Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible lithiation gradients of bulk MoS2 in lithium-ion coin cells†
Alexandar D. Marinov ab,
Ami R. Shah
ab,
Ami R. Shah a,
Christopher A. Howard
a,
Christopher A. Howard c and
Patrick L. Cullen
c and
Patrick L. Cullen *d
*d
aElectrochemical Innovations Laboratory (EIL), Department of Chemical Engineering, University College London, London, WC1E 6BT, UK
bCIC EnergiGUNE, Basque Research and Technology Alliance (BRTA), Alava Technology Park, Albert Einstein 48, 01510 Vitoria-Gasteiz, Spain
cDepartment of Physics & Astronomy, University College London, London, WC1E 6BT, UK
dSchool of Engineering and Materials Science, Queen Mary University, London, E1 4NS, UK. E-mail: p.cullen@qmul.ac.uk
First published on 10th July 2025
Abstract
Molybdenum disulfide (MoS2) is sought out to replace graphite as the negative electrode in lithium-ion batteries (LIBs), due to the higher theoretical capacity of bulk MoS2 (670 mAh g−1) and its natural abundance. However, upon deep discharge (0.01 V) MoS2 suffers from rapid loss of performance. To further the understanding of the MoS2 degradation mechanism, ex situ scanning electron microscopy (SEM), X-ray diffraction (XRD), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) are carried out in the first lithiation and delithiation cycle. The study reveals that visible concentric coloured rings form in MoS2 coin cells, whereby the electrode centre is underused unlithiated 2H MoS2, the middle ring is partially lithiated 1T/2H LixMoS2, and the outer ring experiences the full electrochemical pathway to form an amorphous product. Since lithiation inhomogeneities complicate MoS2 lithiation mechanism studies in coin cells, we propose the use of thin (∼10 μm) coatings and low current densities (∼10 mA g−1) to enable uniform lithiation.
Introduction
With the ever-growing global impact of humankind's reliance on fossil fuels1 and the political disruption of the global supply chains for critical energy storage materials,2 many countries have become dependent on the decisions of a few key exporters to meet their climate goals. As the global community aims to transition away from fossil fuel dependency, the demand and development of long range low-cost electric vehicles (EVs) is surging.3 However, to compensate for lithium-ion battery (LIB) grade graphite powder supply chains being used as political leverage, high energy density materials with established large-scale supply-chains (∼5280 T y−1 in 2020),4 that can easily be inserted into current LIB manufacturing lines to diversify the anode side graphite monopoly in LIBs,5 are becoming of greater scientific and societal interest.6Molybdenum disulfide (MoS2) is a heavily researched transition metal dichalcogenide, involved in LIB research since the 1980s.7 From 2010 onwards, research involving MoS2 as an anode in LIBs has expanded significantly6 due to the higher theoretical capacity of MoS2 (670 mAh g−1)8 calculated for a four-electron pathway relative to the commercially successful graphite (360 mAh g−1). However, MoS2 suffers from poor cycling stability,8–14 unless synthesised with a small particle size, special morphology, or combined in a carbon-based composite.15–18
Experimental MoS2 LIBs are typically cycled under constant current densities (∼100 mA g−1) within a shallow (3.00–0.80 V) or deep (3.00–0.01 V) discharge voltage window vs. a Li+/Li counter electrode.8,10–14 In the shallow case, the commercial MoS2 material reversibly intercalates and deintercalates lithium8,9,19–21 (eqn (1)) achieving a stable capacity of 135 mAh g−1 at the 100th cycle.8 The material characterisation data from ex situ, in situ, and operando studies with X-ray diffraction (XRD),8,9,19–21 Raman spectroscopy,8,20 and Mo K-edge, Mo L-edge, or S K-edge X-ray absorption (XAS)8,9,21 are conclusive that the MoS2 crystal structure is retained, despite some irreversibly trapped lithium and slight altering of the structure via lowered crystallinity.8,9,19–21
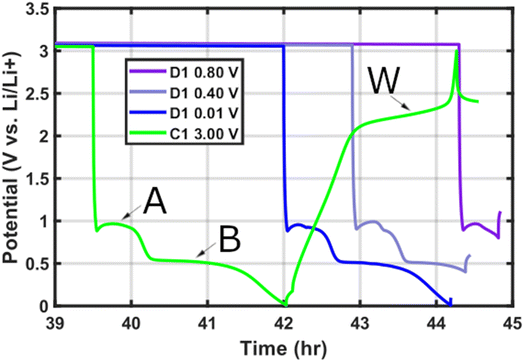
If deep discharge is employed the commercial MoS2 initial cycles display a high capacity (694 mAh g−1) that recedes within the first 100 cycles to 180 mAh g−1.8 During the first discharge (lithiation) (Fig. 1), following the LixMoS2 lithiation plateau A at ∼0.8 V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 it is widely agreed that plateau B at ∼0.4 V is the conversion reaction of LixMoS2 into amorphous Li2S and elemental Mo metal8–10,12,13,19,21–28 (eqn (2)). This has been confirmed with ex situ and in situ high resolution transmission electron microscopy (HRTEM),12,15,22,24,27–30 Raman spectroscopy,8,10,27,29 XRD,8,9,19,28 and S and Mo edge XAS.8,27–29 Additionally, following deep discharge a gel-like layer develops across the electrode surface as seen with scanning electron microscopy (SEM).8–10,31
19 it is widely agreed that plateau B at ∼0.4 V is the conversion reaction of LixMoS2 into amorphous Li2S and elemental Mo metal8–10,12,13,19,21–28 (eqn (2)). This has been confirmed with ex situ and in situ high resolution transmission electron microscopy (HRTEM),12,15,22,24,27–30 Raman spectroscopy,8,10,27,29 XRD,8,9,19,28 and S and Mo edge XAS.8,27–29 Additionally, following deep discharge a gel-like layer develops across the electrode surface as seen with scanning electron microscopy (SEM).8–10,31
However, upon first charge (delithiation) the literature sources begin to deviate from one another over the reaction taking place at plateau W (∼2.3 V)8 (Fig. 1). The leading MoS2 deep discharge mechanism hypotheses consider: (i) reversible formation and conversion of MoS2 and Li2S + Mo on every charge and discharge,10,12,15,24,27,32,33 respectively (eqn (3)). (ii) Irreversible formation of Li2S + Mo during the first discharge, followed by Li–S chemistry (Li2S/Li + S8) operation in all subsequent cycles8,13,19,22,29,34–37 (eqn (4)). (iii) Mixed mechanism operation that considers simultaneous reversible MoS2 formation and Li–S chemistry.28,38,39 (iv) Surface limited gel-layer passivation of the electrode beyond the 50th cycle after initial mixed mechanism operation.10
| MoS2 + xLi+ + xe− ⇌ LixMoS2 | (1) |
| LixMoS2 + (4 − x)Li+ + (4 − x)e− → xLi2S + Mo | (2) |
| Mo + 2Li2S ⇌ MoS2 + 4Li+ + 4e− | (3) |
| 8Li2S ⇌ S8 + 16Li+ + 16e− | (4) |
For bulk MoS2 extracted through mining and refinement or synthetically produced MoS2 to be commercially viable for LIBs, it is necessary to maintain the material's high initial cycle capacities long-term by stabilising the reaction pathway without incurring exceptional production or manufacturing costs. To move towards this goal, it is crucial to understand the lithiation and material degradation mechanism of bulk MoS2 in the pivotal early LIB cycles.
Therefore, herein we lithiated MoS2 electrodes (coating thickness ∼12 μm, ∼36 μm, and ∼89 μm) to various depths of discharge (D1 0.80 V, D1 0.40 V, and D1 0.01 V) or charge (C1 3.00 V) in the first cycle, to observe the effects on the material coating. Visually, all coin cell electrodes displayed concentric coloured rings, which through ex situ characterisation with SEM, XRD, Raman spectroscopy, and XPS depth-profiling show a radial lithiation gradient favouring the electrode exterior. Whereby, the electrode centre remains almost pristine without signs of lithiation, suggesting a severe active material underuse in MoS2 coin cells despite the specific capacity being only 27% short of the theoretical limit (670 mAh g−1).8
Our findings highlight a MoS2 lithiation inhomogeneity taking place in coin cells, which can greatly affect mechanism studies and showcases the multi-level reactions complicating the understanding of MoS2. To tackle the lithiation inhomogeneity, we recommend the use of thin films (∼10 μm) of commercial bulk MoS2 for future mechanism studies and lower current densities (∼10 mA g−1) to enable a uniform electrochemical reaction across the electrode and facilitate cross-study comparison.
Experimental details
Materials
Top-down precursor MoS2 (CAS: 1317-33-5) with an average flake size of ∼6 μm and a maximum size of 40 μm, also referred to as commercial or bulk MoS2 (98+%), was purchased from Sigma-Aldrich. Carbon black conductive additive (Super P™, 99+%) was purchased from Alfa Aesar (CAS: 1333-86-4). PVDF with the commercial name Solef® 5130 was purchased from Solvay®. Copper foil was purchased from Cambridge Energy Solutions, with an average thickness of 11 μm. Anhydrous N-methyl-2-pyrrolidone (NMP; CAS: 872-50-4) was purchased from Sigma Aldrich. 1 M LiPF6 in EC/DMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 vol) LIB electrolyte was purchased from PuriEL under the commercial name of SoulBrain MI.
7 vol) LIB electrolyte was purchased from PuriEL under the commercial name of SoulBrain MI.
Electrode manufacture
Pristine MoS2 battery electrodes were made by slurry-casting, with a coating composition of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 wt% of MoS2, Super P, and PVDF. In a typical process, 3.0 g of PVDF are mixed with 57 mL of NMP (≈58.7 g) and stirred for 24 hours at 400 rpm, to make a 5 wt% PVDF/NMP solution. 500 mg of MoS2 powder, 63 mg of Super P powder, and 1250 mg of PVDF (5 wt%)/NMP solution (≈63 mg PVDF) are added to a plastic tub.
10 wt% of MoS2, Super P, and PVDF. In a typical process, 3.0 g of PVDF are mixed with 57 mL of NMP (≈58.7 g) and stirred for 24 hours at 400 rpm, to make a 5 wt% PVDF/NMP solution. 500 mg of MoS2 powder, 63 mg of Super P powder, and 1250 mg of PVDF (5 wt%)/NMP solution (≈63 mg PVDF) are added to a plastic tub.
The mixture is stirred using a planetary mixer (THINKY ARE-250), with a two-step process. The first step is 0.5 min at 500 rpm to degas the mixture, whilst the second step is 15 min at 1500 rpm to homogenise the slurry. Additional NMP can be added between mixing iterations, to adjust the consistency. The goal is a paint-like smooth flowing slurry.
The slurry is coated onto clean copper foil using a doctor blade and an automatic film coater (Elcometer 4340). The formed electrode is dried on a hot plate at 60 °C until the coating is visibly dry. The coating thickness is measured with a Mitutoyo height gauge. Five points across the electrode surface are sampled to attain an average thickness. In some cases, individual coin cell electrodes are measured as reported in Table SI 1.†
Coin cell assembly & testing
Electrode discs of 15 mm diameter are punched out with a press. The average mass of blank copper 15 mm discs is ∼15.3 mg (∼8.70 mg cm−2), whereas the average active mass loading of the thin (∼12 μm), medium (∼36 μm), and thick (∼89 μm) electrodes are ∼1.11 mg cm−2, ∼3.43 mg cm−2, and ∼8.15 mg cm−2, respectively. The electrode discs are dried at 60 °C for 24 hours in a Buchi tube furnace and taken directly into an inert argon glovebox atmosphere (O2, H2O <0.5 ppm).Individual electrodes are weighed (Kern ABT 120-4NM) inside the glovebox (Table SI 1†) and assembled in Hohsen CR2032 coin cells, using a 19 mm diameter and 25 μm thick Celgard separator, 100 μL of 1 M LiPF6 in EC/DMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 vol) electrolyte, a lithium foil counter (MTI Corporation) with a larger diameter than the working electrode, and a 1 mm thick spacer.
7 vol) electrolyte, a lithium foil counter (MTI Corporation) with a larger diameter than the working electrode, and a 1 mm thick spacer.
Coin cells were cycled using a BioLogic BCS cycler, between the voltages of 3.00–0.80 V, 0.40 V, and 0.01 V, at current densities of 100–200 mA g−1. Coin cells were taken off the cycler and disassembled inside an argon-filled glovebox within 20 min of cycle termination. The only exception is the ∼12 μm C1 3.00 V cell, which was opened at a stable voltage of 2.46 V. Working electrodes were washed with clean di-methoxyethane (DME) to remove remnants of LiPF6 salt. The counter electrodes were not washed.
All cell capacities (mAh g−1) provided are normalised by the mass of active material (80 wt%) in the electrode.
Material characterisation
All digital images were taken with a Google Pixel 3a camera, with ex situ samples and pristine lithium foil being photographed through the glovebox window.SEM was conducted with a Zeiss EVO MA 10 SEM with a tungsten thermionic electron source and a scintillator-type Everhart–Thornley secondary electron detector. The accelerating voltage was set to 10 kV. Multicoloured ex situ sample strips were taken out of the inert glovebox atmosphere and transferred into the SEM within 2 min to minimise air exposure.
XRD was conducted in reflection geometry with a Bruker D2 Phaser benchtop diffractometer with a Cu source (30 kV and 10 mA) and scanned with a 50 s per degree exposure (1–80°). Ex situ samples were cut with scissors according to surface colour and attached with carbon tape onto the Si holder. XRD was carried out after several days of exposure to air and is therefore most prone to changes induced by oxygen and moisture.
Raman spectroscopy was conducted with a Renishaw InVia Reflex microscope with a 785 nm (300 mW) laser, operated at 1% power with a ×40 long distance working objective. The device is used with a 2400 L mm−1 diffraction grating giving a laser spot size of approximately 3 μm. Data collection is carried out with a multichannel charge-coupled device camera detector. Ex situ measurements were conducted air-free on multicoloured sample strips by using a custom glass holder with a Swagelok™ cap to create an air-tight seal.
XPS was conducted with a K-Alpha Thermo Fisher Scientific (Al Kα) spectrometer. Pristine powder and electrode samples were vacuum dried at 60 °C for 24 hours prior to XPS measurements. Ex situ electrodes were cut with scissors according to surface colour and loaded directly onto an air-free XPS transfer stage inside the glovebox. Ar+ etching was conducted at a 3000 eV ion energy and a high ion current.
All XPS regions were fitted with a U2 Tougaard background in CasaXPS. The MoS2 Mo4+ 3d3/2 peak was restricted by a separation of +3.1 eV, 66.7% area, and 110% FWHM relative to its major split orbit peak Mo4+ 3d5/2 for all phases. Similarly, the MoS2 S2− 2p1/2 peak was restricted by a separation of +1.16 eV, 50% area, and a 100% FWHM relative to its S2− 2p3/2 split orbit peak. More detailed XPS fitting procedures can be found in Note II in the ESI.†
Results & discussion
Pristine electrodes are made by casting a slurry of commercial MoS2 flakes (∼6 μm), carbon Super P, and PVDF binder with a common literature formulation of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 wt% (Table SI 2†) with a doctor blade. The as-made electrodes display a greyish-blue hue (Fig. SI 1†), a flake-like morphology in SEM (Fig. SI 2†), crystalline MoS2 XRD patterns (Fig. SI 3†), and 2H phase MoS2 in Raman (Fig. SI 4†) and XPS spectra (Fig. SI 5†). Homogenous electrode coating thickness (Table SI 3†) and mass (Table SI 4†) is ensured by sample statistics.
10 wt% (Table SI 2†) with a doctor blade. The as-made electrodes display a greyish-blue hue (Fig. SI 1†), a flake-like morphology in SEM (Fig. SI 2†), crystalline MoS2 XRD patterns (Fig. SI 3†), and 2H phase MoS2 in Raman (Fig. SI 4†) and XPS spectra (Fig. SI 5†). Homogenous electrode coating thickness (Table SI 3†) and mass (Table SI 4†) is ensured by sample statistics.
The first cycle gives rise to a highly repeatable voltage profile (Fig. 1) with clear discharge plateaus A (0.96 V)8,9,19,28,30,40 and B (0.51 V),8,9,19,28,30,40 and a single charge plateau W (2.1–2.4 V).8,9,19,28,30,40 The discharge profile is consistent irrespective of electrode thickness, only varying in the delivered first discharge specific capacity (Fig. SI 6†) as thin electrodes (∼12 μm) are favoured in terms of mass-based capacity41 (∼785 mAh g−1) relative to the thicker electrodes (∼36 μm; ∼487 mAh g−1 and ∼89 μm; ∼471 mAh g−1). The long-term performance over 500 cycles of the different coating thicknesses is verified across 3 cells per thickness to ensure sample consistency (Fig. SI 7–SI 9†).
Upon opening coin cells on their first cycle within 20 minutes of achieving the desired voltage (Fig. 2), concentric coloured rings are observed across the working electrodes. The inhomogeneous colour distribution worsens as the electrode coating thickness increases (Fig. 2E–L), but nonetheless even the thin electrodes (∼12 μm) are affected (Fig. 2A–D).
In most cases three rings are present across the electrode surface (Fig. 2), although in the shallow discharge (D1 0.8 V) the middle rings are extremely faint. Only in the thin (∼12 μm) electrodes D1 0.01 V and C1 3.00 V the middle rings are absent altogether, as the electrodes become almost uniform in colour (Fig. 2C and D).
The lithium counter electrodes show varying degrees of lithium usage depending on the MoS2 working electrode thickness (Fig. SI 10†). In contrast to the smooth pristine lithium (Fig. SI 11A†), the cycled counter electrodes display indications of lithium involvement. In the cells with the thin working electrodes, a roughening of the lithium surface occurs (Fig. SI 10†), whereas in the thicker electrode cells (≥36 μm) a darkening of the counter electrode surface takes place.
Notably, in the thicker MoS2 cells the lithium darkening only occurs in the outer areas of the counter electrodes (Fig. SI 11†), matching the dark areas of the MoS2 working electrodes. Suggesting, that the dark outer regions of the thicker MoS2 working electrodes experience the greatest contact and interaction with the lithium counter electrodes.
On the other hand, the centre of the lithium counter electrodes appears similar to the pristine lithium metal (Fig. SI 11A†). Likewise, the inner most region of the cycled MoS2 electrodes (Fig. SI 11†) appears similar to the pristine MoS2 electrode (Fig. SI 1†). Suggesting, that the interior of the MoS2 working electrode is increasingly underused as the electrode coating thickness increases (Fig. 2).
Cycling ∼36 μm thick MoS2 electrodes within the deep discharge voltage range for up to 50 cycles does not lead to the disappearance of the inhomogeneous concentric rings (Fig. SI 12†). Instead, the peculiar shapes appearing on the long-term cycled MoS2 electrodes leave an identical dark imprint on their respective lithium counters (Fig. SI 12†). Therefore, strongly reinforcing the notion that electrochemical contact between the electrodes is the key reason for the colour inhomogeneity.
To facilitate ex situ material characterisation, we introduce the following nomenclature for the ∼36 μm MoS2 working electrodes, to differentiate the coloured rings based on the electrochemical plateaus A, B, and W (Fig. 1) preceding their observation (Fig. SI 13†), respectively. For instance, in the shallow discharge (D1 0.80 V) the outer ring is AX1, the middle ring is AX2, and the electrode centre is AX3. The same logic is applied to the D1 0.01 V (BX1–BX3) and C1 3.00 V (WX1–WX3) electrodes.
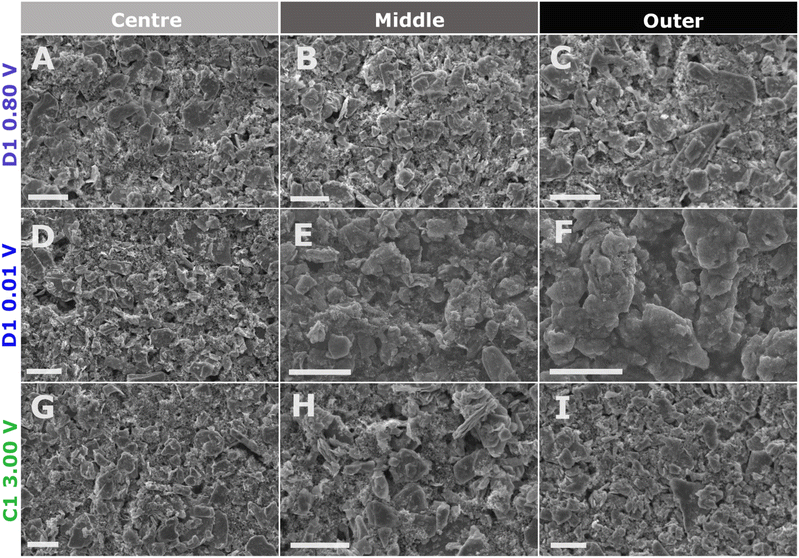
SEM
We applied ex situ SEM to all multi-coloured areas of the cycled ∼36 μm MoS2 working electrodes to verify whether the electrode morphology changes based on lithiation and delithiation in the first cycle.For the shallow discharge (D1 0.80 V), there is no change in the MoS2 flake morphology (Fig. 3A–C) despite the visible change in the electrode colour (Fig. 2E). All three coloured rings (AX1–AX3) display the preserved MoS2 flake morphology, as was observed in the pristine electrode (Fig. SI 2†).
Following a deep discharge (D1 0.01 V) a disparity in morphology takes place between the coloured rings (Fig. 3D–F). The electrode centre (BX3) retains the pristine electrode MoS2 flake-like morphology (Fig. 3D). However, the middle ring (BX2) exhibits a mix of preserved pristine MoS2 flakes (Fig. SI 14A†) and the formation of a gel-like layer10,13,31,42 that covers the electrode surface (Fig. 3E and SI 14B†). Nevertheless, most of the middle ring surface contains the preserved flake morphology. Only the outer ring (BX1) is completely covered by the gel-like layer throughout and experiences the apparent disintegration of the MoS2 flake structure (Fig. 3F and SI 15†).
The morphology of the fully delithiated electrode (C1 3.00 V) following a deep discharge (D1 0.01 V) exhibits only pristine MoS2 flakes (Fig. 3G–I) across all three coloured rings (WX1–WX3) (Fig. 2). Surprisingly, the outer ring (Fig. 3I) has no gel-like layer presence despite its dark colour (Fig. 2L) and the preceding electrochemical plateau resulting in BX1 being entirely covered by the gel-like layer.10,13,31,42 To the best of our knowledge, this is the first time that the removal of the MoS2 gel-like layer has been demonstrated.
Despite, the appearance of the darkest colour alongside the formation of the gel-like layer in D1 0.01 V (BX1), the gel-like layer itself is not responsible for the dark colour. Since, in C1 3.00 V (WX1) the dark colour remains (Fig. 2H) despite the gel-like layer disappearing (Fig. 3I). It is also unlikely for the dark colour to be due to coin cell geometry, as in that case the dark colour would be observed across all states of discharge and charge for a given thickness. Therefore, the chemical reaction taking place in the outer ring at the lower voltages D1 (>0.40 V), where electrochemical contacting and the current experienced is greatest, is responsible for the dark colour in the outer ring.
XRD
To compare the crystallographic properties of the outer, middle, and central regions of each coin cell electrode, appropriate sections of each ex situ electrode were cut with scissors and studied with XRD.The electrode centres AX3, BX3, and WX3 (Fig. 4) perfectly match the pristine MoS2 electrode, despite having been present during lithiation/delithiation within a LIB. The only distinction between the pristine electrode and the cycled electrode centres, is the slight upshift in peak 2θ values due to the introduction of carbon tape beneath the samples (Fig. 4). Unfortunately, the Bruker D2 Phaser diffractometer cannot account for height calibration, but the copper current collector peaks also upshift by the same amount for similar values of 2θ (Fig. SI 16†), validating that the change in sample height is responsible for the diffraction pattern upshift and not a change in the material d-spacings.
The lack of change in the diffraction patterns of the ex situ electrode centres supports the visual observations from the working electrodes (Fig. 2) and the lack of involvement in their corresponding lithium metal counter electrode centres (Fig. SI 10†). Again, suggesting that the MoS2 working electrode and lithium counter electrode centres are underused and do not participate in the lithiation mechanism.
The middle rings (AX2, BX2, and WX2) show significant variability based on the depth of discharge. The AX2 region of the shallow discharge retains the 2H MoS2 character of the pristine electrode (Fig. 4), despite peaks reducing in intensity as the coating becomes irreversibly more amorphous as has been observed previously.8,9,19,21,40
However, the deep discharge BX2 region exhibits a large change with most of the 2H MoS2 indices (103, 006, and 105) becoming extremely faint whilst the rest of the higher angle MoS2 peaks vanish altogether.8,9 Even the intensity of the dominant 002 MoS2 index severely decreases, noticeable by the relative increase in the constant copper current collector indices (Fig. SI 17†).
At the same time, the dominant 002 MoS2 index splits into a doublet (Fig. 4), whereby the downshifted 002* index indicates the expanded d-spacing of MoS2 due to lithium intercalation8,9,19,40 (LixMoS2). In the case of the charged WX2 region, all MoS2 diffraction peaks vanish (Fig. 4) aside from a heavily decreased intensity 002 index (Fig. SI 17†).
In the outer most coloured rings (AX1, BX1, and WX1), the shallow discharge AX1 region retains the 002, 103, and 105 MoS2 indices (Fig. 4), although their intensity is severely reduced as much of the 2H MoS2 crystalline structure is lost (Fig. SI 18†). Nevertheless, the observations are in agreement with previous shallow discharge XRD studies.8,9,19,40
At the same time, the deep discharge ring BX1 is completely void of any MoS2 peaks, does not contain any copper current collector indices as the coating delaminated from the substrate, and is instead identified as Li2MoO4 (Fig. 4). However, it should be noted that exposure of lithiated MoS2 to atmospheric conditions will result in the formation of Li2MoO4 crystals on the electrode surface (see Note I†), which are not present immediately following LIB operation (Fig. SI 19–SI 21†).
On the other hand, the charged electrode ring WX1 is completely void of any material indices aside from the copper current collector peaks (Fig. 4). This is unexpected from the preserved flakes observed with SEM (Fig. 3G–I).
Due to the large degree of amorphousness of the outer ring, especially following deep discharge, no conclusive outcome can be established. Previous studies have also struggled to support proposed mechanisms conclusively with solely XRD.22,36,37
Herein, the stark crystallographic difference between the coloured regions within a single coin cell electrode have been highlighted. This is especially problematic, as depending on which region of the electrode is analysed, strongly opposing lithiation mechanism conclusions can be reached in the absence of sufficient information.
Raman spectroscopy
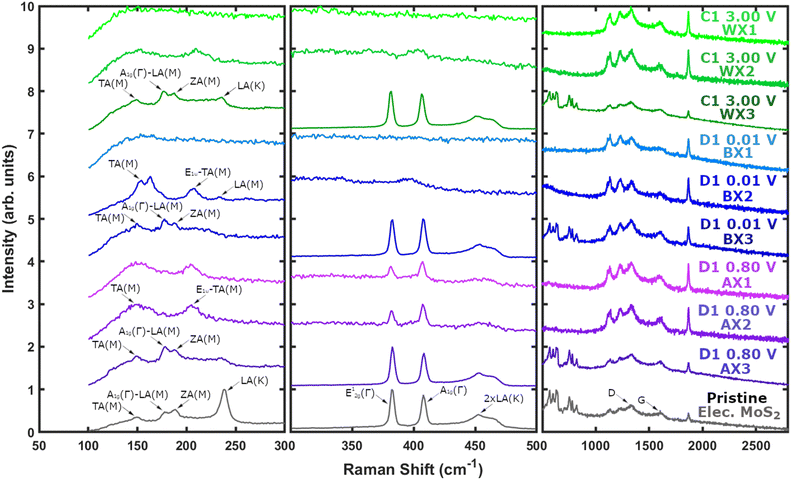
To investigate the multi-coloured electrode surfaces, cycled MoS2 electrodes are cut into tri-coloured strips and studied with air-free ex situ Raman spectroscopy by using a custom Swagelok sample holder.The electrode centres (AX3, BX3, and WX3) clearly demonstrate there is no difference between the pristine electrode and the cycled centres irrespective of the cutoff voltage used (Fig. 5 and SI 21–SI 24†). Strongly reinforcing the previous data from SEM and XRD, validating that the electrode centres do not participate in lithiation/delithiation.
Within the middle coloured rings (AX2, BX2, and WX2), the shallow discharge (AX2) is the only segment that retains some degree of 2H phase MoS2 (Fig. 5 and SI 25†) with the presence of the E12g(Γ) (382 cm−1) and A1g(Γ) (407 cm−1) modes, alongside the rise of the 1T phase lower end TA(M) (149 cm−1) and E1u(M)–TA(M) (205 cm−1) modes associated with the formation of lithiated8,40 LixMoS2. At the same time, the low intensity higher shift (500–900 cm−1) pristine 2H MoS2 modes vanish in all three states.
On the other hand, the deep discharge middle BX2 ring only displays 1T phase LixMoS2 peaks (Fig. 5 and SI 26†), exhibiting the clearest 1T signal out of all regions scanned. Whilst the charge WX2 region shows only a faint 1T presence (Fig. 5 and SI 27†).
For the outer most rings (AX1, BX1, and WX1), the deep discharge (BX1) and charge (WX1) areas are completely void of any MoS2 presence (Fig. 5 and SI 28, SI 29†). Only the shallow discharge ring AX1 displays LixMoS2 Raman modes (Fig. 5 and SI 30†), with the presence of weak mixed phase 1T/2H MoS2 peaks as were observed previously in AX2 (Fig. 5 and SI 25†). Nevertheless, out of all positions scanned in AX1, the majority exhibited only 1T peaks (Fig. SI 30†), indicating a greater degree of lithiation than in the middle ring AX2 (Fig. SI 25†).
The findings agree with previous studies showing the formation of LixMoS2 during shallow discharge,8,10,27,40 followed by the disappearance of Raman signal during deep discharge.10,27 However, in contrast to previous studies after charge the electrochemically active regions (WX2 and WX1) do not reversibly recover MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10,27 nor irreversibly form sulfur.8,29
10,27 nor irreversibly form sulfur.8,29
XPS
To gain elemental insight into the multi-coloured electrode surfaces, air-free ex situ XPS is carried out by cutting coloured sections from the cycled electrodes with scissors and using a custom air-tight sample transfer stage.XPS analysis clearly denotes all three electrode centres (AX3, BX3, and WX3) as 2H phase MoS identified through high intensity Mo 3d and S 2p peaks closely resembling the pristine electrode (Fig. 6 and SI 31, SI 32†). The AX3 and BX3 peaks upshift to higher binding energies (229.6 eV & 162.4 eV) than the pristine electrode (229.4 eV & 162.2 eV), whereas the WX3 peaks remain almost unchanged (229.3 eV & 162.1 eV).
However, in all three electrode centres the binding energy separation (ΔBE) between the Mo 3d5/2 A and S 2p3/2 A peaks (67.18 eV) is conserved ∼±0.02 eV, indicating that any changes in peak binding energies are due to either surface charging within the XPS spectrometer43 or calibration differences. At the same time, the charge centre (WX3) peaks broaden out significantly and decrease in intensity (FWHM 1.04 eV) relative to the pristine electrode (FWHM 0.85 eV), indicating a slight increase in material disorder44 following exposure to the LIB environment for a full cycle.
Even though the central Mo 3d and S 2p peaks remaining relatively unchanged (Fig. 6), the surface electrolyte interphase (SEI) layer related species fluorine, oxygen, and carbon dominate the XPS survey spectra (Fig. SI 32†). Therefore, an SEI layer develops within the LIB cell even in the absence of MoS2 activity due to poor electrode contacting.
Irrespective of depth of discharge, the middle electrode rings (AX2, BX2, and WX2) exhibit a binding energy downshift for both the Mo 3d and S 2p regions (Fig. 6 and SI 33†), with dominant 1T phase MoS2 peaks at approximately 228.4 eV (Mo 3d5/2) and 161.5 eV (S 2p3/2) due to the formation of LixMoS2.6,15,45–47 In all middle rings, weaker 2H phase MoS2 peaks can also be found on the electrode surface, highlighting that lithiation in the middle rings is incomplete with some of the active material remaining unused.
Additionally, the middle ring Mo 3d and S 2p peaks partially shift in binding energy (229.5 eV & 162.1 eV) relative to the pristine electrode (229.4 eV & 162.2 eV) and possess a ΔBE (67.43 eV) with a greater variation (±0.17 eV) than the central regions. This is caused by the difficulty in fitting low intensity XPS signals with an increased number of MoS2 phases (Fig. 6). The MoS2 and LixMoS2 peak intensities in the middle rings are significantly lower than the pristine electrode and the electrode centres (Fig. 6), exhibiting a sharp reduction in MoS2 presence in the middle ring in favour of SEI growth through fluorine, oxygen, carbon, and lithium species (Fig. SI 34†).
Within the outer ring (AX1, BX1, and WX1), the XPS signal varies greatly between the three states of discharge/charge (Fig. 6 and SI 35, SI 36†). The shallow discharge AX1 ring closely resembles the middle ring AX2, with a strong 1T phase MoS2 presence but expresses a reduction in 2H phase MoS2. Therefore, suggesting that greater electrode contacting in the outer region of the shallow cell results in a higher degree of LixMoS2 and less underused active material remaining as 2H MoS2.
The deep discharge BX1 region is void of sufficient Mo or S presence to garner sample fitting (Fig. 6), showcasing an absence of MoS2, Mo, or Li2S8–10,12,13,19,21–28 on the electrode surface. This finding is confirmed by scanning two additional locations on the sample surface for consistency (Fig. SI 35†). Therefore, neither supporting the reversible10,12,15,24,27,32,33 nor irreversible8,13,19,22,29,34–37 mechanism hypotheses.
Following charge, the outer region (WX1) exhibits presence of 2H phase MoS2 (229.8 eV), 1T MoS2 (228.5 eV), MoO3 (231.6 eV), and sulphates (SOx ∼166–169 eV) (Fig. 6 and SI 35†). No significant amount of elemental sulfur (∼163.9 eV)48,49 can be found in WX1 to support the irreversible mechanism hypothesis, nor is the MoS2 presence strong enough to warrant justification for reversible formation. Overall, the surface WX1 contribution of both molybdenum and sulfur compounds is minimal, collectively Mo + S account for 2.1 at%, whilst the SEI species fluorine (13.7 at%), oxygen (18.3 at%), carbon (45.6 at%), and lithium (20.0 at%) dominate the surface layer (Fig. SI 36†).
Etching of the cycled electrodes results in the modulation of the sample, due to the preferential removal44,50–55 of sulfur from MoS2 (Fig. SI 37 and SI 38†). However, in all rings and regions etching reveals the presence of MoS2 below the surface (Fig. SI 37 and SI 38†). Even the most electrochemically active outer region (BX1), shows noticeable amounts of MoS2 underneath the surface. Therefore, suggesting that MoS2 remains inactive during the first cycle not just radially but with depth as well.
Unfortunately, the phase and composition of the unearthed MoS2 cannot be established as the Mo 3d and S 2p binding energies downshift across all electrode regions (Fig. SI 37 and SI 38†) as a result of argon ion bombardment.44,50–55 Hence, it remains unknown whether the material is 2H or 1T prior to etching.
Overall, XPS confirms the Raman spectroscopy observation that MoS2 lithiates progressively further moving radially outwards from the electrode centre (Fig. 6), due to the improved electrochemical contacting between the working electrode and the lithium counter electrode. In the shallow discharge this results in a greater presence of LixMoS2 relative to inactive 2H MoS2, whereas in the deep discharge MoS2 vanishes altogether and does not recover significantly after charge.
It is important to highlight, that even in the pristine electrode fluorine (18.0 at%), oxygen (4.0 at%), and carbon (58.3 at%) make up most (80.3 at%) of the electrode surface (MoS2; 16.4 at%), whereby their contribution increases further with the first cycle when considering the addition of lithium (6.6–25.4 at%) to the SEI species (88.9–100.0 at%) and the severe reduction of the MoS2 presence (0.0–5.2 at%). Therefore, it is highly probably that the unstable development of an SEI layer might be a leading cause for long-term performance deterioration10 (Fig. SI 7 and SI 12†), as suggested by the observation of deposition (Fig. 2F) and removal (Fig. 2I) of the gel-like layer on discharge and charge, respectively.
Surplus capacity
Fig. 7 provides an overarching summary of all the information gathered from ex situ material characterisation (SEM, XRD, Raman, and XPS). Regardless of the depth of discharge or state of charge, the first cycle electrode centre remains as pristine 2H MoS2 identified through morphology, crystallinity, phase, and chemical signature.Within the middle rings (AX2, BX2, and WX2) MoS2 is partially lithiated with some amount remaining as 2H MoS2, whilst depth profiling reveals the existence of MoS2 underneath all surface layers irrespective of voltage. Hence, for a medium thick (∼36 μm) MoS2 electrode in a CR2032 coin cell, a significant percentage of the active material acts as dead mass.
Therefore, despite the MoS2 active mass loading of 3.43 mg cm−2 a smaller fraction of the active material contributes to the 102 mAh g−1 and ∼487 mAh g−1 capacity delivered within the first shallow (3.00–0.80 V) and deep discharge (3.00–0.01 V), respectively.
Part of the added deep discharge capacity can be attributed to the consumption of electrolyte to form the gel-like layer observed in BX1 (Fig. 2F), but nonetheless a significant amount of MoS2 in the electrode remains non-lithiated or partially lithiated (LixMoS2 ∼100 mAh g−1) (Fig. 6). Hence, suggesting there exists a source of surplus capacity enabling the active MoS2 in the outer ring to exceed its one-electron shallow (167 mAh g−1) or four-electron deep discharge (670 mAh g−1)8 pathway theoretical capacity.
To reduce the impact of MoS2 lithiation inhomogeneity within mechanism studies, we suggest the use of thin electrode coatings (∼10 μm) cycled at low current densities (∼10 mA g−1), and at least ex situ visual colour homogeneity validation. Low loadings and currents will enable the active material to fully lithiate (Fig. 2A–D), achieving the full storage capacity, facilitating the identification of the electron transfer number required.
Additionally, utilising a widely available commercial bulk MoS2 material will enable for studies to be replicable, directly comparable, and explored further across different research facilities.
Conclusions
MoS2 lithiation in CR2032 coin cells is not uniform but occurs as a lithiation gradient from the outside of the electrode disc moving inwards as electrode contacting deteriorates in the centre. Increasing the electrode coating thickness, causes the lithiation gradient to affect cell performance negatively as the electrode centre stops contributing to electrochemical storage. Therefore, a substantial amount of active material acts as dead mass and results in a lower mass-based performance (∼487 mAh g−1) than the theoretical capacity of deep discharge MoS2 (670 mAh g−1).Uniting four ex situ material characterisation perspectives (SEM, XRD, Raman, and XPS), shows the electrode centre to be almost identical to the pristine 2H MoS2 electrode, the middle ring to be heterogenous 1T/2H phase LixMoS2/MoS2, and the outer ring to vary depending on the cutoff voltage (0.80 V, 0.01 V, and 3.00 V). As the cycle progresses, the electrode centre remains mostly unaltered. Within the middle ring 1T/2H phase LixMoS2/MoS2 forms for all voltages, whereby the MoS2 structure crystallinity and 2H phase contribution progressively reduces with cycle progression. Nonetheless, the middle ring contains 2H MoS2, indicating that not all the active material contributes towards the cell storage capacity.
In the outer most ring, the material fully participates in the lithiation mechanism. For the shallow discharge (D1 0.80 V), the region is low crystalline heterogenous 1T/2H phase LixMoS2/MoS2 with a predominant 1T contribution. On the other hand, the deep discharge (D1 0.01 V) section is covered by a gel-like layer in SEM and is completely amorphous. Therefore, it cannot be penetrated by the Raman laser and is distinguishable with XPS due to sample phase modulation induced by depth-profiling. Following charge (C1 3.00 V), the outer ring remains amorphous, does not exhibit a gel-like layer, and presents weak 1T/2H MoS2, MoO3, and sulphate signals in XPS.
In order to avoid further complication of the study of the MoS2 lithiation mechanism in LIB, we suggest the use of thin coatings (∼10 μm) of commercial MoS2 and the application of low current densities (∼10 mA g−1) to enable the coin cell electrodes to uniformly lithiate. Without electrode homogeneity there is an unidentified surplus source of capacity within the cell, considering that not all the MoS2 present lithiates to deliver ∼487 mAh g−1. Additionally, sample inhomogeneities can result in the existence of misleading evidence for competing MoS2 reaction mechanisms depending on the coloured ring analysed.
In all instances, the cycled electrode surface is dominated by the SEI species fluorine, oxygen, carbon, and lithium (88.9–100.0 at%), which already accounted for most of the surface (without lithium) even before cell cycling (80.3 at%). Therefore, alongside the study of the MoS2 lithiation mechanism, the manufacture of well dispersed MoS2 electrode coatings and the study of MoS2 LIB SEI layer development should be the focal point of future studies to aid MoS2 long-term stability without incurring large production or processing costs.
Data availability
Data for this article, including digital images, electrochemical data (.mpt), SEM images, spectroscopy data (Raman & XPS), and XRD diffraction patterns are available at a Science Data Bank repository at https://www.scidb.cn/en/s/7VBBbu.Author contributions
ADM and PLC were responsible for the project conceptualisation. ADM and ARS developed the cell methodology and cycled the cells. ADM collected and analysed the data. ADM wrote the initial draft. ARS, CAH, and PLC reviewed and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The UK EPSRC (CAH and PLC; EP/S001298/2) and the Faraday Institution (https://Faraday.ac.uk; EP/S003053/1) supported this work. The authors (ADM and ARS) would like to acknowledge the Faraday Institution for funding the energy storage work at the Electrochemical Innovation Lab through the LiSTAR (FIRG0014) and Degradation (FIRG001) projects. Additionally, the authors acknowledge the National Physical Laboratory (NPL) for funding the PhD Studentship of ADM.References
- K. Abbass, M. Z. Qasim, H. Song, M. Murshed, H. Mahmood and I. Younis, Environ. Sci. Pollut. Res., 2022, 29, 42539–42559 CrossRef PubMed.
- C. P. Bown, Peterson Institute for International Economics Working Paper 24-2, 2024, pp. 1–44 Search PubMed.
- L. S. Martins, L. F. Guimarães, A. B. Botelho Junior, J. A. S. Tenório and D. C. R. Espinosa, J. Environ. Manage., 2021, 295, 113091 CrossRef PubMed.
- M. Hachhach, H. Akram, M. Hanafi, O. Achak and T. Chafik, Chem. Prod. Process Model., 2023, 18, 355–368 CrossRef CAS.
- Y. Deng, J. Li, T. Li, J. Zhang, F. Yang and C. Yuan, Energy, 2017, 123, 77–88 CrossRef CAS.
- A. D. Marinov, L. Bravo Priegue, A. R. Shah, T. S. Miller, C. A. Howard, G. Hinds, P. R. Shearing, P. L. Cullen and D. J. L. Brett, ACS Nano, 2023, 17, 5163–5186 CrossRef CAS PubMed.
- L. S. Selwyn, W. R. McKinnon, U. von Sacken and C. A. Jones, Solid State Ionics, 1987, 22, 337–344 CrossRef CAS.
- L. Zhang, D. Sun, J. Kang, J. Feng, H. A. Bechtel, L. W. Wang, E. J. Cairns and J. Guo, Nano Lett., 2018, 18, 1466–1475 CrossRef CAS PubMed.
- Z. Zhu, Y. Tang, W. R. Leow, H. Xia, Z. Lv, J. Wei, X. Ge, S. Cao, Y. Zhang, W. Zhang, H. Zhang, S. Xi, Y. Du and X. Chen, Angew. Chem., 2019, 131, 3559–3564 CrossRef.
- T. Liu, G. Melinte, O. Dolotko, M. Knapp and B. Mendoza-Sánchez, J. Energy Chem., 2022, 78, 56–70 CrossRef.
- J. Bai, B. Zhao, J. Zhou, J. Si, Z. Fang, K. Li, H. Ma, J. Dai, X. Zhu and Y. Sun, Small, 2019, 15, 1–11 Search PubMed.
- L. Wang, Q. Zhang, J. Zhu, X. Duan, Z. Xu, Y. Liu, H. Yang and B. Lu, Energy Storage Mater., 2019, 16, 37–45 CrossRef.
- K. K. Halankar, B. P. Mandal, M. K. Jangid, A. Mukhopadhyay, N. Abharana, C. Nayak, K. Dasgupta and A. K. Tyagi, J. Alloys Compd., 2020, 844, 156076 CrossRef CAS.
- C. Deng, H. Wang and S. Wang, J. Mater. Chem. A, 2021, 9, 15734–15743 RSC.
- K. Leng, Z. Chen, X. Zhao, W. Tang, B. Tian, C. T. Nai, W. Zhou and K. P. Loh, ACS Nano, 2016, 10, 9208–9215 CrossRef CAS PubMed.
- F. Pan, J. Wang, Z. Yang, L. Gu and Y. Yu, RSC Adv., 2015, 5, 77518–77526 RSC.
- Y. M. Chen, X. Y. Yu, Z. Li, U. Paik and X. W. Lou, Sci. Adv., 2016, 2, 1–9 Search PubMed.
- Y. Jiao, A. Mukhopadhyay, Y. Ma, L. Yang, A. M. Hafez and H. Zhu, Adv. Energy Mater., 2018, 8, 1–9 Search PubMed.
- X. Fang, C. Hua, X. Guo, Y. Hu, Z. Wang, X. Gao, F. Wu, J. Wang and L. Chen, Electrochim. Acta, 2012, 81, 155–160 CrossRef CAS.
- G. Wang, Y. Zhang, H. S. Cho, X. Zhao, F. Kim and J. Zou, ACS Appl. Energy Mater., 2021, 4, 14180–14190 CrossRef CAS.
- C. D. Quilty, L. M. Housel, D. C. Bock, M. R. Dunkin, L. Wang, D. M. Lutz, A. Abraham, A. M. Bruck, E. S. Takeuchi, K. J. Takeuchi and A. C. Marschilok, ACS Appl. Energy Mater., 2019, 2, 7635–7646 CrossRef CAS.
- X. Fang, X. Guo, Y. Mao, C. Hua, L. Shen, Y. Hu, Z. Wang, F. Wu and L. Chen, Chem.–Asian J., 2012, 7, 1013–1017 CrossRef CAS PubMed.
- K. Leng, Z. Chen, X. Zhao, W. Tang, B. Tian, C. T. Nai, W. Zhou and K. P. Loh, ACS Nano, 2016, 10, 9208–9215 CrossRef CAS PubMed.
- Q. Su, S. Wang, M. Feng, G. Du and B. Xu, Sci. Rep., 2017, 7, 7275 CrossRef PubMed.
- L. Wang, Z. Xu, W. Wang and X. Bai, J. Am. Chem. Soc., 2014, 136, 6693–6697 CrossRef CAS PubMed.
- U. K. Sen, P. Johari, S. Basu, C. Nayak and S. Mitra, Nanoscale, 2014, 6, 10243–10254 RSC.
- Z. Zhu, S. Xi, L. Miao, Y. Tang, Y. Zeng, H. Xia, Z. Lv, W. Zhang, X. Ge, H. Zhang, J. Wei, S. Cao, J. Chen, Y. Du and X. Chen, Adv. Funct. Mater., 2019, 29(42), 1904843 CrossRef CAS.
- W. Choi, Y. S. Choi, H. Kim, J. Yoon, Y. Kwon, T. Kim, J. H. Ryu, J. H. Lee, W. Lee, J. Huh, J. M. Kim and W. S. Yoon, Chem. Mater., 2021, 33, 1935–1945 CrossRef CAS.
- U. K. Sen, P. Johari, S. Basu, C. Nayak and S. Mitra, Nanoscale, 2014, 6, 10243–10254 RSC.
- L. Wang, Z. Xu, W. Wang and X. Bai, J. Am. Chem. Soc., 2014, 136, 6693–6697 CrossRef CAS PubMed.
- Z. Zhu, Y. Tang, Z. Lv, J. Wei, Y. Zhang, R. Wang, W. Zhang, H. Xia and M. Ge, Angew. Chem., 2018, 130, 3718–3722 CrossRef.
- K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252 RSC.
- X. Cao, Y. Shi, W. Shi, X. Rui, Q. Yan, J. Kong and H. Zhang, Small, 2013, 9, 3433–3438 CrossRef CAS PubMed.
- Y. Liu, X. He, D. Hanlon, A. Harvey, U. Khan, Y. Li and J. N. Coleman, ACS Nano, 2016, 10, 5980–5990 CrossRef CAS PubMed.
- L. Hu, Y. Ren, H. Yang and Q. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 14644–14652 CrossRef CAS PubMed.
- J. Xiao, X. Wang, X. Yang, S. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21(15), 2840–2846 CrossRef CAS.
- T. Wang, C. Sun, M. Yang, G. Zhao, S. Wang, F. Ma, L. Zhang, Y. Shao, Y. Wu, B. Huang and X. Hao, J. Alloys Compd., 2017, 716, 112–118 CrossRef CAS.
- L. Yang, S. Wang, J. Mao, J. Deng, Q. Gao, Y. Tang and O. G. Schmidt, Adv. Mater., 2013, 25, 1180–1184 CrossRef CAS PubMed.
- Y. Teng, H. Zhao, Z. Zhang, Z. Li, Q. Xia, Y. Zhang, L. Zhao, X. Du, Z. Du, P. Lv and K. Świerczek, ACS Nano, 2016, 10, 8526–8535 CrossRef CAS PubMed.
- G. Wang, Y. Zhang, H. S. Cho, X. Zhao, F. Kim and J. Zou, ACS Appl. Energy Mater., 2021, 4, 14180–14190 CrossRef CAS.
- W. Xie, Z. Zhang and X. Gao, Electrochim. Acta, 2024, 493, 144396 CrossRef CAS.
- S. K. Das, R. Mallavajula, N. Jayaprakash and L. A. Archer, J. Mater. Chem., 2012, 22, 12988–12992 RSC.
- K. N. Wood and G. Teeter, ACS Appl. Energy Mater., 2018, 1, 4493–4504 CrossRef CAS.
- M. A. Baker, R. Gilmore, C. Lenardi and W. Gissler, Appl. Surf. Sci., 1999, 150, 255–262 CrossRef CAS.
- N. H. Attanayake, A. C. Thenuwara, A. Patra, Y. V. Aulin, T. M. Tran, H. Chakraborty, E. Borguet, M. L. Klein, J. P. Perdew and D. R. Strongin, ACS Energy Lett., 2018, 3, 7–13 CrossRef CAS.
- W. Chen, J. Gu, Q. Liu, R. Luo, L. Yao, B. Sun, W. Zhang, H. Su, B. Chen, P. Liu and D. Zhang, ACS Nano, 2018, 12, 308–316 CrossRef CAS PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- M. Wahlqvist and A. Shchukarev, J. Electron Spectrosc. Relat. Phenom., 2007, 156–158, 310–314 CrossRef CAS.
- Z. Wang, Y. Dong, H. Li, Z. Zhao, H. Bin Wu, C. Hao, S. Liu, J. Qiu and X. W. D. Lou, Nat. Commun., 2014, 5, 5002 CrossRef CAS PubMed.
- A. Santoni, F. Rondino, C. Malerba, M. Valentini and A. Mittiga, Appl. Surf. Sci., 2017, 392, 795–800 CrossRef CAS.
- S. Bae, N. Sugiyama, T. Matsuo, H. Raebiger, K. I. Shudo and K. Ohno, Phys. Rev. Appl., 2017, 7, 1–7 CAS.
- R. Addou, S. McDonnell, D. Barrera, Z. Guo, A. Azcatl, J. Wang, H. Zhu, C. L. Hinkle, M. Quevedo-Lopez, H. N. Alshareef, L. Colombo, J. W. P. Hsu and R. M. Wallace, ACS Nano, 2015, 9, 9124–9133 CrossRef CAS PubMed.
- J. Luxa, V. Mazánek, A. Mackova, P. Malinsky, S. Akhmadaliev and Z. Sofer, Appl. Mater. Today, 2019, 14, 216–223 CrossRef.
- L. H. Isherwood, Z. Hennighausen, S. K. Son, B. F. Spencer, P. T. Wady, S. M. Shubeita, S. Kar, C. Casiraghi and A. Baidak, 2D Mater., 2020, 7(3), 035011 CrossRef CAS.
- T. Grünleitner, A. Henning, M. Bissolo, M. Zengerle, L. Gregoratti, M. Amati, P. Zeller, J. Eichhorn, A. V. Stier, A. W. Holleitner, J. J. Finley and I. D. Sharp, ACS Nano, 2022, 16, 20364–20375 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta02290h |
| This journal is © The Royal Society of Chemistry 2025 |