-(2′-ethylhexyl)-4-octyl-4H-cyclopenta[2,1-b:3,4-b′]dithiophene (126 mg, 0.173 mmol) and Pd(PPh3)4 (10 mg, 0.0087 mmol) were dissolved in a mixture of dry DMF (2 mL) and dry toluene (2 mL). The solution was purged with N2 gas for 30 min and heated to 110 °C for 15 h. The resulting mixture was allowed to cool to room temperature and was precipitated in methanol. After filtration, the crude material was further purified by column chromatography (silica, petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane, 60
dichloromethane, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) and recycling prep-SEC and the pure material was collected as a dark blue solid (185 mg, 78%). 1H NMR (400 MHz, CDCl3): δ 8.24 (s, 1H), 8.22 (s, 1H), 7.97 (d, J = 1.3 Hz, 2H), 7.73 (dd, J = 13.4, 1.3 Hz, 2H), 7.06 (d, J = 3.6 Hz, 2H), 6.77 (d, J = 3.6 Hz, 2H), 2.84 (q, J = 7.5 Hz, 8H), 2.16–1.93 (m, 4H), 1.80–1.65 (m, 8H), 1.50–1.27 (m, 25H), 1.24–1.10 (m, 10H), 1.10–0.95 (m, 10H), 0.95–0.83 (m, 12H), 0.83–0.73 (m, 3H), 0.71–0.59 (m, 6H); HRMS (ESI): calcd for C77H96F2N4S8Na [M + Na]+: 1393.5261, found: 1393.5240; MS (MALDI-TOF) m/z: 1370.5 ([M]+).
40) and recycling prep-SEC and the pure material was collected as a dark blue solid (185 mg, 78%). 1H NMR (400 MHz, CDCl3): δ 8.24 (s, 1H), 8.22 (s, 1H), 7.97 (d, J = 1.3 Hz, 2H), 7.73 (dd, J = 13.4, 1.3 Hz, 2H), 7.06 (d, J = 3.6 Hz, 2H), 6.77 (d, J = 3.6 Hz, 2H), 2.84 (q, J = 7.5 Hz, 8H), 2.16–1.93 (m, 4H), 1.80–1.65 (m, 8H), 1.50–1.27 (m, 25H), 1.24–1.10 (m, 10H), 1.10–0.95 (m, 10H), 0.95–0.83 (m, 12H), 0.83–0.73 (m, 3H), 0.71–0.59 (m, 6H); HRMS (ESI): calcd for C77H96F2N4S8Na [M + Na]+: 1393.5261, found: 1393.5240; MS (MALDI-TOF) m/z: 1370.5 ([M]+).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3 3
CDCl3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 8.90–8.40 (br, 2H), 7.79 (s, 2H), 7.48 (d, J = 13.6 Hz, 2H), 6.91 (d, J = 3.5 Hz, 2H), 6.68 (d, J = 3.5 Hz, 2H), 3.42 (quint, J = 6.5 Hz, 1H), 2.81 (t, J = 7.7 Hz, 4H), 2.67 (t, J = 8.0 Hz, 4H), 2.20–2.05 (m, 2H), 1.97–1.77 (m, 2H), 1.77–1.15 (m, 47H), 1.04 (t, J = 7.2 Hz, 3H), 0.95 (t, J = 6.4 Hz, 12H); HRMS (ESI): calcd for C68H77F2N5OS8Na [M + Na]+: 1296.3754, found: 1296.3750; MS (MALDI-TOF) m/z: 1273.9 ([M]+).
1) δ 8.90–8.40 (br, 2H), 7.79 (s, 2H), 7.48 (d, J = 13.6 Hz, 2H), 6.91 (d, J = 3.5 Hz, 2H), 6.68 (d, J = 3.5 Hz, 2H), 3.42 (quint, J = 6.5 Hz, 1H), 2.81 (t, J = 7.7 Hz, 4H), 2.67 (t, J = 8.0 Hz, 4H), 2.20–2.05 (m, 2H), 1.97–1.77 (m, 2H), 1.77–1.15 (m, 47H), 1.04 (t, J = 7.2 Hz, 3H), 0.95 (t, J = 6.4 Hz, 12H); HRMS (ESI): calcd for C68H77F2N5OS8Na [M + Na]+: 1296.3754, found: 1296.3750; MS (MALDI-TOF) m/z: 1273.9 ([M]+).
1H NMR (400 MHz, CS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3 3
CDCl3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δ 8.59 (s, 2H), 7.99 (s, 2H), 7.66 (d, J = 13.4 Hz, 2H), 6.97 (d, J = 3.6 Hz, 2H), 6.72 (d, J = 3.6 Hz, 2H), 2.85 (t, J = 7.6 Hz, 4H), 2.79 (t, J = 7.8 Hz, 4H), 1.79–1.67 (m, 8H), 1.52–1.32 (m, 26H), 1.00–0.91 (m, 12H); HRMS (ESI): calcd for C58H62F2N4S8Na [M + Na]+: 1131.2600, found: 1131.2565; MS (MALDI-TOF) m/z: 1108.3 ([M]+).
1) δ 8.59 (s, 2H), 7.99 (s, 2H), 7.66 (d, J = 13.4 Hz, 2H), 6.97 (d, J = 3.6 Hz, 2H), 6.72 (d, J = 3.6 Hz, 2H), 2.85 (t, J = 7.6 Hz, 4H), 2.79 (t, J = 7.8 Hz, 4H), 1.79–1.67 (m, 8H), 1.52–1.32 (m, 26H), 1.00–0.91 (m, 12H); HRMS (ESI): calcd for C58H62F2N4S8Na [M + Na]+: 1131.2600, found: 1131.2565; MS (MALDI-TOF) m/z: 1108.3 ([M]+).
Solar cell and FET preparation and characterization
Solar cells in standard architecture were prepared with a layout glass/ITO/PEDOT:PSS/small molecule:methanofullerene/Ca/Al. Substrates with pre-patterned ITO on glass were purchased from Kintec (100 nm, 20 Ohm per sq.) and cleaned through sonication in soap, deionized water, acetone and isopropyl alcohol before proceeding with the spin-coating of PEDOT:PSS (Heraeus Clevios AI 4083). Substrates were subsequently brought inside a N2 filled glovebox and annealed during 10 min at 130 °C to remove residual humidity. All subsequent processing and characterization steps were conducted in inert atmosphere. The active layers in the various blend compositions (see Table 2 and ESI†) were spin-cast on the PEDOT:PSS layer. The optimal concentrations were found to be 35 mg mL−1 for CPDT(FBTTh2)2, DTP(FBTTh2)2 and TT(FBTTh2)2 (in chlorobenzene). In case of additives present in the processing solution, the films were kept under vacuum for a minimum of 2 h to remove residual solvent or additive remainders before thermally evaporating Ca/Al (30/80 nm) stacks as top contacts, defining device areas of 0.03 cm2 through the use of masks. Electrical characterization was carried out under illumination from a Newport class A solar simulator (model 91195A), calibrated with a silicon solar cell to give a 1 sun AM 1.5G spectrum. EQE measurements were performed with a Newport Apex illuminator (100 W xenon lamp, 6257) as light source, a Newport Cornerstone 130 monochromator and a Stanford SR830 lock-in amplifier for the current measurements. A silicon-calibrated FDS-100 photodiode was employed as a reference cell. JEQE values calculated from the EQE spectra were 8.86, 7.81 and 9.81 mA cm−2 for CPDT(FBTTh2)2, DTP(FBTTh2)2-pure and TT(FBTTh2)2, respectively. PeakForce Tapping™ AFM images were acquired with a Bruker Multimode 8 AFM, employing ScanAsyst™. The silicon nitride tip had a spring constant of 4 N m−1. FETs were prepared by spin-coating solutions of CPDT(FBTTh2)2, DTP(FBTTh2)2-pure and TT(FBTTh2)2 in chlorobenzene with a concentration of 15, 15 and 8 mg mL−1, respectively, on 200 nm of thermally grown SiO2. The gate contact consisted of highly n-doped Si. Interdigitated source and drain electrodes were pre-patterned, comprising of a stack of Ti/Au (10/100 nm). FET substrates were acquired from Philips. The channel length was 10 μm. Two Keithley 2400 source meters were used to measure the IDS and correct it for leakage through the gate electrode. All FET preparations and characterizations were carried out in a N2 filled glovebox. Fourier transform photocurrent spectroscopy (FTPS) was performed using a Thermo Nicolet 8700 FTIR with an external detector. The spectra were recorded with a quartz beamsplitter and appropriate optical bandpass filters to improve the signal to noise ratio. All spectra were corrected for the frequency response. More information can be found in literature.31Acknowledgements
This work was supported by the IAP 7/05 project FS2 (Functional Supramolecular Systems), granted by the Science Policy Office of the Belgian Federal Government (BELSPO). We are also grateful for financial support by the Research Programme of the Research Foundation – Flanders (FWO) (project G.0415.14N and M.ERA-NET project RADESOL). P. Verstappen and T. Vangerven acknowledge the Agency for Innovation by Science and Technology in Flanders (IWT) for their PhD grants. I. Cardinaletti thanks Hasselt University for her PhD scholarship. The authors are grateful to B. Van Mele, N. Van den Brande and M. Defour for thermal analysis. We further acknowledge Hercules for providing the funding for the LTQ Orbitrap Velos Pro mass spectrometer. Hasselt University and IMO-IMOMEC are partners within the Solliance network, the strategic alliance for research and development in the field of thin-film PV energy in the Eindhoven-Leuven-Aachen region.Notes and references
- Recent reviews on organic solar cells: (a) M. Jørgensen, K. Norrman, S. A. Gevorgyan, T. Tromholt, B. Andreasen and F. C. Krebs, Adv. Mater., 2012, 24, 580 CrossRef PubMed; (b) H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS; (c) Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed; (d) Y. Su, S. Lan and K. Wei, Mater. Today, 2012, 15, 554 CrossRef CAS; (e) R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36 CrossRef; (f) R. A. J. Janssen and J. Nelson, Adv. Mater., 2013, 25, 1847 CrossRef CAS PubMed; (g) S. Lizin, S. Van Passel, E. De Schepper, W. Maes, L. Lutsen, J. Manca and D. Vanderzande, Energy Environ. Sci., 2013, 6, 3136 RSC; (h) T. Xu and L. Yu, Mater. Today, 2014, 17, 11 CrossRef CAS; (i) J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821 CrossRef CAS PubMed; (j) K. A. Mazzio and C. K. Luscombe, Chem. Soc. Rev., 2015, 44, 78 RSC.
- (a) G. Yu, J. Gao, J. Hummelen, F. Wudl and A. Heeger, Science, 1995, 270, 1789 CAS; (b) A. J. Heeger, Adv. Mater., 2014, 26, 10 CrossRef CAS PubMed.
- (a) C. B. Nielsen, S. Holliday, H.-Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803 CrossRef CAS PubMed; (b) G. Sauvé and R. Fernando, J. Phys. Chem. Lett., 2015, 6, 3770 CrossRef PubMed; (c) S. M. McAfee, J. M. Topple, I. G. Hill and G. C. Welch, J. Mater. Chem. A, 2015, 3, 16393 RSC; (d) D. Sun, D. Meng, Y. Cai, B. Fan, Y. Li, W. Jiang, W. Huo, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2015, 137, 11156 CrossRef CAS PubMed; (e) Y.-J. Hwang, B. A. E. Courtright, A. S. Ferreira, S. H. Tolbert and S. A. Jenekhe, Adv. Mater., 2015, 27, 4578 CrossRef CAS PubMed; (f) H. Lin, S. Chen, Z. Li, J. Yuk Lin Lai, G. Yang, T. McAfee, K. Jiang, Y. Li, Y. Liu, H. Hu, J. Zhao, W. Ma, H. Ade and H. Yan, Adv. Mater., 2015, 27, 7299 CrossRef CAS PubMed.
- (a) K. M. Coakley and M. D. McGehee, Chem. Mater., 2004, 16, 4533 CrossRef CAS; (b) S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef PubMed; (c) B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed.
- (a) L. Ye, S. Zhang, W. Zhao, H. Yao and J. Hou, Chem. Mater., 2014, 26, 3603 CrossRef CAS; (b) Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed; (c) Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS; (d) J. Subbiah, B. Purushothaman, M. Chen, T. Qin, M. Gao, D. Vak, F. H. Scholes, X. Chen, S. E. Watkins, G. J. Wilson, A. B. Holmes, W. W. H. Wong and D. J. Jones, Adv. Mater., 2015, 27, 702 CrossRef CAS PubMed; (e) W. Yue, R. Shahid Ashraf, C. B. Nielsen, E. Collado-Fregoso, M. R. Niazi, S. Amber Yousaf, M. Kirkus, H.-Y. Chen, A. Amassian, J. R. Durrant and I. McCulloch, Adv. Mater., 2015, 27, 4702 CrossRef CAS PubMed; (f) G. Pirotte, J. Kesters, P. Verstappen, S. Govaerts, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, ChemSusChem, 2015, 8, 3228 CrossRef CAS PubMed.
- (a) R. L. Uy, S. C. Price and W. You, Macromol. Rapid Commun., 2012, 33, 1162 CrossRef CAS PubMed; (b) M. Jeffries-EL, B. M. Kobilka and B. J. Hale, Macromolecules, 2014, 47, 7253 CrossRef CAS.
- (a) A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020 CrossRef CAS PubMed; (b) Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645 CrossRef CAS PubMed.
- (a) Y. Liu, C.-C. Chen, Z. Hong, J. Gao, Y. M. Yang, H. Zhou, L. Dou, G. Li and Y. Yang, Sci. Rep., 2013, 3, 3356 Search PubMed; (b) A. K. K. Kyaw, D. H. Wang, V. Gupta, J. Zhang, S. Chand, G. C. Bazan and A. J. Heeger, Adv. Mater., 2013, 25, 2397 CrossRef CAS PubMed; (c) B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529 CrossRef CAS PubMed; (d) B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886 CrossRef CAS PubMed; (e) K. Sun, Z. Xiao, S. Lu, W. Zajaczkowski, W. Pisula, E. Hanssen, J. M. White, R. M. Williamson, J. Subbiah, J. Ouyang, A. B. Holmes, W. W. H. Wong and D. J. Jones, Nat. Commun., 2015, 6, 6013 CrossRef CAS PubMed; (f) L. Yuan, Y. Zhao, J. Zhang, Y. Zhang, L. Zhu, K. Lu, W. Yan and Z. Wei, Adv. Mater., 2015, 27, 4229 CrossRef CAS PubMed.
- (a) T. S. van der Poll, J. A. Love, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2012, 24, 3646 CrossRef CAS PubMed; (b) A. K. K. Kyaw, D. H. Wang, V. Gupta, W. L. Leong, L. Ke, G. C. Bazan and A. J. Heeger, ACS Nano, 2013, 7, 4569 CrossRef CAS PubMed; (c) J. E. Coughlin, Z. B. Henson, G. C. Welch and G. Bazan, Acc. Chem. Res., 2014, 47, 257 CrossRef CAS PubMed.
- (a) J. Zhou, Y. Zuo, X. Wan, G. Long, Q. Zhang, W. Ni, Y. Liu, Z. Li, G. He, C. Li, B. Kan, M. Li and Y. Chen, J. Am. Chem. Soc., 2013, 135, 8484 CrossRef CAS PubMed; (b) Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645 CrossRef CAS PubMed.
- (a) J. Min, Y. N. Luponosov, A. Gerl, M. S. Polinskaya, S. M. Peregudova, P. V. Dmitryakov, A. V. Bakirov, M. A. Shcherbina, S. N. Chvalun, S. Grigorian, N. Kaush-Busies, S. A. Ponomarenko, T. Ameri and C. J. Brabec, Adv. Energy Mater., 2014, 5, 1301234 CrossRef; (b) V. S. Gevaerts, E. M. Herzig, M. Kirkus, K. H. Hendriks, M. M. Wienk, J. Perlich, P. Müller-Buschbaum and R. A. J. Janssen, Chem. Mater., 2014, 26, 916 CrossRef CAS; (c) M. Jung, Y. Yoon, J. H. Park, W. Cha, A. Kim, J. Kang, S. Gautam, D. Seo, J. H. Cho, H. Kim, J. Y. Choi, K. H. Chae, K. Kwak, H. J. Son, M. J. Ko, H. Kim, D.-K. Lee, J. Y. Kim, D. H. Choi and B. Kim, ACS Nano, 2014, 8, 5988 CrossRef CAS PubMed; (d) H. Qin, L. Li, F. Guo, S. Su, J. Peng, Y. Cao and X. Peng, Energy Environ. Sci., 2014, 7, 1397 RSC.
- (a) W. R. Mateker, J. D. Douglas, C. Cabanetos, I. T. Sachs-Quintana, J. A. Bartelt, E. T. Hoke, A. El Labban, P. M. Beaujuge, J. M. J. Fréchet and M. D. McGehee, Energy Environ. Sci., 2013, 6, 2529 RSC; (b) J. Kong, S. Song, M. Yoo, G. Y. Lee, O. Kwon, J. K. Park, H. Back, G. Kim, S. H. Lee, H. Suh and K. Lee, Nat. Commun., 2014, 5, 5688 CrossRef CAS PubMed.
- W. L. Leong, G. C. Welch, L. G. Kaake, C. J. Takacs, Y. Sun, G. C. Bazan and A. J. Heeger, Chem. Sci., 2012, 3, 2103 RSC.
- (a) J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS PubMed; (b) A. P. Zoombelt, S. G. J. Mathijssen, M. G. R. Turbiez, M. M. Wienk and R. A. J. Janssen, J. Mater. Chem., 2010, 20, 2240 RSC; (c) S. Albrecht, S. Janietz, W. Schindler, J. Frisch, J. Kurpiers, J. Kniepert, S. Inal, P. Pingel, K. Fostiropoulos, N. Koch and D. J. Neher, J. Am. Chem. Soc., 2012, 134, 14932 CAS; (d) S. Van Mierloo, A. Hadipour, M.-J. Spijkman, N. Van den Brande, B. Ruttens, J. Kesters, J. D'Haen, G. Van Assche, D. M. de Leeuw, T. Aernouts, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, Chem. Mater., 2012, 24, 587 CrossRef CAS; (e) M. Horie, J. Kettle, C.-Y. Yu, L. A. Majewski, S.-W. Chang, J. Kirkpatrick, S. M. Tuladhar, J. Nelson, B. R. Saunders and M. L. Turner, J. Mater. Chem., 2012, 22, 381 RSC.
- (a) W. Vanormelingen, J. Kesters, P. Verstappen, J. Drijkoningen, J. Kudrjasova, S. Koudjina, V. Liégeois, B. Champagne, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, J. Mater. Chem. A, 2014, 2, 7535 RSC; (b) J. Kesters, P. Verstappen, W. Vanormelingen, J. Drijkoningen, T. Vangerven, L. Marin, B. Champagne, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, Sol. Energy Mater. Sol. Cells, 2015, 136, 70 CrossRef CAS.
- (a) H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. S. Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272 CrossRef CAS PubMed; (b) J. C. Bijleveld, R. A. M. Verstrijden, M. M. Wienk and R. A. J. Janssen, J. Mater. Chem., 2011, 21, 9224 RSC; (c) Y. S. Choi and W. H. Jo, Org. Electron., 2013, 14, 1621 CrossRef CAS.
- C.-Y. Kuo, W. Nie, H. Tsai, H.-J. Yen, A. D. Mohite, G. Gupta, A. M. Dattelbaum, D. J. William, K. C. Cha, Y. Yang, L. Wang and H.-L. Wang, Macromolecules, 2014, 47, 1008 CrossRef CAS.
- (a) R. L. Danley, P. A. Caulfield and S. R. Aubuchon, Am. Lab., 2008, 40, 9 CAS; (b) T. Ghoos, N. Van den Brande, M. Defour, J. Brassinne, C.-A. Fustin, J.-F. Gohy, S. Hoeppener, U. S. Schubert, W. Vanormelingen, L. Lutsen, D. J. Vanderzande, B. Van Mele and W. Maes, Eur. Polym. J., 2014, 53, 206 CrossRef CAS.
- B. Ebenhoch, S. A. J. Thomson, K. Genevičius, G. Juška and I. D. W. Samuel, Org. Electron., 2015, 22, 62 CrossRef CAS.
- L. J. A. Koster, V. D. Mihailetchi and P. W. M. Blom, Appl. Phys. Lett., 2006, 88, 052104 CrossRef.
- A. Salleo and R. A. Street, J. Appl. Phys., 2003, 94, 471 CrossRef CAS.
- J.-C. Bolsée and J. Manca, Synth. Met., 2011, 161, 789 CrossRef.
- (a) P. Espinet and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704 CAS; (b) A. E. Rudenko and B. C. Thompson, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 135 CrossRef CAS.
- (a) K. H. Hendriks, W. Li, G. H. L. Heintges, G. W. P. van Pruissen, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2014, 136, 11128 CrossRef CAS PubMed; (b) L. Lu, T. Zheng, T. Xu, D. Zhao and L. Yu, Chem. Mater., 2015, 27, 537 CrossRef CAS; (c) T. Vangerven, P. Verstappen, J. Drijkoningen, W. Dierckx, S. Himmelberger, A. Salleo, D. Vanderzande, W. Maes and J. V. Manca, Chem. Mater., 2015, 27, 3726 CrossRef CAS; (d) J. Kudrjasova, J. Kesters, P. Verstappen, J. Brebels, T. Vangerven, I. Cardinaletti, J. Drijkoningen, H. Penxten, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, J. Mater. Chem. A, 2016, 4, 791 RSC.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Nat. Mater., 2009, 8, 904 CrossRef CAS PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 125204 CrossRef.
- K. R. Graham, P. Erwin, D. Nordlund, K. Vandewal, R. Li, G. O. N. Ndjawa, E. T. Hoke, A. Salleo, M. E. Thompson, M. D. McGehee and A. Amassian, Adv. Mater., 2013, 25, 6076 CrossRef CAS PubMed.
- T. M. Burke, S. Sweetnam, K. Vandewal and M. D. McGehee, Adv. Energy Mater., 2015, 5, 1500123 CrossRef.
- (a) J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, 2nd edn, 2001 Search PubMed; (b) S. Trasatti, Pure Appl. Chem., 1986, 58, 955 CAS.
- P. Verstappen, J. Kesters, W. Vanormelingen, G. H. L. Heintges, J. Drijkoningen, T. Vangerven, L. Marin, S. Koudjina, B. Champagne, J. Manca, L. Lutsen, D. Vanderzande and W. Maes, J. Mater. Chem. A, 2015, 3, 2960 CAS.
- K. Vandewal, L. Goris, I. Haeldermans, M. Nesládek, K. Haenen, P. Wagner and J. V. Manca, Thin Solid Films, 2008, 516, 7135 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06146j |
| This journal is © The Royal Society of Chemistry 2016 |