Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solution and solid-state characterization of rare silyluranium(III) complexes†
Nathan J.
Lin
 ,
Matthias
Zeller
and
Suzanne C.
Bart
,
Matthias
Zeller
and
Suzanne C.
Bart
 *
*
H. C. Brown Laboratory, Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: sbart@purdue.edu
First published on 29th February 2024
Abstract
A uranium(III) silylate complex [K(DME)4][UI2{(Si(SiMe3)2SiMe2)2O}] (1) was stabilized by the addition of 18-crown-6, forming [K(18-crown-6)][UI2{(Si(SiMe3)2SiMe2)2O}] (1-crown). Crystallization under multiple conditions resulted in three distinct molecular structures. Compound 1-crown was further characterized in the solution state via1H, 13C, and 29Si NMR spectroscopy, and electronic absorption spectroscopy.
Interactions of uranium and silicon have been elucidated in different chemical environments, both from anthropogenic and natural sources.1 For instance, spent nuclear fuels are sometimes stored in silica based glasses, as these materials are more effective at withstanding high temperatures than plastic or concrete containers and are more durable than metals.2 Naturally, uranium and silicon also intermix at high temperatures, and can be found together in the glasses of volcanic rocks proximal to uranium ore deposits.3 Shales in Europe and Asia have also been discovered to be rich in uranium and silicon as well.4 As such, understanding the basic bonding of these two elements is critical for future energy needs where extraction and separation are utilized.
These two elements, U and Si, also happen to be important in the field of organometallic chemistry, where uranium mediated hydrosilylation catalysis of unsaturated alkyls has been demonstrated.5 Silyl based ligands have also provided a robust framework to support catalytically active uranium ions for hydroamination chemistry.6 Mazzanti has recently used siloxide ligands for uranium, showing that these ligands can support highly reduced uranium centres capable of small molecule activation.7,8 Molecular systems that contain both uranium and silicon such as these offer a unique opportunity to study U–Si interactions at the fundamental level, rather than the macroscale that is found in naturally occurring systems.
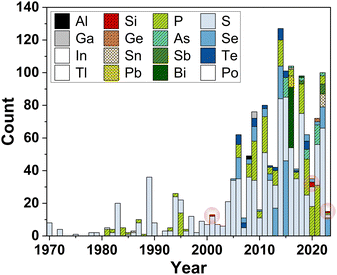
The field of coordination chemistry involving uranium and p-block main group elements has grown significantly since the 1970s and continues (Fig. 1).9 Yet, uranium complexes featuring the heavy Group 14 (tetrel) elements, including Si, Ge, Sn, and Pb, are significantly less common as compared to their lighter congener, carbon,10 and their pnictogen and chalcogen counterparts. With the abundance of organouranium coordination complexes,11,12 silicon serves as the knowledge bridge between carbon and the heavier tetrel congeners for understanding their fundamental bonding principles with uranium.13
The debut of U–Si interactions in a molecular system was first reported in 1989 by Porchia and co-workers, who synthesized Cp3UIV(SiPh3) (Cp = η5-C5H5) during their exploration of uranium-tetrel complexes (Chart 1).14–16 King and Marks reported the first use of Si(SiMe3)3 for U(IV) systems as a ligand to study U–Si reactivity.17 In 2001, Cummins and co-workers reported the first structurally characterized molecular U–Si complex though X-ray diffraction.17 Kaltsoyannis, Liddle, Mills, and co-workers compared U and Th analogs Cp′3AnIV(Si(SiMe3)3) (Cp′ = η5-C5H4SiMe3, An = Th, U).18 Arnold and co-workers reported the first uranium(III) silylene complexes.19 More recently Chilton, Liddle, Mills, and co-workers reported Cp′′2UIIISi(SiMe3)3 (Cp′′ = η5-C5H3(SiMe3)2) as the first U(III) silanide complex to be studied through EPR spectroscopy and SQUID magnetometry.20
Baumgartner, Marschner and co-workers have observed solvent activation as a major byproduct of forming rare-earth-silicon bonds, which encouraged their design of a chelating silyl ligand, [Si(SiMe3)2Si(Me)2]2O (L), to stabilize low-valent rare-earth silyl complexes.21–23 Based on the success of this ligand to support trivalent rare earth complexes, we hypothesized this ligand may also be a robust framework to isolate low-valent uranium–silicon bonds. Because of the importance of trivalent uranium in small molecule activation24 and its presence in spent nuclear fuels,25 this represents an area where additional understanding would have broad scientific impacts. Herein, we report a family of uranium(III)–silyl derivatives stabilized by [Si(SiMe3)2Si(Me)2]2O (L). These species were isolated and fully characterized via1H, 13C, and 29Si NMR spectroscopy, electronic absorption spectroscopy, and single-crystal X-ray crystallography.
Our studies commenced with generating the potassium silanide form of the ligand, [KSi(SiMe3)2Si(Me)2]2O (K2L). This was done by treating (Me3Si)2L with two equivalents of KOtBu, followed by 2 equivalents of 18-crown-6. Following workup, a solution of [K(18-crown-6)]2L was isolated and added dropwise to a 1,2-dimethoxyethane (DME) solution of UI3(dioxane)1.5 at −35 °C. A blue-green powder was isolated from workup and identified as [K(18-crown-6)][UI2{(Si(SiMe3)2SiMe2)2O}] (1-crown). Compound 1-crown can be synthesized in 88% yield and was pure based on combustion analysis. If 18-crown-6 is not introduced during the synthesis, [K(DME)4][UI2{(Si(SiMe3)2SiMe2)2O}] (1) can still be made; however, the longevity of 1 is shortened to a few hours in solution and upon drying it readily decomposes into an intractable brown substance. Alternatively an equivalent of 18-crown-6 can be added to a crude sample of 1 to generate 1-crown (Scheme 1).
The 1H NMR spectrum of 1-crown in THF-d8 shows a large resonance at 2.7 ppm that corresponds to the protons of the [K(18-crown-6)]+ cation. There are also two paramagnetically shifted singlets at −0.79 (SiMe3) and 8.76 ppm (SiMe2) that correspond to the silane arms of the ligand (Fig. S5, ESI†). 13C NMR spectra were also collected, revealing a upfield shifted resonance at −13.0 ppm, likely corresponding to the SiMe2 arm (Fig. S6, ESI†). The 29Si NMR spectrum (collected as its polarization transfer INEPT spectrum) shows a singlet at −50.0 ppm likely corresponding to the SiMe3 arm (Fig. 2). The 29Si resonances of 1-crown are shifted in comparison to the isostructural rare-earth analogs which have a 29Si shift range of −4.9 to −9.0 ppm for SiMe3 and 13.3 to 42.9 ppm for SiMe2.21
The 29Si signal of 1-crown is shifted downfield as compared to previously reported silicon-containing U(III) complexes (−120 to −250 ppm) as tabulated by Windorff and Evans.26 Furthermore, this is a common instance where 29Si NMR signals could not be detected for a U–Si interaction, as many paramagnetic M–Si compounds have Si atoms that are not observable by both 29Si INEPT and 29Si{1H} NMR spectroscopy.18,20,21,26
To confirm the molecular structures of the new U(III) silyl species, single crystals of 1 and 1-crown suitable for X-ray diffraction were grown at −35 °C in three separate solvent systems (Table 1 and Fig. 3). Collection and refinement of the diffraction data reveal that the uranium ions in all three systems are 7-coordinate distorted pentagonal bipyramids and the molecular fragments are isostructural to the rare-earth silylate compounds crystallographically characterized by Baumgartner, Marschner and co-workers (Y, Ce, Pr, Sm, Tb, and Dy).211 was grown from diffusion of pentane into a DME solution of 1, 1-crown (DME) adduct was grown from pentane diffusion into an Et2O solution of 1-crown, and 1-crown (THF adduct) was grown from pentane diffusion into a THF solution of 1-crown.
| Growth conditions | Coord. solv. | Space group | U–Si (Å) | Si–U–Si (°) | U–OSi (Å) | U–Osolv (Å) | U–I (Å) | I–U–I (°) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DME/pentane | DME |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
3.1281(12) | 128.94(5) | 2.528(4) | 2.553(18) | 3.1065(4) | 159.157(19) |
| 3.1457(13) | 129.96(5) | 2.587(4) | 2.71(2) | 3.1319(3) | 162.177(16) | ||||
| 1-crown | Et2O/pentane | DME | P2/c | 3.1149(6) | 130.014(16) | 2.5613(13) | 2.6149(17) | 3.1125(6) | 164.321(6) |
| 3.1276(6) | 3.1256(2) | ||||||||
| 1-crown | THF/pentane | THF | C2/c | 3.1713(14) | 129.59(4) | 2.554(3) | 2.579(3) | 3.0845(10) | 166.017(13) |
| 3.1377(15) | 2.578(3) | 3.1086(11) |
The U–I distances range from 3.0845(10) to 3.1256(2) Å, and are in agreement with the U–I bonds in UI3(THF)4 (3.05–3.17 Å), thus corroborating a U(III) oxidation state.27 The siloxane oxygen atom is datively interacting with the uranium ion with U–OSi distances ranging from 2.528(4) to 2.587(4) Å for 1 and 2.554(3) to 2.5613(13) Å for 1-crown. The outer-sphere potassium countercation of 1-crown crystallized from DME and THF is sequestered by 18-crown-6 and is weakly coordinated to an iodide ligand of the uranium anion with K⋯I distances of 3.4608(5) and 3.3535(13) Å, respectively, which is similar to (18-crown-6)K–I (3.4131(7) Å).28 Without 18-crown-6, as in the structure of 1, the K+ ion is coordinated to four DME molecules.
The potassium cation is not permanently tethered to a uranium-bound iodide ligand, as suggested by the symmetric structure observed in the 1H and 13C NMR spectra. The U(III)–Si distances range from 3.1149(6) to 3.1713(14) Å, comparable to Cp′′2UIIISi(SiMe3)3 (3.116(2) Å), with only a difference of approx. 0.001 to 0.06 Å (Table 2).20 These U(III)–Si bonds are approx. 0.02 to 0.1 Å longer than the U(IV)–Si bonds found in Cp′3UIVSi(SiMe3)3 (3.0688(8) Å)18 and [Me2C6H3(tBu)N]3UIVSi(SiMe3)3 (3.091(3) Å), due to the difference in ionic radius of UIV and UIII, and they are significantly longer than the sum of the Pyykkö covalent radii of U–Si single bonds (2.86 Å).29 The U–Si distances in 1 and 1-crown in comparison to U(III) interactions with N-heterocyclic silylenes (formally Si(II)) (3.1637(7) to 3.1750(6) Å) differ by approx. 0.008 to 0.06 Å,19 indicating that U–Si distances are more affected by the ionic radius of uranium rather than that of silicon.
The electronic absorption spectrum of 1-crown collected from 300 to 1600 nm in THF shows a broad ligand-based absorbance at 390 (ε = 2930 M−1 cm−1). The color-producing band, which is responsible for the blue-green hue of 1-crown, is likely at 582 nm, which absorbs in the red-orange region. The absorbances at 580 (ε = 1250 M−1 cm−1) and 750 (ε = 820 M−1 cm−1) are assigned as the 5f to 6d transitions commonly reported for U(III).30 The NIR portion of the spectrum is consistent with the oxidation state assignment as U(III); the broad near-infrared bands at 910 (ε = 120 M−1 cm−1), 1040 (ε = 60 M−1 cm−1), 1090 (ε = 60 M−1 cm−1), and 1200 nm (ε = 100 M−1 cm−1) correspond to f–f transitions of the three uranium based electrons and are in agreement with known U(III) compounds (Fig. 4).31
The synthesis of the uranium(III) silyl species reported here is significant as previous derivatives have only featured a very bulky monodentate silyl ligand. Compounds 1 and 1-crown are rare in that two concurrent silyl bonds are formed trans to each other, and there is a relatively unhindered uranium ion that is capable of further chemistry. In these cases, the chelate effect from the bidentate ligand serves to stabilize these unusual silyl species.
In summary, new uranium(III) coordination compounds featuring uranium–silicon bonds have been isolated and fully characterized. The salt metathesis reaction between UI3(dioxane)1.5 and [K(18-crown-6)]2L resulted in the formation of the silyluranium species, 1-crown. Electronic absorption spectroscopy confirms the presence of a U(III), f3 ion due to the strong f–f transitions noted in the near-infrared region of the spectrum. 1H and 13C NMR spectroscopy reveals that 1-crown is C2v symmetric in THF-d8. Although rare in the presence of a paramagnetic ion, a 29Si NMR spectroscopic signal was detectable for 1-crown. The uncrowned species, 1, where K+ is sequestered by DME solvent molecules, is less stable than 1-crown, but both were able to be characterized by X-ray crystallography. The geometries around the uranium ions are analogous to those of the previously reported rare-earth silyl species, but the actinide examples crystallize in different unit cells depending on both the coordinated solvent and crystallization conditions. When compared to known U–Si bond lengths, the U–Si distance of 1-crown indicates that the ionic radius of uranium (vs. silicon) has a greater effect on the U–Si distance, as a comparison of ionic U–Si vs. silylene dative interactions. Compounds 1 and 1-crown represent the only examples where more than one bond to silicon is present on the same uranium center, and where a bidentate ligand encapsulates a uranium(III) ion. Future work will focus on the reactivity of the low-valent uranium ion towards small molecules, and to determine what role the silyl groups, if any, play in this activation.
The authors acknowledge Dr. John Harwood for assistance with 29Si NMR spectroscopy. The authors acknowledge a grant from the National Science Foundation (CHE-2247452, grant to S.C.B.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Plášil, Minerals, 2018, 8, 551 CrossRef.
- J. H. Simmons, P. B. Macedo, A. Barkatt and T. A. Litovitz, Nature, 1979, 278, 729–731 CrossRef CAS.
- V. V. Poluektov, V. A. Petrov, M. I. Ojovan and S. V. Yudintsev, Ceramics, 2023, 6, 1152–1163 CrossRef CAS.
- Z. Li, K. Zhang, Y. Song, X. Liu, Z. Jiang, S. Jiang, M. Wen, Y. Huang, C. Jia, W. Liu, X. Wang, X. Li, Z. Chen, L. Tang, P. Wang, T. Liu and X. Xie, Open Geosci., 2019, 11, 89–100 Search PubMed.
- E. Barnea and M. S. Eisen, Coord. Chem. Rev., 2006, 250, 855–899 CrossRef CAS.
- D. R. Hartline and K. Meyer, JACS Au, 2021, 1, 698–709 CrossRef CAS PubMed.
- M. Falcone, L. Barluzzi, J. Andrez, F. Fadaei Tirani, I. Zivkovic, A. Fabrizio, C. Corminboeuf, K. Severin and M. Mazzanti, Nat. Chem., 2019, 11, 154–160 CrossRef CAS PubMed.
- C. Camp, J. Pécaut and M. Mazzanti, J. Am. Chem. Soc., 2013, 135, 12101–12111 CrossRef CAS PubMed.
- D. Patel and S. T. Liddle, Rev. Inorg. Chem., 2012, 32, 1–22 CAS.
- S. T. Liddle, Angew. Chem., Int. Ed., 2015, 54, 8604–8641 CrossRef CAS PubMed.
- M. Gregson, A. J. Wooles, O. J. Cooper and S. T. Liddle, Comments Inorg. Chem., 2015, 35, 262–294 CrossRef CAS.
- T. W. Hayton, Dalton Trans., 2010, 39, 1145–1158 RSC.
- A. R. Fox, S. C. Bart, K. Meyer and C. C. Cummins, Nature, 2008, 455, 341–349 CrossRef CAS PubMed.
- M. Porchia, N. Brianese, U. Casellato, F. Ossola, G. Rossetto, P. Zanella and R. Graziani, J. Chem. Soc., Dalton Trans., 1989, 0, 677–681 RSC.
- M. Porchia, F. Ossola, G. Rossetto, P. Zanella and N. Brianese, J. Chem. Soc., Chem. Commun., 1987, 550–551 RSC.
- M. Porchia, U. Casellato, F. Ossola, G. Rossetto, P. Zanella and R. Graziani, J. Chem. Soc., Chem. Commun., 1986, 1034–1035 RSC.
- P. L. Diaconescu, A. L. Odom, T. Agapie and C. C. Cummins, Organometallics, 2001, 20, 4993–4995 CrossRef CAS.
- B. L. L. Réant, V. E. J. Berryman, J. A. Seed, A. R. Basford, A. Formanuik, A. J. Wooles, N. Kaltsoyannis, S. T. Liddle and D. P. Mills, Chem. Commun., 2020, 56, 12620–12623 RSC.
- J. Arnold, I. J. Brackbill, D. Lussier, M. Boreen, L. Maron and I. Douair, Chem. – Eur. J., 2020, 26, 2360–2364 CrossRef PubMed.
- G. K. Gransbury, B. L. L. Réant, A. J. Wooles, J. Emerson-King, N. F. Chilton, S. T. Liddle and D. P. Mills, Chem. Sci., 2023, 14, 621–634 RSC.
- A. Pöcheim, C. Marschner and J. Baumgartner, Inorg. Chem., 2021, 60, 8218–8226 CrossRef PubMed.
- R. Zitz, J. Baumgartner and C. Marschner, Organometallics, 2019, 38, 1159–1167 CrossRef CAS PubMed.
- R. Zitz, J. Hlina, M. Aghazadeh Meshgi, H. Krenn, C. Marschner, T. Szilvási and J. Baumgartner, Inorg. Chem., 2017, 56, 5328–5341 CrossRef CAS PubMed.
- B. M. Gardner and S. T. Liddle, Eur. J. Inorg. Chem., 2013, 3753–3770 CrossRef CAS.
- N. L. Banik, B. Brendebach and C. M. Marquardt, J. Radioanal. Nucl. Chem., 2014, 300, 177–183 CrossRef CAS.
- C. J. Windorff and W. J. Evans, Organometallics, 2014, 33, 3786–3791 CrossRef CAS.
- T. V. Fetrow, J. P. Grabow, J. Leddy and S. R. Daly, Inorg. Chem., 2021, 60, 7593–7601 CrossRef CAS PubMed.
- C. Kleeberg and C. Borner, Eur. J. Inorg. Chem., 2013, 2799–2806 CrossRef CAS.
- P. Pyykkö, J. Phys. Chem. A, 2015, 119, 2326–2337 CrossRef PubMed.
- S. T. Liddle, The Renaissance of Non-Aqueous Uranium Chemistry, Angew. Chem. Int. Ed., 2015, 54(30), 8604–8641 CrossRef CAS PubMed.
- N. J. Lin, D. Perales, E. M. Matson, M. Zeller and S. C. Bart, Organometallics, 2023, 42, 641–650 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and spectroscopic data. CCDC 2325809, 2325807 and 2325811. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc00655k |
| This journal is © The Royal Society of Chemistry 2024 |