Soft template induced phase selective synthesis of Fe2O3 nanomagnets: one step towards peroxidase-mimic activity allowing colorimetric sensing of thioglycolic acid†
Anindita
Roy
,
Ramkrishna
Sahoo
,
Chaiti
Ray
,
Soumen
Dutta
and
Tarasankar
Pal
*
Department of Chemistry, Indian Institute of Technology, Kharagpur 721302, India. E-mail: tpal@chem.iitkgp.ernet.in
First published on 22nd March 2016
Abstract
Different Schiff's base (SB) templates have been obtained to mediate phase selective synthesis of α- or γ-Fe2O3 from the same temperature condition. The inherent phase transformation tendency (γ-Fe2O3 to α-Fe2O3) is found to be inhibited. Again, the phase selective evolution emerges out of the binding modes of SBs which has been illustrated here. The nature of SBs governs the shape and size distribution of Fe2O3 NPs. As both the phases are magenetically active, easy magnetic separation of them widens their applicability in catalysis. Magnetically active both α- and γ-Fe2O3 mimic interesting peroxidase like activity by oxidising colourless 3,3′,5,5′-tetramethylbenzidine (TMB) to blue coloured oxidised product (Ox-TMB) in aqueous H2O2. These results prompted us to improvise further to detect thioglycolic acid (TGA) at a micromolar level which provides commercial applicability.
Introduction
Iron oxides occur ubiquitously in environmental, geological, planetary, and technological settings. Amongst the polymorphs of ferric oxides, hematite (α-Fe2O3) is the most stable form and being an n-type semiconductor, it attracts a great deal of attention for water treatment, catalysis, electrocatalysis, energy storage and gas sensing.1 However; the antiferromagnetic (AF) nature of α-Fe2O3 restricts the application pertaining to its separation with an external magnet. The importance of a magnetically separable catalyst finds innumerable applications in biology, radiology, therapeutics, electronics etc.2 Hence, there comes the usage of maghemite i.e. γ-Fe2O3 in different fields. Being cation-deficient spinel (Fd3), γ-Fe2O3 shows ferromagnetism and the charge neutrality is rendered by cation vacancies, with a Curie temperature (TC) of 928 K. Due to canted AF property, rhombohedral α-Fe2O3 (R3c), below ∼260 K (Morin temperature-TM), shows weak ferromagnetism (WF) and that is explained by the Dzyaloshinsky3–Moriya4 mechanism (TC 950 K). Tailoring of magnetic property of nanocrystals relates to well-defined size, shape and self-assembly which has become a topic of intensive research because of the enormous scope for applications of these materials as storage media, biomedical devices, optical devices, sensors and magnetic fluids. Transformation of magnetically active meta-stable γ-Fe2O3 into the most stable magnetically inactive α-Fe2O3 is a consequence of temperature, time domain and precursor salt variations.5 Now-a-days, template assisted synthesis has become very popular for obtaining monodispersed crystalline iron oxide nanoparticles (NPs). Among these, carbon NPs,6 carbon nanotubes (CNTs),7 mesoporous silica8etc. are successfully employed as the well suited templates. Self-aggregation of NPs can be prevented in the templates due to compartmentalization of the particles within the restricted domain of the template cavity.In case of hard template mediated multistep synthesis,5 Ohkoshi et al. showed that template cavity helps the oxidation of the precursor under confinement at high temperature (∼600–1200 °C) and subsequent template dissolution finally set free the desired oxides. In this regard, iron complexes like acetylacetonates, acetates, oleates,2 iron containing different metal–organic framework, ferritin proteins etc. are used extensively as template2 which upon controlled pyrolysis produce template free magnetic NPs. Using specially designed three-dimensional mesoporous silica (KIT-6), Bruce et al.9 showed the synthesis of different mesoporous iron oxides at different reaction conditions (150–650 °C). A conceptually new strategy has been adopted by Wei et al.10 for growing atomically thin α-Fe2O3 nanomaterial by bringing in transition metal oxide (TMO) sheet as host material. Again, biological template assisted synthesis of iron oxide NPs has also found their relevance towards synthesis as well as biocompatibility.2
Herein, understanding the great utility of template assisted synthesis, Schiff base (SB), a condensation product of aldehyde and amine, draws our attention to improvise a prospective template. Considering the donor–acceptor property of SB, iron(II/III) chelation is evident and then NPs stabilization is realized in the subsequent steps. Thus, four different SBs, generated from the permutative combination of two different aldehydes (glyoxal and glutaraldehyde) and two different amines (ethylenediamine and triethylenetetramine) have been employed to serve the purpose of template for the synthesis of morphologically different iron oxide. Mohr's salt was used as iron source and hydrazine as hydrolyzing reagent (Scheme 1) at a refluxing temperature of 373 K. Under this condition, morphologically different Fe2O3 NPs were produced in 1 h time as a consequence of dehydration.11
 | ||
| Scheme 1 Schematic illustration of Schiff base template mediated synthesis of various phases of Fe2O3 NPs using Mohr's salt as precursor salt. | ||
The as-synthesised magnetic nanoparticles (MNPs) exhibit property as peroxidase-mimicking agent catalysing the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) to blue coloured oxidised product (Ox-TMB) in presence of H2O2 which has similar activity of horseradish peroxidase (HRP), a natural enzyme. Moreover, these enzyme-mimicking nanomaterials or nanozymes show several advantages over natural enzymes, such as cost effectiveness, tuneable catalytic activities, and high stability against rigorous conditions, thus being used in various ways and means.12,13 Utility of Fe3O4 as magnetic peroxidase-like nanozyme follows a well known pathway considering Fenton's catalysis.14 But Fe2O3 lacks this ability due to insignificant proportion of Fe2+ ions at the NPs surface. Here the SB template mediated growth of Fe2O3 MNPs leaves a higher propensity of Fe2+ ions at the surface transcending it into an effective peroxidase-like nanozyme. Meanwhile, because of the magnetic nature the nanozyme can easily be separated, concentrated and thus reusability of MNPs also popularise the system towards TMB and H2O2 detection. In general, to evade the sophisticated instrumentation and naked eye detection of different analytes, the colorimetric detection technique is well accepted.15–19 So, we further extent our TMB oxidation catalysis on colorimetric sensing of thioglycolic acid (TGA) applying the peroxidase mimicking property of Fe2O3.
Experimental section
Materials used and instrumentation are described in ESI as S1 and S2† respectively.Synthesis
Well defined α- and γ-phases of Fe2O3 NPs were synthesized by impregnation of Fe2+ precursor; Mohr's salt into SB templates followed by hydrolysis of iron-SB templates using hydrazine as hydrolyzing agent. The general strategy can be described as follows. An aliquot of the chosen aldehyde and amine (5 × 10−2 M) were mixed together in 10 ml of ethanol. The mixture was stirred for ∼1 h using a magnetic stirrer to produce SB template (Fig. S2a:† FTIR gives the evidence of prepared Schiff base). After 1 h of stirring, 10 ml of ethanolic solution was mixed with 90 ml aqueous Mohr's salt solution (5 × 10−2 M) to obtain an effective reaction mixture. Then 0.5 ml hydrazine hydrate (0.16 M) was added into the reaction mixture and the solution was refluxed, until (∼1 h) the supernatant became colourless. TemFe A (from glyoxal and ethylenediamine), TemFe B (from glutaraldehyde and ethylenediamine), TemFe C (from glyoxal and triethylenetetramine) and TemFe D (from glutaraldehyde and triethylenetetramine) were prepared by the same procedure. After the preparation of four sets, the products were thoroughly washed with ethanol, water and finally separated simply by employing an external door magnet. Thorough washing of the product removed all the adhering remnants from the surface making it pure enough for further characterisation and application in catalysis.Catalytic oxidation of TMB
The peroxidase-like catalytic activities of both α- or γ-Fe2O3 are described out of the oxidation of TMB in presence of H2O2. In this study, 25 μl (10−2 M) ethanolic solution of TMB was oxidised by 100 μl (30%) H2O2 in presence of 0.1 mg of dispersed catalyst in acetate buffer medium (pH = 4.0). The steady state kinetic study has been carried out at room temperature (RT). The UV-vis spectra have been recorded at 5 minute interval at RT for performing the kinetic study. With completion of the reaction, colourless TMB solution has been found to be oxidised to blue coloured oxidised product Ox-TMB which generated a broad peak at 652 nm. Thus, formation of Ox-TMB leads to study kinetically following spectroscopic observation. The Michaelis–Menten parameter has been determined from double reciprocal Lineweaver–Burk plot.| 1/V0 = 1/Vmax + Km/Vmax[S0] | (1) |
Detection of thioglycolic acid (TGA) concentration
In a general procedure, freshly prepared aqueous thioglycolic acid (TGA) solution (0.033–0.67 mM) was introduced into the previously mentioned reaction mixture containing TMB. Keeping other reagents unaltered, we only varied the TGA solution in the reaction mixture maintaining the final volume (3 ml) constant. Successive colour variation, arising from the concentration gradient of TGA solution, was studied thoroughly by UV-vis spectral measurements.Results and discussion
The crystal structure, shape, size and size distribution of NPs is the resultant effect of controlled crystallisation process. The theoretical approach to understand the mechanism of crystal formation has been explained. Lowering the high Gibbs' free energy in supersaturated solution thermodynamically favours the formation of nucleation and growth of crystal. The Gibbs' free energy per unit volume of the solid phase (ΔGv) is dependent on the concentration of the solute and is represented as, | (2) |
| r* = −2γ/ΔGv | (3) |
Nucleation is the key in progress of crystallisation.20 Under refluxing condition, large numbers of nuclei are formed and remain homogeneously distributed throughout the bulk solution. Cao et al. also enriched the crystallisation mechanism by proposing the stronger effect of bubble formed during this refluxing condition in nucleation process, thus crystal formation takes place.21 So, refluxing condition leads to high-yielding route to synthesis NPs. In our case, the refluxing condition for an hour and the N2 bubble formed from the decomposition of hydrazine effects the crystallisation process by formation of large number of nucleation sites in the reaction medium. This facile synthesis leads to gram level production of phase pure crystalline γ-Fe2O3 or α-Fe2O3, depending upon the variable SB templates' nature. Oxidation of Fe2+ to Fe3+ is a spontaneous process in the ambient condition. Considering Mohr's salt as precursor, we wished to control the transformation of Fe2+ ions, which further act as catalyst showing peroxidase-like activity. The effect of SB templates in the phase specific formation of Fe2O3 has been elaborated here. Advantageous imine group in SBs accelerates the hydrolysis of the metal hydroxide.22 This supports the benefit of SB template for the synthesis of Fe2O3 nanoparticles under refluxing condition. The PXRD patterns of TemFe A, B, C (see ESI, PXRD pattern of TemFe B and C; Fig. S1†) were indexed to γ-Fe2O3 justified with JCPDS file no. 04-0755. For TemFe D, the PXRD pattern suggests it as α-Fe2O3, supported by JCPDS file no. 84-0311 (Fig. 1).
 | ||
| Fig. 1 PXRD patterns of TemFe A and TemFe D, represent existence of γ-Fe2O3 and α-Fe2O3 respectively. | ||
The sharp peaks indicate the crystallinity of α- or γ-Fe2O3. Absence of other peaks confirm the phase purity in the system. In the present investigation, we find that the nature of the SB governs the formation of specific phases along with definite size distribution of particles. The preferential adsorption of four different SBs with different iminic bonding motif could alter the surface energy and restricts the crystal growth in certain orientation by attachment of ions, introducing formation of different phases. There is a natural tendency of greater interaction of metal ion with tridentate imine ligands. Thus, the hydrolysis is acceletared locally in presence of the imine group attached to the iron ion. So, facile formation of maghemite occurs in case of TemFe A, B and C. But in case of TemFe D, due to the larger chain length of both aldehyde and amine used in SB (TemD), particle size increases which leads to the formation of most stable hematite phase. With increase in particle size preferential nucleation to form thermodynamically stable hematite phase occur due to the nanomization of the free surface energy.23 Also tenacious hold of water on the surface of nanoparticle leads to the formation of hematite.24 Increasing the size of template, depending upon the chain length of aldehydes and amines in the SB skeletons, the particle size increases which is confirmed from the FESEM (Fig. S3†) and TEM (Fig. 2) images. The average particle size of TemFe A and B lies within 20–50 nm, whereas for TemFe C it varies from 100–120 nm and for nanorod (TemFe D), the length is about 400–500 nm with an aspect ratio ∼20. Larger available free space of the templates support surface energy driven oriented attachment25 of the evolved Fe2O3 nanoparticles. Fringe spacing in Fig. 2 shows that TemFe A has plane orientation along (311) and that of TemFe D is (116) proven by presence of fringe spacing 0.25 nm and 0.16 nm respectively. The crystalline nature of α-Fe2O3 is also supported by the hexagonal bright spot in selected area electron diffraction (SAED) pattern (Fig. 2). Formation of rings in the SAED patterns (Fig. 2) of the γ-Fe2O3 signifies the polycrystalline nature.
FTIR analysis (Fig. S2b†) was performed to confirm the formation of different phases of iron oxide NPs illustrated by the vibrational modes. Generally in maghemite, being inverse spinel, half of the Fe3+ ions occupy the tetrahedral interstices and remaining half of Fe3+ in octahedral sites whereas in hematite each Fe3+ occupy the octahedral coordination site. Vibration of Fe3+ ions in tetrahedral positions was attributed to the high frequency band and low frequency band was attributed to the vibration of Fe3+ ions in octahedral positions.26
According to Waldron,26 the band appeared at 350–450 cm−1 is attributed to the stretching vibration of Fe3+–O2− in the octahedral complexes and at 500–600 cm−1 to that of bending vibrations in tetrahedral complexes. The presence of intense peak at 500–600 regions confirms the presence of Fe3+ ions in tetrahedral position which supports the formation of γ-Fe2O3 in case of TemFe A, B and C. Again absence of such intense peak at that region in TemFe D also suggests the evolution of α-Fe2O3. This association of SB template together with Fe2O3 was established from the shift of imine stretching frequency (Fig. S2b†) towards lower frequency region (1556 and 1628 cm−1) which is due to the lowering in bond order in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N because of the drifted electronic cloud towards Fe3+ ions. This suggests the formation of coordination bond between imine π-electron cloud and Fe3+ ion. The tenacious adsorption of SBs as protective layer stabilises the meta stable γ-Fe2O3. All these stretching vibrations in FTIR analysis (Fig. S2b†) are attributed to fingerprint vibrational modes of SBs, indicating their adherence at the surface of the NPs.
N because of the drifted electronic cloud towards Fe3+ ions. This suggests the formation of coordination bond between imine π-electron cloud and Fe3+ ion. The tenacious adsorption of SBs as protective layer stabilises the meta stable γ-Fe2O3. All these stretching vibrations in FTIR analysis (Fig. S2b†) are attributed to fingerprint vibrational modes of SBs, indicating their adherence at the surface of the NPs.
The XPS spectra (Fig. S4†) have been measured considering adventitious carbon (284.0 eV) as reference material. The XPS peak position at 711.0 eV (Fe2p3/2), 724.0 eV (Fe2p1/2) and 529.0 eV (O1s) confirms the presence of Fe3+ and O2− in Fe2O3. We also observed the presence of Fe2+ (709.6 eV) ions in as-synthesised TemFes after deconvulation of the XPS spectra. The peak area ratio of Fe2+ and Fe3+ has also been obtained from the deconvulated XPS peak (Fig. 3) of iron. The [Fe2+]/[Fe3+] of TemFe A, B, C and D are calculated from the peak area respectively. Thus, the surface attached Fe2+ ions and their relative concentration helps to establish the mechanism and increasing order of the catalysis. The data related to deconvulated XPS peaks for TemFe A, B, C and D are given in ESI, Table S1.†
Though the [Fe2+]/[Fe3+] of TemFe B and D were similar; but the critical observation of deconvulated peak position of TemFe D denotes that the peak corresponds to Fe2+ (709.0) is shifted to 710.0 eV which reflects the formation of Fe3+ ion. So, the order of the catalysis is justified. Because the peak position denotes that conversion from Fe2+ to Fe+ took place. So, absence of Fe2+ ions lowers the catalytic activity of TemFe D as peroxidase-mimic catalyst.
Magnetic property
Presence of magnetic domain in material is responsible for magnetic property in them. As the particle size decreases to nanoregime, tinier than the magnetic domain; NPs are considered to be completely magnetised.27 Because in nanoregime the multi-magnetic-domain organization is energetically unfavourable, thus particles with single-magnetic-domain are formed.28 The term “superparamagnetism” is not very accurate but is commonly used to emphasis the magnetic behaviour for paramagnetic nanoparticles which arises from the coupling of several thousands of atoms in comparison to the paramagnetism of a single atom. This magnetization can reach nearly the saturation magnetization of ferromagnetic iron oxide, but in contrast to ferromagnetic iron the particles no longer show magnetic interactions after elimination of the magnetic field. According to the theory of magnetism for exhibiting superparamagnetic behaviour, required minimum NP volume (VP) is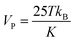 | (4) |
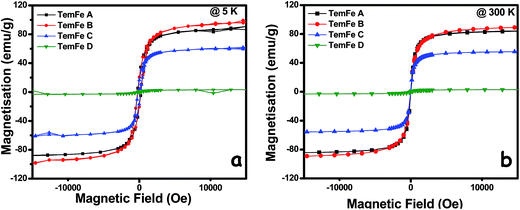 | ||
| Fig. 4 Comparison of saturation magnetization (MS) data from M − H curves of as-synthesised TemFe A, B, C and D at both 5 K and 300 K. | ||
| Sample (2 T) | Saturation magnetisation (MS) (emu g−1) | Remnant magnetisation (MR) (emu g−1) | Coercive field (Oe) | Squareness (MR/MS) | Nature | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5 K | 300 K | 5 K | 300 K | 5 K | 300 K | 5 K | 300 K | ||
| TemFe A | 90.44 | 83.63 | 19.6 | — | 255 | — | 0.217 | — | γ-Fe2O3 |
| TemFe B | 97.32 | 89.2 | 20.3 | — | 234 | — | 0.208 | — | γ-Fe2O3 |
| TemFe C | 60.62 | 55.64 | 16.6 | — | 182 | — | 0.274 | — | γ-Fe2O3 |
| TemFe D | 3.73 | 3.74 | 0.89 | — | 402 | — | 0.238 | — | α-Fe2O3 |
In rhombohedral hexagonal crystal of hematite, Fe3+ occupy the octahedral coordination site, making it magnetically inactive, whereas in maghemite, the unequal distribution of Fe3+ in octahedral and tetrahedral voids in inverse spinel structure arouse net magnetisation. The natural tendency of magnetisation in nanoparticle is less than that of their massive parent materials. The reason behind such reduction in magnetization in NPs is often attributed to the finite size effects such as surface spin disorder, spin canting as well as magnetically dead layer on the surface of synthesized NPs.32 But here in nanoregime, the inherited magnetic property of γ-Fe2O3 is enhanced by the presence of uncompensated surface spin at grain boundary region. The uncompensated surface spins can overcome the AF property of the core part, so α-Fe2O3 NPs can also exhibit a net magnetisation which is reported here. The concentration of unsaturated spins on the surface of NPs depends on the size and shape of NPs which is modulated by the variable SB templates.
The anisotropy factor in eqn (5) also explains the enhanced magnetic property.
| Ea = KaV | (5) |
In the present investigation for all synthesized NPs, magnetic aggregation is almost absent which is in agreement with the individual particle coating and the pseudo self-assembly due to the presence of the various SB templates. So, ceasing the spin alignment with variation of temperature is observed. Thus, there is no distinct change in saturation magnetisation occurs amongst any of these TemFes at 5 and 300 K respectively. Presence of greater anisotropy in both magnetic and morphological nature of nanorod than in sphere causes spin alignment to generate net magnetism. The convergent nature of zero-field-cooling (ZFC) and field-cooling (FC) curves (Fig. S7†) were obtained under 100 Oe magnetic fields within the temperature window 5–300 K.
The soft paramagnetic nature of all template-directed NPs has been explained both by M − H curve and ZFC–FC curve. Being soft magnetic materials, it is easy to align the magnetic domains in a definite direction. We observed that AF hematite (TemFe D) also shows unnatural saturation magnetisation (MS) 3.74 emu g−1 at RT. The WF property associated with (TemFe D) α-Fe2O3 can be explained by first-order transition observed at TM ∼ 110 °C, in Fig. S7,† which is much less than that of bulk α-Fe2O3 (263 K) in Dzyaloshinsky–Moriya3,4 mechanisms. In this transition, the spin-flip occurs from the spin orientation along the symmetry axis to perpendicular direction with a canted angle giving rise to weak ferromagnetism. Decrease in TM with rise in temperature attributed to the anisotropy effect, lattice strain, crystal defect etc. occurred in α-Fe2O3.33
The decrease in MS of γ-Fe2O3 with temperature increase can be illustrated due to thermal fluctuations, resulting disturbance in spin alignment. But the unchanged MS of α-Fe2O3 can be supported by change in spin-axis from c-axis to c-plane due to increase in thermal fluctuations. There is also a possibility of self-grown oxygen vacancies, destroying the AF interactions between the sublattices,34 results in net magnetism for α-Fe2O3. Increased temperature facilitates the transition of large fraction of clusters from ferromagnetic to superparamagnetic. The soft nature of the entire template stabilized Fe2O3 at RT can also be explained by the squareness value. In absence of template, the as-synthesised γ-Fe2O3 showed as usual saturation magnetisation of ∼68 and ∼60 emu g−1 respectively at 5 K and 300 K (Fig. S5†), which draws special attention emphasising the template mediated synthesis technique.
Peroxidase-like activity
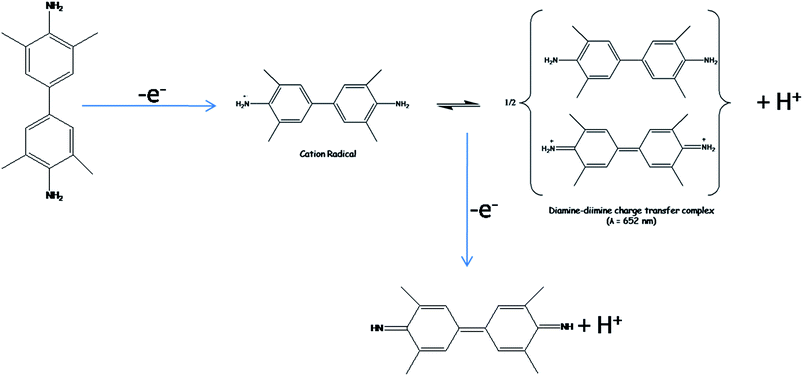
To observe the natural enzyme mimic property of catalyst, TMB is used as a chromogenic substance. In presence of H2O2, our as-synthesised TemFes catalyse the oxidation of TMB to produce blue-coloured Ox-TMB at RT. This crucial finding makes the system very important for analytical purposes which are enhanced by the magnetic separation technique. We have performed the catalysis with all of the TemFes and SB template free γ-Fe2O3. We found that amongst all catalyst variation, TemFe A act as best catalyst towards TMB oxidation reaction. This can be explained by the size variation of the catalysts. With the smallest size, the boon of high surface to volume ratio comes into the picture and the phenomenon is also true in this case, which is observed from the rate of TMB oxidation. Generally Fe2+ ions are responsible for the Fenton's reaction with H2O2, which here drives the catalysis of TMB oxidation. The enhanced presence of surface-attached Fe2+ ions in our synthesised catalyst surface due to the SB template which is directly related with the small size of catalyst, widen the probability of catalysis. Amongst all TemFes, the order of catalysis was found; TemFe A > TemFe B > TemFe C > TemFe D (Fig. S8†).In presence of 0.1 mg catalyst (1 mg dispersed in 1 ml H2O), 0.1 ml (30%) H2O2 in acetate buffer medium (pH = 4.0) efficiently oxidises (0.025 ml, 10−2 M) TMB to blue-coloured Ox-TMB generating a peak at 652 nm at RT. The oxidised TMB products become stable at acidic pH condition. The partially oxidised, one electron, TMB produces cation radical species which is in equilibrium with the diamine–diamine species forming blue coloured charge transfer complex (∼652 nm), is stable at pH = 4.0. Upon increasing the pH, the stability of the spectroscopic observable peak (∼652 nm) decreases and thus we observe a decrease in intensity. The mechanism of oxidation of TMB35 is given in Fig. 5.
Kinetic study with the variation of both TMB concentration (80–320 μM) and H2O2 concentration (40–80 mM) has been performed with TemFe A, relatively better catalyst than other, establishing its peroxidase-like property (Fig. 6).
Mechanism of peroxidase-like activity
The peroxidase-like catalytic activity in TemFe may be generated from its surface-attached Fe2+ ions. The mechanism may follow Fenton's reaction. The reaction can be written as (6) and (7), where k1 and k2 are reaction rate constants.| Fe2+ + H2O2 = Fe3+ + ˙OH + OH−; k1 = 76 l mol−1 S−1 | (6) |
| Fe3+ + H2O2 = Fe2+ + ˙OOH + H+; k2 = 0.002 l mol−1 S−1 | (7) |
Step (7) has a low rate constant and thus become a rate-limiting reaction process. So, the possible reaction mechanism can be elaborated following two cascade steps. At first, H2O2 get attached to the surface of the catalyst. Then Fenton's reaction takes place at the NPs surface. The Fe2+ ion generates the highly active ˙OH (hydroxyl radical) species which catalyses the oxidation of TMB.36 After oxidation, the blue-coloured Ox-TMB has been thoroughly investigated spectroscopically. To justify the proposed mechanistic pathway, we have also performed oxidation of terephthalic acid (TA) by H2O2 under same experimental condition in presence of catalyst. Though TA is a non-fluorescent compound, but reaction with highly reactive ˙OH radical, it generates 2-hydroxyterephthalic acid (HTA) which is a highly fluorescent compound.37 In presence of catalyst, we observed enhanced fluorescence due to HTA which justifies the enhanced production of ˙OH radical in presence of catalyst (Fig. S9†). Thus, ˙OH radical mediated mechanism for the oxidation of TMB with catalyst has been justified unequivocally. A direct surface attached Fe2+ concentration dependent reaction mechanism for TMB oxidation catalysis can be explained explicitly from the XPS spectra (Fig. 3). The [Fe2+]/[Fe3+] ratio give the conclusive explanation of higher catalytic activity of TemFe A with respect to others when the TMB oxidation reaction goes according to Fenton's reaction as proposed. In presence of H2O2 and TemFe catalyst in acetate buffer (pH = 4.0) medium, TMB catalytically oxidises into blue-coloured Ox-TMB arising a peak at ∼652 nm in 20 minutes time duration. It is important that presence of both catalyst and H2O2 can oxidise TMB in this reaction condition. In absence of any of these, oxidation of TMB into Ox-TMB has become futile. It was also observed that rate of oxidation is dependent upon the concentration of H2O2. So, we can also estimate spectroscopically the concentration of H2O2 observing the catalytic oxidation of TMB.
Steady state kinetic study
The steady state kinetics of TMB oxidation has thoroughly done by using TemFe A as catalyst at RT keeping the concentration of one substrate constant during the variation of the concentration of other substrate. The kinetic study of this nanozymatic TMB oxidation reaction has been studied thoroughly using UV-vis spectrophotometer. Following the Lambert–Beer's law, the concentration of the Ox-TMB product with the molar extinction coefficient of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 is calculated from the absorbance value obtained from the UV-vis spectra with time variation. The obtained data was observed to well-fit into Michaelis–Menten equation for both the substrate, TMB and H2O2 (Fig. S10†).
000 M−1 cm−1 is calculated from the absorbance value obtained from the UV-vis spectra with time variation. The obtained data was observed to well-fit into Michaelis–Menten equation for both the substrate, TMB and H2O2 (Fig. S10†). | (8) |
The Michaelis–Menten equation describes the relationship between the rate of substrate conversion by an enzyme and the concentration of the substrate. In this equation, V is the velocity or rate of conversion, Vmax is the maximum rate of conversion, [S] is the substrate concentration, and Km is the Michaelis–Menten constant.
V max is the direct parameter for ensuring the enzymatic catalytic activity. Km is also an important parameter in enzymatic reaction to define the binding affinity of substrate and enzyme. Lower the value of Km, higher is the binding affinity of substrate towards corresponding enzyme and vice versa. We have also determined the Km and Vmax values of both individual substrate, H2O2 and TMB, considering TemFe A as enzyme mimic catalyst from double reciprocal Lineweaver–Burk plot (Fig. 6a and b). Comparison of the Km values between horseradish peroxidase (HRP)38 and TemFe A has been tabled (Table 2) out.
| Catalyst | TMB | H2O2 | ||
|---|---|---|---|---|
| K m (mM) | V max (M s−1) | K m (mM) | V max (M s−1) | |
| TemFe A | 0.0887 | 0.97 × 10−8 | 157.19 | 1.284 × 10−8 |
| HRP | 5.90 | 1.83 × 10−6 | 0.63 | 0.225 × 10−6 |
This value helped us for discussing about peroxidase mimicking activity of as-synthesised catalyst.
The Km value calculated for TMB as substrate using TemFe A 0.0887 is much less than that of HRP 5.9. This shows the higher affinity of TemFe A towards TMB with respect to HRP, a natural enzyme. This result can be explained by the presence of more number of active sites at the surface of TemFe A whereas only one iron site is present in natural enzyme, HRP. Magnetically active TemFe A can also be separated from the reaction medium using simply an external magnet which also increases the efficiency. But we observed significantly higher Km value for H2O2 using TemFe A with respect to HRP. This describes that higher concentration of H2O2 is required to achieve maximal catalytic activity. We have calculated the limit of detection (LOD) for both TMB and H2O2 by varying TMB concentration from 0–0.32 mM and H2O2 concentration from 0–80 mM respectively.
Thioglycolic acid (TGA) detection
Thioglycolic acid (TGA) or mercaptoacetic acid (MAA) has commercial importance from drug to cosmetic industries due its applications as chemical depilatory, hair perming agent etc.39 Among various compounds which are applicable as relaxers for hard hair (e.g. cysteine, cysteamine etc.), TGA was found to possess greater efficiency in terms of reduction potential at all pH values. The chemistry behind its activity is the tendency of breaking of disulfide bond easily. The whole hardening and softening process of hair has been elaborated as follows:| K–S–S–K + 2R–SH → 2K–SH + R–S–S–R | (9) |
| K–SH + O2 → K–S–S–K + H2O | (10) |
Mechanism
TGA has a redox active thiol side linkage which is prone to oxidation to form disulphide linkage spontaneously. In this study, the presence of TGA hinders the usual oxidation of TMB by H2O2 to blue coloured Ox-TMB and leads to a path for sensing TGA. The hindrance in blue colour evolution occurs due to the thermodynamically more spontaneous oxidation of TGA by H2O2 instead of TMB. The proclamation of feasibility of the oxidation of TGA instead of TMB by H2O2 is explained by the redox potential values, shown in Table 3.43Thus, thermodynamics drives the oxidation of TGA to generate disulphide linkage by H2O2 in presence of TMB. The phenomenon is TGA concentration dependent.
Thus, with increase in concentration of TGA, tendency of formation of blue coloured Ox-TMB decreases. So, concentration gradient of TGA is responsible for the UV-vis spectra variation and a calibration curve can easily be obtained. Thus, leads to its application for colorimetric sensing of TGA.
Conclusion
In conclusion, different magnetically active phase pure α- and γ-Fe2O3 synthesis at gram level has been illustrated without entertaining temperature change and tedious hard template dissolution strategies. The main attraction of this work lies in the fact that the synthesis of varying morphology, phase and magnetism, of all the Fe2O3 are described simply as the exposition of the nature of SB templates with variable chelating imine donor sites and chain length variation keeping other factors constant. Again SB-modified α- and γ-Fe2O3 driven peroxidase mimicking activity leads to the catalysis of oxidation of TMB. This fact leads to the importance of TemFes in analytical usage of colorimetric TGA sensing with much improved magnetic character. Thus, transcending the traditional barrier of templates, SBs as soft template will play an important role in near future.Acknowledgements
CSIR and DST, New Delhi and Indian Institute of Technology, Kharagpur are greatly acknowledged for financial and instrumental support. We are grateful to Prof. A. Sundaresan and Mr Chandan Dey (JNCSAR) for their generous support in the magnetic data measurement.References
- V. Urbanova, M. Magro, A. Gedanken, D. Baratella, F. Vianello and R. Zboril, Chem. Mater., 2014, 26, 6653 CrossRef CAS.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS PubMed.
- I. Dzyaloshinsky, J. Phys. Chem. Solids, 1958, 4, 241 CrossRef CAS.
- T. Moriya, Phys. Rev., 1960, 120, 91 CrossRef CAS.
- S. Sakurai, A. Namai, K. Hashimoto and S. Ohkoshi, J. Am. Chem. Soc., 2009, 131, 18299 CrossRef CAS PubMed.
- J. H. Bang and K. S. Suslick, J. Am. Chem. Soc., 2007, 129, 2242 CrossRef CAS PubMed.
- Z. Sun, H. Yuan, Z. Liu, B. Han and X. Zhang, Adv. Mater., 2005, 17, 2993 CrossRef CAS.
- K. V. Manukyan, Y. S. Chen, S. R. P. Li, X. Li, S. Dong, X. Liu, J. K. Furdyna, A. Orlov, G. H. Bernstein, W. Porod, S. Roslyakov and A. Mukasyan, J. Phys. Chem. C, 2014, 118, 16264 CAS.
- F. Jiao, J. Jumas, M. Womes, A. V. Chadwick, A. Harrison and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS PubMed.
- W. Cheng, J. He, T. Yao, Z. Sun, Y. Jiang, Q. Liu, S. Jiang, F. Hu, Z. Xie, B. He, W. Yan and S. Wei, J. Am. Chem. Soc., 2014, 136, 10393 CrossRef CAS PubMed.
- Z. X. Deng, C. Wang, X. M. Sun and Y. D. Li, Inorg. Chem., 2002, 41, 869 CrossRef CAS PubMed.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060 RSC.
- W. He, W. Wamer, Q. Xia, J. J. Yin and P. P. Fu, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2014, 32, 186 CrossRef CAS PubMed.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang and S. Perrett, Nat. Nanotechnol., 2007, 2, 577 CrossRef CAS PubMed.
- S. Wang, Z. Chen, J. Choo and L. Chen, Anal. Bioanal. Chem., 2016, 408, 1015 CrossRef CAS PubMed.
- Z. Zhang, J. Zhang, C. Qu, D. Pan, Z. Chen and L. Chen, Analyst, 2012, 137, 2682 RSC.
- L. Chen, X. Fu, W. Lu and L. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 284 CAS.
- Z. Chen, R. Liu, S. Wang, C. Qu, L. Chen and Z. Wang, RSC Adv., 2013, 3, 13318 RSC.
- Z. Zhang, Z. Chen, C. Qu and L. Chen, Langmuir, 2014, 30, 3625 CrossRef CAS PubMed.
- V. K. Lamer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS.
- J. Lynch, J. Zhuang, T. Wang, D. LaMontagne, H. Wu and Y. C. Cao, J. Am. Chem. Soc., 2011, 133, 12664 CrossRef CAS PubMed.
- C. Castro, J. Ramos, A. Millán, J. González-Calbet and F. Palacio, Chem. Mater., 2000, 12, 3681 CrossRef CAS.
- A. Navrotsky, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12096 CrossRef CAS PubMed.
- M. Lin, H. R. Tan, J. P. Y. Tan and S. Bai, J. Phys. Chem. C, 2013, 117, 11242 CAS.
- S. Mitra, S. Das, K. Mandal and S. Chaudhuri, Nanotechnology, 2007, 18, 275608 CrossRef.
- R. D. Waldron, Phys. Rev., 1955, 99, 1727 CrossRef CAS.
- F. Jiao, J. Jumas, M. Womes, A. V. Chadwick, A. Harrison and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS PubMed.
- N. Amin, S. Arajs and E. Matijevic, Phys. Status Solidi A, 1985, 104, K65 CrossRef.
- B. D. Cullity and C. D. Graham, Introduction to Magnetic Materials, 2nd edn, 2008, ISBN: 978-0-471-47741 Search PubMed.
- A. B. Salunkhe, V. M. Khot, J. M. Ruso and S. I. Patil, RSC Adv., 2015, 5, 18420 RSC.
- T. Sugano, M. Nomura, K. Awaga, L. K. Poh, T. Ohta and M. Kinoshita, Bull. Chem. Soc. Jpn., 1986, 59, 2615 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schth, Angew. Chem., 2007, 46, 1222 CrossRef CAS PubMed.
- M. Nidhin, M. Vedhanayagam, S. Sangeetha, M. S. Kiran, S. S. Nazeer, R. S. Jayasree, K. J. Sreeram and B. U. Nair, Nat. Sci. Rep., 2014, 4, 5968 CAS.
- Y. L. Chueh, M. W. Lai, J. Q. Liang, L. J. Chou and Z. L. Wang, Adv. Funct. Mater., 2006, 16, 2243 CrossRef CAS.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 1982, 257, 3669 CAS.
- X. Sun, S. Guo, C. S. Chung, W. Zhu and S. Sun, Adv. Mater., 2013, 25, 132 CrossRef CAS PubMed.
- S. Dutta, C. Ray, S. Mallick, S. Sarkar, R. Sahoo, Y. Negishi and T. Pal, J. Phys. Chem. C, 2015, 119, 23790 CAS.
- C. Lu, X. Li, Y. Liu, F. Yu, L. Tang, Y. Hu and Y. Ying, ACS Appl. Mater. Interfaces, 2015, 7, 15395 CAS.
- Y. Labat, Thioglycolic Acid. Kirk-Othmer Encyclopedia of Chemical Technology, 2000, DOI:10.1002/0471238961.2008091512010201.a01.
- D. Oikawa, W. Takeuchi, S. Murata, K. Takahashi and Y. Sekine, J. Occup. Health, 2013, 54, 370 CrossRef.
- C. Buffet and J. Rao, Skin Therapy Letter, 2013, 9, 8 Search PubMed.
- N. Xie, X. Ding, X. Wanga, P. Wanga, S. Zhao and Z. Wang, J. Pharm. Biomed. Anal., 2014, 88, 509 CrossRef CAS PubMed.
- L. D. Freedman and A. H. Corwin, J. Biol. Chem., 1949, 181, 601 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, FTIR, FESEM, XPS spectra, UV-vis spectra, ZFC-FC, M − H curve of TemFes and template free γ-Fe2O3. See DOI: 10.1039/c6ra00963h |
| This journal is © The Royal Society of Chemistry 2016 |