Emergence of graphene as a novel nanomaterial for cardiovascular applications
Paniz
Memarian†
ab,
Zohreh
Bagher†
cd,
Sheida
Asghari
e,
Mina
Aleemardani
 *fg and
Alexander
Seifalian
*fg and
Alexander
Seifalian
 *a
*a
aNanotechnology and Regenerative Medicine Commercialization Centre, London BioScience Innovation Centre, London, UK. E-mail: alex@nanoregmed.com; p.memarian48@gmail.com; a.seifalian@gmail.com; Tel: +447985380797
bDepartment of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran
cENT and Head and Neck Research Center and Department, The Five Senses Health Institute, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. E-mail: baharebagher@gmail.com
dDepartment of Tissue Engineering & Regenerative Medicine, Iran University of Medical Sciences, Tehran, Iran
eLife Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran. E-mail: sh.asghari24@gmail.com
fBiomaterials and Tissue Engineering Group, Department of Materials Science and Engineering, Kroto Research Institute, The University of Sheffield, Sheffield, S3 7HQ, UK. E-mail: maleemardani1@sheffield.ac.uk
gDepartment of Translational Health Science, Bristol Medical School, University of Bristol, Bristol BS1 3NY, UK. E-mail: mina.aleemardani@bristol.ac.uk
First published on 19th June 2024
Abstract
Cardiovascular diseases (CDs) are the foremost cause of death worldwide. Several promising therapeutic methods have been developed for this approach, including pharmacological, surgical intervention, cell therapy, or biomaterial implantation since heart tissue is incapable of regenerating and healing on its own. The best treatment for heart failure to date is heart transplantation and invasive surgical intervention, despite their invasiveness, donor limitations, and the possibility of being rejected by the patient's immune system. To address these challenges, research is being conducted on less invasive and efficient methods. Consequently, graphene-based materials (GBMs) have attracted a great deal of interest in the last decade because of their exceptional mechanical, electrical, chemical, antibacterial, and biocompatibility properties. An overview of GBMs’ applications in the cardiovascular system has been presented in this article. Following a brief explanation of graphene and its derivatives’ properties, the potential of GBMs to improve and restore cardiovascular system function by using them as cardiac tissue engineering, stents, vascular bypass grafts,and heart valve has been discussed.
1. Introduction
Cardiovascular diseases (CDs) are the leading cause of death worldwide.1,2 Among the most common diseases in this category are myocardial infarctions (MIs) and strokes, which can permanently and irreversibly damage the heart muscle.2,3 In spite of recent advances in therapeutic interventions, such as balloon angioplasty, bypass grafts, revascularization surgery, and pharmacological treatments, all challenges and limitations for each method to restore the function of injured heart muscles have not yet been overcome.4–8 A potential solution to address these shortcomings is to develop engineered 3-dimensional (3D) cardiac tissue, which has drawn scientists’ interest in conducting several research studies on a variety of synthetic and natural biomaterials in order to develop the desired structure.5,9 There are several factors that should be considered for using biomaterials in cardiac applications, including mimicking native tissue constructions, providing chemical, mechanical, and electrical properties, as well as ensuring biocompatibility and hemocompatibility.10–12 Previous studies indicate that carbon-based biomaterials are one of the most promising biomaterials in cardiac applications due to their exceptional properties.Among all the allotropes of carbon, graphene (G) has gained extensive attention and is extensively used in biomedical applications. Due to its two-dimensional (2D) structure, which is composed of a single layer of hybridized sp2 carbon atoms arranged in a honeycomb lattice pattern and long-range conjugation bonds, it represents extraordinary physical and chemical properties, such as, 100 times superior mechanical properties than steel, light weighted, excellent electrical and thermal conductivity, and high surface area.13–15 Several studies show that G has good biocompatibility in a specific range and antibacterial properties as well.16–21 Different synthesis methods have been used to create GBMs to include a range of properties. These methods have been extensively reviewed and summarized from multiple perspectives, particularly emphasizing the scalable production, classification, and application of GBMs.22,23 There are two types of strategies for synthesizing G, top-down and bottom-up. The top-down approach includes mechanical or chemical exfoliation; through the process of exfoliation, a few layers of bulk material are separated from adjacent layers by overcoming the strong van der Waals attractions.24,25 The bottom-up approach consists of pyrolysis or epitaxial growth of G and the chemical vapor deposition (CVD) process, as shown in Fig. 1. Pyrolysis decomposes carbon precursors like methane to synthesize G directly, while epitaxial growth deposits G on crystalline substrates such as silicon carbide for controlled alignment and high-quality synthesis. The CVD method enables the production of large-scale, high-quality G layers by promoting carbon atom self-organization on crystal surfaces through thermal hydrocarbon decomposition, followed by monolayer segregation on metal substrates.25–29
 | ||
| Fig. 1 Various methods of graphene production. Reproduced from ref. 189 and 254 with permission from [MDPI] and [Nature], copyright [2022] and [2012]. | ||
G also has some drawbacks limiting its use in biomedicine; these include its hydrophobic nature, unstable chemical structure, and incompatibility in some ranges. In order to address these concerns, G derivatives, including graphene oxide (GO), reduced graphene oxide (rGO), and functionalized graphene oxide (FGO), have been developed.15,30,31 The atomic structure of GO is similar to that of G, but it has a more hydrophilic structure with a larger number of functional groups, such as carboxyl, hydroxyl, and epoxy groups.31 The reduction of GO by thermal, chemical, or electrochemical treatments results in the production of rGO, which has fewer oxygen function groups and better conductivity.32 Additionally, functionalization of GO with amine groups makes FGO.30
This review focuses mainly on the development of graphene-based materials (GBMs) for cardiac tissue applications. The study summarizes some of the most important cardiovascular disease research that has been conducted so far, as well as classifying some of the most common treatment methods, such as cardiac tissue engineering, vascular grafts, stents, and heart valves.
2. The properties of GBMs
The mechanical characteristics of the heart muscle are crucial due to its continuous load-bearing nature. The native myocardium has a Young's modulus and tensile strength ranging from 0.2–0.5 MPa and 3–15 kPa, respectively.33–35 Studies suggest that GBMs with their mechanical features could serve as promising materials for reinforcing scaffolds in cardiovascular systems. The unique honeycomb structure of G, with carbon atoms arranged in sp2 hybrid form, contributes to its exceptional mechanical properties, including a Young's modulus, fracture strength, and elastic modulus in the range of 1 TPa, 130 GPa, and 32 GPa, respectively.36,37 The strong C–C bonding in G also results in a hardness higher than that of diamond.36 However, factors such as GBMs’ dispersion can negatively impact the uniformity of their mechanical structures. Overcoming issues like sheet van der Waals interactions and surface energy by applying parallel and perpendicular counter forces is crucial to inhibit G sheet aggregation and ensure proper dispersion.38 Parameters such as solvent properties, the presence of functional groups, and the hydrophobic nature of the G family must be considered to achieve optimal dispersion. Studies have indicated that the dispersity of GO is higher than rGO and pristine G (GO > rGO > G), possibly due to the presence of oxygen-containing functional groups.38–40As the hydrophobicity of GBMs increases, the need for using surfactant to enhance dispersity also rises. While surfactants have been shown to efficiently improve GBMs’ dispersity, their potential cytotoxicity necessitates careful removal post-dispersion.38 Other methods such as microwave heating, shear mixing, surface modification, and sonication can also aid in improving dispersity.40–43 For instance, studies have shown that G quantum dots and low oxygen-containing GO exhibit better dispersion when subjected to microwave heating. Sonication has been found to be more effective for dispersing rGO, GO, and pristine G, with the duration of sonication playing a crucial role. Shear mixing has shown more promising results for dispersing pristine G compared to sonication.39,40 Surface modification through covalent and non-covalent methods is another viable approach to improving GBMs’ dispersity, with covalent techniques demonstrating more promising outcomes in preserving stability during prolonged circulation and showing better loading capacity.40,44
The native myocardium functions by generating its own electrical signals at a rate of 60–100 times per minute, which are then propagated throughout the heart tissue, allowing the myocardium to function as a single unit.45 The conductivity of the tissue is approximately 5 × 10−5 S cm−1 across and 1.6 × 10−3 S cm−1 along the tissue.33,46 Any disruption in the cell signaling pathway can lead to alterations in ion-channel activities, resulting in heart dysfunctions.47,48 To address issues related to disrupted cell signaling pathways, electroconductive materials have emerged as a novel approach for use in cardiovascular applications. Various studies have explored the use of electroconductive biomaterials such as polypyrrole (PPy), polyaniline, poly(3,4-ethylenedioxythiophene), and carbon nanomaterials.49 Among electroconductive materials, GBMs have garnered much attention from researchers due to their electrical conductivity and their ability to facilitate intracellular coupling. The π–π conjugation system and presence of free electrons in G structures provide superior electrical conductivity (∼104 S cm−1) and unique charge carrier properties for GBMs, making them highly suitable for various biomedical applications.50
In addition to their conductivity, the high volume-to-surface ratio of GBMs makes them ideal candidates for use in electrical and electrochemical sensors.51 They can effectively bond to ions and molecules, acting as receptors to detect specific molecules. For example, during ischemic stroke or myocardial infarction (MI), the levels of platelet-derived microparticles significantly increase, and a GO-based sensor could accurately identify patients at high risk based on these biomarkers.52
Another remarkable property of GBMs is their ability to absorb radiation, particularly near-infrared rays (NIR), and convert it into heat.44 This characteristic makes GBMs suitable for applications in photothermal therapy, where cancer cells can be targeted and killed through hyperthermia.53 GBMs’ stability under NIR for extended periods makes it a promising technique for fabricating cardiac patches and applying them in clinical settings through minimally invasive surgical methods.54
In addition to their photothermal properties, GBMs also exhibit antibacterial and antioxidant properties. The antibacterial properties of G family materials are influenced by factors such as size, number of layers, and functional groups.51,55,56 At the molecular level, GBMs’ antibacterial features may be attributed to two mechanisms: physical disruption of bacterial membranes by their sharp edges and chemical mediation of oxidative stress, leading to bacterial cell death.57,58 While combining GBMs with other antibacterial agents has shown potential to enhance antibacterial activities, research on the antibacterial properties of GBMs for cardiovascular applications is relatively limited.33,59 Furthermore, GBMs demonstrate antioxidant properties due to their sp2 hybridization structures.60 In the context of myocardial infarction (MI), where levels of reactive oxygen species (ROS) and proinflammatory cytokines increase in the injured area, the use of antioxidant agents such as GBMs can effectively reduce the excess ROS, thereby decreasing myocardial and vascular cell death and promoting the healing and remodeling processes.33,61 This antioxidant property of GBMs holds promise for mitigating the detrimental effects of oxidative stress in cardiovascular conditions.
The bio and hemocompatibilities of GBMs are crucial and challenging aspects in their biomedical applications. Extensive studies have been conducted to evaluate the cytotoxicity of G materials, yet a consensus has not been reached on this topic. The toxicity of GBMs is influenced by various factors, including size, charge, number of layers, oxygen content, hydrophilicity, dose, dispersity, exposure duration, and specific applications.33,50,51,62 The exact mechanisms underlying the cytotoxicity of GBMs remain unclear, but five possible routes have been proposed: generation of reactive oxygen species (ROS), depletion of mitochondrial membrane potential (MMP), cell starvation, physical damage, and genotoxicity.40,63–67 One effective strategy to enhance the biocompatibility of GBMs is through functional modification, which can help adjust the aforementioned parameters and reduce the risk of cellular toxicity.44,67–69 Given GBMs are inorganic nanomaterials, they have inherent resistance to self-degrade by the biological system.38 Such modifications could modulate their biodegradability, effectively prevent bioaccumulation in tissues, thereby mitigating potential long-term toxicity risks associated with GBM exposure.38,70 Tailoring GBMs’ properties also positively impact the angiogenic properties, leading to positive impacts on angiogenic properties.71–75 This enhancement can promote endothelial cell proliferation and migration, ultimately facilitating the formation of new blood vessels—a critical step in tissue remodeling and regeneration processes. In the subsequent sections, various examples of utilizing functionalized GBMs and investigating their bio and hemocompatibility have been further examined. These studies have demonstrated significant potential in promoting tissue regeneration, enhancing vascularization, and potentially addressing cardiovascular conditions through cutting-edge biomedical applications.
Fig. 2 presents a concise schematic representation of the distinctive characteristics of GBMs that are paramount in the realm of cardiovascular research. Drawing upon existing literature, the figure also delineates several prevalent fabrication methodologies commonly employed in this field.
 | ||
| Fig. 2 Highlights the techniques, characteristics, and uses of graphene in cardiovascular applications. | ||
3. Incorporation of GBM in tissue-engineered scaffolds for cardiac tissue
3.1. Cardiac tissue engineering
The heart has insufficient capability to self-repair and regeneration due to the limited number and growth of heart tissue stem cells.76,77 In the realm of scientific inquiry, cardiac tissue engineering has emerged as a promising avenue for restoring function at injured sites and improving blood flow, thereby potentially reducing the necessity for heart transplants, invasive procedures, and mortality rates.48,77,78 Despite ongoing efforts, the development of fully functional cardiac tissue through cardiac tissue engineering remains a challenge, necessitating careful consideration of various factors before clinical application, including the provision of native-like physical, mechanical, and chemical cues for cultured cells.48,79 Studies have revealed that cardiac muscles exhibit an electrical conductivity, with electrical signals being generated and propagated 60–100 times per minute.45,48 The myocardium's organized myofibers contribute to its unique structural design, offering essential mechanical support for the rhythmic and spontaneous contractions of the heart.45,78,80 Therefore, the selection of suitable biomaterials in cardiac tissue engineering is critical for constructing and engineering the microarchitecture of native myocardium, facilitating the alignment and elongation of cells, as well as restoring cardiac conductivity, contractility, uniform tissue structure, and function.33,81,82While a variety of biocompatible natural and synthetic polymers have been utilized in engineering cardiac tissues resembling the native myocardium's microarchitecture, challenges persist regarding topography, biophysical, chemical, and mechanical properties.83–87 GBMs, known for their exceptional mechanical, electrical, and biocompatible properties, hold promise in meeting these critical requirements for mimicking cardiac tissue and enhancing the expression of cardiac-specific genes and proteins, including TrPT-2, Actn4, connexin 43, among others.30,31,33,80,88–95
In contrast to studies focusing on hydrogels with high modulus, such as the work by Burdic et al.,97 this study proposed a soft injectable hydrogel with fatigue resistance and adequate conductivity for transmitting mechanical and electrical signals to coordinate myocardial tissue contraction.46 The hydrogel was created by combining PEGDA700-Melamine (PEG-MEL) with GO (Fig. 3). The resulting hydrogel exhibited a young modulus of 25 Pa and demonstrated enhanced conductivity due to the π–π conjugation, surpassing the conductivity of PEGDA hydrogel (PEG-MELL/GO: 2.84 × 10−4 S cm−1, PEGDA: 3.47 × 10−9 S cm−1). Moreover, this hydrogel displayed excellent cytocompatibility, and when adipose tissue-derived stromal cells (ADSCs) were encapsulated within it, the cells maintained their initial spindle shape. In an in vivo experiment involving MI rat models, the injection of this hydrogel led to improvements in ejection fraction (EF) and fractional shortening (FS). The GO-incorporated hydrogel also exhibited superior neovascularization compared to other samples. Furthermore, the results indicated that PEG-MELL/GO hydrogels reduced infarction size and fibrosis area, suggesting a promotion of cardiac function recovery.
 | ||
| Fig. 3 Schematic illustration of PEG-MEL/HA-SH/GO hydrogel system encapsulating ADSCs for cardiac repair. (A) Cardiac repair by different treatments after 28 days (Masson's trichrome staining for collagen (blue) and muscle (red)). Reproduced from ref. 46 with permission from [Elsevier], copyright [2017]. | ||
In a study, gelatin methacryloyl (GelMA) hydrogel was fabricated by incorporating rGO at concentrations of 0, 1, 3, and 5 mg mL−1.99 Previous research has suggested that gelatin can act as a biocompatible surfactant for the suspension of G due to van der Waals and π–π stacking interactions. This property of GelMA helps inhibit the agglomeration of rGO, resulting in a homogeneous hydrogel containing rGO. The incorporation of rGO led to a significant improvement in the mechanical and electrical properties of the hydrogel, with a Young's modulus of 22.6 kPa and an impedance value of 4 kΩ. Biocompatibility evaluations demonstrated that the rGO/GelMA hydrogel was non-toxic to cardiac cells. Additionally, the expression of cardiac markers α-actinin and Cx-43 indicated that the rGO-containing hydrogel provided a structure with better organization and cell-cell coupling, leading to proper contractile properties. In vivo assessments revealed that cardiomyocytes on rGO-GelMA hydrogels exhibited greater contractility, a faster spontaneous beating rate, and better biological activities compared to pure GelMA hydrogels. The study suggested that higher electrical conductivity and structural uniformity of the hydrogel could create an appropriate environment for cardiomyocytes, improving charge redistribution and propagation of action potential, thereby promoting synchronized beating throughout the engineered cardiac tissue. The results of this study were promising, and rGO has since garnered attention for cardiac tissue engineering applications, particularly in the development of cardiopatches, due to its ability to mimic cardiac properties effectively.
In another study, researchers aimed to replicate the anisotropic electrical signal propagation observed in the native myocardium by using aligned and random electrospun silk fibroin functionalized with rGO (rGO/silkA/R) as depicted in Fig. 4.100 The conductivity of the rGO/silkA/R patches was found to be significantly higher parallel to the aligned nanofibers compared to perpendicular to them. The conductivity ratios in the parallel direction were 1.7, which closely matched the native myocardial anisotropic conductivity range of 1.1–2.6. After four weeks of implanting the patches, the isotropic conductive rGO/silkA/R patches demonstrated remarkable repair of the infarcted myocardium and alleviated adverse myocardial remodeling. This was evidenced by a reduction in scar size, decreased susceptibility to arrhythmias, improved left ventricular wall thickness, enhanced cardiomyocyte survival, and increased capillary angiogenesis. The outcomes of the study highlighted the potential of the anisotropic conductive rGO/silkA/R biomaterial as a promising candidate for reconstructing the electrical myocardial microenvironment following myocardial infarction. This biomaterial could play a crucial role in restoring the electrical properties of the heart tissue post-infarction and potentially improve overall cardiac function and remodeling.
 | ||
| Fig. 4 Schematics illustrating the fabrication of rGO/silk A/R scaffolds: (A) fabrication of aligned and random rGO/silk scaffold by electrospinning method and then deposition in GO solution through vacuum-filtration; (B) cross-sectional SEM images demonstrating the structure of the rGO/silkA/R scaffolds consisting of a random, aligned-random, aligned nanofibrous layer, providing anisotropic conductive cardiac patch. Reproduced from ref. 100 with permission from [Elsevier], copyright [2022]. | ||
GO, has also gained much attention in several cardiac patch studies.92 In a study, GO-gold nanosheets (GO-Au) were incorporated into a chitosan solution at concentrations of 0.1%, 0.25%, and 0.5% w/v to create a conductive and biodegradable patch.101 The addition of GO-Au resulted in a two-fold increase in conductivity, exceeding 10 × 10−5 S cm−1, while maintaining controlled swelling and degradation properties. After 5 weeks of implantation, the patch showed significant improvements in average QRS intervals and the QRS complex. Moreover, the implanted patches enhanced cardiac tissue function and electrical coupling by notably increasing fractional shortening, ejection fraction, left ventricular diameter dimensions, expression of Cx43, cardiac contractility, and the heart's ability to pump blood more efficiently, suggesting the enhance of the conductivity of the patch make it a promising candidate for applications in cardiac tissue engineering and myocardial repair.
Hydrogel containing oligo (polyethylene glycol) fumarate (OPF) incorporated with varying concentrations of GO were synthesized (0.3, 0.6, and 1.0 mg mL−1 GO/OPF).89 The addition of GO to the scaffolds resulted in a significant improvement in electrical conductivity, with the highest concentration achieving a 4-fold increase, reaching 4.235 × 10−3 S cm−1. The GO-loaded hydrogel showed enhanced adhesion of rat cardiac fibroblast cells compared to the control group. Four weeks after implantation, the GO nanoparticles facilitated the expression of intercalated disk (ID) related proteins such as N-cadherin (NC), plakoglobin (PG), and desmoplakin (DP), which play crucial roles in cardiac function and integrity. The OPF samples treated with GO exhibited improved gap junction remodeling, as evidenced by the increased expression levels of related genes (Cx43, Cx40, Cx37, and Cx45). This enhancement was attributed to the presence of GO, which was found to elevate the phosphorylation levels of GSK-3β (pGSK-3β), subsequently activating β-catenin signaling and leading to an upregulation of Cx43 expression as displayed in Fig. 5. Furthermore, the treated samples showed a reduction in scar tissue and an increase in myocardial tissue within the infarcted area, indicating improved heart structure and functional recovery. The OPF-GO samples also exhibited higher mRNA levels for smooth muscle actin (SMA) and vascular endothelial growth factor (VEGF), suggesting positive angiogenic effects potentially associated with the enhancement of gap junctions (Fig. 6). These results were attributed to the presence of an electrically conductive network within the hydrogel, highlighting the potential of GO-incorporated OPF hydrogels for cardiac tissue engineering applications.89,102
 | ||
| Fig. 5 (G, H, I and J) The formation of gap junction-associated proteins and their mechanism in the infarcted region. (K, L, M, N, O and P) Masson's trichrome staining and echocardiographic measurements demonstrating the effects of OPF/GO hydrogel on the morphology and myocardial functional recovery of infarcted hearts. Reproduced from ref. 89 with permission from [Springer], copyright [2019]. | ||
 | ||
| Fig. 6 (A and B) OPF/GO enhanced cytoskeletal structure 4 weeks after MI. (C, D, E and F) Immunofluorescence staining and western blot results of ID-related proteins in the hearts of PBS-, OPF-, OPF/GO-injected groups at 4 weeks post MI. Reproduced from ref. 89 with permission from [Springer], copyright [2019]. | ||
Study by Kayat et al. have highlighted the potential for GBMs, particularly GO, to induce cell and tissue damage, especially in organs like the heart and liver, potentially leading to inflammation and fibrosis.114 This can occur due to the distribution of GBMs in these organs, triggering local and systemic inflammatory responses.115,116 Furthermore, research by Kanakia et al. has indicated that high concentrations of G (over 250 mg mL−1) can lead to histopathological changes.117 Functionalizing GBMs with different agents and functional groups, such as polyethylene glycol (PEG), aptamers, antibodies, enzymes, and magnetic nanoparticles, has been proposed as a promising strategy to enhance their applications and mitigate negative effects in bio-applications.118–122
In a study aimed at reducing the cytotoxicity of GO, researchers utilized Arginine (Arg), Lysine (Lys), and Ginsenoside Rh2 (Rh2) to functionalize GO, creating nanostructures with excellent anti-cancer activity.122 The results demonstrated that functionalizing GO with hydrophilic groups could enhance cytotoxicity and hemocompatibility. By incorporating Arg and Lys, the nanostructure size was increased, and the surface charge was improved, reducing the interaction between the negative charge of GO and the positive charge of phosphatidylcholine in red blood cells (RBCs).123 This led to a decrease in RBC toxicity and hemolytic activity. The anti-cancer activity of these nanostructures was assessed using the childhood acute lymphoblastic leukemia (ALL) cancer cell line (K562), revealing that GO-Arg-Rh2 and GO-Lys-Rh2 exhibited the highest anticancer activity. In rat models injected with these nanostructures at GO concentrations of 200 and 1000 mg mL−1, histological examination of heart tissue showed that the presence of Rh2 resulted in a decrease in side effects on the heart tissue and blood system, typically associated with chemotherapy for blood cancers, highlighting the potential of these nanostructures in mitigating such side effects.
Minimally invasive surgery (MIS) is gaining attention in the medical field due to its potential to reduce mortality rates, shorten recovery times, and decrease operation durations.124,125 In a recent study, researchers developed a near-infrared (NIR)-triggered self-unfolding microneedle (MN) patch made of GO-poly (vinyl alcohol) (GO-PVA) with varying mass ratios (0%, 0.2%, 0.4%, 0.8%, and 1.6%) loaded with vascular endothelial growth factor (VEGF) as shown in Fig. 7.54 The utilization of GO, known for its excellent absorption in the NIR region, endowed the patch with a remarkable shape memory effect and enhanced VEGF loading and release efficiency.126 Biocompatibility assessments using rat cardiomyocytes derived from H9c2 cells revealed low cytotoxicity and over 80% cellular viability. The 0.8% GO-PVA sample, chosen for its optimal mechanical strength, controllable local sustained drug release, and good biocompatibility, was implanted into rats with MI. A week post-implantation, significant improvements were observed, including increased neovascularization (confirmed by CD31 and VEGF staining), anti-inflammatory effects, reduced scar size, and decreased levels of a-SMA, VWF, and collagen-1. Statistical analyses demonstrated superior left ventricular ejection fraction (LVEF) and left ventricular short-axis shortening rate (LVFS) compared to the control group, indicating promising prospects for MIS applications.
 | ||
| Fig. 7 A general view and various steps of the MIS for treating MI with GO-PVA microneedle patches (1 and 4). The SEM image of the patch and microneedles morphology (2 and 3). Reproduced from ref. 54 with permission from [ACS], copyright [2021]. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, or 1
3, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) into a poly (hydroxyethyl methacrylate) cryogel, followed by reduction into p(HEMA)-rhGO/pPy cryogels (HrhGPs).127 Among the samples tested, the formulation containing pPy/hGO at a ratio of 0.5
5) into a poly (hydroxyethyl methacrylate) cryogel, followed by reduction into p(HEMA)-rhGO/pPy cryogels (HrhGPs).127 Among the samples tested, the formulation containing pPy/hGO at a ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w demonstrated excellent mechanical and electrical properties comparable to native myocardium, with a Young's modulus of 31.59 ± 0.44 kPa and an electrical conductivity of 0.071 ± 0.013 S m−1. Biocompatibility assessments revealed that HrhGP0.5 supported the survival of over 80% of CMs and exhibited favorable cytocompatibility. A 3D biomimetic vessel structure was created in the patch using a coronary artery casting as a template as depicted in Fig. 8, which was subsequently removed after cryopolymerization. Rat aortic endothelial cells (RAECs) were seeded inside and outside the channels under dynamic conditions to establish fully vascularized channels (v-HrhGP0.5). Co-culturing CMs in v-HrhGP0.5 (v-HrhGP0.5 ECP) demonstrated enhanced CM functionalization and synchronous contraction. In vivo evaluations showed a 1.5-fold increase in microvessels and arterioles, with substantial anastomoses formed between v-HrhGP0.5 ECP and host vessels, promoting electrical integration and conduction velocity in the infarcted heart. Furthermore, RNA sequencing analyses indicated that the conductive and dynamic microenvironment created by v-HrhGP0.5 ECP enhanced the repair of myocardial infarction (MI) by upregulating mRNA expression related to cardiac muscle contraction and ATP biosynthesis.
1 w/w demonstrated excellent mechanical and electrical properties comparable to native myocardium, with a Young's modulus of 31.59 ± 0.44 kPa and an electrical conductivity of 0.071 ± 0.013 S m−1. Biocompatibility assessments revealed that HrhGP0.5 supported the survival of over 80% of CMs and exhibited favorable cytocompatibility. A 3D biomimetic vessel structure was created in the patch using a coronary artery casting as a template as depicted in Fig. 8, which was subsequently removed after cryopolymerization. Rat aortic endothelial cells (RAECs) were seeded inside and outside the channels under dynamic conditions to establish fully vascularized channels (v-HrhGP0.5). Co-culturing CMs in v-HrhGP0.5 (v-HrhGP0.5 ECP) demonstrated enhanced CM functionalization and synchronous contraction. In vivo evaluations showed a 1.5-fold increase in microvessels and arterioles, with substantial anastomoses formed between v-HrhGP0.5 ECP and host vessels, promoting electrical integration and conduction velocity in the infarcted heart. Furthermore, RNA sequencing analyses indicated that the conductive and dynamic microenvironment created by v-HrhGP0.5 ECP enhanced the repair of myocardial infarction (MI) by upregulating mRNA expression related to cardiac muscle contraction and ATP biosynthesis.
 | ||
| Fig. 8 The preparation process of the vascularized conductive elastic ECP and its effect after implantation in MI rats; (1) using the incorporation of rhGO/pPy and poly (HEMA) to fabricate HrhGPs cryogel after reduction and cryopolymerization; (2) preparation of v-HrhGP and culturing of CMs and RAECs; (3) the patches were implanted into the MI hearts of rats, and their efficacy was explored. Reproduced from ref. 127 with permission from [Wiley], copyright [2022]. | ||
Numerous studies have been conducted to improve angiogenesis in tissue engineering.128–132 However, G-based materials were shown to upregulate the expression of angiogenic genes, including CD34, MMP9, SDF-1, and VEGF, and positively promote angiogenesis.71,129,131,133 In a study involving polyvinyl alcohol and carboxymethyl cellulose scaffolds loaded with varying concentrations of rGO nanoparticles (0.0025%, 0.005%, 0.0075%, 0.01% w/v), it was observed that during cell culture, the rGO-loaded scaffolds seeded with endothelial cells (EA. hy926) did not exhibit cytotoxic effects or morphological changes.129 Moreover, the presence of rGO nanoparticles led to an increase in the proliferation rate of the cells. In an in vivo chick chorioallantoic membrane assay, it was found that the number of blood vessels significantly increased when the concentration of rGO exceeded 0.0075% (w/v) by 62%, according to Fig. 9. This phenomenon, known as arteriogenesis, was also observed during the assay period, as the average thickness of the vessels increased by 51.7%, indicating blood vessel maturation.129,134 In another study, the angiogenic effects of GO were investigated by analyzing angiogenic-related gene expression (biocompatibility). A chitosan-GO hydrogel was synthesized by freezing and lyophilization technique.135 The study showed that the incorporation of GO (up to 150 mg L−1) improved the uniformity of the hydrogel structure and enhanced its mechanical properties. Specifically, the elastic modulus, tensile strength, and elongation at the break increased from 0.829 to 22.026 MPa, from 1.363 to 7.153 MPa, and from 61.3% to 161.5%, respectively. When GO was added to endothelial progenitor cells (EPCs) extracted from umbilical cord blood, it led to enhanced tube formation and angiogenesis. Western blot analysis of angiogenesis-related genes revealed an upregulation of CD34, VEGF, MMP9, and SDF-1 in the GO-loaded hydrogel. This suggests that GO may influence the angiogenic ability of EPCs through the SDF-1/VEGF signaling pathway.135–137
 | ||
| Fig. 9 (a) Image of chick chorioallantoic membrane assay; (b) The percentage of number and thickness of blood vessels. Reproduced from ref. 129 with permission from [Royal Society open science], copyright [2018]. | ||
Norahan et al. conducted a study where they fabricated scaffolds based on collagen that were covalently coated with various concentrations of GO (5, 15, 30, 45, 75, 90 μg ml−1).10 The scaffolds exhibited randomly oriented interconnected pores with GO flakes properly distributed in the pore walls. To enhance the biocompatibility of the scaffolds, monolayer GO flakes with a lateral size of about 300–500 nm and a thickness of 2 nm were used, as studies have indicated lower cytotoxicity and inflammation response of nano-sized GO compared to micro-sized GO.138 Mechanical and electrical analyses showed that with increasing concentrations of GO, the tensile strength, Young's modulus, and electrical conductivity of the scaffold increased. For example, the tensile strength and Young's modulus increased from 75 kPa and 160 kPa for Col-GO-5 to 162 kPa and 750 kPa for Col-GO-90, respectively. Although GO itself did not exhibit high conductivity, the electrical conductivity was altered from 3.8 ± 0.09 × 10−4 S m−1 for Col-rGO-5 to 29 ± 0.18 × 10−4 S m−1 for Col-rGO-90 after reduction. Biocompatibility results indicated that both Col-GO-90 and Col-rGO-90 significantly enhanced cell proliferation rate and adhesion, with no observed cytotoxicity on human umbilical vein endothelial cells (HUVECs) due to the surface properties and interaction of functional groups on GO and rGO, leading to the adsorption of serum proteins. Gene expression evaluation on rat neonatal cardiomyocytes (CMs) revealed that the expression of cardiac markers such as Cx43 and Actn-4 increased approximately two-fold in Col-rGO-90 samples compared to Col samples, while the expression of TrpT-2 was upregulated 3.2-fold, indicating an improvement in extracellular matrix-cell interactions. Furthermore, the optimal sample (Col-rGO-90) was subcutaneously implanted in mice, showing higher cell migration and newly formed blood vessels, highlighting the angiogenic characteristics of GO (Fig. 10).
 | ||
| Fig. 10 (1) Immunohistology analysis of Col and Col–rGO of scaffolds after implantations; (2) and (3): morphology and H & E staining of the scaffolds after 2 (A and B) and 4 (C and D) weeks after subcutaneous implantation. Reproduced from ref. 10 with permission from [Wiley], copyright [2019]. | ||
While GBMs have been extensively studied for their angiogenic effects, some studies have suggested that they could also act as anti-angiogenic agents and play a role in tumor growth and metastasis by targeting angiogenic markers.139,140 In certain studies, G and its derivatives have been shown to activate macrophages and dendritic cells, leading to the stimulation of the immune system to produce cytokines that can effectively target cancerous cells.139 For example, a study on bovine serum albumin-capped GO demonstrated that it can strongly bind to VEGF-A, a crucial proangiogenic factor, and inhibit its function.141 This blocking of VEGF-A suppresses angiogenic signaling processes in chick chorioallantoic cells and hinders neovascularization in rabbit corneal cells, making it a potential therapeutic anti-angiogenic agent.141 Furthermore, in another study, GO was incorporated into a nanocomposite with monoclonal antibodies (mAbs) targeting FSHR, a marker of tumor vasculature expressed during the early stages of tumor metastasis.140 This approach shows promise in targeting tumor vasculature and inhibiting metastatic spread.
In a study investigating the optimal niches for human umbilical cord-derived mesenchymal stem cells (hUC-MSCs), the effect of GO flake sizes (100–200 nm, 0.2–2 μm) and the reduction level of rGO (low and high) with different thicknesses (5, 10, 15, and 25 μg cm−2) was evaluated.32 The study found that increasing the size of GO flakes enhanced cell proliferation by up to 17% in aqueous and 14% in ethanol solutions. Additionally, the number of hUC-MSCs improved at lower levels of rGO reduction. However, increased thicknesses of both GO and rGO induced oxidative stress, leading to the generation of reactive oxygen species and activation of apoptosis. The study identified 10 and 15 μg cm−2 as the most suitable thicknesses. Furthermore, due to their hydrophilic properties, GO-based substrates were found to facilitate MSC migration in terms of cell speed and distance. While cells adhered more rapidly to GO samples than to rGO samples initially, after 2, 3, and 4 hours, cells adhered to both types of samples similarly. The study also revealed that the expression levels of angiogenic-related genes GATA-2, ENDOGLIN, and VE-CADHERIN were significantly elevated with both GO and rGO compared to control groups. Moreover, both GO and rGO promoted capillary formation as well as differentiation into cardiomyocyte and endothelial-like cells. Interestingly, the study found that GO did not require any external factors to differentiate into angiogenic cells, whereas rGO did. Additionally, both GO and rGO increased the expression levels of cardiomyogenic genes, such as GATA-4 and ACTC1, and myocyte-specific markers (ME22C) without the need for biological induction of differentiation.
In a recent study, human bone marrow mesenchymal stem cells (hBM-MSCs) were encapsulated in conductive hydrogels composed of alginate (ALG) and reduced rGO.98 These hydrogels were then implanted into rat models with chronic ischemic cardiomyopathy. The results of the study showed significant improvements in various cardiac parameters in rGO-ALG-MSC group compared to the control group and other treatment groups. Specifically, the rGO-ALG-MSC group exhibited enhanced fractional shortening (FS), ejection fraction (EF), wall thickness, and internal diameters, indicating improved cardiac function and structure. Morphological observations revealed increased blood vessel formation, particularly in the rGO-ALG-MSC group, 8 weeks post-transplantation. Histological analyses further supported the positive outcomes, showing reduced collagen fibrosis and increased neovascularization in the group treated with rGO-ALG-MSC. These findings suggest that the use of rGO/ALG hydrogel encapsulated with BMSCs is both safe and effective for the treatment of myocardial infarction, offering promising results for cardiac repair and regeneration. In another study, a hybrid composite of collagen type 1, which is the most abundant protein in the body, and G at concentrations of 8% and 32% by weight was evaluated.153 The study demonstrated that as the G content increased, the stiffness and electrical conductivity of the scaffold significantly increased. The enhanced hydrophilicity and roughness of the scaffold due to the presence of G led to increased cell adhesion, with improvements of 26% and 45% observed for samples with 8% and 32% G content, respectively. Furthermore, the addition of G resulted in a reduction in bacterial cell binding to the surface by up to 17.6%. In addition to these findings, the study applied 48 hour external electrical stimulation at 25 V cm−1 and 1 Hz. The results, as indicated by immune fluorescent staining of sarcomeric myosin (MF20) and cardiac troponin (cTNT), showed that the addition of G improved the cross-striation structure and alignment of cells. This enhancement further promoted the elongation, maturation, and alignment of embryonic stem cell-derived cardiomyocytes (ESC-CMs), suggesting that the hybrid composite scaffold has the potential to support and enhance the development of functional cardiac tissue (Table 1).
| Biomaterial(s) | Concentration of G | Fabrication technique | In vitro (cell type) | In vivo (animal model), duration | Outcomes | Year, Ref. |
|---|---|---|---|---|---|---|
| Polyurethane/rGO (PU/rGO) | 5, 10, 15 and 20 wt% | Electrospinning and electrospraying | H9c2 and HUVEC | Not conducted | ↑ Tensile strength | 2023, 154 |
| ↑ H9c2 and HUVEC cells viability and attachment, particularly PU/RGO10 and PU/RGO15 | ||||||
| Holey GO/polypyrroleincorporated poly hydroxyethyl methacrylate | Hybrid suspension with a hGO to pPy mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1 0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, or 1 3, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Cryogelation | CMs | MI rat model, 4 weeks | ↑ Cell viability over 80% | 2022, 127 |
| In vivo: ↑ microvessels and arterioles up to 1.5-fold | ||||||
| ↑ Electrical integration and conduction velocity | ||||||
| ↑ Cardiac muscle contraction and ATP biosynthesis mRNA express | ||||||
| ↓ Infarcted area (∼32%) | ||||||
| ↑ cGMP-PKG signaling pathway | ||||||
| ↑ Expression of functionalization-related genes | ||||||
| PGS and G | 0.5, 1, 2, 4 (wt%) | Micropatterned film by solvent evaporation | H9c2 rat CMs | MI rat model, 4 weeks | Tensile strength (0.6 ± 0.1–3.2 ± 0.08 MPa) | 2022, 155 |
| ↑ Conductive to 5.8 × 10−7 S m−1 | ||||||
| promote cell proliferation in H9c2 | ||||||
| In vivo: ↓ infarct size and degree of myocardial fibrosis | ||||||
| ↓ Collagen deposition | ||||||
| ↓ Left ventricular. | ||||||
| ↑ Fractional shortening and ejection fraction | ||||||
| ↑ CX43 protein expression | ||||||
| Poly (ethylene glycol) and few-layer GO (FLGO), or GO | 1% w/v FLGO and 4% w/v GO | In situ crosslinking | HUVECs | Not conducted | ↑ MP | 2022, 96 |
| Optimum sample: 4% w/v GO | ||||||
| Possess anti-adhesive and anti-bacterial properties | ||||||
| No cytotoxicity | ||||||
| Alginate and GO | 2 g | Dual cross linking | MSCs and L929 fibroblasts | Not conducted | ↑ MP and toughness | 2022, 156 |
| ↑ L929 cell viability and adhesion | ||||||
| Regulate the fate of encapsulated | ||||||
| MSCs | ||||||
| ↑ MSCs differentiation markers (1.30-times for TNT and 1.21-times for GATA4) | ||||||
| rGO and Alginate | 10 μg mL−1 | Using crosslinking agent | hBM-MSCs and CMs | Rat model of chronic ischemic cardiomyopathy, 8 weeks | ↑ Cell viability | 2022, 98 |
| ↑ Cardiac marker expression (TrpT-2, Conx43, and Actn4) | ||||||
| In vivo: ↑ EF, FS, wall thickness ≫ ↑ cardiac function | ||||||
| ↑ Formation of blood vessels | ||||||
| rGO and silk | 0.01 mg rGO per 1 cm2 patch | Electrospinning (aligned/random) | CMs and MFs | Acute MI rat model, 4 weeks | Anisotropic conductivity | 2021, 100 |
| In vivo: ↑ repairing infarcted myocardium. | ||||||
| ↑ Pumping function | ||||||
| Thickened left ventricular walls. | ||||||
| ↓ Susceptibility to arrhythmias | ||||||
| ↑ CD31-positive microvessels ↓ density of α-SMA | ||||||
| PEGDA hydrogels and G nanoplatelets | 15% | 4D printing | hiPSC-CMs, hMSCs, and HUVECs | Not conducted | Dynamic and remotely controllable transformation in a spatiotemporal manner | 2021, 157 |
| ↑ Rhythmic and directional beating behavior | ||||||
| ↑ Myocardial protein (α-actinin and cTnI) expression of the nascent myocardial tissue | ||||||
| ↑ Degree of alignment and elongation for hMSCs and hiPSC-CMs | ||||||
| PCL/PGS/G | 0.25, 0.75 and 1 wt%; Optimum: 1 wt% | Electrospinning | HCMs | Not conducted | ↑ Tensile strength: 21.51 to 11.41 MPa | 2021, 158 |
| cell clusters | ||||||
| ↑ CPA | ||||||
| ↑ Cell viability: 98.79% (≫1 wt%) | ||||||
| (GO)-poly (vinyl alcohol)-VEGF loaded | 0, 0.2, 0.4, 0.8, and 1.6 wt% | Freeze–thaw | CMs derived from H9c2 | MI mice model, 2 weeks | ↑ Cellular viabilities >80% | 2021, 54 |
| ↓ Expressed α-SMA, VWF, and collagen-1 | ||||||
| In vivo: ↑ neovascularization | ||||||
| ↑ New blood vessels | ||||||
| ↓ Myocardial fibrosis | ||||||
| GO and Chitosan | 0.1, 0.5, and 1.0 wt% | Using crosslinking agent and freeze dry | EPCs | Not conducted | ↑ Hardness and strength | 2021, 159 |
| ↑ The expressions of angiogenesis-related genes at optimum content of GO (CD34, VEGF, MMP9, and SDF-1) ≫ ↑ angiogenesis | ||||||
| Oxidized alginate (OA), myocardial extracellular matrix (ECM), and amine-rGO | 25 μg ml−1 | Using crosslinking agent | HUVECs | Not conducted | ↑ MP: 41.1 kPa | 2021, 160 |
| ↑ EC: 2.8 × 10−4 S m−1 | ||||||
| Well distributed amine-rGO | ||||||
| ↑ Cell viability | ||||||
| pHEMA and GO | 1% w/v | Using crosslinking agent | RBCs and HUVECs | porcine arteriovenous-shunt model, 30 min | MP (YM): 1.80 × 103 kPa | 2021, 17 |
| Possess anti-adhesion against HUVECs, bacteria, and platelets | ||||||
| ↑ Hemocompatibility & cytocompatibility | ||||||
| In vivo: ↓ Blood component adhesion | ||||||
| GO and rGO | 5, 10, 15, or 25 μg cm−2 | Coating | hUC-MSCs | Not conducted | No change of phenotype, cell cycle progression | 2020, 73 |
| ↑ Cell proliferation in sample with large flakes of GO | ||||||
| ↑ Cardiomyogenic and angiogenic differentiation | ||||||
| Functionalized G (carboxyl group), PCL/chitosan/polyPyrrole (PCP) and heparin | 0.5, 1.5, and 2.5 wt% | Electrospinning | ESC | Not conducted | ↑ Hydrophilicity | 2020, 161 |
| ↑ MP: | ||||||
| 0.032 ± 0.001 to 0.098 ± 0.001 MPa | ||||||
| ↑ EC: | ||||||
| 1.12 ± 0.045 to 5.33 ± 1.16 S cm−1 | ||||||
| ↓ Bovine serum albumin (BSA) adsorption (↑ hemocompatibility) | ||||||
| Embryoid bodies (EB) formation | ||||||
| Cardiomyocytes differentiation | ||||||
| Silk, GO, and growth factor | 0.25 mg mL−1 and 0.5 mg mL−1 | Using gelation agent (PEG) | CPCs and CMs | MI mouse model | Uniform distribution of RGO and porous structure | 2020, 162 |
| ↑ Cytocompatibility, cell adhesion and proliferation rate | ||||||
| ↑ The expression of cardiac markers (SAC, Cx43 and cTnl) | ||||||
| In vivo: ↑ LV wall thickness | ||||||
| ↓ Scar thickness and infarct size | ||||||
| ↓ Pro-inflammatory cytokines | ||||||
| Positive effect on cardiomyocytes differentiations | ||||||
| rGO and collagen | 200, 400, 600 and 800 μg ml−1; Optimum: 400 μg ml−1 | Coating | HUVECs | Not conducted | ↑ Cell viability | 2019, 163 |
| Cardiac gene expression upregulation like Cx43, troponin-T and actinin-4 (≫400 μg ml−1) | ||||||
| ↑ MP: 340 ± 20 kPa | ||||||
| ↑ EC: 2.2 ± 0.3 × 10−5 S m−1 | ||||||
| Antibacterial properties | ||||||
| GO and polyethylene terephthalate (PET) | 0.5 mg ml−1 | Electrospinning | HUVECs | Not conducted | Cardiomyocyte elongated and spreading morphology | 2019, 164 |
| ↑ EC | ||||||
| Polyurethane and rGO | 0% and 0.025% of PU | Electrospinning | Satellite cells from skeletal muscle | Not conducted | ↑ MP and the fibers’ orientation | 2019, 88 |
| Well dispersion of rGO nanoparticles | ||||||
| ↑ Cell growth and differentiation on aligned fibers | ||||||
| ↑ The expression of Troponin I cardiac-specific gene on aligned fibers | ||||||
| Gelatin, poly caprolactone (PCL), and G | 0%, 0.3%, 0.5%, 0.8%, and 1% of Gt/PCL | Electrospinning | Neonatal rat ventricular CMs (NRVCMs) and CMs | subcutaneous in rat model, 6 weeks | Optimum sample: 0.5%G | 2019, 93 |
| ↑ EC & ↑ MP | ||||||
| ↑ Cell viability and adhesion | ||||||
| In vivo: No cell necrosis and tissue damage | ||||||
| Chitosan and GO | 50, 100, 150, 600 mg L−1 | Freezing and lyophilization technique | H9C2 rat MCs | Not conducted | Optimum sample: 150 mg L−1 GO | 2019, 135 |
| ↑ Uniformity | ||||||
| ↑ EC: 0.134 S m−1 | ||||||
| ↑ Cell viability and proliferation | ||||||
| ↑ The expression of CX-43 | ||||||
| Polyvinyl alcohol, carboxymethyl cellulose, and rGO | 0.0025%, 0.005%, 0.0075%, 0.01% w/v | Freezing and lyophilization technique | Endothelial cells (EA. hy926), fibroblasts (NIH3T3), and endothelial-like cells (ECV304) | Chick chorioallantoic membrane model, 10 days | No cytotoxicity and morphology change | 2018, 129 |
| ↑ Proliferation rate | ||||||
| In vivo: ↑ Angiogenesis and arteriogenesis | ||||||
| Chitosan and GO-Au | 0.1%, 0.25%, 0.5% (w/v) | Freeze dry | SMCs, fibroblasts and hiPSC-CMs | MI rat model, 5 weeks | Two-fold increase in EC | 2018, 101 |
| ↑ iPSC derived cardiomyocytes attachment | ||||||
| In vivo: ↑QRS interval, conduction velocity and contractility in infracted zone | ||||||
| ↑ connexin 43 levels | ||||||
| GO, Arginine, Lysine, and Ginsenoside Rh2 | 5, 25, 100, 200, 400, 1000 μg ml−1 | Esterification and radical reaction | RBCs and cancer cell line (K562) | Injection in rat model, 2 weeks | ↑ Nanostructure size and charge ≫ ↓ toxicity to RBCs ≫ ↑Hemocompatibility & ↑ anti-cancer activity | 2018, 122 |
| In vivo: ↓ Heart tissue damage | ||||||
| GO and Collagen | 5, 15, 30, 45, 75, and 90 μg mL−1 | Freeze dry | HUVECs and rat newborn CMs | subcutaneous implantation on mice, 4 weeks | Well disperse GO | 2018, 10 |
| ↑ MP (YM): 750 kPa & ↑UTS: 162 kPa | ||||||
| EC: 29 × 10−4 S m−1 | ||||||
| ↑ The expression of Cx43, Actn-4, and TrpT-2 | ||||||
| ↑ Biocompatibility | ||||||
| ↓ Cytotoxicity and inflammation reaction | ||||||
| In vivo: ↑ Angiogenesis | ||||||
| Oligo (polyethylene glycol) fumarate (OPF) and GO | 0.3, 0.6, and 1.0 mg mL−1 GO/OPF | Using crosslinking agent | Cardiac fibroblast cells | MI rat model, 4 weeks | ↑ EC: 4.235 × 10−3 S cm−1 | 2018, 116 |
| ↑Cell adhesion & no cytotoxicity | ||||||
| In vivo: ↑ The expression of NC, PG, and DP ≫ ↑ cardiac function and integrity | ||||||
| ↑ The expression of Cx43, Cx40, Cx37, and Cx45 ≫ ↑ gap junction remodeling | ||||||
| ↑ Angiogenesis | ||||||
| PEGDA700-Melamine (PEG-MEL) and GO | 0.5 mg ml−1 | Using crosslinking agent | ADSCs | MI rat model, 4 weeks | ↑ EC: 2.84 × 10–4 S cm−1 | 2016, 46 |
| MP: 25 Pa | ||||||
| ↑ Cytocompatibility | ||||||
| In vivo: ↑ EF, FS, and neovascularization | ||||||
| ↓ Infarction size and fibrosis area | ||||||
| rGO and GelMA | 0, 1, 3 and 5 mg ml−1 | Hydrogel fabrication | NIH-3T3 cells and rat CMs | Not conducted | ↑ Tensile strength | 2016, 165 |
| ↑ DNA content ⇒ no significant toxicity | ||||||
| ↑ Cardiac markers expression | ||||||
| bovine serum albumin-capped GO (BSA/GO) | 30, 100, and 150 μg mL−1 | Mixing | HUVECs | Chick chorioallantoic membrane and rabbit corneal neovascularization (CNV) models, 2 weeks | Strong bonding to VEGF-A | 2016, 141 |
| ↑ Inhibition the proliferation, migration of cells and tube formation | ||||||
| In vivo: ↓ blood vessel formation, growth, and density ≫ ↑ anti-angiogenic properties |
3.2. Vascular stents and grafts
Vascular diseases are among the leading causes of death around the globe.166 With increasing demands for curing these diseases, various vascular therapeutic strategies have been developed, including percutaneous and surgical revascularization procedures.167 Stent implantation as an advanced part of these protocols has drawn scientists’ attention since 1980.168 Nowadays, there are three distinct generations of stents: bare metal stents, drug-eluting stents, and biodegradable stents; however, they still have several major drawbacks, including the biocompatibility and hemocompatibility of biomaterials, their mechanical properties, and their design, which inhibit them from being considered as a long-term and completely successful clinical treatment.169 To mitigate these adverse effects, stents must undergo various modifications to improve their properties and meet clinical requirements. G and its family are among the most sought-after materials due to their chemically inert nature, atomic smoothness, durability, and biocompatibility properties, which have been successfully used for surface or bulk stent modifications as shown in Table 2.170,171| Biomaterial(s) | Graphene-based materials concentration(s) | Technique | Target and cell type | Outcomes | Year, Ref. | |
|---|---|---|---|---|---|---|
| Vascular stents | Zn/PEG grafted G nanoplatelets (GNPs) | 25, 50, 100 mg L−1 of f-GNP | Zn/functionalized-GNP nanocomposite prepared by electro co-deposition (ECD) | HaCaT | ↑ Compressive yield stress ranges from 182.3 ± 7.9 MPa (f-GNP: 25 mg L−1) to 284.9 MPa (f-GNP: 100 mg L−1) | 2022, 206 |
| ↓ Friction coefficient and wear loss of Zn/f-GNP (100 mg L−1) nanocomposites (58.1% and 47.36%) | ||||||
| ↑ Antibacterial | ||||||
| Performance (S. aureus and E. coli) | ||||||
| ↑ CPAM | ||||||
| AZ31b Mg/GO/PTMC | 0, 0.2, 1, 3, 6, and 10 wt% | Spin coating | HUECs and HUSMCs | ↑ Anti-corrosion ability (up to 6 wt%) | 2022, 207 | |
| ↓ Cytotoxicity | ||||||
| ↑ CPAM | ||||||
| ↓ Adhesive number of platelets ⇒ ↓ thrombosis risk | ||||||
| Good blood compatibility | ||||||
| Optimum: 6 wt% GO | ||||||
| HEMA/methacrylate graphene oxide (MeGO) and loaded with RSL | 0.1 g | 3D printing | HUVECs | ↑ Elastic stiffness of the stent (0.29 ± 0.01 × 10−2 MPa) | 2022, 182 | |
| ↑ NO release from HUVECs ⇒ Suppress platelet adhesion and activation | ||||||
| No cytotoxicity | ||||||
| ↓ Cell apoptosis (5-fold) | ||||||
| ↓ TNF-α release | ||||||
| ↓ H2O2-induced ROS ⇒ recover endothelial cell functions | ||||||
| Mg alloy/chitosan functionalized GO (GOCS) loaded with Zn2+ and propranolol | 1 mg mL−1 GO | Covalently immobilized coated by immersing in GOCS@Zn/Pro solution (1 mg mL−1) | ECs | ↑ Corrosion resistance | 2020, 208 | |
| ↓ Degradation rate | ||||||
| ↓ Platelet adhesion and activation ≫ ↑ hemocompatibility | ||||||
| ↑ Clotting time ⇒ excellent anticoagulation | ||||||
| ↑ Cell adhesion and proliferation | ||||||
| ↑ Nitric oxide (NO) and VEGF | ||||||
| Expression ⇒ ↑ surface endothelialization | ||||||
| ↓Bacterial adhesion and proliferation | ||||||
| AZ31B Mg alloy /chitosan-functionalized graphene oxide (GOCS)/hep | 1 mg ml−1 | coated by immersing in GOCS and heparin solution | ECs | ↓ pH value of solution ⇒ ↑ corrosion resistance | 2020, 169 | |
| ↑ Coating thickness ⇒ ↑ corrosion resistance ↓ | ||||||
| Adhered platelets | ||||||
| ↓ Hemolysis rate by multilayer coating | ||||||
| ↑ CPAM | ||||||
| ↑ Expression of VEGF and NO in endothelial cells ⇒ stimulates the growth of the endothelial cells ⇒ ↑cGMP in platelets ⇒ | ||||||
| ↓ Intracellular Ca2+ ⇒ preventing the adhesion and activation of platelets and fibrinogen | ||||||
| ↑ Blood compatibility | ||||||
| SUS316L stainless steel/EDOT /GO/PSS /Hep | 0.01 g | Coated by electrochemically copolymerizing | Mouse embryonic fibroblasts (NIH 3T3 cells) | Exhibited anti-fouling ability | 2019, 209 | |
| ↓ Protein adsorption | ||||||
| GO contributed most of the anti-adsorption effect | ||||||
| ↑ Blood coagulation time | ||||||
| No cytotoxicity | ||||||
| 316L stainless steel/DA/(GO) loaded with docetaxel (DTX)/carboxymethyl chitosan (CMC) loaded with Hep | 2 mg ml−1 | Coated by ultrasonic spray system | VSMCs | ↓ Hemolysis rate | 2019, 210 | |
| ↓ Adhered platelets appear to be spherical and inactivated | ||||||
| No platelets and fibrin (after 12-week implantation) ⇒ ↑Antithrombotic ability | ||||||
| No toxicity (zebrafish embryos and larvae) | ||||||
| ↑ Inhibit the proliferation of VSMCs | ||||||
| Inhibits intimal hyperplasia and thrombosis and promotes endothelialization (in vivo rabbit study) | ||||||
| PCL-rGO | 0.1, 0.3, 0.6, and 1.0% (wt/wt) | The 3D printing technique of melt electrowriting (MEW) | HUVECs | ↑ Mechanical Properties (for 0.1% rGO: a 2.5-fold ↑Young's modulus (520 ± 39 MPa) and a 1.5-fold) | 2019, 211 | |
| ↑ Ultimate tensile strength (21 ± 2 MPa) | ||||||
| ↑ CPAM ⇒ ↑endothelialization | ||||||
| ↓ Platelet adhesion for 0.1% rGO | ||||||
| Stainless steel/G/chitosan/TiO2 nanoparticles (TiO2NPs) | 7(wt.%) | Coated by immersing in Ch-G-TiO2NPs | RBCs and WBCs | No blood platelets adhesion when exposed to healthy and diabetic human blood | 2018, 212 | |
| No negative effect on RBCs and WBCs count | ||||||
| Stainless steel (316L) coated by G | Not mentioned | Coated by using the PMMA-based transfer method | HPCAEC | ↑ CPAM on | 2018, 213 | |
| G-overlaid discs over 100% | ||||||
| No cytotoxic effect | ||||||
| ↑ Metabolic activity | ||||||
| ↓ Fibroblast phenotype cells | ||||||
| ↑ Endothelium phenotype cells | ||||||
| ↓ Platelets attached (two times) | ||||||
| Diminishing the endothelial to mesenchymal transition ⇒ ↓ in-stent restenosis risk | ||||||
| Pure titanium coated by DA and heparin-loaded GO | 0.5 mg ml−1 GO solution | Coated by immersing into the GO solution | ECs | ↑ Surface hydrophilicity | 2016, 214 | |
| ↑ CPAM | ||||||
| ↓ Adhered platelets | ||||||
| ↑ Blood compatibility | ||||||
| ↑ Clotting time | ||||||
| NiTi coated by G | Not mentioned | G layer Coated by using the PMMA-based transfer method | RAECs and RASMCs | ↓ Stress gradients in cells ⇒ better cells morphology | 2012, 170 | |
| Supporting cells proliferation | ||||||
| No cytotoxicity | ||||||
| G coatings inhibit any charge transfer from fibrinogen (subsequent blood clotting) ⇒ ↓fibrinogen/albumin ratio ⇒ ↑ hemo-compatibility | ||||||
| Hemo-compatibility of G is layer-independent. | ||||||
| Vascular graft | Thermoplastic polyurethane (TPU)/GO | 0%, 0.5%, 1%, and 2% of TPU | Electrospinning | HUVECs, Mouse fibroblast (3T3) | ↑ Tensile modulus and tensile strengt | 2015, 215 |
| ↑ CPAM | ||||||
| ↑ Fibroblasts viability (0.5% GO) | ||||||
| ↓ Water contact angle | ||||||
| Oxygen plasma treatment ⇒ ≫ ↑hydrophilicity | ||||||
| ↑ HUVECs attachment | ||||||
| 0.5% GO scaffolds ≫ ↑ live cells ∼(99% viability) | ||||||
| 5% GO sample exhibited high flexibility ⇒ ↑ recovery from the cyclical force loading | ||||||
| Proper suture retention strength for use in small-diameter vascular graft applications | ||||||
| ↓ Platelets attached (round morphology) ⇒ ↓ thrombosis | ||||||
| Polyvinyl alcohol (PVA)/G | 1, 2, and 3 wt% OF PVA | Electrospinning | ECs | ↓ Young's modulus from 65.3 to 42.7MPa | 2020, 216 | |
| ↑ Elongation (∼3 times for 3% G) and tensile strength | ||||||
| ↑ G concentration ⇒ ↑water contact angle and hydrophobicity | ||||||
| No toxicity | ||||||
| ↑ CPAM | ||||||
| Decellularized Arteries (Human placental and umbilical cord tissues)/GO | 0.5 mg ml−1 | Recirculating perfusion system | HUVECs | ↑ Burst pressure of the umbilical cord decellularized arteries by 29%, strain by 25%, and compliance by 10% | 2021, 187 | |
| ↑ Compliance by 10% for placental chorion decellularized arteries | ||||||
| ↓ Adhered platelets (both) ≫ in umbilical cord arteries | ||||||
| ↓Bacterial adhesion | ||||||
| Collagen-coated vascular matrix/apyrase and 5′-nucleotidase (5′-NT)/rGO | Not mentioned | Coated by dispersion in RGO-enzyme complexes | ECs | ↑ Stability of the enzyme by binding to Rgo | 2017, 188 | |
| ↓ ADP concentrations | ||||||
| ↑ AMP and adenosine ⇒ enzymes good activity and could transform ADP into antiplatelet AMP and adenosine ⇒ Inhibit platelet aggregation | ||||||
| ↑ CPAM | ||||||
| ↑ Patency rate of 90% in in vivo study | ||||||
| Heart Valve | Acellular pulmonary valve/rGO | 100 μg ml−1 | Coated by immersing in rGO solution | Fibroblast cell | No effect on CPAM (indirect) | 2015, 1 |
| No significant difference in platelet activation, adhesion, DNA fragmentation | ||||||
| Functionalized GO (FGO)/poly(carbonate-urea) urethane (PCU) (the trade name Hastalex)/GORE-TEX | 5% of prepolymer | Mixing | ECs (Ea.hy 926) | ↑ Mechanical properties (ultimate tensile strength and elongation of Hastalex: 2.5, 3.5, and 5-fold ≫ GORE-TEX in the longitudinal direction, transverse direction, and uniform elongation) | 2020, 205 | |
| ↑ Contact angle (both shiny and opaque surface) | ||||||
| ↑ Smooth and structured surface | ||||||
| ↑ CPAM (2.2 folds than control sample and 7.5 folds than GORE-TEX) | ||||||
| ↓ Adherent platelets (2-folds) | ||||||
| ↓ Calcium deposits of the connective tissue capsules | ||||||
| ↓ Thickness of the fibrous capsule around |
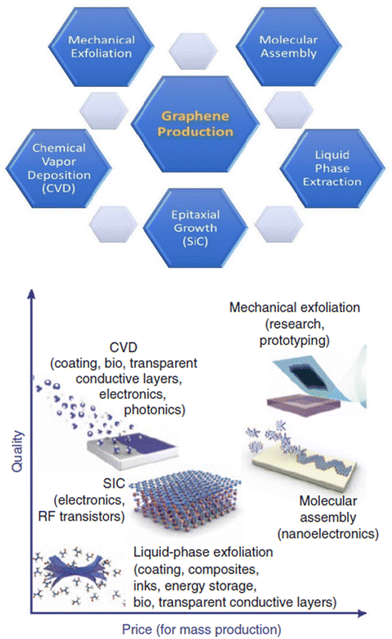
For example, in one study, AZ31B Mg alloy surfaces were modified by chitosan-functionalized GO (GOCS) and then immersed in a heparin solution, and five coating layers were fabricated by repeating this cycle five times (Fig. 11).169 According to the results of electrochemical corrosion tests, GO anti-permeability and negatively charged heparin inhibited the penetration of molecules and ions, providing high corrosion resistance. An analysis of platelet adhesion, cGMP, and hemolysis rate was performed to determine the blood compatibility of the Mg-GOCS/heparin stent. Prior studies showed that fibrinogen adhesion decreased with increasing wettability, which resulted in a reduction in adhered platelets and thrombus formation.172,173 Therefore, almost no adhered platelets were reported on the Mg-GOCS/heparin stent, since chitosan and GO were proven to be blood compatible and heparin increased the hydrophilicity of the surface. Further, the high level of cGMP expression suggests low platelet activation, and markedly higher cGMP levels were detected in modified samples versus unmodified Mg alloy stent samples.174,175
 | ||
| Fig. 11 (1) The schematic of the corrosion resistance mechanism of Mg alloys with GOCS/Hep coating; (2) the SEM image and the number of adhered platelets on different samples ((a) Mg, (b) Mg–OH, (c) Mg-16, (d) Mg-GOCS, (e) Mg-GOCS/Hep); (3) the results of the concentration cGMP expression and hemolysis ratio in samples; (4) fluorescent images of the endothelial cells cultured on the different samples for 1d and 3d (green is the cytoplasm and blue is the nucleus). Reproduced from ref. 169 with permission from [Elsevier], copyright [2020]. | ||
If red blood cells are to perform normally when interacting with biomaterials, their hemolysis rate should be below 5%. Nevertheless, a 30% hemolysis rate was observed in the unmodified sample due to its poor corrosion resistance and the production of high amounts of Mg2+ and hydrogen peroxide, which resulted in blood cells rupturing and hemolysis.176 On the other hand, the hemolysis rate of Mg-GOCS/heparin stents decreased significantly to about 3%. The biocompatibility of stents was assessed by cultivating endothelial cells, and Mg-GOCS/heparin stents enhanced cell proliferation significantly with better-spreading morphologies. In addition, modified stents also contain higher levels of VEGF, a highly specific vascular endothelial growth factor, which improves endothelial cell differentiation and maintenance.177 Lastly, nitric oxide (NO) was measured as an indicator of endothelial cells’ health and normal physiological processes.178,179 A GOCS/heparin coating enhances NO expression, increasing the compatibility of Mg alloy stents since heparin combined with NO reduced platelet activation further and enhanced endothelial cell proliferation.180,181
A 3D-printed cardiovascular stent was developed using a hydrogel polymeric composite composed of 2-hydroxyethyl methacrylate (HEMA) and methacrylate GO (MeGO) with loaded resveratrol (RSL).182 MeGO was found to effectively crosslink the hydrogel, thereby decreasing swelling and maintaining the integrity of the stent. MeGO also provided excellent mechanical properties (the elastic stiffness of the stent was 0.29 × 10−2 MPa (F-ratio = 1.46) and enhanced load-bearing efficiency). There have been numerous studies showing that RSL reduces oxidative stress-induced endothelial apoptosis and increases NO production, preventing platelet adhesion and restoring endothelial function.175,183,184 According to the RSL release data, the addition of MeGO caused the pores in the structure to be smaller, providing sustained RSL release without initial burst release.185 The NO release assessment also showed that this stent suppressed platelet adhesion and activation by significantly promoting NO production. In terms of viability results, there was no cytotoxicity observed on HUVECs, and the apoptosis of the cells was reduced up to fivefold.
There are also several vascular diseases that can be treated and revascularized with vascular grafts, including coronary artery disease (CAD), peripheral arterial disease (PAD), abdominal aortic aneurysm (AAA), and aortic dissection (AD).186 Given autologous blood vessels’ limitations, such as their poor quality, non-availability due to patients’ diseases, and challenges in surgical interventions and techniques, vascular tissue engineering offers some promising solutions.187,188 Vascular tissue engineering involves designing and creating artificial blood vessels that can be implanted in vivo and provide functional vessels for patients. This can be accomplished with GBM's antibacterial properties and other unique features, which make it perfect for fabricating vascular grafts and alleviating complications after implanting.19,189 Some of these research results are mentioned in Table 2.
As an example, GO has been used as a coating to promote the mechanical and biological features of decellularized placental and umbilical cord arteries.187 In vascular grafts, mechanical properties play a key role and should mimic native vessel properties as much as possible; therefore, any mismatch in strain, compliance, and strength or integrity has a significant impact on the success or failure of the implant and the patency rate.190–192 According to the mechanical evaluation, GO coating did not significantly affect decellularized placental arteries except for its compliance, which increased by 10%. However, GO coating promoted all measured parameters for umbilical cord arteries, including Fmax (the maximum force for rupturing placental and umbilical cord decellularized arteries), burst pressure, strain, and compliance by 27%, 29%, 25%, and 10% respectively. As such, GO coating provides desired mechanical properties with similar values to the native saphenous vein.193 For both placental and umbilical cord decellularized arteries, platelet adhesion analysis indicated a high value of adhered platelets leading to clot formation. In spite of this, the number of adhered platelets decreased significantly for GO-coated arteries, particularly for umbilical cord arteries. Additionally, antibacterial activity results indicated a drastic reduction of bacterial adhesion (S. aureus) for both, which was more pronounced in the placental decellularized arteries. Furthermore, a biological assessment was conducted with human umbilical vein endothelial cells (HUVECs). In accordance with the cytocompatibility results, GO coating not only had no cytotoxic effect on HUVEC adhesion and proliferation, but also, according to previous studies, increased endothelial cell proliferation by forming reactive oxygen and nitrogen species within the cells as well as activating phospho-eNOS and phospho-Akt (Fig. 12).14,187
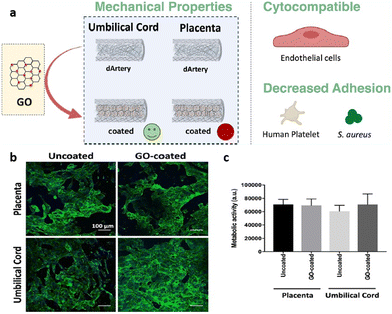 | ||
| Fig. 12 (a) Schematics of GO coating on decellularized placental and umbilical cord arteries; (b) immunofluorescent images of HUVECs on treated and untreated decellularized placental chorion and umbilical cord arteries HUVECs adherent to uncoated and GO-coated placental chorion and umbilical cord decellularized; (c) the evaluation of metabolic activity of cells by resazurin assay. Reproduced from ref. 187 with permission from [ACS], copyright [2021]. | ||
3.3. Graphene in the development of heart valve
Heart valve failure has encompassed a great number of patients suffering from cardiovascular disease.2 Many attempts have been made to manufacture prosthetic heart valves with the same function and design to treat these kinds of disorders. There are three types of heart valve prosthetics: mechanical, biosynthetic valves, and polymeric heart valves.194–196A mechanical valve demonstrates excellent mechanical properties and long-term durability. Nevertheless, due to their geometry and rigid synthetic materials, they increase the risk of thrombosis, requiring patients to take lifelong anticoagulation therapy.197 Bioprosthetic valves offer better hemodynamic and hemocompatibility than mechanical valves without requiring daily anticoagulants; however, they have some limitations, including leaflet calcification and weak mechanical performance.198,199 In recent years, polymeric heart valves have been developed to overcome many drawbacks associated with two other types of heart valves by offering a nature-like structure in addition to excellent hemo and biocompatibility. Even so, clinically applying them can still be challenging because of their degradation rate.200 So far, several research studies have been conducted to develop and improve all types of heart valve prosthetics through various modifications according to Table 2.201 In terms of biomaterials that can promote the functionality and applicability of heart valve prosthetics in clinics, GBM family members have attracted scientists’ attention due to their unique properties for modifying mechanical or bioprosthetic valves, as well as fabricating polymeric valves.
A study, for instance, modified acellular pulmonary valve tissue with rGO as a biosynthetic valve.1 In the experiment, four groups were defined: poly-L lysine-modified, fibronectin-modified, rGO-modified, and acellular tissue without coating.1 Platelet activation was studied under dynamic conditions by analyzing markers involved in platelet adhesion and activation, such as CD42a, CD42b, CD41a, CD40, and CD62P. According to the results, there was no significant difference between the rGO-modified sample and the other samples, suggesting that rGO does not activate platelets and can impart thrombo-resistance through rGO. Further, direct contact cytotoxicity results showed 100 g ml−1 of rGO significantly reduced L929 fibroblast viability after 24 h. Various studies have reported that cytotoxicity may occur due to disrupted membranes or the formation of reactive oxygen species.202–204 In the indirect test, however, all samples had approximately 100% viability, and no cytotoxicity was observed. As a result, it can be concluded that rGO does not induce ROS and mechanical damage to the cells’ membrane is the main cause of viability reduction in the direct test. By studying DNA fragmentation using TUNEL assays, it was also found that rGO did not cause DNA damage.
A polymeric heart valve is also being developed based on functionalized GO (FGO) and poly(carbonate-urea) urethane known as “Hastalex”, whose characteristics have been tested against those of GORE-TEX, a commercial composite for cardiovascular applications.205 According to the results, Hastalex displayed superior mechanical properties both transversely and longitudinally (3.5-fold and 2.5-fold higher ultimate tensile strength, respectively). Hence, this composite can be more efficient in manufacturing thinner leaflets, as it has higher rigidity and deformability. Regarding biocompatibility, the highest number of viable endothelial cells (Ea.hy 926) exceeded 2.2-fold and 7.5-fold over control and GORE-TEX samples, respectively. The number of adhered platelets in both samples was not significantly different based on the blood platelet adhesion assessment. However, the GORE-TEX surface showed higher platelet deformation than the Hastalex surface. As a result of 60 days of implantation, Hastalex formed a thinner fibrous capsule and was more resistant to calcification, indicating that it is a more biocompatible implant than GORE-TEX.205
4. Problems, solutions and future opportunities for GBMs in cardiovascular applications
Questions about the safety of nanoparticles in biomedicine are not new. Despite extensive efforts to understand their safety and toxicity, many questions remain unanswered.217,218 Similarly, while GBMs possess remarkable properties, concerns persist regarding their biocompatibility and long-term toxicity. The biocompatibility of GBMs may dramatically change by the size, concentration, shape or surface chemistry of the 2D sheet.219 Many studies have reported conflicting results about the cytotoxic effects of GBMs. If GBMs cannot properly biodegrade, they may accumulate within our bodies.27 For example, accumulation of GBMs in the heart tissue may trigger an inflammatory response, leading to chronic inflammation and fibrosis.220 Another potential outcome of GBM accumulation could involve the disruption of the heart's normal electrical activity, given GBMs distinctive properties. Other limitations of GBMs include their potential to interact with cell membranes, leading to membrane damage by forming reactive oxygen species (ROS).221 Among the cells in our body, red blood cells stand out as particularly sensitive, making them significant considerations for cardiac applications. Red blood cells possess limited repair capabilities and exhibit diminished responsiveness to oxidative stress. Therefore, the premature breakdown of red blood cells (haemolysis) serves as a sensitive indicator of damage induced by GBMs.222–225 Moreover, several studies have indicated that GO is more prone to inducing thrombosis and platelet activation compared to rGO.25,226 Research by Singh's group suggests that GO triggers platelet aggregation by activating nonreceptor protein tyrosine kinases of the Src family and releasing intracellular free calcium from cytosolic stores.227Enhancing the biocompatibility of GBMs involves functionalizing their surface; however, it is crucial to consider the long-term stability of these functionalization methods such as surface coatings.228–230 If the coatings degrade over time, the toxicity may differ significantly from short-term exposure results. Extended studies are necessary to assess whether longer treatment durations influence the nanotoxic potential of GBMs. One strategy that can improve GBM biocompatibility is PEGylation.231–233 In a study, it was shown that GO may bind to the complement protein C3, which could then be cleaved, inducing activation of the complement cascade. However, through PEGylation of GO, this process impaired the normal interaction between C3a and its receptor, thereby reducing the adverse complement response to G.232 PEG has attracted significant attention among various polymeric structures, largely due to its approval by the US Food and Drug Administration (FDA).234,235 Renowned for its non-toxic, non-immunogenic, and non-antigenic properties, along with its high water solubility, PEGylation effectively reduces reticuloendothelial clearance, extends circulation time, and improves material stability. Current literature indicates that PEGylation decreases the cytotoxicity of various GBMs while enhancing their physiological stability and pharmacokinetic characteristics.224,236–242 In addition to PEG, various hydrophilic polymers, ranging from natural polymers like dextran to synthetic ones such as polyvinyl alcohol (PVA) and cationic polymers like polyethyleneimine (PEI), polypropyleneimine (PPI), and poly(amidoamine) (PAMAM), have been reported to enhance dispersity and stability and reduce toxicity of GBMs.25,225,243,244
Therefore, future research should focus on elucidating the mechanisms underlying GBM-cell interactions and developing strategies to enhance biocompatibility while minimizing toxicity. Furthermore, to enhance the accuracy and comparability of studies, comprehensive physicochemical characterization and standardization are essential in all future investigations concerning the toxicity of GBMs. Experimental descriptions of GBMs should include details such as their size, morphology, surface area, concentration, charge, surface modifications, purity, and agglomeration.25,163,245–249 The toxicity and biocompatibility of GBMs are largely influenced by these physicochemical factors; therefore, researchers should consider single-factor experimental designs and avoid excluding other interfering parameters. Moreover, conducting various cell experiments in combination with primary cells is essential for cardiac applications, enabling a comprehensive evaluation of the physicochemical properties and toxicity of GBMs.25,246,247,250 It is essential to investigate the in vivo biocompatibility of these nanomaterials through both short-term and long-term studies before utilizing them in biomedical applications.251–253 Another challenging aspect related to GBMs for cardiac applications is the limited availability of reports on their in vivo biocompatibility. Additionally, the majority of existing in vivo studies have been conducted in small animals, which presents an additional obstacle.10,241,245,247,249
5. Conclusion
Cardiovascular diseases (CVDs) are among the most common health concerns worldwide, so heart transplantation remains the most effective and invasive method of fully recovering heart function.78 In spite of recent advances in repairing cardiac tissue and restoring its complete function, recent developments have yet to address the unique structural, physical, mechanical, and chemical characteristics of the heart and blood vessels.8 Many studies have been conducted in order to mimic native-like constructs by applying various types of materials, cells, and fabrication methods.9 Among several biomaterials to meet this purpose, GBMs may be one of the most promising ones due to their remarkable high electrical, mechanical, and biocompatible properties, as well as their inert chemical properties and compatibility with various fabrication methods that make them an ideal candidate.4,15,91 This study reviewed the recent applications of GBMs in cardiovascular medicine, including cardiac tissue engineering, vascular grafts, and heart valves. It is expected that GBMs will be used in clinics as effective treatments for cardiovascular diseases, and perhaps will become a great alternative to heart transplants based on the above-mentioned information, despite some serious concerns about their long-term biocompatibility range.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the help of Nicholas Seifalian in reading through the table and references for accuracy.References
- P. Wilczek, R. Major, L. Lipinska, J. Lackner and A. Mzyk, Thrombogenicity and biocompatibility studies of reduced graphene oxide modified acellular pulmonary valve tissue, Mater. Sci. Eng., C, 2015, 53, 310–321, DOI:10.1016/j.msec.2015.04.044.
- R. A. Rippel, H. Ghanbari and A. M. Seifalian, Tissue-engineered heart valve: future of cardiac surgery, World J. Surg., 2012, 36, 1581–1591 CrossRef PubMed.
- J. R. Venugopal, M. P. Prabhakaran, S. Mukherjee, R. Ravichandran, K. Dan and S. Ramakrishna, Biomaterial strategies for alleviation of myocardial infarction, J. R. Soc., Interface, 2012, 9(66), 1–19 CrossRef CAS PubMed.
- K. N. Alagarsamy, et al., Carbon nanomaterials for cardiovascular theranostics: Promises and challenges, Bioact. Mater., 2021, 6(8), 2261–2280, DOI:10.1016/j.bioactmat.2020.12.030.
- X. Yan, H. Sun and P. Yang, Recent Advances on Electroconductive Hydrogels Used in Heart Repair and Regeneration, Adv. Mater. Sci. Eng., 2022, 1, 6042137, DOI:10.1155/2022/6042137.
- D. Sharma, M. Ferguson, T. J. Kamp and F. Zhao, Constructing biomimetic cardiac tissues: a review of scaffold materials for engineering cardiac patches, Emergent Mater., 2019, 2(2), 181–191, DOI:10.1007/s42247-019-00046-4.
- Z. Shao, et al., Recent progress in biomaterials for heart valve replacement: Structure, function, and biomimetic design, View, 2021, 2(6), 20200142, DOI:10.1002/VIW.20200142.
- V. Sharma, et al., Recent advances in cardiac tissue engineering for the management of myocardium infarction, Cells, 2021, 10(10), 2538, DOI:10.3390/cells10102538.
- S. Saghebasl, A. Akbarzadeh, A. M. Gorabi, N. Nikzamir, M. SeyedSadjadi and E. Mostafavi, Biodegradable functional macromolecules as promising scaffolds for cardiac tissue engineering, Polym. Adv. Technol., 2022, 33(7), 2044–2068, DOI:10.1002/pat.5669.
- M. H. Norahan, M. Amroon, R. Ghahremanzadeh, M. Mahmoodi and N. Baheiraei, Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering, J. Biomed. Mater. Res., Part A, 2019, 107(1), 204–219, DOI:10.1002/jbm.a.36555.
- K. Ashtari, et al., Electrically conductive nanomaterials for cardiac tissue engineering, Adv. Drug Delivery Rev., 2019, 144, 162–179 CrossRef CAS PubMed.
- M. Tallawi, et al., Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review, J. R. Soc., Interface, 2015, 12(108), 20150254 CrossRef PubMed.
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, A roadmap for graphene, Nature, 2012, 490(7419), 192–200 CrossRef CAS PubMed.
- S. Stankovich, et al., Graphene-based composite materials, Nature, 2006, 442(7100), 282–286 CrossRef CAS PubMed.
- A. Savchenko, R. T. Yin, D. Kireev, I. R. Efimov and E. Molokanova, Graphene-Based Scaffolds: Fundamentals and Applications for Cardiovascular Tissue Engineering, Front. Bioeng. Biotechnol., 2021, 9, 797340, DOI:10.3389/fbioe.2021.797340.
- R. N. Gomes, et al., Antimicrobial graphene nanoplatelets coatings for silicone catheters, Carbon, 2018, 139, 635–647 CrossRef CAS.
- A. T. Pereira, et al., Graphene-based materials: The key for the successful application of pHEMA as a blood-contacting device, Biomater. Sci., 2021, 9(9), 3362–3377 RSC.
- A. T. Pereira, P. C. Henriques, P. C. Costa, M. C. L. Martins, F. D. Magalhaes and I. C. Goncalves, Graphene oxide-reinforced poly (2-hydroxyethyl methacrylate) hydrogels with extreme stiffness and high-strength, Compos. Sci. Technol., 2019, 184, 107819 CrossRef CAS.
- P. C. Henriques, I. Borges, A. M. Pinto, F. D. Magalhaes and I. C. Goncalves, Fabrication and antimicrobial performance of surfaces integrating graphene-based materials, Carbon, 2018, 132, 709–732 CrossRef CAS.
- I. E. P. Souza, L. V. Cambraia, V. S. Gomide and E. H. M. Nunes, Short review on the use of graphene as a biomaterial –prospects, and challenges in Brazil, J. Mater. Res. Technol., 2022, 19, 2410–2430, DOI:10.1016/j.jmrt.2022.05.170.
- N. Amiryaghoubi and M. Fathi, Bioscaffolds of graphene based-polymeric hybrid materials for myocardial tissue engineering, BioImpacts, 2024, 14(1) DOI:10.34172/bi.2023.27684.
- W.-W. Liu, S.-P. Chai, A. R. Mohamed and U. Hashim, Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments, J. Ind. Eng. Chem., 2014, 20(4), 1171–1185, DOI:10.1016/j.jiec.2013.08.028.
- Y. M. Manawi, Ihsanullah, A. Samara, T. Al-Ansari and M. A. Atieh, A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method, Materials, 2018, 11(5), 822, DOI:10.3390/ma11050822.
- J.-H. Lee, S.-J. Park and J.-W. Choi, Electrical property of graphene and its application to electrochemical biosensing, Nanomaterials, 2019, 9(2), 297 CrossRef CAS PubMed.
- Y. Xiao, et al., Synthesis and functionalization of graphene materials for biomedical applications: recent advances, challenges, and perspectives, Adv. Sci., 2023, 10(9), 2205292 CrossRef CAS PubMed.
- J. Li, H. Zeng, Z. Zeng, Y. Zeng and T. Xie, Promising graphene-based nanomaterials and their biomedical applications and potential risks: A comprehensive review, ACS Biomater. Sci. Eng., 2021, 7(12), 5363–5396 CrossRef CAS PubMed.
- P. Zare, M. Aleemardani, A. Seifalian, Z. Bagher and A. M. Seifalian, Graphene Oxide: Opportunities and Challenges in Biomedicine, Nanomaterials, 2021, 11(5), 1083 CrossRef CAS PubMed.
- L. Yao, A. Chen, L. Li and Y. Liu, Preparation, properties, applications and outlook of graphene-based materials in biomedical field: a comprehensive review, J. Biomater. Sci., Polym. Ed., 2023, 34(8), 1121–1156 CrossRef CAS PubMed.
- F. Kuang, T. Hui, Y. Chen, M. Qiu and X. Gao, Post–Graphene 2D Materials: Structures, Properties, and Cancer Therapy Applications, Adv. Healthc. Mater., 2024, 13(5), 2302604 CrossRef CAS PubMed.
- M. Aleemardani, P. Zare, A. Seifalian, Z. Bagher and A. M. Seifalian, Graphene-based materials prove to be a promising candidate for nerve regeneration following peripheral nerve injury, Biomedicines, 2021, 10(1), 73 CrossRef PubMed.
- P. Zare, M. Aleemardani, A. Seifalian, Z. Bagher and A. M. Seifalian, Graphene oxide: Opportunities and challenges in biomedicine, Nanomaterials, 2021, 11(5), 1083 CrossRef CAS PubMed.
- M. Sekuła-Stryjewska, et al., Graphene-based materials enhance cardiomyogenic and angiogenic differentiation capacity of human mesenchymal stem cells in vitro – Focus on cardiac tissue regeneration, Mater. Sci. Eng., C, 2021, 119, 111614, DOI:10.1016/j.msec.2020.111614.
- F. Edrisi, N. Baheiraei, M. Razavi, K. Roshanbinfar, R. Imani and N. Jalilinejad, Potential of graphene-based nanomaterials for cardiac tissue engineering, J. Mater. Chem. B, 2023, 11(31), 7280–7299, 10.1039/D3TB00654A.
- H. Jawad, N. N. Ali, A. R. Lyon, Q. Z. Chen, S. E. Harding and A. R. Boccaccini, Myocardial tissue engineering: a review, J. Tissue Eng. Regener. Med., 2007, 1(5), 327–342 CrossRef CAS PubMed.
- Q.-Z. Chen, S. E. Harding, N. N. Ali, A. R. Lyon and A. R. Boccaccini, Biomaterials in cardiac tissue engineering: ten years of research survey, Mater. Sci. Eng., R, 2008, 59(1–6), 1–37 CrossRef.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Measurement of the elastic properties and intrinsic strength of monolayer graphene, Science, 2008, 321(5887), 385–388 CrossRef CAS PubMed.
- R. Geetha Bai, N. Ninan, K. Muthoosamy and S. Manickam, Graphene: A versatile platform for nanotheranostics and tissue engineering, Prog. Mater. Sci., 2018, 91, 24–69, DOI:10.1016/j.pmatsci.2017.08.004.
- G. Reina, J. M. González-Domínguez, A. Criado, E. Vázquez, A. Bianco and M. Prato, Promises, facts and challenges for graphene in biomedical applications, Chem. Soc. Rev., 2017, 46(15), 4400–4416, 10.1039/c7cs00363c.
- D. W. Johnson, B. P. Dobson and K. S. Coleman, A manufacturing perspective on graphene dispersions, Curr. Opin. Colloid Interface Sci., 2015, 20(5–6), 367–382 CrossRef CAS.
- Y. Xiao, et al., Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, Challenges, and Perspectives, Adv. Sci., 2023, 10(9), 2205292, DOI:10.1002/advs.202205292.
- C. N. R. Rao, et al., A study of the synthetic methods and properties of graphenes, Sci. Technol. Adv. Mater., 2010, 11(5), 054502 CrossRef CAS PubMed.
- X. Wang, Y. Wei, H. Zhou, Q. Liu and L. Zhu, Synthesis of graphene nanosheets by the electrical explosion of graphite powder confined in a tube, Ceram. Int., 2021, 47(15), 21934–21942 CrossRef CAS.
- J. Chen, et al., One-step reduction and PEGylation of graphene oxide for photothermally controlled drug delivery, Biomaterials, 2014, 35(18), 4986–4995 CrossRef CAS PubMed.
- Y. Qu, et al., Advances on graphene-based nanomaterials for biomedical applications, Mater. Sci. Eng., C, 2018, 90, 764–780, DOI:10.1016/j.msec.2018.05.018.
- V. C. Scanlon and T. Sanders, Essentials of anatomy and physiology, FA Davis, 2018 Search PubMed.
- R. Bao, B. Tan, S. Liang, N. Zhang, W. Wang and W. Liu, A π–π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction, Biomaterials, 2017, 122, 63–71, DOI:10.1016/j.biomaterials.2017.01.012.
- T. Doenst, T. D. Nguyen and E. D. Abel, Cardiac metabolism in heart failure: implications beyond ATP production, Circ. Res., 2013, 113(6), 709–724 CrossRef CAS PubMed.
- M. Solazzo, F. J. O'Brien, V. Nicolosi and M. G. Monaghan, The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering, APL Bioeng., 2019, 3(4) DOI:10.1063/1.5116579.
- A. Ul Haq, et al., Extrinsically conductive nanomaterials for cardiac tissue engineering applications, Micromachines, 2021, 12 (8), 914, DOI:10.3390/mi12080914.
- X. Wu, S. J. Ding, K. Lin and J. Su, A review on the biocompatibility and potential applications of graphene in inducing cell differentiation and tissue regeneration, J. Mater. Chem. B, 2017, 5(17), 3084–3102, 10.1039/c6tb03067j.
- J. Arkowski, M. Obremska, K. Kędzierski, A. Sławuta and M. Wawrzyńska, Applications for graphene and its derivatives in medical devices: Current knowledge and future applications, Adv. Clin. Exp. Med., 2021, 29(12), 1497–1504, DOI:10.17219/ACEM/130601.
- J. Kailashiya, N. Singh, S. K. Singh, V. Agrawal and D. Dash, Graphene oxide-based biosensor for detection of platelet-derived microparticles: a potential tool for thrombus risk identification, Biosens. Bioelectron., 2015, 65, 274–280 CrossRef CAS PubMed.
- P. Kalluru, R. Vankayala, C.-S. Chiang and K. C. Hwang, Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors, Biomaterials, 2016, 95, 1–10 CrossRef CAS PubMed.
- Z. Fan, et al., Near-infrared light-triggered unfolding microneedle patch for minimally invasive treatment of myocardial ischemia, ACS Appl. Mater. Interfaces, 2021, 13(34), 40278–40289 CrossRef CAS PubMed.
- F. Perreault, A. F. De Faria, S. Nejati and M. Elimelech, Antimicrobial properties of graphene oxide nanosheets: why size matters, ACS Nano, 2015, 9(7), 7226–7236 CrossRef CAS PubMed.
- O. Akhavan and E. Ghaderi, Toxicity of graphene and graphene oxide nanowalls against bacteria, ACS Nano, 2010, 4(10), 5731–5736 CrossRef CAS PubMed.
- J. Wang, Y. Wei, X. Shi and H. Gao, Cellular entry of graphene nanosheets: the role of thickness, oxidation and surface adsorption, RSC Adv., 2013, 3(36), 15776–15782 RSC.
- Y. Tu, et al., Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets, Nat. Nanotechnol., 2013, 8(8), 594–601 CrossRef CAS PubMed.
- S. Ullah, et al., Tobramycin mediated silver nanospheres/graphene oxide composite for synergistic therapy of bacterial infection, J. Photochem. Photobiol., B, 2018, 183, 342–348 CrossRef CAS PubMed.
- J. Han, et al., Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair, ACS Nano, 2018, 12(2), 1959–1977 CrossRef CAS PubMed.
- G. Choe, et al., Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: mesenchymal stem cell encapsulation and regeneration of infarcted hearts, Biomaterials, 2019, 225, 119513 CrossRef CAS PubMed.
- A. M. Pinto, et al., Smaller particle size and higher oxidation improves biocompatibility of graphene-based materials, Carbon, 2016, 99, 318–329 CrossRef CAS.
- M. Orecchioni, D. Bedognetti, F. Sgarrella, F. M. Marincola, A. Bianco and L. G. Delogu, Impact of carbon nanotubes and graphene on immune cells, J. Transl. Med., 2014, 12, 1–11 CrossRef PubMed.
- S. P. Mukherjee, et al., Next–Generation Sequencing Reveals Differential Responses to Acute versus Long–Term Exposures to Graphene Oxide in Human Lung Cells, Small, 2020, 16(21), 1907686 CrossRef CAS PubMed.
- G. Duan, et al., Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane, Nanoscale, 2015, 7(37), 15214–15224 RSC.
- Y. Li, et al., The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways, Biomaterials, 2012, 33(2), 402–411 CrossRef CAS PubMed.
- E. Demirel, E. Karaca and Y. Y. Durmaz, Effective PEGylation method to improve biocompatibility of graphene derivatives, Eur. Polym. J., 2020, 124, 109504 CrossRef CAS.
- Y. Li, et al., Surface coating–dependent cytotoxicity and degradation of graphene derivatives: Towards the design of non–toxic, degradable nano–graphene, Small, 2014, 10(8), 1544–1554 CrossRef CAS PubMed.
- K. Yang, Y. Li, X. Tan, R. Peng and Z. Liu, Behavior and toxicity of graphene and its functionalized derivatives in biological systems, Small, 2013, 9(9–10), 1492–1503 CrossRef CAS PubMed.
- J. Li, H. Zeng, Z. Zeng, Y. Zeng and T. Xie, Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review, ACS Biomater. Sci. Eng., 2021, 7(12), 5363–5396, DOI:10.1021/acsbiomaterials.1c00875.
- J. Park, et al., Graphene potentiates the myocardial repair efficacy of mesenchymal stem cells by stimulating the expression of angiogenic growth factors and gap junction protein, Adv. Funct. Mater., 2015, 25(17), 2590–2600 CrossRef CAS.
- A. K. Barui, A. Roy, S. Das, K. Bhamidipati and C. R. Patra, Therapeutic applications of graphene oxides in angiogenesis and cancers, Nanoparticles and their biomedical applications, 2020, pp. 147–189 Search PubMed.
- M. Sekuła-Stryjewska, et al., Graphene-based materials enhance cardiomyogenic and angiogenic differentiation capacity of human mesenchymal stem cells in vitro–focus on cardiac tissue regeneration, Mater. Sci. Eng., C, 2021, 119, 111614 CrossRef PubMed.
- S. Mukherjee, et al., Graphene oxides show angiogenic properties, Adv. Healthc. Mater., 2015, 4(11), 1722–1732 CrossRef CAS PubMed.
- E. Chavakis and S. Dimmeler, Regulation of endothelial cell survival and apoptosis during angiogenesis, Arterioscler., Thromb., Vasc. Biol., 2002, 22(6), 887–893 CrossRef CAS PubMed.
- F. Weinberger, I. Mannhardt and T. Eschenhagen, Engineering cardiac muscle tissue: a maturating field of research, Circ. Res., 2017, 120(9), 1487–1500 CrossRef CAS PubMed.
- N. Kiaie, A. M. Gorabi, S. H. Ahmadi Tafti and S. Rabbani, Pre-vascularization Approaches for Heart Tissue Engineering, Regener. Eng. Transl. Med., 2021, 7(4), 450–459, DOI:10.1007/s40883-020-00172-0.
- M. D. Dozois, L. C. Bahlmann, Y. Zilberman and X. (Shirley) Tang, Carbon nanomaterial-enhanced scaffolds for the creation of cardiac tissue constructs: A new frontier in cardiac tissue engineering, Carbon, 2017, 120, 338–349, DOI:10.1016/j.carbon.2017.05.050.
- H. M. El-Husseiny, E. A. Mady, W. A. El-Dakroury, A. S. Doghish and R. Tanaka, Stimuli-responsive hydrogels: smart state of-the-art platforms for cardiac tissue engineering, Front. Bioeng. Biotechnol., 2023, 11, 1174075, DOI:10.3389/fbioe.2023.1174075.
- S. R. Shin, et al., Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators, ACS Nano, 2013, 7(3), 2369–2380 CrossRef CAS PubMed.
- Y. Wu, L. Wang, B. Guo and P. X. Ma, Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy, ACS Nano, 2017, 11(6), 5646–5659 CrossRef CAS PubMed.
- M. Maleki, et al., Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering, Biomol. Concepts, 2021, 11(1), 182–200, DOI:10.1515/bmc-2020-0017.
- P. Memarian, A. Solouk, Z. Bagher, S. Akbari and M. H. Nazarpak, Ionic conductive nanocomposite based on poly (l-lactic acid)/poly (amidoamine) dendrimerelectrospun nanofibrous for biomedical application, Biomed. Mater., 2021, 17(1), 015007 CrossRef PubMed.
- O. Lozano, A. Torres-Quintanilla and G. García-Rivas, Nanomedicine for the cardiac myocyte: where are we?, J. Controlled Release, 2018, 271, 149–165 CrossRef CAS PubMed.
- M. Farokhi, M. Aleemardani, A. Solouk, H. Mirzadeh, A. H. Teuschl and H. Redl, Crosslinking strategies for silk fibroin hydrogels: Promising biomedical materials, Biomed. Mater., 2021, 16(2), 022004 CrossRef CAS PubMed.
- S. K. Jaganathan, E. Supriyanto, S. Murugesan, A. Balaji and M. K. Asokan, Biomaterials in cardiovascular research: applications and clinical implications, BioMed Res. Int., 2014, 1, 459465, DOI:10.1155/2014/459465.
- L. Vogt, F. Ruther, S. Salehi and A. R. Boccaccini, Poly (glycerol sebacate) in biomedical applications—A review of the recent literature, Adv. Healthc. Mater., 2021, 10(9), 2002026 CrossRef CAS PubMed.
- M. Azizi, M. Navidbakhsh, S. Hosseinzadeh and M. Sajjadi, Cardiac cell differentiation of muscle satellite cells on aligned composite electrospun polyurethane with reduced graphene oxide, J. Polym. Res., 2019, 26(11), 1–9, DOI:10.1007/s10965-019-1936-9.
- J. Zhou, et al., Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct, Theranostics, 2018, 8(12), 3317–3330, DOI:10.7150/thno.25504.
- M. Kavousi Heidari, et al., Wound dressing based on PVA nanofiber containing silk fibroin modified with GO/ZnO nanoparticles for superficial wound healing: In vitro and in vivo evaluations, Biotechnol. Prog., 2023, 39(3), e3331, DOI:10.1002/btpr.3331.
- W. Xue, et al., Preparation, Properties, and Application of Graphene-Based Materials in Tissue Engineering Scaffolds, Tissue Eng., Part B, 2022, 28(5), 1121–1136, DOI:10.1089/ten.teb.2021.0127.
- S. Tiwari, R. Patil, S. K. Dubey and P. Bahadur, Graphene nanosheets as reinforcement and cell-instructive material in soft tissue scaffolds, Adv. Colloid Interface Sci., 2020, 281, 102167 CrossRef CAS PubMed.
- X. Chen, et al., Characteristics and toxicity assessment of electrospun gelatin/PCL nanofibrous scaffold loaded with graphene in vitro and in vivo, Int. J. Nanomed., 2019, 14, 3669–3678, DOI:10.2147/IJN.S204971.
- S. Bahrami, et al., Three-dimensional graphene foam as a conductive scaffold for cardiac tissue engineering, J. Biomater. Appl., 2019, 34(1), 74–85, DOI:10.1177/0885328219839037.
- A. Ghofrani, L. Taghavi, B. Khalilivavdareh, A. Rohani Shirvan and A. Nouri, Additive manufacturing and advanced functionalities of cardiac patches: A review, Eur. Polym. J., 2022, 174, 111332, DOI:10.1016/j.eurpolymj.2022.111332.
- H. P. Ferreira, et al., Using Graphene-Based Materials for Stiff and Strong Poly(ethylene glycol) Hydrogels, Int. J. Mol. Sci., 2022, 23(4), 2312, DOI:10.3390/ijms23042312.
- J. L. Ifkovits, et al., Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(25), 11507–11512 CrossRef CAS PubMed.
- N. Karimi Hajishoreh, N. Baheiraei, N. Naderi, M. Salehnia and M. Razavi, Left Ventricular Geometry and Angiogenesis Improvement in Rat Chronic Ischemic Cardiomyopathy following Injection of Encapsulated Mesenchymal Stem Cells, Cell J., 2022, 24(12), 741–747, DOI:10.22074/CELLJ.2022.557257.1040.
- S. R. Shin, et al., Reduced graphene oxide–gelMA hybrid hydrogels as scaffolds for cardiac tissue engineering, Small, 2016, 12(27), 3677–3689 CrossRef CAS PubMed.
- G. Zhao, et al., Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity, Acta Biomater., 2022, 139, 190–203, DOI:10.1016/j.actbio.2021.03.073.
- S. Saravanan, et al., Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart, Sci. Rep., 2018, 8(1), 15069, DOI:10.1038/s41598-018-33144-0.
- V. Martinelli, et al., Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes, Nano Lett., 2012, 12(4), 1831–1838 CrossRef CAS PubMed.
- B. D. Ratner, The catastrophe revisited: blood compatibility in the 21st century, Biomaterials, 2007, 28(34), 5144–5147 CrossRef CAS PubMed.
- B. D. Ratner, A. S. Hoffman, F. J. Schoen and J. E. Lemons, Biomaterials science: an introduction to materials in medicine, Elsevier, 2004 Search PubMed.
- Y. Shapira, M. Vaturi and A. Sagie, Hemolysis associated with prosthetic heart valves: a review, Cardiol. Rev., 2009, 17(3), 121–124 CrossRef PubMed.
- A. Sasidharan, et al., Hemocompatibility and macrophage response of pristine and functionalized graphene, Small, 2012, 8(8), 1251–1263 CrossRef CAS PubMed.
- R. N. Urankar, et al., Expansion of cardiac ischemia/reperfusion injury after instillation of three forms of multi-walled carbon nanotubes, Part. Fibre Toxicol., 2012, 9, 1–16 CrossRef PubMed.
- H. B. Bostan, et al., Cardiotoxicity of nano-particles, Life Sci., 2016, 165, 91–99 CrossRef CAS PubMed.
- R. K. Roy, et al., Hemocompatibility of surface-modified, silicon-incorporated, diamond-like carbon films, Acta Biomater., 2009, 5(1), 249–256 CrossRef CAS PubMed.
- J. F. Hecker and L. A. Scandrett, Roughness and thrombogenicity of the outer surfaces of intravascular catheters, J. Biomed. Mater. Res., 1985, 19(4), 381–395 CrossRef CAS PubMed.
- M. I. Jones, I. R. McColl, D. M. Grant, K. G. Parker and T. L. Parker, Protein adsorption and platelet attachment and activation, on TiN, TiC, and DLC coatings on titanium for cardiovascular applications, J. Biomed. Mater. Res., 2000, 52(2), 413–421 CrossRef CAS PubMed.
- T. Hasebe, et al., Effects of surface roughness on anti-thrombogenicity of diamond-like carbon films, Diamond Relat. Mater., 2007, 16(4–7), 1343–1348 CrossRef CAS.
- X. Meng, et al., Enhanced Hemocompatibility of a Direct Chemical Vapor Deposition-Derived Graphene Film, ACS Appl. Mater. Interfaces, 2021, 13(4), 4835–4843, DOI:10.1021/acsami.0c19790.
- J. Kayat, V. Gajbhiye, R. K. Tekade and N. K. Jain, Pulmonary toxicity of carbon nanotubes: a systematic report, Nanomedicine, 2011, 7(1), 40–49 CrossRef CAS PubMed.
- P. A. Stapleton, et al., Impairment of coronary arteriolar endothelium-dependent dilation after multi-walled carbon nanotube inhalation: a time-course study, Int. J. Mol. Sci., 2012, 13(11), 13781–13803 CrossRef CAS PubMed.
- S. Murugesan, S. A. Mousa, L. J. O'Connor, D. W. Lincoln II and R. J. Linhardt, Carbon inhibits vascular endothelial growth factor-and fibroblast growth factor-promoted angiogenesis, FEBS Lett., 2007, 581(6), 1157–1160 CrossRef CAS PubMed.
- S. Kanakia, et al., Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations, Biomaterials, 2014, 35(25), 7022–7031 CrossRef CAS PubMed.
- H. Fan, et al., Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites, Biomacromolecules, 2010, 11(9), 2345–2351 CrossRef CAS PubMed.
- V. Georgakilas, et al., Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications, Chem. Rev., 2012, 112(11), 6156–6214 CrossRef CAS PubMed.
- L. Zhang, Z. Lu, Q. Zhao, J. Huang, H. Shen and Z. Zhang, Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI–grafted graphene oxide, Small, 2011, 7(4), 460–464 CrossRef CAS PubMed.
- H. Kim, R. Namgung, K. Singha, I.-K. Oh and W. J. Kim, Graphene oxide–polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool, Bioconjugate Chem., 2011, 22(12), 2558–2567 CrossRef CAS PubMed.
- H. Zare-Zardini, A. Taheri-Kafrani, M. Ordooei, A. Amiri and M. Karimi-Zarchi, Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: In vitro and in vivo studies, J. Taiwan Inst. Chem. Eng., 2018, 93, 70–78, DOI:10.1016/j.jtice.2018.08.010.
- K.-H. Liao, Y.-S. Lin, C. W. Macosko and C. L. Haynes, Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts, ACS Appl. Mater. Interfaces, 2011, 3(7), 2607–2615 CrossRef CAS PubMed.
- A. Lakoma and E. S. Kim, Minimally invasive surgical management of benign breast lesions, Gland Surg., 2014, 3(2), 142 Search PubMed.
- L. G. Svensson, et al., Minimally invasive versus conventional mitral valve surgery: a propensity-matched comparison, J. Thorac. Cardiovasc. Surg., 2010, 139(4), 926–932 CrossRef PubMed.
- T. Li, Y. Li, X. Wang, X. Li and J. Sun, Thermally and near-infrared light-induced shape memory polymers capable of healing mechanical damage and fatigued shape memory function, ACS Appl. Mater. Interfaces, 2019, 11(9), 9470–9477 CrossRef CAS PubMed.
- W. Xiong, et al., A Vascularized Conductive Elastic Patch for the Repair of Infarcted Myocardium through Functional Vascular Anastomoses and Electrical Integration, Adv. Funct. Mater., 2022, 32(19), 2111273 CrossRef CAS.
- C. Hirschhäuser, et al., Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning, Basic Res. Cardiol., 2021, 116, 1–18 CrossRef PubMed.
- S. Chakraborty, T. Ponrasu, S. Chandel, M. Dixit and V. Muthuvijayan, Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications, R. Soc. Open Sci., 2018, 5(5), 172017, DOI:10.1098/rsos.172017.
- H. Bae, et al., Building vascular networks, Sci. Transl. Med., 2012, 4(160), 160ps23 Search PubMed.
- M. Lovett, K. Lee, A. Edwards and D. L. Kaplan, Vascularization strategies for tissue engineering, Tissue Eng., Part B, 2009, 15(3), 353–370 CrossRef CAS PubMed.
- F. Edrisi, N. Baheiraei, M. Razavi, K. Roshanbinfar, R. Imani and N. Jalilinejad, Potential of graphene-based nanomaterials for cardiac tissue engineering, J. Mater. Chem. B, 2023, 11, 7280–7299, 10.1039/D3TB00654A.
- M. Sekuła-Stryjewska, et al., Graphene-based materials enhance cardiomyogenic and angiogenic differentiation capacity of human mesenchymal stem cells in vitro – Focus on cardiac tissue regeneration, Mater. Sci. Eng., C, 2021, 119, 111614, DOI:10.1016/j.msec.2020.111614.
- M. Heil, I. Eitenmüller, T. Schmitz-Rixen and W. Schaper, Arteriogenesis versus angiogenesis: similarities and differences, J. Cell. Mol. Med., 2006, 10(1), 45–55 CrossRef CAS PubMed.
- L. Jiang, et al., Preparation of an Electrically Conductive Graphene Oxide/Chitosan Scaffold for Cardiac Tissue Engineering, Appl. Biochem. Biotechnol., 2019, 188(4), 952–964, DOI:10.1007/s12010-019-02967-6.
- A. Paul, et al., Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair, ACS Nano, 2014, 8(8), 8050–8062 CrossRef CAS PubMed.
- H. Sun, et al., Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the β1-integrin-mediated signaling pathway, Biomaterials, 2015, 55, 84–95 CrossRef CAS PubMed.
- A. Bianco, Graphene: safe or toxic? The two faces of the medal, Angew. Chem., Int. Ed., 2013, 52(19), 4986–4997 CrossRef CAS PubMed.
- J. Saleem, L. Wang and C. Chen, Carbon-Based Nanomaterials for Cancer Therapy via Targeting Tumor Microenvironment, Adv. Healthcare Mater., 2018, 7(20), 1800525, DOI:10.1002/adhm.201800525.
- D. Yang, et al., In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide, Biomaterials, 2016, 104, 361–371 CrossRef CAS PubMed.
- P. X. Lai, et al., Ultrastrong trapping of VEGF by graphene oxide: Anti-angiogenesis application, Biomaterials, 2016, 109, 12–22, DOI:10.1016/j.biomaterials.2016.09.005.
- J. Müller-Ehmsen, et al., Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium, J. Mol. Cell Cardiol., 2002, 34(2), 107–116 CrossRef PubMed.
- G. Choe, et al., Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: mesenchymal stem cell encapsulation and regeneration of infarcted hearts, Biomaterials, 2019, 225, 119513 CrossRef CAS PubMed.
- J. Müller-Ehmsen, et al., Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction, J. Mol. Cell Cardiol., 2006, 41(5), 876–884 CrossRef PubMed.
- D. M. Nelson, Z. Ma, K. L. Fujimoto, R. Hashizume and W. R. Wagner, Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges, Acta Biomater., 2011, 7(1), 1–15 CrossRef CAS PubMed.
- H. Zhang, et al., Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution, J. Thorac. Cardiovasc. Surg., 2007, 134(5), 1234–1240 CrossRef CAS PubMed.
- R. Lakshmanan and N. Maulik, Development of next generation cardiovascular therapeutics through bio-assisted nanotechnology, J. Biomed. Mater. Res., Part B, 2018, 106(5), 2072–2083, DOI:10.1002/jbm.b.34000.
- R. S. Mahla, Stem cells applications in regenerative medicine and disease therapeutics, Int. J. Cell Biol., 2016, 1, 6940283, DOI:10.1155/2016/6940283.
- H. Arzaghi, et al., Nanomaterials modulating stem cell behavior towards cardiovascular cell lineage, Mater. Adv., 2021, 2(7), 2231–2262, 10.1039/d0ma00957a.
- N. Karimi Hajishoreh, N. Baheiraei, N. Naderi and M. Salehnia, Reduced graphene oxide facilitates biocompatibility of alginate for cardiac repair, J. Bioact. Compat. Polym., 2020, 35(4–5), 363–377, DOI:10.1177/0883911520933913.
- J. Park, et al., Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules, Adv. Healthc. Mater., 2014, 3(2), 176–181 CrossRef CAS PubMed.
- E. Bressan, et al., Graphene based scaffolds effects on stem cells commitment, J. Transl. Med., 2014, 12(1), 1–15 CrossRef PubMed.
- A. J. Ryan, et al., Electroconductive Biohybrid Collagen/Pristine Graphene Composite Biomaterials with Enhanced Biological Activity, Adv. Mater., 2018, 30(15), 1706442, DOI:10.1002/adma.201706442.
- A. Najafi Tireh Shabankareh, H. Ghanbari and P. Samadi Pakchin, Development of a New Electroconductive Nanofibrous Cardiac Patch Based on Polyurethane-Reduced Graphene Oxide Nanocomposite Scaffolds, 2023, DOI:10.2139/ssrn.4339940.
- M. Shi, et al., Micropatterned conductive elastomer patch based on poly (glycerol sebacate)-graphene for cardiac tissue repair, Biofabrication, 2022, 14(3), 035001, DOI:10.1088/1758-5090/ac59f2.
- S. Soltani, et al., Development of an Injectable Shear-Thinning Nanocomposite Hydrogel for Cardiac Tissue Engineering, Gels, 2022, 8(2), 121, DOI:10.3390/gels8020121.
- Y. Wang, et al., 4D Printed Cardiac Construct with Aligned Myofibers and Adjustable Curvature for Myocardial Regeneration, ACS Appl. Mater. Interfaces, 2021, 13(11), 12746–12758, DOI:10.1021/acsami.0c17610.
- A. Fakhrali, et al., Biocompatible graphene–embedded PCL/PGS–based nanofibrous scaffolds: A potential application for cardiac tissue regeneration, J. Appl. Polym. Sci., 2021, 138(40), 51177 CrossRef CAS.
- L. Zhang, et al., Biocompatibility and Angiogenic Effect of Chitosan/Graphene Oxide Hydrogel Scaffolds on EPCs, Stem Cells Int., 2021, 1, 5594370, DOI:10.1155/2021/5594370.
- A. Mousavi, S. Mashayekhan, N. Baheiraei and A. Pourjavadi, Biohybrid oxidized alginate/myocardial extracellular matrix injectable hydrogels with improved electromechanical properties for cardiac tissue engineering, Int. J. Biol. Macromol., 2021, 180, 692–708, DOI:10.1016/j.ijbiomac.2021.03.097.
- A. Talebi, S. Labbaf, F. Karimzadeh, E. Masaeli and M.-H. N. Esfahani, Electroconductive graphene-containing polymeric patch: a promising platform for future cardiac repair, ACS Biomater. Sci. Eng., 2020, 6(7), 4214–4224 CrossRef CAS PubMed.
- Z. Yuan, Q. Qin, M. Yuan, H. Wang and R. Li, Development and novel design of clustery graphene oxide formed Conductive Silk hydrogel cell vesicle to repair and routine care of myocardial infarction: Investigation of its biological activity for cell delivery applications, J. Drug Delivery Sci. Technol., 2020, 60, 102001, DOI:10.1016/j.jddst.2020.102001.
- M. H. Norahan, M. Pourmokhtari, M. R. Saeb, B. Bakhshi, M. S. Zomorrod and N. Baheiraei, Electroactive cardiac patch containing reduced graphene oxide with potential antibacterial properties, Mater. Sci. Eng., C, 2019, 104, 109921 CrossRef CAS PubMed.
- A. Ghasemi, R. Imani, M. Yousefzadeh, S. Bonakdar, A. Solouk and H. Fakhrzadeh, Studying the potential application of electrospun polyethylene terephthalate/graphene oxide nanofibers as electroconductive cardiac patch, Macromol. Mater. Eng., 2019, 304(8), 1900187 CrossRef.
- S. R. Shin, et al., Reduced graphene oxide–gelMA hybrid hydrogels as scaffolds for cardiac tissue engineering, Small, 2016, 12(27), 3677–3689 CrossRef CAS PubMed.
- A. Sen Gupta, Nanomedicine approaches in vascular disease: A review, Nanomedicine, 2011, 7(6), 763–779, DOI:10.1016/j.nano.2011.04.001.
- C. Spadaccio, et al., Preventing treatment failures in coronary artery disease: what can we learn from the biology of in-stent restenosis, vein graft failure and internal thoracic arteries? Coronary artery disease: preventing graft failure, Cardiovasc. Res., 2020, 116(3), 505–519, DOI:10.1093/cvr/cvz214/5545542.
- R. Holzer, et al., Stenting of aortic coarctation: Acute, intermediate, and long–term results of a prospective multi–institutional registry—Congenital Cardiovascular Interventional Study Consortium (CCISC), Catheter. Cardiovasc. Interv., 2010, 76(4), 553–563 CrossRef PubMed.
- F. Gao, et al., Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility, Bioact. Mater., 2020, 5(3), 611–623, DOI:10.1016/j.bioactmat.2020.04.016.
- R. Podila, T. Moore, F. Alexis and A. M. Rao, Graphene coatings for enhanced hemo-compatibility of nitinol stents, RSC Adv., 2013, 3(6), 1660–1665, 10.1039/c2ra23073a.
- V. Berry, Impermeability of graphene and its applications, Carbon, 2013, 62, 1–10 CrossRef CAS.
- W. Tsai, J. M. Grunkemeier, C. D. McFarland and T. A. Horbett, Platelet adhesion to polystyrene–based surfaces preadsorbed with plasmas selectively depleted in fibrinogen, fibronectin, vitronectin, or von Willebrand's factor, J. Biomed. Mater. Res., 2002, 60(3), 348–359 CrossRef CAS PubMed.
- L. Zhang, et al., The influence of surface chemistry on adsorbed fibrinogen conformation, orientation, fiber formation and platelet adhesion, Acta Biomater., 2017, 54, 164–174 CrossRef CAS PubMed.
- O. Ros, et al., SponGee: a genetic tool for subcellular and cell-specific cGMP manipulation, Cell Rep., 2019, 27(13), 4003–4012 CrossRef CAS PubMed.
- Z. Yang, et al., Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents, Biomaterials, 2015, 63, 80–92 CrossRef CAS PubMed.
- H. Zhang, et al., Catechol/polyethyleneimine conversion coating with enhanced corrosion protection of magnesium alloys: potential applications for vascular implants, J. Mater. Chem. B, 2018, 6(43), 6936–6949 RSC.
- G. Liu, et al., A VEGF delivery system targeting MI improves angiogenesis and cardiac function based on the tropism of MSCs and layer-by-layer self-assembly, Biomaterials, 2017, 127, 117–131 CrossRef CAS PubMed.
- A. W. Carpenter and M. H. Schoenfisch, Nitric oxide release: Part II, Therapeutic applications, Chem. Soc. Rev., 2012, 41(10), 3742–3752 RSC.
- A. de Mel, F. Murad and A. M. Seifalian, Nitric oxide: a guardian for vascular grafts?, Chem. Rev., 2011, 111(9), 5742–5767 CrossRef CAS PubMed.
- D. J. Suchyta, H. Handa and M. E. Meyerhoff, A nitric oxide-releasing heparin conjugate for delivery of a combined antiplatelet/anticoagulant agent, Mol. Pharm., 2014, 11(2), 645–650 CrossRef CAS PubMed.
- H. Qiu, et al., Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy, Biomaterials, 2019, 207, 10–22 CrossRef CAS PubMed.
- K. Veerubhotla and C. H. Lee, Design of biodegradable 3D-printed cardiovascular stent, Bioprinting, 2022, 26, e00204, DOI:10.1016/j.bprint.2022.e00204.
- T. Wallerath, et al., Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase, Circulation, 2002, 106(13), 1652–1658 CrossRef CAS PubMed.
- Z. Ungvari, et al., Resveratrol increases vascular oxidative stress resistance, Am. J. Physiol.: Heart Circ. Physiol., 2007, 292(5), H2417–H2424 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater., 2016, 1(12), 1–17 CrossRef PubMed.
- E. He, S. Serpelloni, P. Alvear, M. Rahimi and F. Taraballi, Vascular Graft Infections: An Overview of Novel Treatments Using Nanoparticles and Nanofibers, Fibers, 2022, 10(2), 12, DOI:10.3390/fib10020012.
- A. T. Pereira, et al., Graphene Oxide Coating Improves the Mechanical and Biological Properties of Decellularized Umbilical Cord Arteries, ACS Appl. Mater. Interfaces, 2021, 13(28), 32662–32672, DOI:10.1021/acsami.1c04028.
- D. Huo, et al., Construction of Antithrombotic Tissue-Engineered Blood Vessel via Reduced Graphene Oxide Based Dual-Enzyme Biomimetic Cascade, ACS Nano, 2017, 11(11), 10964–10973, DOI:10.1021/acsnano.7b04836.
- V. Srimaneepong, H. E. Skallevold, Z. Khurshid, M. S. Zafar, D. Rokaya and J. Sapkota, Graphene for antimicrobial and coating application, Int. J. Mol. Sci., 2022, 23(1), 499, DOI:10.3390/ijms23010499.
- K. H. Schneider, et al., Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts, Acta Biomater., 2016, 29, 125–134 CrossRef CAS PubMed.
- C.-H. Lin, K. Hsia, H. Ma, H. Lee and J.-H. Lu, In vivo performance of decellularized vascular grafts: a review article, Int. J. Mol. Sci., 2018, 19(7), 2101 CrossRef PubMed.
- S. Pashneh-Tala, S. MacNeil and F. Claeyssens, The tissue-engineered vascular graft—past, present, and future, Tissue Eng., Part B, 2016, 22(1), 68–100 CrossRef CAS PubMed.
- G. Konig, et al., Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery, Biomaterials, 2009, 30(8), 1542–1550 CrossRef CAS PubMed.
- N. Slepičková Kasálková, S. Rimpelová and C. Vacek, et al., Surface activation of Hastalex by vacuum argon plasma for cytocompatibility enhancement., Heliyon, 2024, 10(6), e27816, DOI:10.1016/j.heliyon.2024.e27816.
- K. Hammermeister, G. K. Sethi, W. G. Henderson, F. L. Grover, C. Oprian and S. H. Rahimtoola, Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial, J. Am. Coll. Cardiol., 2000, 36(4), 1152–1158 CrossRef CAS PubMed.
- W. R. E. Jamieson, et al., Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after mitral valve replacement, J. Thorac. Cardiovasc. Surg., 2005, 129(6), 1301–1308 CrossRef CAS PubMed.
- Y. Misawa, Valve-related complications after mechanical heart valve implantation, Surg. Today, 2015, 45(10), 1205–1209 CrossRef PubMed.
- S. Lee, et al., Calcification and oxidative modifications are associated with progressive bioprosthetic heart valve dysfunction, J. Am. Heart Assoc., 2017, 6(5), e005648 CrossRef PubMed.
- R. A. Manji, B. Ekser, A. H. Menkis and D. K. C. Cooper, Bioprosthetic heart valves of the future, Xenotransplantation, 2014, 21(1), 1–10 CrossRef PubMed.
- D. Bezuidenhout, D. F. Williams and P. Zilla, Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices, Biomaterials, 2015, 36, 6–25 CrossRef CAS PubMed.
- A. G. Kidane, et al., Current developments and future prospects for heart valve replacement therapy, J. Biomed. Mater. Res., Part B, 2009, 88(1), 290–303, DOI:10.1002/jbm.b.31151.
- Y. Chang, et al., In vitro toxicity evaluation of graphene oxide on A549 cells, Toxicol. Lett., 2011, 200(3), 201–210 CrossRef CAS PubMed.
- G. Qu, et al., Graphene oxide induces toll-like receptor 4 (TLR4)-dependent necrosis in macrophages, ACS Nano, 2013, 7(7), 5732–5745 CrossRef CAS PubMed.
- S. Goenka, V. Sant and S. Sant, Graphene-based nanomaterials for drug delivery and tissue engineering, J. Controlled Release, 2014, 173, 75–88 CrossRef CAS PubMed.
- E. A. Ovcharenko, et al., A New Nanocomposite Copolymer Based On Functionalised Graphene Oxide for Development of Heart Valves, Sci. Rep., 2020, 10(1), 5271, DOI:10.1038/s41598-020-62122-8.
- A. Owhal, M. Choudhary, A. D. Pingale, S. U. Belgamwar, S. Mukherjee and J. S. Rathore, Non-cytotoxic zinc/f-graphene nanocomposite for tunable degradation and superior tribo-mechanical properties: Synthesized via modified electro co-deposition route, Mater. Today Commun., 2023, 34, 105112, DOI:10.1016/j.mtcomm.2022.105112.
- Z. Zhao, et al., Strengthened corrosion control of biodegradable poly(trimethylene carbonate) coating on bioabsorbable Mg alloy by introducing graphene oxide, Surf. Coat. Technol., 2022, 451, 129052, DOI:10.1016/j.surfcoat.2022.129052.
- C. Pan, et al., Immobilization of bioactive complex on the surface of magnesium alloy stent material to simultaneously improve anticorrosion, hemocompatibility and antibacterial activities, Colloids Surf., B, 2021, 199, 111541, DOI:10.1016/j.colsurfb.2020.111541.
- M. C. Yang, et al., Electrochemical polymerization of PEDOT-graphene oxide-heparin composite coating for anti-fouling and anti-clotting of cardiovascular stents, Polymers, 2019, 11(9), 1520, DOI:10.3390/polym11091520.
- S. Ge, et al., Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility, Regener. Biomater., 2019, 6(5), 299–309, DOI:10.1093/rb/rbz010.
- K. Somszor, et al., Personalized, Mechanically Strong, and Biodegradable Coronary Artery Stents via Melt Electrowriting, ACS Macro Lett., 2020, 9(12), 1732–1739, DOI:10.1021/acsmacrolett.0c00644.
- A. M. ElSawy, N. F. Attia, H. I. Mohamed, M. Mohsen and M. H. Talaat, Innovative coating based on graphene and their decorated nanoparticles for medical stent applications, Mater. Sci. Eng., C, 2019, 96, 708–715, DOI:10.1016/j.msec.2018.11.084.
- M. Wawrzyńska, et al., Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface, BioMed Res. Int., 2018, 1(2018), 2758347, DOI:10.1155/2018/2758347.
- C. J. Pan, et al., Anticoagulation and endothelial cell behaviors of heparin-loaded graphene oxide coating on titanium surface, Mater. Sci. Eng., C, 2016, 63, 333–340, DOI:10.1016/j.msec.2016.03.001.
- X. Jing, H. Y. Mi, M. R. Salick, T. M. Cordie, X. F. Peng and L. S. Turng, Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications, Mater. Sci. Eng., C, 2015, 49, 40–50, DOI:10.1016/j.msec.2014.12.060.
- S. Karimi Alavije, M. Kokabi and M. Soleimani, Endothelial cells performance on 3D electrospun PVA/graphene nanocomposite tubular scaffolds, Polym. Bull., 2021, 78(9), 4797–4815, DOI:10.1007/s00289-020-03340-y.
- W. Najahi-Missaoui, R. D. Arnold and B. S. Cummings, Safe nanoparticles: are we there yet?, Int. J. Mol. Sci., 2020, 22(1), 385 CrossRef PubMed.
- S. Medici, M. Peana, A. Pelucelli and M. A. Zoroddu, An updated overview on metal nanoparticles toxicity, in Seminars in cancer biology, Elsevier, 2021, pp. 17–26 Search PubMed.
- C. McCallion, J. Burthem, K. Rees-Unwin, A. Golovanov and A. Pluen, Graphene in therapeutics delivery: Problems, solutions and future opportunities, Eur. J. Pharm. Biopharm., 2016, 104, 235–250 CrossRef CAS PubMed.
- J. Han, et al., Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair, ACS Nano, 2018, 12(2), 1959–1977 CrossRef CAS PubMed.
- K. Ghosal, P. Mondal, S. Bera and S. Ghosh, Graphene family nanomaterials-opportunities and challenges in tissue engineering applications, FlatChem, 2021, 30, 100315 CrossRef CAS.
- S. K. Singh, M. K. Singh, P. P. Kulkarni, V. K. Sonkar, J. J. A. Grácio and D. Dash, Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications, ACS Nano, 2012, 6(3), 2731–2740 CrossRef CAS PubMed.
- X. Zhang, et al., Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration, Carbon, 2011, 49(3), 986–995 CrossRef CAS.
- L. Ou, et al., Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms, Part. Fibre Toxicol., 2016, 13(1), 1–24 CrossRef PubMed.
- A. T. Pereira, et al., Graphene-based materials: The key for the successful application of pHEMA as a blood-contacting device, Biomater. Sci., 2021, 9(9), 3362–3377 RSC.
- C. N. R. Rao and A. K. Sood, Graphene: synthesis, properties, and phenomena, John Wiley & Sons, 2013 Search PubMed.
- S. K. Singh, et al., Thrombus inducing property of atomically thin graphene oxide sheets, ACS Nano, 2011, 5(6), 4987–4996 CrossRef CAS PubMed.
- J. Mu, F. Gao, G. Cui, S. Wang, S. Tang and Z. Li, A comprehensive review of anticorrosive graphene-composite coatings, Prog. Org. Coat., 2021, 157, 106321 CrossRef CAS.
- M. Schriver, W. Regan, W. J. Gannett, A. M. Zaniewski, M. F. Crommie and A. Zettl, Graphene as a long-term metal oxidation barrier: worse than nothing, ACS Nano, 2013, 7(7), 5763–5768 CrossRef CAS PubMed.
- A. C. Ferrari, et al., Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems, Nanoscale, 2015, 7(11), 4598–4810 RSC.
- A. Sasidharan, et al., Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study, Carbon, 2015, 95, 511–524 CrossRef CAS.
- X. Tan, et al., Functionalization of graphene oxide generates a unique interface for selective serum protein interactions, ACS Appl. Mater. Interfaces, 2013, 5(4), 1370–1377 CrossRef CAS PubMed.
- E. Demirel and Y. Y. Durmaz, PEGylated reduced graphene oxide as nanoplatform for targeted gene and drug delivery, Eur. Polym. J., 2023, 186, 111841 CrossRef CAS.
- F. Liu, Y. Sun, C. Kang and H. Zhu, Pegylated drug delivery systems: from design to biomedical applications, Nano LIFE, 2016, 6(03n04), 1642002 CrossRef CAS.
- M. Aleemardani, L. Johnson, M. Z. Trikić, N. H. Green and F. Claeyssens, Synthesis and characterisation of photocurable poly (glycerol sebacate)-co-poly (ethylene glycol) methacrylates, Mater. Today Adv., 2023, 19, 100410 CrossRef CAS.
- R. K. Thapa, Y. S. Youn, J.-H. Jeong, H.-G. Choi, C. S. Yong and J. O. Kim, Graphene oxide-wrapped PEGylated liquid crystalline nanoparticles for effective chemo-photothermal therapy of metastatic prostate cancer cells, Colloids Surf., B, 2016, 143, 271–277 CrossRef CAS PubMed.
- M. Xu, et al., Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: poly (acrylic acid)-functionalization is superior to PEGylation, ACS Nano, 2016, 10(3), 3267–3281 CrossRef CAS PubMed.
- E. Demirel, E. Karaca and Y. Y. Durmaz, Effective PEGylation method to improve biocompatibility of graphene derivatives, Eur. Polym. J., 2020, 124, 109504 CrossRef CAS.
- E. A. Chiticaru and M. Ionita, Graphene toxicity and future perspectives in healthcare and biomedicine, FlatChem, 2022, 35, 100417 CrossRef CAS.
- C. Xu, et al., Long circulating reduced graphene oxide–iron oxide nanoparticles for efficient tumor targeting and multimodality imaging, Nanoscale, 2016, 8(25), 12683–12692 RSC.
- H.-J. Im, et al., Accelerated blood clearance phenomenon reduces the passive targeting of PEGylated nanoparticles in peripheral arterial disease, ACS Appl. Mater. Interfaces, 2016, 8(28), 17955–17963 CrossRef CAS PubMed.
- C. G. England, et al., Re-assessing the enhanced permeability and retention effect in peripheral arterial disease using radiolabeled long circulating nanoparticles, Biomaterials, 2016, 100, 101–109 CrossRef CAS PubMed.
- R. Kumar, et al., Graphene-based materials for biotechnological and biomedical applications: Drug delivery, bioimaging and biosensing, Mater. Today Adv., 2023, 33, 101750 CAS.
- Z. Gao, S. Qin, C. Ménard-Moyon and A. Bianco, Applications of graphene-based nanomaterials in drug design: The good, the bad and the ugly, Expert Opin. Drug Discovery, 2023, 18(12), 1321–1332 CrossRef PubMed.
- N. Baheiraei, M. Razavi and R. Ghahremanzadeh, Reduced graphene oxide coated alginate scaffolds: potential for cardiac patch application, Biomater. Res., 2023, 27(1), 109 CrossRef CAS PubMed.
- C.-H. Kim, S.-Y. Lee, K. Y. Rhee and S.-J. Park, Carbon-based composites in biomedical applications: a comprehensive review of properties, applications, and future directions, Adv. Compos. Hybrid Mater., 2024, 7(2), 55 CrossRef CAS.
- Z. Niknam, et al., Recent advances and challenges in graphene–based nanocomposite scaffolds for tissue engineering application, J. Biomed. Mater. Res., Part A, 2022, 110(10), 1695–1721 CrossRef CAS PubMed.
- X. Jing, H. Y. Mi, B. N. Napiwocki, X. F. Peng and L. S. Turng, Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering, Carbon, 2017, 125, 557–570, DOI:10.1016/j.carbon.2017.09.071.
- C. J. Bullock and C. Bussy, Biocompatibility considerations in the design of graphene biomedical materials, Adv. Mater. Interfaces, 2019, 6(11), 1900229 CrossRef.
- J. Lee, et al., Nanoparticle-based hybrid scaffolds for deciphering the role of multimodal cues in cardiac tissue engineering, ACS Nano, 2019, 13(11), 12525–12539 CrossRef CAS PubMed.
- H. Kaur, et al., Progress and challenges of graphene and its congeners for biomedical applications, J. Mol. Liq., 2022, 368, 120703 CrossRef CAS PubMed.
- A. N. Banerjee, Graphene and its derivatives as biomedical materials: Future prospects and challenges, Interface Focus, 2018, 8(3), 20170056 CrossRef PubMed.
- P. Orsu and A. Koyyada, Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: a comprehensive review, Mater. Today Commun., 2020, 22, 100823 CrossRef CAS.
- K. S. Novoselov, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, A roadmap for graphene, Nature, 2012, 490(7419), 192–200 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
