 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanically-sensitive fluorochromism by molecular domino transformation in a Schiff base crystal†
Toshiyuki
Sasaki
 *a,
Takanori
Nakane
*a,
Takanori
Nakane
 b,
Akihiro
Kawamoto
b,
Akihiro
Kawamoto
 b,
Yakai
Zhao
b,
Yakai
Zhao
 cd,
Yushi
Fujimoto
e,
Tomohiro
Nishizawa
cd,
Yushi
Fujimoto
e,
Tomohiro
Nishizawa
 f,
Nabadeep
Kalita
g,
Seiji
Tsuzuki
f,
Nabadeep
Kalita
g,
Seiji
Tsuzuki
 *h,
Fuyuki
Ito
*h,
Fuyuki
Ito
 *e,
Upadrasta
Ramamurty
*cd,
Ranjit
Thakuria
*e,
Upadrasta
Ramamurty
*cd,
Ranjit
Thakuria
 *g and
Genji
Kurisu
*g and
Genji
Kurisu
 *bij
*bij
aJapan Synchrotron Radiation Research Institute (JASRI), 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5198, Japan. E-mail: toshiyuki.sasaki@spring8.or.jp
bInstitute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan
cSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore 639798, Singapore
dInstitute of Materials Research and Engineering, Agency for Science, Technology and Research (A*STAR), Singapore 138634, Singapore
eDepartment of Chemistry, Institute of Education, Shinshu University, 6-ro, Nishinagano, Nagano, 380-8544, Japan
fDepartment of Biological Sciences, Yokohama City University, 1-7-29 Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan
gDepartment of Chemistry, Gauhati University, Guwahati 781014, Assam, India
hDepartment of Applied Physics, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo, 113-8656, Japan
iJEOL YOKOGUSHI Research Alliance Laboratories, Graduate School of Frontier Biosciences, Osaka University, 1–3 Yamadaoka, Suita, Osaka 565-0871, Japan
jInstitute for Open and Transdisciplinary Research Initiatives, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
First published on 31st May 2024
Abstract
The ability to make large changes in properties against small external stimuli is one of the key factors in sensing materials. Molecular domino transformation, i.e., polymorphic transformation starting at a stimulated point and extending to the whole crystal, is an attractive phenomenon from this viewpoint. We recently found such a transformation for the first time in an organic crystal of 4-nitro-N-salicylideneaniline as one of the Schiff bases. In this study, quantitative evaluations were conducted on the mechanical stimulus and emission properties in the transformation of the crystal. Our results demonstrate the potential applicability of the crystal to the detection of even less than a few μN mechanical stimuli as an emission color change. A molecular level transformation mechanism revealed by microcrystal electron diffraction also contributes to the future development of the transformation-based materials.
Introduction
Molecular crystals can alter their properties in response to external stimuli by conversion of molecular structures, molecular arrangements, and/or crystal's components.1–3 Various kinds of external stimuli, such as thermal,4–9 mechanical,10–15 light,16–18 and vapor-based stimuli,19–21 have been used to induce the conversion. Remarkable property changes and high sensitivity to external stimuli are reported; the tailoring of such changes can lead to fascinating sensing and actuation applications.Molecular domino transformation (MDT), discovered and named by Ito et al. in a gold(I) complex in 2013,11 is one such possible mechanism to achieve the desired characteristics. Not only is the transformation inducible by a small mechanical stimulus, but it also progresses from the initial point through the entire crystal autocatalytically. Such domino-like amplification of the response to mechanical stimulus enables the molecular crystals to detect mechanical stimuli with high sensitivity. However, MDT is reported only in a small number of molecular crystals hitherto.9,11,12 Therefore, research on it has depended mostly on serendipity. For further rational development of sensing materials that use MDT as the main mechanism, fundamental understanding of it and in a wider variety of molecular systems is necessary.
Salicylideneaniline derivatives have been selected as a model system for this purpose in this study because of their remarkable polymorph-dependent fluorescence properties22,23 as well as their easy availability via a condensation reaction of anilines and salicylaldehydes. Herein, we characterize MDT in a crystal of 4-nitro-N-salicylideneaniline (1). In addition to the evaluation of the emission characteristics by fluorescent microscopy, the mechanical sensitivity and the molecular level mechanism of the transformation are investigated using nanoindentation24–27 and microcrystal electron diffraction (MicroED) techniques,28–35 respectively.
Results and discussion
Crystals of 1 were synthesized by a condensation reaction of 4-nitroaniline and salicylaldehyde in ethanol. Recrystallization of 1 from THF resulted in columnar orange crystals (Fig. 1b). Two types of crystals were observed. Upon UV (365 nm) light irradiation, one type emits strong yellow color while the other emits weak orange color. Hereafter, these two crystal polymorphs are referred as 1Y and 1O, respectively (Fig. 1c(i)). Such remarkable emission color differences between polymorphs are often observed in molecular crystals showing excited-state intramolecular proton transfer (ESIPT): an intramolecular proton transfer from an excited enol to an excited keto form causes an emission spectral redshift.16,23,36–38 Their emission spectra were recorded by a fluorescent microscope (IX71, Olympus Co., Ltd.) with a spectrometer (USB4000, Ocean Optics Co., Ltd.) as shown in Fig. 1c(ii). 1Y exhibits two emission peaks at 567 and 606 nm while 1O at 614 nm with a shoulder at 580 nm. A shorter fluorescence lifetime of 1O than that of 1Y indicates that non-radiative relaxation pathways have a larger contribution to the fluorescence of 1O than 1Y (Fig. S4, ESI†). In addition, spatially resolved fluorescent measurements by a hyperspectral camera (SI-108, EBA JAPAN Co., Ltd.), which can get emission spectra at a specific area of a single crystal, revealed their non-uniform emission properties (Fig. 1d). The experimental setup is the same as in the previous report.39 The relative intensity of the emission peaks around 570 and 610 nm changes depending on measurement points in each single crystal. Note that emission from the edge of 1Y (region 3 in Fig. d(i)) shows a spectrum resembling that of 1O when compared to the middle region (region 2 in Fig. d(i)). We surmise that the emission heterogeneity is due to the formation of a mixed crystal of 1Y with a small portion of 1O. The ratio of 1O at the edge is larger than that at the middle. Such kind of intergrowth polymorphs have been investigated by Desiraju and coworkers on aspirin40 and felodipin25 molecular crystals.Interestingly, 1O shows an emission color change upon mechanical stimuli (Fig. 1e and Movie S1, ESI†). Orange emission from 1O transformed into yellow at the stimulated area. The yellow emission area gradually spreads over the whole crystal, suggesting the operation of MDT in the crystal11 that transforms it from 1O to 1Y. Propagation of MDT from the stimulated area to an area within a radius of ca. 30 μm occurred within one minute. In the next 20 seconds, MDT rapidly proceeded over 200 μm along the long axis of the 1O. The transformation was also confirmed by spatially resolved emission spectra recorded by a hyperspectral camera and fluorescence lifetime measurements (Fig. S5, ESI†). The emission spectrum obtained at the regions 3 of 1O in Fig. 1e has strong and relatively weak peaks at 610 and 580 nm, respectively. The relative intensities of the peaks become the opposite after the transformation and the resulting spectrum nearly-corresponds to that of the as-prepared 1Y. Moreover, increase of the emission intensity by 9.4 times as well as elongation of the fluorescence lifetime after the transformation were confirmed.
Sensitivity of 1O against mechanical stimuli was investigated using nanoindentation (Fig. 2 and Fig. S8, ESI†).24–271O for nanoindentation studies was prepared by evaporation of the THF solution dropped on a glass plate under air at room temperature. More than 20 crystals of 1O prepared on glass plates were stimulated by a diamond indenter tip with the maximum applied load ranging from 1 to 3000 μN. The transformation of the stimulated crystals was confirmed by emission color change. The large variation in the force required for triggering the transformation could have origins in a variety of factors such as the crystal size, shape, local surface topology, how firmly the crystal is attached to the substrate, and so on. Some crystals showed high mechanical sensitivities, i.e., they underwent the transformation at forces as low as 1 μN.
 | ||
| Fig. 2 Photographic images of 1 under UV light before and after nanoindentation (Hysitron TI980, Bruker Corp.). Mechanically stimulated 1O is circled in red. | ||
The origins of the different emission properties and structural stabilities of 1O and 1Y were investigated based on their crystal structures.41,42 Due to 1O's mechanical sensitivity to transform into 1Y, large single crystals of 1O could not be mounted on a conventional X-ray diffractometer. Thus, the crystal structures of 1O and 1Y were determined by microcrystal electron diffraction (MicroED) (Fig. 3 and 4).28–35 For this purpose, microcrystalline 1O and 1Y were prepared directly on a copper EM grid (Quantifoil R1.2/1.3 Cu 200 mech) by ‘painting’ the THF solution, not crystal suspension, using a brush to prevent any possible mechanical stimulation of the crystals and loaded onto a Talos Arctica microscope (Thermo Fisher Scientific) equipped with a Ceta detector (CMOS 4k × 4k, Thermo Fisher Scientific). Data collection was performed using SerialEM43 with a strategy described in the literature.34,35,44,45 Diffraction patterns from more than 1150 crystals were measured at 200 kV with an electron flux of 0.05 e− Å−2 s−1. Diffraction patterns were indexed, integrated, and scaled using DIALS.46–48 First, crystals were indexed without using any prior cell information and the Bravais lattice constraints. The distribution of the resulting unit cell parameters indicated the presence of two crystal forms (monoclinic and orthorhombic) in the sample (ESI,† Fig. S2). Next, the dataset was reprocessed twice, using the cell parameter and lattice type of each crystal form. Crystals in each group were further clustered by xia2.multiplex49 to find the best sets of crystals for final merging (Tables S1 and S2, ESI†). 697 crystals were indexed in the monoclinic crystal form and 55 good crystals were merged. The structure was solved in the space group P21/c and was identical to the 1Y structure solved using X-ray diffraction (XRD) in previous studies.50,51 The powder XRD pattern of 1Y agrees well with that simulated from the solved structure (Fig. S3, ESI†). The other 90 crystals were indexed in the new orthorhombic crystal form and the 4 best crystals gave a structure in the Pna21 space group. We assigned this to be 1O. The crystal structures were solved using SHELXT52 and refined kinematically by SHELXL53 in Olex2 GUI (Table S3, ESI†).54,55
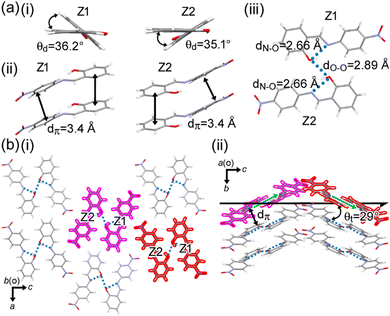 | ||
| Fig. 3 (a) (i) Dihedral angle, θd, between the two phenyl rings, (ii) distances between π-stacked molecules, (iii) hydrogen-bonding dimer, (b) (i) packing diagram, and (ii) side view of a right-handed two-fold helix by neighboring columns in 1O. The helical handedness is defined by tilt (green arrow) of the dimer according to the supramolecular-tilt-chirality method.60–62 | ||
 | ||
| Fig. 4 (a) (i) Dihedral angle between the two phenyl rings, (ii) distance between π-stacked molecules, (iii) hydrogen-bonding dimer, (b) (i) packing diagram, and (ii) side view of a right-handed two-fold helix by neighbouring columns in 1Y. The helical handedness is defined by tilt (green arrow) of the dimer according to the supramolecular-tilt-chirality method.60–62 | ||
There are two crystallographically independent molecules of Z1 and Z2 in 1O; the dihedral angles between the two phenyl groups in them are θd = 36.2° and 35.1°, respectively (Fig. 3a(i)). Such large θd could be one of the origins of the low emission intensity due to twisted intramolecular charge transfer.23,37,56–59 The π–π distance (dπ) between Z1 (or Z2) molecules is ca. 3.4 Å (Fig. 3a(ii)). In addition to the intramolecular hydrogen-bonding between hydroxyl and imino groups (N⋯O distance of dN–O = 2.66 Å), 1 forms an intermolecular hydrogen bond between the hydroxyl groups (O⋯O distance of dO–O = 2.89 Å) of Z1 and Z2 to afford a hydrogen-bonded dimer (Fig. 3a(iii)). The dimer tilts ca. 29° against the c axis and stacks along the b axis with a distance of dπ = 3.4 Å to form a columnar assembly (Fig. 3b). Two-fold helices in 1O are perpendicular to the stacking direction.
In contrast to 1 in 1O, 1 in 1Y is almost planar, i.e., the two phenyl groups are nearly parallel (θd = 3.8°) (Fig. 4a(i)). This value is much smaller than that noted in 1O (θd = 36.2°, 35.1°). Consistent with a previous report by Borbone et al.23θd and fluorescence lifetime are correlated, i.e., smaller dihedral angles result in longer fluorescence lifetime. dπ between Z1 molecules is 3.0–3.5 Å (Fig. 4a(ii)). Similar to 1O, 1 forms a hydrogen-bonding dimer with intra- and inter-molecular hydrogen-bonding N⋯O (dN–O = 2.66 Å) and O⋯O (dO–O = 3.00 Å), respectively (Fig. 4a(iii)). The dimer tilts ca. θt = 25° against the b axis and stacks with an inversion symmetry at dπ = 3.0–3.5 Å along the b axis to form a columnar assembly. Neighbouring columns are related by a two-fold helical symmetry along the stacking direction in a herringbone manner (Fig. 4b). The π-stacking manner of 1 is parallel in 1O while that in 1Y is antiparallel.
Based on the crystallographic studies, the domino transformation from 1O to 1Y coupled with an emission change is described by molecular conformational changes as well as molecular rearrangement in the crystal lattice. Phenyl rings rotate more than 30° during MDT, i.e., from θd = 36.2° and 35.1° in 1O to θd = 3.8° in 1Y. One of the possible molecular movements is schematically shown in Fig. 5. Hydrogen-bonding dimers in a column rotate clockwise or counterclockwise along with the conformational change. As a result, the dimers in a column and those in the neighbouring column stack alternately to form 1Y. Interestingly, electron microscope images of 1Y crystals often had cracks in the middle and/or jagged ends, while 1O crystals tended to be straighter (Fig. S1, ESI†). This might have resulted from internal stress caused by the transformation.
The domino transformation from 1O to 1Y by mechanical stimuli suggests that 1Y is more stable than 1O. The relative stability was evaluated by DFT calculations using the Quantum ESPRESSO program (Fig. S7 and Tables S4, S5, ESI†).63–65 The calculated lattice energies (Elattice) of 1O (n = 8) and 1Y (n = 4) are 228.3 and 116.4 kcal mol−1, respectively. The Elattice values per molecule (Elattice/n) for 1O and 1Y are 28.5 and 29.1 kcal mol−1, respectively, which shows that 1Y is more stable than 1O. Heating under an optical microscope showed that 1O melts at lower temperature (ca. 120 °C) than 1Y (ca. 160 °C), also suggesting the stability order.
Effects of the conformation (1 is planar in 1Y, while twisted in 1O) on fluorescence spectra were studied by time dependent DFT calculations using the Gaussian program.66 The torsional angles of four rotatable bonds were fixed in the geometry optimizations in the excited state. The optimized structures show that 1 has a keto form in the excited state (Fig. S7, ESI†). Although the calculated wavelengths of the fluorescence spectra are 50–70 nm longer than the experimental ones, the calculated wavelength in 1O is longer than that in 1Y, which agrees with experiments (Table S6, ESI†). This result indicates a crucial role of molecular conformation (twisting of two benzene rings) on the emission spectral redshift in 1O.
Conclusions
In conclusion, we discovered and characterized a Schiff base crystal of 1 showing mechanically-induced molecular domino transformation (MDT). Spectrometry revealed changes in emission color and intensity by the transformation (peak top shift of ca. 50 nm and over 9-fold increase of the intensity). Nanoindentation studies quantified the absolute values of the mechanical force necessary for the transformation (1 μN at the smallest). 1 is attractive as a sensing material because of the mechanical sensitivity and large emission change. MDT amplifies a local stimulus to a response by the whole crystal. Atomic level insights of the transformation mechanism were obtained by solving the crystal structure of a metastable 1O by MicroED. Other series of Schiff bases, which show polymorph-dependent emission differences, are under investigation to rationally improve and control properties suitable for sensing applications based on the fundamental understanding of MDT.Author contributions
TS, TN, AK, TN and GK contributed in sample preparation and crystallographic studies. YF and FI contributed in fluorescent measurements. YZ and UR contributed in nanoindentation measurements. ST contributed in DFT calculations. NK contributed in 1H NMR, 13C NMR and HR-MS. TS and RT initiated the project. TS wrote the draft and all of the authors contributed in finalizing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 22K05054 for T. S. and Research Support Project for Life Science and Drug Discovery (BINDS) from AMED under Grant Number JP22ama121001. R. T. thanks the Science and Engineering Research Board for funding under the Teachers Associateship for Research Excellence (TARE) grant (Project No. TAR/2021/000251). We thank Dr Yanagisawa and Dr Yamashita for their advice on semi-automatic MicroED data collection with SerialEM macros. We are also grateful to Dr Suzuki for pre-evaluation of crystals for MicroED by a fluorescence microscope.Notes and references
-
P. Bamfield and M. G. Hutching, Chromic Phenomena: Technological Applications of Colour Chemistry, Royal Society of Chemistry, 2nd edn, 2010 Search PubMed
.
- M. Kato, H. Ito, M. Hasegawa and K. Ishii, Chem. – Eur. J., 2019, 25, 5105 CrossRef CAS PubMed
.
- Y. Zheng, X. Jia, K. Li, J. Xu and X.-H. Bu, Adv. Energy Mater., 2021, 2100324 Search PubMed
.
- M. D. Cohen, G. M. J. Schmidt and S. Flavian, J. Chem. Soc., 1964, 2041 RSC
.
- J. Bregman, L. Leiserowitz and G. M. J. Schmidt, J. Chem. Soc., 1964, 2068 RSC
.
- T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685 CrossRef CAS PubMed
.
- T. Mutai, H. Tomoda, T. Ohkawa, Y. Yabe and K. Araki, Angew. Chem., Int. Ed., 2008, 47, 9522 CrossRef CAS PubMed
.
- Y. Hino and S. Hayashi, Chem. – Eur. J., 2021, 27, 17595 CrossRef CAS PubMed
.
- M. Fukushima, K. Sato, Y. Fujimoto, F. Ito and R. Katoh, Cryst. Growth Des., 2022, 22, 2071 CrossRef CAS
.
- Y. Sagara, T. Mutai, I. Yoshikawa and K. Araki, J. Am. Chem. Soc., 2007, 129, 1520 CrossRef CAS PubMed
.
- H. Ito, M. Muromoto, S. Kurenuma, S. Ishizaka, N. Kitamura, H. Sato and T. Seki, Nat. Commun., 2013, 4, 2009 CrossRef PubMed
.
- M. Jin, T. Sumitani, H. Sato, T. Seki and H. Ito, J. Am. Chem. Soc., 2018, 140, 2875 CrossRef CAS PubMed
.
- S. Hayashi and T. Koizumi, Angew. Chem., Int. Ed., 2016, 55, 2701 CrossRef CAS PubMed
.
- T. Mutai, T. Sasaki, S. Sakamoto, I. Yoshikawa, H. Houjou and S. Takamizawa, Nat. Commun., 2020, 11, 1824 CrossRef CAS PubMed
.
- B. Bhattacharya, D. Roy, S. Dey, A. Puthuvakkal, S. Bhunia, S. Mondal, R. Chowdhury, M. Bhattacharya, M. Mandal, K. Manoj, P. K. Mandal and C. M. Reddy, Angew. Chem., Int. Ed., 2020, 59, 19878 CrossRef CAS PubMed
.
- E. Hadjoudis and I. M. Mavridis, Chem. Soc. Rev., 2004, 33, 579 CAS
.
- K. Amimoto and T. Kawato, J. Photochem. Photobiol., C, 2005, 6, 207 CrossRef CAS
.
- S. Kobatake, S. Takami, H. Muto, T. Ishikawa and M. Irie, Nature, 2007, 446, 778 CrossRef CAS PubMed
.
- M. Kato, A. Omura, A. Toshikawa, S. Kishi and Y. Sugimoto, Angew. Chem., Int. Ed., 2002, 41, 3183 CrossRef CAS PubMed
.
- T. Ogoshi, Y. Shimada, Y. Sakata, S. Akine and T. A. Yamagishi, J. Am. Chem. Soc., 2017, 139, 5664 CrossRef CAS PubMed
.
- I. Hisaki, Y. Suzuki, E. Gomez, Q. Ji, N. Tohnai, T. Nakamura and A. Douhal, J. Am. Chem. Soc., 2019, 141, 2111 CrossRef CAS PubMed
.
- Z. Zhang, X. Song, S. Wang, F. Li, H. Zhang, K. Ye and Y. Wang, J. Phys. Chem. Lett., 2016, 7, 1697 CrossRef CAS PubMed
.
- F. Borbone, A. Tuzi, B. Panunzi, S. Piotto, S. Concilio, R. Shikler, S. Nabha and R. Centore, Cryst. Growth Des., 2017, 17, 5517 CrossRef CAS
.
- S. Varughese, M. S. R. N. Kiran, U. Ramamurty and G. R. Desiraju, Angew. Chem., Int. Ed., 2013, 52, 2701 CrossRef CAS PubMed
.
- M. K. Mishra, G. R. Desiraju, U. Ramamurty and A. D. Bond, Angew. Chem., Int. Ed., 2014, 53, 13102 CrossRef CAS PubMed
.
- M. K. Mishra, U. Ramamurty and G. R. Desiraju, Curr. Opin. Solid State Mater. Sci., 2016, 20, 361 CrossRef CAS
.
- R. Devarapalli, S. B. Kadambi, C.-T. Chen, G. R. Krishna, B. R. Kammari, M. J. Buehler, U. Ramamurty and C. M. Reddy, Chem. Mater., 2019, 31, 1391 CrossRef CAS
.
- T. Gruene, J. T. C. Wennmacher, C. Zaubitzer, J. J. Holstein, J. Heidler, A. Fecteau-Lefebvre, S. De Carlo, E. Müller, K. N. Goldie, I. Regeni, T. Li, G. Santiso-Quinones, G. Steinfeld, S. Handschin, E. van Genderen, J. A. van Bokhoven, G. H. Clever and R. Pantelic, Angew. Chem., Int. Ed., 2018, 57, 16313 CrossRef CAS PubMed
.
- S. Ito, F. J. White, E. Okunishi, Y. Aoyama, A. Yamano, H. Sato, J. D. Ferrara, M. Jasnowski and M. Meyer, CrystEngComm, 2021, 23, 8622 RSC
.
- C. G. Jones, M. W. Martynowycz, J. Hattne, T. J. Fulton, B. M. Stoltz, J. A. Rodriguez, H. M. Nelson and T. Gonen, ACS Cent. Sci., 2018, 4, 1587 CrossRef CAS PubMed
.
- J. A. Newman, L. Iuzzolino, M. Tan, P. Orth, J. Bruhn and A. Y. Lee, Mol. Pharm., 2022, 19, 2133 CrossRef CAS PubMed
.
- M. Lightowler, S. Li, X. Ou, X. Zou, M. Lu and H. Xu, Angew. Chem., Int. Ed., 2022, 61, e202114985 CrossRef CAS PubMed
.
- J. Hitchen, I. Andrusenko, C. L. Hall, E. Mugnaioli, J. Potticary, M. Gemmi and S. R. Hall, Cryst. Growth Des., 2022, 22, 1155 CrossRef CAS
.
- T. Sasaki, T. Nakane, A. Kawamoto, T. Nishizawa and G. Kurisu, CrystEngComm, 2023, 25, 352 RSC
.
- D. Gogoi, T. Sasaki, T. Nakane, A. Kawamoto, H. Hojo, G. Kurisu and R. Thakuria, Cryst. Growth Des., 2023, 23, 5821 CrossRef CAS
.
- T. Mutai, H. Shono, Y. Shigemitsu and K. Araki, CrystEngComm, 2014, 16, 3890 RSC
.
- H. Konoshima, S. Nagao, I. Kiyota, K. Amimoto, N. Yamamoto, M. Sekine, M. Nakata, K. Furukawa and H. Sekiya, Phys. Chem. Chem. Phys., 2012, 14, 16778 RSC
.
- V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169 RSC
.
- S. Katsumi, M. Saigusa and F. Ito, J. Phys. Chem. B, 2022, 126, 976 CrossRef CAS PubMed
.
- A. D. Bond, R. Boese and G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 618 CrossRef CAS PubMed
.
- Deposition numbers CCDC 2279131/COD-3000450 (for 1O) and CCDC 2279132/COD-3000451 (for 1Y) contain the supplementary crystallographic data for this paper†.
- Raw diffraction images of 1O and 1Y XRDa-142 are provided free of charge by Xtal Raw Data Archive (XRDa). Scripts for MicroED data collection and processing are available at https://github.com/GKLabIPR/MicroED.
- C. J. H. Smalley, H. E. Hoskyns, C. E. Hughes, D. N. Johnstone, T. Willhammar, M. T. Young, C. J. Pickard, A. J. Logsdail, P. A. Midgley and K. D. M. Harris, Chem. Sci., 2022, 13, 5277 RSC
.
- H. Lu, T. Nakamuro, K. Yamashita, H. Yanagisawa, O. Nureki, M. Kikkawa, H. Gao, J. Tian, R. Shang and E. Nakamura, J. Am. Chem. Soc., 2020, 142, 18990 CrossRef CAS PubMed
.
- H. Hamada, T. Nakamuro, K. Yamashita, H. Yanagisawa, O. Nureki, M. Kikkawa, K. Harano, R. Shang and E. Nakamura, Bull. Chem. Soc. Jpn., 2020, 93, 776 CrossRef
.
- G. Winter, D. G. Waterman, J. M. Parkhurst, A. S. Brewster, R. J. Gildea, M. Gerstel, L. Fuentes-Montero, M. Vollmar, T. Michels-Clark, I. D. Young, N. K. Sauter and G. Evans, Acta Crystallogr., Sect. D: Struct. Biol., 2018, 74, 85 CrossRef CAS PubMed
.
- M. T. B. Clabbers, T. Gruene, J. M. Parkhurst, J. P. Abrahams and D. G. Waterman, Acta Crystallogr., Sect. D: Struct. Biol., 2018, 74, 506 CrossRef CAS PubMed
.
- J. Beilsten-Edmands, G. Winter, R. Gildea, J. Parkhurst, D. Waterman and G. Evans, Acta Crystallogr., Sect. D: Struct. Biol., 2020, 76, 385 CrossRef CAS PubMed
.
- R. J. Gildea, J. Beilsten-Edmands, D. Axford, S. Horrell, P. Aller, J. Sandy, J. Sanchez-Weatherby, C. D. Owen, P. Lukacik, C. Strain-Damerell, R. L. Owen, M. A. Walsh and G. Winter, Acta Crystallogr., Sect. D: Struct. Biol., 2022, 78, 752 CrossRef CAS PubMed
.
- J. Burgess, J. Fawcett, D. R. Russell, S. R. Gilani and V. Palma, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 1707 CrossRef
.
- T. Kondori, N. Akbarzadeh-T, M. Fazli, B. Mir, M. Dušek and V. Eigner, J. Mol. Struct., 2021, 1226, 129395 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef PubMed
.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed
.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Cryst., 2009, 42, 339 CrossRef CAS
.
- L. J. Bourhis, O. V. Dolomanov, R. J. Gildea, J. A. K. Howard and H. Puschmann, Acta Crystallogr., Sect. A: Found. Crystallogr., 2015, 71, 59 CrossRef CAS PubMed
.
- M. I. Knyazhansky, A. V. Metelitsa, A. J. Bushkov and S. M. Aldoshin, J. Photochem. Photobiol., A, 1996, 97, 121 CrossRef CAS
.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef PubMed
.
- S. Sasaki, G. P. C. Drummen and G.-I. Konishi, J. Mater. Chem. C, 2016, 4, 2731 RSC
.
- J. Shi, S.-J. Yoon, L. Viani, S. Y. Park, B. Milián-Medina and J. Gierschner, Adv. Opt. Mater., 2017, 5, 1700340 CrossRef
.
- I. Hisaki, T. Sasaki, N. Tohnai and M. Miyata, Chem. – Eur. J., 2012, 18, 10066 CrossRef CAS PubMed
.
- T. Sasaki, I. Hisaki, T. Miyano, N. Tohnai, K. Morimoto, H. Sato, S. Tsuzuki and M. Miyata, Nat. Commun., 2013, 4, 1787 CrossRef PubMed
.
- M. Miyata, N. Tohnai, I. Hisaki and T. Sasaki, Symmetry, 2015, 7, 1914 CrossRef CAS
.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed
.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. Buongiorno Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. Dal Corso, S. de Gironcoli, P. Delugas, R. A. DiStasio, A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H.-Y. Ko, A. Kokalj, E. Küçükbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H.-V. Nguyen, A. Otero-de-la-Roza, L. Paulatto, S. Poncé, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, J. Phys.: Condens. Matter, 2017, 29, 465901 CrossRef CAS PubMed
.
- T. Sasaki, T. Nakane, A. Kawamoto, Y. Zhao, Y. Fujimoto, T. Nishizawa, S. Tsuzuki, F. Ito, U. Ramamurty, R. Thakuria and G. Kurisu, Data for “Mechanically-Sensitive Fluorochromism by Molecular Domino Transformation in a Schiff Base Crystal”, zenodo, 2024 DOI:10.5281/zenodo.10476969
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision A.03, Gaussian Inc., Wallingford CT, 2016 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Cryo-EM images, distribution of unit cell parameters, crystallographic data, powder X-ray diffraction patterns, theoretical calculation results, fluorescence lifetimes, and Movie S1 (molecular domino transformation of 1O). CCDC 2279131 and 2279132. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc00406j |
| This journal is © The Royal Society of Chemistry 2024 |


