Open Access Article
Open Access ArticleDual control of stereoregularity and molecular weight in cationic polymerization of vinyl ether by tunable TADDOLs/TiCl4 initiating systems†
Hironobu
Watanabe
 ,
Yuji
Mishima
,
Arihiro
Kanazawa
,
Yuji
Mishima
,
Arihiro
Kanazawa
 and
Sadahito
Aoshima
and
Sadahito
Aoshima
 *
*
Department of Macromolecular Science, Osaka University, Machikaneyama, Toyonaka, Osaka 560-0043, Japan. E-mail: aoshima@chem.sci.osaka-u.ac.jp
First published on 7th February 2024
Abstract
The effects of aryl substituents on α,α,α′,α′-tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL) ligands were investigated to develop a TADDOL/TiCl4 system that simultaneously controls the stereoregularity and molecular weight of poly(isobutyl vinyl ether). Substituents were appropriately designed to generate polymers with relatively high m values (80–90%) and narrow molecular weight distributions (Mw/Mn = 1.1–1.4) at 0 °C and −78 °C. The steric and electronic effects of TADDOL substituents on the stereoregularity and molecular weight were investigated. Interestingly, conventional fast equilibrium between the dormant C–Cl bonds and active species was likely absent at −78 °C, while the polymerization was mediated by long-lived species, resulting in a linear increase in molecular weights with monomer consumption. This study provides significant insight for the development of high-performance polymerization catalysts through which multiple polymer structures can be simultaneously controlled.
Introduction
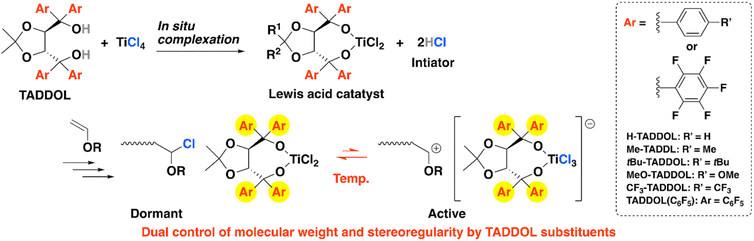
Over the past few decades, numerous efforts have been dedicated to precisely controlling properties of polymer structures, such as their molecular weight (MW), stereoregularity, monomer sequence, and topology, because these properties are closely linked with the properties and functions of polymers.1 MW and stereoregularity are essential elements of polymer structures but are difficult to simultaneously control. Living polymerization2–4 and stereospecific polymerization5–8 are difficult to achieve in natural cationic polymerization of vinyl monomers because the propagating species are highly reactive planar sp2 carbocations. Nevertheless, progress in polymerization methodologies has enabled various systems to perform living cationic polymerization by suppressing side reactions with appropriate initiators, catalysts, and additives.9–18 A crucial step in controlling MW is to promote a fast equilibrium between the active and dormant species, through which all propagating chains can evenly propagate at similar rates. Performing stereospecific cationic polymerization is more difficult, although several research groups have created novel stereospecific cationic polymerization systems.19–37 Examples of systems that can simultaneously control stereoregularity and MW in cationic polymerization of vinyl monomers are limited. A few examples have been achieved by combining stereospecific cationic polymerization and degenerative chain-transfer (DT) mechanisms, such as the reversible addition-fragmentation chain transfer (RAFT) mechanism.28,31,34 In these cases, the counteranions that formed from special initiators and the chain transfer agents separately control the stereoregularity and MW, respectively. On the other hand, a novel high-performance catalyst could control the stereoregularity by forming a special counteranion and control the MW by an atom-transfer mechanism. These catalysts could contribute to the development of polymer chemistry but have been rarely reported in cationic polymerization.22,23,33Previously, we reported the stereospecific cationic polymerization of vinyl ethers (VEs) by using a new initiating system that combined TiCl4 and a tartaric acid-derived ligand, α,α,α′,α′-tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL).26 Through this initiating system, stereospecific polymerization of VEs (m = 83–94%) was achieved, but MW control of the resultant polymers was not achieved. We also reported stereospecific living cationic polymerization of N-vinylcarbazole by designing zinc-based polymerization catalysts.23,33 These results motivated us to further develop the TADDOL/TiCl4 initiating system to simultaneously control the stereoregularity and MW of poly(VE)s. The excellent tunability of TADDOL substituents38,39 will greatly help researchers design initiating systems.
Here, by investigating the effect of TADDOL substituents and reaction conditions, we simultaneously controlled the stereoregularity and MW of poly(VE) with TADDOL/TiCl4 systems. In this system, the initiator and catalyst are generated by mixing the TADDOL ligands and TiCl4 (Scheme 1).40 We show that the TADDOL substituents and the polymerization temperature are the key factors that control the stereoregularity and MW. We also examine the relationship between the electronic/steric effect of TADDOL substituents and the MW/stereoregularity of the resultant polymers.
Experimental section
See the ESI† for the procedure for TADDOLs synthesis and characterization.Materials
Isobutyl vinyl ether (IBVE) (TCI; >99.0%) and ethyl vinyl ether (EVE) (TCI; >98.0%) were washed with 10% aqueous sodium hydroxide solution and then with water, dried overnight over potassium hydroxide, and distilled twice over calcium hydride before use. (4S,5S)-(−)-2,2-Dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol (H-TADDOL) (TCI; >97.0%) was used without further purification. Ethyl acetate (Wako; >99.5%) was distilled twice over calcium hydride. Commercially obtained TiCl4 (Aldrich; 1.0 M solution in toluene) was used without further purification. 1-(Isobutoxy)ethyl chloride [IBVE–HCl; CH3CH(OiBu)Cl] was prepared via an addition reaction of IBVE with dry HCl, according to a reported method.41 Hexane (Wako; 96.0%), toluene (Wako; 99.5%), and dichloromethane (Wako; 99.0%) were dried by passage through solvent purification columns (glass contour).Procedure for TADDOLs synthesis
TADDOLs were synthesized via the Grignard reaction according to the reported method (Scheme 2).42,43 The typical procedure is described in the ESI.†Polymerization procedure
The typical polymerization procedure is as follows. A glass tube equipped with a three-way stopcock was dried using a heat gun (Ishizaki, PJ-206A; blow temperature ∼450 °C) under dry nitrogen. Hexane and IBVE were sequentially added to the tube using dry syringes. In another tube, a TADDOL solution was added to a solution of TiCl4 in toluene, and the mixture was left at −78 °C for at least 30 min. The polymerization was initiated by adding the Ti-TADDOLate solution to the monomer solution at −78 °C. After a predetermined time, the reaction was terminated by adding a methanol solution that contained a small amount of aqueous ammonia. The quenched mixture was washed with water. Then, the volatiles were removed under reduced pressure at 50 °C to yield the polymer. The monomer conversion was determined by gas chromatography (column packing material: PEG-20MUniPort B; GL Sciences Inc.) using hexane as an internal standard.Results and discussion
Effects of polymerization temperature on polymerization using the HT-ADDOL/TiCl4 initiating system
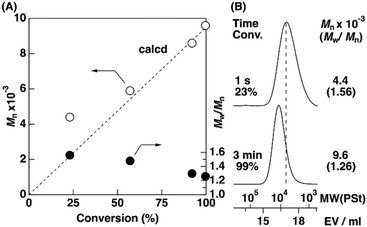
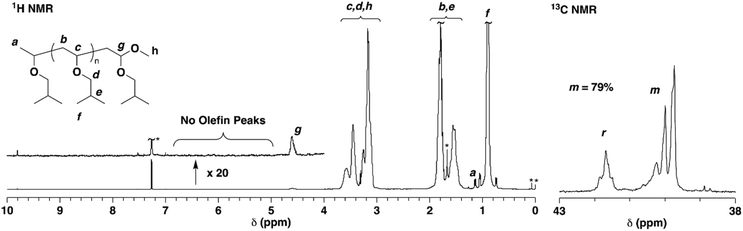
In cationic polymerization, the MWs of polymers are typically controlled via a fast equilibrium between dormant and active species. We assumed that the interconversion between the dormant and active species in the TADDOL/TiCl4 initiating system would change depending on the polymerization temperature. Therefore, the cationic polymerization of IBVE was conducted with the H-TADDOL/TiCl4 initiating system (Scheme 1) in hexane/toluene (9/1 v/v) at various temperatures (−78 to 30 °C). Fig. 1 (upper) shows the MWD curves of the obtained polymers. A polymer generated at −78 °C exhibited a high MW and broad molecular weight distribution (MWD) (Mn = 60.5 × 103, Mw/Mn = 4.75), as we reported previously.26 When the polymerization was conducted at −40 °C, the number-average molecular weight (Mn) approached the theoretical value (7.7 × 103), while the MWD remained broad (Mn = 13.6 × 103, Mw/Mn = 3.26). Notably, the polymers obtained at 0 °C possessed MWs close to the theoretical value and narrow MWDs (Mn = 8.1 × 103, Mw/Mn = 1.24; Fig. 1C). The MWs of the polymers increased with increasing monomer conversion (Fig. 2). Structures resulting from side reactions were not observed by 1H NMR even for the polymers obtained at quantitative conversion (Fig. S1†). MW control was achieved with the TADDOL/TiCl4 system even at 30 °C (Mn = 8.0 × 103, Mw/Mn = 1.19; Fig. 1D).The isotacticity of the generated polymers increased with decreasing temperature {up to m = 87% at −78 °C, Fig. 1 (bottom) and Fig. S2†}. The polymer obtained at 0 °C had a relatively high m ratio of 80%, demonstrating that MW and stereoregularity were simultaneously controlled. From these results, we found that the polymerization temperature greatly impacted the ability to control MW and stereoregularity. Therefore, we further investigated the cationic polymerization at different temperatures, namely, at 0 °C and −78 °C, by using various TADDOLs.
Investigation of TADDOL substituents in cationic polymerization of IBVE at 0 °C
Cationic polymerization of IBVE was conducted at 0 °C using TADDOLs with various substituents. TADDOLs with different Ar groups {Ar = 4-R′C6H4 (R′ = Me, tBu, OMe, CF3) or C6F5, Scheme 1} were synthesized via Grignard reactions between a tartaric acid-derived ester and the corresponding aryl bromides (Scheme 2).42,43 The substituents of TADDOL greatly affected the polymerization behavior (Table 1). In particular, the Mn values of the poly(IBVE)s obtained at the early stage of the polymerization with Me-TADDOL were closer to the theoretical values (Fig. 3A) than those with H-TADDOL (Fig. 2A). In addition, the poly(IBVE)s obtained with Me-TADDOL exhibited obviously narrower MWDs (Mw/Mn ∼1.10) than those obtained with H-TADDOL. These characteristics indicate that efficient, fast initiation reactions occurred when Me-TADDOL was used. Side reactions were not observed by 1H NMR (Fig. 4). Furthermore, the living nature of the polymerization with Me-TADDOL was confirmed by a monomer-addition experiment (Fig. 3B). On the other hand, the MWs of polymers obtained with tBu-, MeO-, and CF3-TADDOLs were uncontrollable and the MWDs of the polymers were broad. Notably, the stereoregularity of polymers obtained with Me- and H-TADDOL (m = 79% and 81%, respectively) was higher than that with other TADDOLs. These results showed the potential of the TADDOL/TiCl4 initiating system for stereospecific living cationic polymerization. The steric and electronic effects of the TADDOL substituent on the stereoregularity and MW are discussed below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Ar | Time | Conv. (%) | M n × 10−3 (calcd)b | M n × 10−3 (obs)c | M w/Mnc | m (%) |
|---|---|---|---|---|---|---|---|
| a [VE]0 = 0.76 M, [TADDOL]0 = 4.4 mM (for entry 1), 4.0 mM (for entries 2, 3, and 5), or 5.0 mM (for entry 4), [TiCl4]0 = 4.0 mM (for entries 1–3 and 5) or 5.0 mM (for entry 4). b Based on the two protons per TADDOL/TiCl4 complex. c Calculated with polystyrene standards. | |||||||
| 1 | C6H5 | 3 min | 99 | 9.5 | 9.6 | 1.26 | 81 |
| 2 | 4-MeC6H4 | 8 min | ∼100 | 9.6 | 11.0 | 1.08 | 79 |
| 3 | 4-tBuC6H4 | 2 h | 10 | 0.1 | 18.6 | 7.90 | 61 |
| 4 | 4-MeOC6H4 | 2 h | 95 | 7.3 | 9.9 | 23.5 | 67 |
| 5 | 4-CF3C6H4 | 2 s | ∼100 | 9.6 | 13.6 | 3.11 | 74 |
Investigation of the TADDOL substituent in the cationic polymerization of IBVE at −78 °C
We next investigated methodologies to control the stereoregularity and MW using TADDOLs with various substituents at −78 °C to attain higher stereoregularity. The polymerization was conducted at lower monomer concentrations ([VE]0 = 0.10 or 0.20 M) because the reaction solution remained homogeneous and the stereoregularity of the resultant polymers increased at low monomer concentrations, as described in the previous paper.26 Various weakly Lewis basic additives,15 such as esters and ethers, that are typically effective for the living cationic polymerization of VEs were not effective in controlling MWs in this initiating system (Table S1†). In contrast, the substituents of TADDOL had a great impact even at this temperature, although the tendency was different from that at 0 °C (Table 2 and Fig. 5). H- and Me-TADDOLs generated polymers with high stereoregularity (m = 90% and 91%, respectively), whereas the MWDs were broad (entries 1 and 2 in Table 2). tBu- and MeO-TADDOLs generated polymers with broad MWDs and lower m values (entries 3 and 4 in Table 2). TADDOL with electron-withdrawing C6F5 groups formed a polymer with an MW closer to the theoretical value with a relatively narrow MWD, while the stereoregularity was low (entry 5 in Table 2). | ||
| Fig. 5 MWD curves of the poly(IBVE)s obtained using various TADDOLs {[IBVE]0 = 0.10 M, [TADDOL]0 = 5.5 mM, [TiCl4]0 = 5.0 mM, in toluene or hexane/toluene (9/1 v/v) at −78 °C }. The data correspond to entries 2, 3, 4, and 5 in Table 2. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Ar | Solvent (v/v) | Time | Conv. (%) | M n × 10−3 (calcd)b | M n × 10−3 (obs)c | M w/Mnc | m (%) |
|---|---|---|---|---|---|---|---|---|
| a [IBVE]0 = 0.10 M, [TADDOL]0 = 5.5 mM, [TiCl4]0 = 5.0 mM. b Based on the amount of TADDOL/TiCl4 complex. c Calculated with polystyrene standards. d Ethyl acetate (5.0 mM) was added. | ||||||||
| 1 | C6H5 | Hexane/toluene (9/1) | 1 min | ∼100 | 1.0 | 84.9 | 2.63 | 90 |
| 2 | 4-MeC6H4 | Hexane/toluene (9/1) | 1 min | ∼100 | 1.0 | 49.9 | 2.54 | 91 |
| 3 | 4-tBuC6H4 | Hexane/toluene (9/1) | 15 min | ∼100 | 1.0 | 6.1 | 17.5 | 79 |
| 4 | 4-MeOC6H4 | Hexane/toluene (9/1) | 2 h | 29 | 0.3 | 14.7 | 2.28 | 81 |
| 5 | C6F5 | Toluene | 30 s | ∼100 | 1.0 | 4.6 | 1.39 | 71 |
| 6 | 4-CF3C6H4 | Hexane/toluene (9/1) | 30 s | ∼100 | 1.0 | 7.3 | 1.81 | 83 |
| 7 | Toluene | 30 s | ∼100 | 1.0 | 7.7 | 1.45 | 84 | |
| 8d | Hexane/toluene (9/1) | 30 min | ∼100 | 1.0 | 66.2 | 1.40 | 90 | |
Notably, CF3-TADDOL generated polymers with relatively narrow MWDs and high stereoregularity at −78 °C (m = 83–84%; entries 6 and 7 in Table 2). Interestingly, adding a small amount of ethyl acetate (entry 8), which functions as a weak Lewis base and potentially interacts with the Ti complex, slowed the reaction and increased the isotacticity (m = 90%, Fig. 6C) although excess ethyl acetate (twice the amounts of Ti) decreased the m value (entry 3 in Table S2†). Moreover, the MWs of the resultant polymers increased with increasing monomer conversion while maintaining relatively narrow MWDs (Mw/Mn = 1.2–1.4; Fig. 6A and B), which indicates that long-lived propagating species formed. Although the initiation efficiency was low, this result demonstrates that MW control and high stereoregularity were simultaneously achieved by designing the TADDOL substituent. We also confirmed a polymer with a higher molecular weight (conv. ∼100%, Mn = 466![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.67) was obtained at a higher IBVE concentration ([IBVE]0 = 0.76 M) (entry 4 in Table S2†) although the reaction mixture became heterogeneous because of the polymer precipitation.
000, Mw/Mn = 1.67) was obtained at a higher IBVE concentration ([IBVE]0 = 0.76 M) (entry 4 in Table S2†) although the reaction mixture became heterogeneous because of the polymer precipitation.
 | ||
| Fig. 6 (A) Mn and Mw/Mn values, (B) MWD curves, and (C) 13C NMR spectrum of the poly(IBVE)s obtained by the CF3-TADDOL/TiCl4/ethyl acetate initiating system at −78 °C {[IBVE]0 = 0.10 M, [CF3-TADDOL]0 = 5.5 mM, [TiCl4]0 = 5.0 mM, [ethyl acetate]0 = 5.0 mM in hexane/toluene (9/1 v/v)}. The data correspond to entry 8 in Table 2. | ||
Steric and electronic effects of TADDOL substituents on the control of stereoregularity and MW
In contrast, the MWs of the obtained polymers were much higher than the theoretical values at any conversion at −78 °C (Fig. 8B), even when IBVE–HCl was used. IBVE–HCl did not affect the MWs of the resultant polymers (Fig. 8B, red), suggesting that dormant C–Cl species were scarcely activated by the catalyst at this temperature and that one Ti-TADDOLate counteranion probably attached to one propagating chain end (Scheme S1B†). Very small amounts of propagating carbocations were rapidly generated by the CF3-TADDOL/TiCl4/ethyl acetate initiating system, and subsequently, the generated carbocations with the bulky Ti-TADDOLate counteranion propagated without undergoing side reactions. These results indicate that MW control may have occurred with “long-lived” propagating cations that do not rely on conventional fast dormant-active equilibrium, and this process occurred in a similar manner to living anionic polymerization.
To reveal the nature of the propagating species, the effect of polymerization conditions was further investigated. Solvents and temperatures exhibited an extraordinary impact on the reaction rate with the TADDOL/TiCl4 initiating system (Table S4†). Surprisingly, the reaction was accelerated with decreasing solvent polarity (dielectric constant) and temperature. This is opposite to the behavior observed in many ionic reactions. The temperature effect suggests that formation of dormant species may be supressed at low temperatures.47 In addition, considering that many titanium catalysts form dimers (oligomers),48–50 lower solvent polarity and lower temperature possibly enhance dimerization or oligomerization of titanium catalysts. These “assembled” catalysts most likely possess higher activity than their monomeric counterparts. Previous studies revealed that the cationic polymerization of isobutene with TiCl4 accelerates at lower temperatures and is catalyzed by two TiCl4 per active species.51–53 The trend is similar to that found in this study, although increasing solvent polarity accelerates the isobutene polymerization reaction. The assembly of the Ti-TADDOLate catalyst may be responsible for the unique nature of the propagating species shown above at −78 °C.
Conclusion
Simultaneous control of the MWs and stereoregularity of poly(IBVE) was achieved with the TADDOL/TiCl4 initiating system by utilizing appropriate TADDOL substituents. H- and Me-TADDOL generated polymers with narrow MWDs and a relatively high stereoregularity at 0 °C. The use of CF3-TADDOL led to stereospecific cationic polymerization of IBVE with long-lived propagating species even at −78 °C, whereas the nature of the propagating species was different from that at 0 °C. An appropriate steric combination of VE pendant and Ti-TADDOLate complex was responsible for the stereospecific polymerization. This study provides advances in the precise design of catalysts as in organic chemistry and coordination polymerization, which has been difficult to achieve in cationic polymerization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI grant numbers 18J20555 and 17H03068.References
- D. J. Walsh, M. G. Hyatt, S. A. Miller and D. Guironnet, ACS Catal., 2019, 9, 11153–11188 CrossRef CAS.
- A. H. E. Müller and K. Matyjaszewski, Controlled and Living Polymerizations: From Mechanisms to Applications, Wiley-VCH, Weinheim, 2009 Search PubMed.
- R. B. Grubbs and R. H. Grubbs, Macromolecules, 2017, 50, 6979–6997 CrossRef CAS.
- G. Polymeropoulos, G. Zapsas, K. Ntetsikas, P. Bilalis, Y. Gnanou and N. Hadjichristidis, Macromolecules, 2017, 50, 1253–1290 CrossRef CAS.
- G. W. Coates, Chem. Rev., 2000, 100, 1223–1252 CrossRef CAS PubMed.
- E. Y. -X. Chen, Chem. Rev., 2009, 109, 5157–5214 CrossRef CAS PubMed.
- K. Hatada and T. Kitayama, Polym. Int., 2000, 49, 11–47 CrossRef CAS.
- A. J. Teator, T. P. Varner, P. C. Knutson, C. C. Sorensen and F. A. Leibfarth, ACS Macro Lett., 2020, 9, 1638–1654 CrossRef CAS PubMed.
- M. Sawamoto, Prog. Polym. Sci., 1991, 16, 111–172 CrossRef CAS.
- J. P. Kennedy and B. Ivan, Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice, Hanser Publishers, New York, 1992 Search PubMed.
- J. P. Kennedy, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2285–2293 CrossRef CAS.
- J. E. Puskas and G. Kaszas, Prog. Polym. Sci., 2000, 25, 403–452 CrossRef CAS.
- P. De and R. Faust, in Macromolecular Engineering. Precise Synthesis, Materials Properties, Applications; ed. K. Matyjaszewski, Y. Gnanou and L. Leibler, Wiley-VCH, Weinheim, 2007 Search PubMed.
- E. J. Goethals and F. Du Prez, Prog. Polym. Sci., 2007, 32, 220–246 CrossRef CAS.
- S. Aoshima and S. Kanaoka, Chem. Rev., 2009, 109, 5245–5287 CrossRef CAS PubMed.
- Y. Chen, L. Zhang, Y. Jin, X. Lin and M. Chen, Macromol. Rapid Commun., 2021, 42, 2100148 CrossRef CAS PubMed.
- M. Uchiyama, K. Satoh and M. Kamigaito, Prog. Polym. Sci., 2022, 124, 101485 CrossRef CAS.
- Examples of recent studies on living cationic polymerization: (a) M. Uchiyama, K. Satoh and M. Kamigaito, Angew. Chem., Int. Ed., 2015, 54, 1924 CrossRef CAS PubMed; (b) V. Kottisch, Q. Michaudel and B. P. Fors, J. Am. Chem. Soc., 2016, 138, 15535 CrossRef CAS PubMed; (c) K. Takagi, K. Yamauchi and H. Murakata, Chem. – Eur. J., 2017, 23, 9495 CrossRef CAS PubMed; (d) V. Kottisch, J. O'Leary, Q. Michaudel, E. E. Stache, T. H. Lambert and B. P. Fors, J. Am. Chem. Soc., 2019, 141, 10605 CrossRef CAS PubMed; (e) R. Haraguchi, T. Nishikawa, A. Kanazawa and S. Aoshima, Macromolecules, 2020, 53, 4185 CrossRef CAS; (f) V. Kottisch, J. Jermaks, J. Mak, R. A. Woltornist, T. H. Lambert and B. P. Fors, Angew. Chem., Int. Ed., 2021, 60, 4535 CrossRef CAS PubMed; (g) K. Takagi, H. Murakata and T. Hasegawa, Macromolecules, 2022, 55, 5756 CrossRef CAS; (h) M. Li, Z. Zhang, Y. Yan, W. Lv, Z. Li, X. Wang and Y. Tao, Nat. Synth., 2022, 1, 815 CrossRef; (i) M. Li, H. Li, X. Zhang, X. Wang and Y. Tao, Angew. Chem., 2023, 135, e202303237 CrossRef.
- M. Ouchi, M. Kamigaito and M. Sawamoto, Macromolecules, 1999, 32, 6407–6411 CrossRef CAS.
- M. Ouchi, M. Kamigaito and M. Sawamoto, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1060–1066 CrossRef CAS.
- M. Ouchi, M. Kamigaito and M. Sawamoto, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1067–1074 CrossRef CAS.
- P. Sudhakar and K. Vijayakrishna, ChemCatChem, 2010, 2, 649–652 CrossRef CAS.
- H. Watanabe, A. Kanazawa and S. Aoshima, ACS Macro Lett., 2017, 6, 463–467 CrossRef CAS PubMed.
- A. J. Teator and F. A. Leibfarth, Science, 2019, 363, 1439–1443 CrossRef CAS PubMed.
- A. J. Teator, T. P. Varner, P. E. Jacky, K. A. Sheyko and F. A. Leibfarth, ACS Macro Lett., 2019, 8, 1559–1563 CrossRef CAS PubMed.
- H. Watanabe, T. Yamamoto, A. Kanazawa and S. Aoshima, Polym. Chem., 2020, 11, 3398–3403 RSC.
- T. P. Varner, A. J. Teator, Y. Reddi, P. E. Jacky, C. J. Cramer and F. A. Leibfarth, J. Am. Chem. Soc., 2020, 142, 17175–17186 CrossRef CAS PubMed.
- M. Uchiyama, K. Satoh and M. Kamigaito, Giant, 2021, 5, 100047 CrossRef CAS.
- P. C. Knutson, A. J. Teator, T. P. Varner, C. T. Kozuszek, P. E. Jacky and F. A. Leibfarth, J. Am. Chem. Soc., 2021, 143, 16388–16393 CrossRef CAS PubMed.
- Z. Yang, X. Zhang, Y. Jiang, Q. Ma and S. Liao, Sci. China: Chem., 2022, 65, 304–308 CrossRef CAS.
- X. Zhang, Z. Yang, Y. Jiang and S. Liao, J. Am. Chem. Soc., 2022, 144, 679–684 CrossRef CAS PubMed.
- C. C. Sorensen and F. A. Leibfarth, J. Am. Chem. Soc., 2022, 144, 8487–8492 CrossRef CAS PubMed.
- H. Watanabe, A. Kanazawa, S. Okumoto and S. Aoshima, Macromolecules, 2022, 55, 4378–4388 CrossRef CAS.
- M. Uchiyama, D. Watanabe, Y. Tanaka, K. Satoh and M. Kamigaito, J. Am. Chem. Soc., 2022, 144, 10429–10437 CrossRef CAS PubMed.
- H. Zhu, Y. Jiang, Z. Yang, X. Zhang and S. Liao, Giant, 2023, 14, 100151 CrossRef CAS.
- C. C. Sorensen, C. T. Kozuszek, M. A. Borden and F. A. Leibfarth, ACS Catal., 2023, 13, 3272–3284 CrossRef CAS.
- C. C. Sorensen, V. Bhat, A. Y. Bello and F. A. Leibfarth, ACS Catal., 2023, 13, 12163–12172 CrossRef CAS.
- D. Seebach, A. K. Beck and A. Heckel, Angew. Chem., Int. Ed., 2001, 40, 92–138 CrossRef CAS PubMed.
- Examples of crystal structure of TADDOL complexes: (a) A. Hafner, R. O. Duthaler, R. Marti, G. Rihs, P. Rothe-Streit and F. Schwarzenbach, J. Am. Chem. Soc., 1992, 114, 2321 CrossRef CAS; (b) D. Seebach, D. A. Plattner, A. K. Beck, Y. M. Wang, D. Hunziker and W. Petter, Helv. Chim. Acta, 1992, 75, 2171 CrossRef CAS; (c) Y. N. Ito, X. Ariza, A. K. Beck, A. Boháč, C. Ganter, R. E. Gawley, F. N. M. Kühnle, J. Tuleja, Y. M. Wang and D. Seebach, Helv. Chim. Acta, 1994, 77, 2071 CrossRef CAS.
- In our previous study (ref. 26), similar polymerization results were obtained from the in situ generated catalyst and an isolated catalyst.
- T. Higashimura, M. Kamigaito, M. Kato, T. Hasebe and M. Sawamoto, Macromolecules, 1993, 26, 2670–2673 CrossRef CAS.
- D. Seebach, A. K. Beck, R. Imwinkelzied, S. Roggo and A. Wonnacott, Helv. Chim. Acta, 1987, 70, 954–974 CrossRef CAS.
- X. Gao, J. Han and L. Wang, Org. Lett., 2015, 17, 4596–4599 CrossRef CAS PubMed.
- A. Verloop, in Drug Design, ed. E. J. Ariens, Academic Press, New York, 1976, vol. III, pp. 133 Search PubMed.
- K. C. Harper, E. N. Bess and M. S. Sigman, Nat. Chem., 2012, 4, 366–374 CrossRef CAS PubMed.
- A. Berkessel, S. S. Vormittag, N. E. Schlörer and J.-M. Neudörfl, J. Org. Chem., 2012, 77, 10145–10157 CrossRef CAS PubMed.
- L. Sipos, P. De and R. Faust, Macromolecules, 2003, 36, 8282–8290 CrossRef CAS.
- M. G. Finn and K. B. Sharpless, J. Am. Chem. Soc., 1991, 113, 113–126 CrossRef CAS.
- T. J. Boyle, N. W. Eilerts, J. A. Heppert and F. Takusagawa, Organometallics, 1994, 13, 2218–2229 CrossRef CAS.
- S. P. Webb and M. S. Gordon, J. Am. Chem. Soc., 1999, 121, 2552–2560 CrossRef CAS.
- Q. A. Thomas and R. F. Storey, Macromolecules, 2003, 36, 10120–10125 CrossRef CAS.
- R. F. Storey and K. R. Choate, Macromolecules, 1997, 30, 4799–4806 CrossRef CAS.
- J. E. Puskas, S. W. P. Chan, K. B. McAuley, S. Shaikh and G. Kaszas, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5394–5413 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, NMR spectra, and polymerization data. See DOI: https://doi.org/10.1039/d3py01353g |
| This journal is © The Royal Society of Chemistry 2024 |