Organocatalytic cationic degenerate chain transfer polymerization of vinyl ethers with excellent temporal control†
Zan
Yang
a,
Wenpei
Xiao
a,
Xun
Zhang
a and
Saihu
Liao
 *ab
*ab
aKey Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), State Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350108, China. E-mail: shliao@fzu.edu.cn
bBeijing National Laboratory for Molecular Science, Beijing 100190, China
First published on 18th April 2022
Abstract
An organocatalytic cationic degenerate chain transfer (DCT) polymerization of vinyl ethers with temporal control under visible light is reported. By using a bisphosphonium salt (BPS) as the photocatalyst and easily prepared thioacetal as the chain transfer agent, well-defined and colorless poly(vinyl ether)s with good molecular weight control and narrow molecular weight distributions can be obtained. The resulting polymers exhibited high chain-end fidelity, allowing further in situ chain extension and chain-end functionalization to synthesize block copolymers and end-functionalized polymers. Notably, the excellent stability and efficiency of BPS enables a strict temporal control over polymer chain growth at high monomer conversion for a long dark period.
Introduction
Living/controlled polymerization represents a highly useful, effective, and widely applicable method for the synthesis of functional polymers with specific physical properties and well-defined structures.1,2 Recently, external stimuli3–5 (such as thermal,6,7 chemical,8–12 photo13–16 and electrochemical17–21) regulated living polymerizations have attracted considerable research interests, due to the possibility to introduce additional control over the polymerization process. Among these external stimuli, visible light, as an unlimited source of renewable and clean energy from nature, has shown many appealing features, particularly, with the capability to impose a spatiotemporal control on the material construction.22–26Recently, photo-controlled cationic polymerization have received increasing attention, and several visible light-regulated polymerizations have been successfully developed, including cationic reversible addition–fragmentation chain transfer (RAFT)27–32 and degenerate chain transfer (DCT)33,34 polymerization, ring opening metathesis polymerization (ROMP),35–37 ring opening polymerization (ROP).38–40 You, Nicewicz and Perkowski reported the first photoredox-initiated living cationic DCT polymerization of 4-methoxystyrene by using a pyrylium salt as the photo-initiator and MeOH as the chain-transfer agent (CTA).33 Later on, the Fors group disclosed a photo-controlled cationic RAFT polymerization of vinyl ethers with methoxy-substituted pyrrlium salt as the photoredox catalyst,27 and achieved much better temporal control later by using iridium complexes as the photocatalyst.29,30 Recently, Zhu and co-workers reported a visible light-induced cationic RAFT polymerization by employing commercially available iron or manganese complexes as a photocatalys.31,32 Very recently, Kamigaito et al. demonstrated that acridinium salts were also suitable photoredox organocatalysts for the visible light-mediated cationic RAFT polymerizations of vinyl ethers, and achieved substantial temporal control via catalyst structure-optimization (Fig. 1A).41
The capability of thioethers to mediate cationic degenerative chain transfer polymerization in the presence of triflic acid was first demonstrated by Kamigaito, which was proposed to proceed through reversibly interchanges between the growing carbocationic and the dormant sulfonium.42,43 Importantly, compared with thiocarbonylthio esters, thioethers can be easily prepared and could afford colorless polymer products. However, the corresponding photo-controlled cationic DCT polymerization with thioethers as the CTA has been proved to be more challenging to achieve a good temporal control.34,41 For example, pyrylium salts and iridium complexes showed excellent temporal control in the cationic RAFT polymerizations of vinyl ethers,29 while in the corresponding degenerate chain-transfer cationic polymerization with thioacetals as the CTA, pyrylium salts almost lost the temporal control.34 Accordingly, the development of an organocatalytic cationic degenerate chain transfer polymerization with strict temporal control remains a challenge task.
Recently, by following a photocatalyst design logic based on heteroatom-doping of polycyclic arenes,44,45 we successfully identified bisphosphonium salts (BPS) as an effective type of organophotocatalysts for the cationic reversible addition–fragmentation chain transfer (RAFT) polymerizations of vinyl ethers.46 Their highly oxidizing excited state potential and suitable ground state redox potential (E* > +2.00 V, E1/2 < −0.60 V vs. SCE), as well as high stability, enabled the polymerization proceed at a low ppm level of catalyst loading with strict temporal control. We conceived BPS could also oxidize the DCT agents (e.g. thioacetals) upon excitation to generate the active carbocation, and the reduced BPS with thiyl radical could re-cap the cationic chain end to regenerate the DCT agent (or the dormant chains), thus establishing a light-regulated degenerative chain-transfer process (Fig. 1B). Here, we report our efforts toward this goal, and the development of an organocatalytic photo-controlled cationic degenerate chain-transfer polymerization of vinyl ethers with a thioacetal as the chain transfer agent with high chain-end fidelity and excellent temporal control.
Experimental methods
Materials and characterization
All monomers (isobutyl vinyl ether (99%, TCI), ethyl vinyl ether (99%, TCI), n-propyl vinyl ether (99%, TCI), n-butyl vinyl ether (98%, TCI), isopropy vinyl ether (98%, Energy Chemical), tert-butyl vinyl ether (98%, TCI), cyclohexyl vinyl ether (>95%, TCI), 2,3-dihydrofuran (99%, TCI), 2-chloroethyl vinyl ether (97%, TCI)) and solvent (DCM (HPLC, J&K)) were dried over calcium hydride (CaH2) for 24 h, collected under reduced pressure, deoxygenized by freeze–pump–thaw cycle three times and stored at −20 °C in the glove box. Butyl(1-isobutoxyethyl)thioether (CTA) was synthesized according to literature.42 Other chemicals were purchased from Energy Chemical or Adamas-beta, and used without further purification. 1H NMR was recorded on a Bruker AVIII 400 MHz spectrometer using TMS as the internal standard. Number-average molecular weight (Mn, GPC) and molecular weight distributions (Đ) were obtained by GPC (Waters 1515 series) in THF at 35 °C, and equipped with two Styragel Column (HR3 and HR4, 7.8 × 300 mm, flow rate = 1.0 mL min−1) using Shodex Polystyrene standards (890 to 5.98 × 105 g mol−1).Typical DCT polymerization procedure
In glove box, 10 mL Schlenk tube equipped with a magnetic stir bar, with IBVE (300 mg, 3.0 mmol, 100 eq., 3.0 M), CTA (30.0 μmol, 1 eq.), BPS (0.03 μmol, 0.001 eq., 10 ppm) in DCM was used. The mixture was stirred under blue LEDs irradiation (6 W, λmax = 460 nm, 30 mW cm−2) at room temperature. Following the desired amount of reaction time, benzene was added as an internal standard to measure the conversions by 1H NMR. Later, the reaction was quenched by the addition of MeOH/NEt3 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) and the reaction mixture stirred at room temperature for another 0.5 h. The pure polymer was obtained by vacuo to remove the solvent, and analyzed by GPC to determine the number average molecular weights (Mn), and molecular weight distributions (Mw/Mn).
1, 1 mL) and the reaction mixture stirred at room temperature for another 0.5 h. The pure polymer was obtained by vacuo to remove the solvent, and analyzed by GPC to determine the number average molecular weights (Mn), and molecular weight distributions (Mw/Mn).
Typical chain-end functionalization procedure
In glove box, 10 mL Schlenk tube equipped with a magnetic stir bar, with IBVE (300 mg, 3.0 mmol, 100 eq., 3.0 M), CTA (30.0 μmol, 1 eq.), BPS (0.03 μmol, 0.001 eq.) in DCM was used. The mixture was stirred under blue LEDs irradiation (6 W, λmax = 460 nm, 30 mW cm−2) at room temperature for 20 min. Upon completion, alcohol (90.0 μmol, 3 eq.), and 2,6-di-tert-butylpyridine (30.0 μmol, 1 eq.) were added, and then exposed to light again for 5 h. Later, the reaction was quenched by the addition of MeOH/NEt3 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) and the reaction mixture stirred at room temperature for another 0.5 h. The polymer was obtained by vacuo to remove the solvent, then precipitated from cold methanol, and dried in vacuo to afford pure polymer, and the polymer was analyzed by GPC and 1H NMR.
1, 1 mL) and the reaction mixture stirred at room temperature for another 0.5 h. The polymer was obtained by vacuo to remove the solvent, then precipitated from cold methanol, and dried in vacuo to afford pure polymer, and the polymer was analyzed by GPC and 1H NMR.
Results and discussion
We commenced our study on the development of a degenerate chain transfer cationic polymerization of isobutyl vinyl ether by using butyl(1-isobutoxyethyl)thioether as the chain transfer agent and bisphosphonium salt (BPS) as the catalyst, under the irradiation of blue LEDs. To our delight, full monomer conversion (>99%) can be achieved in 1 h, in the presence of 100 ppm of BPS only, affording poly(IBVE), 8.6 kg mol−1 with a narrower Đ of 1.35 (Table 1, entry 1). Although a higher loading of BPS (200 ppm) resulted in a broader dispersity (Đ 1.46, entry 2), decreasing the catalyst loading to 10 ppm delivered even a better control, giving poly(IBVE) with good control of molecular weight and low dispersity (Đ 1.30, entry 5). Full monomer conversion and good control were also achieved when decreasing the catalyst loading to 5 ppm (entry 6). Control experiments indicated that the photocatalyst, light irradiation, and CTA were all important to ensure the polymerization proceeding in a controlled manner (entries 8–10). Moreover, controlled molecular weights and narrow molecular weight distributions were observed with different ratio of [M]0/[CTA]0 (35/1 to 200/1, entries 11–13).| Entry | [M]0/[CTA]0/[BPS] | Conv.b (%) | M n,GPC (kg mol−1) | Đ |
|---|---|---|---|---|
| a [M]0/[CTA]0/[BPS]0 ratio as shown in the table, in DCM (3 M), 6 W Blue LEDs, 1 h. b Determined by 1H NMR using benzene as an internal standard. c M n and Đ were measured by GPC with polystyrene standards. d 6 W Blue LEDs for 3 h. e 2.0 M of IBVE was carried out. f Carried out in dark. | ||||
| 1 | 100/1/0.01 | >99 | 8.6 | 1.35 |
| 2 | 100/1/0.02 | >99 | 8.1 | 1.46 |
| 3 | 100/1/0.005 | >99 | 9.0 | 1.34 |
| 4 | 100/1/0.002 | >99 | 9.2 | 1.33 |
| 5 | 100/1/0.001 | >99 | 10.0 | 1.30 |
| 6d | 100/1/0.0005 | >99 | 9.0 | 1.40 |
| 7e | 100/1/0.001 | >99 | 9.1 | 1.34 |
| 8 | 100/1/— | — | — | — |
| 9 | 100/—/0.001 | 95 | 20.2 | 2.0 |
| 10f | 100/1/0.001 | — | — | — |
| 11 | 35/1/0.001 | >99 | 3.8 | 1.21 |
| 12 | 50/1/0.001 | >99 | 5.1 | 1.20 |
| 13 | 200/1/0.001 | 98 | 17.5 | 1.38 |
The BPS-mediated degenerative chain transfer cationic polymerization also worked well with other vinyl ether monomers, such as ethyl vinyl ether (EVE), n-propyl vinyl ether (NPVE), n-butyl vinyl ether (NBVE) and isopropy vinyl ether (IPVE) (Table 2). In the presence of 10 ppm of BPS as the photocatalyst, high conversions were achieved after 1 hour of irradiation with blue LEDs at room temperature, affording the corresponding poly(vinyl ether)s with narrow molecular weight distribution, together with a good control over the molecular weight (entries 1–7). Bulky vinyl ethers, such as tert-butyl vinyl ether (TBVE) and cyclohexanyl vinyl ether (CyVE), could also polymerize under the standard conditions, albeit with relatively broader dispersity (entries 8 and 9). These results may indicate that increased steric hinderance could decrease the degenerative chain transfer rate. Further, the polymerization of 2,3-dihydrofuran (DHF) afforded poly(DHF) as white solids also with good control over molecular weight and dispersity. Moreover, 2-chloroethyl vinyl ether (Cl-EVE) could be polymerized with 50 ppm of BPS catalyst for 2 h, delivering the polymer product with a dispersity of 1.32 (entry 11).
| Entry | M | [M]0/[CTA]0 | Conv.a (%) | M n,theo (kg mol−1) | M n,GPC (kg/mol) | Đ |
|---|---|---|---|---|---|---|
| a Determined by 1H NMR analysis with benzene as an internal standard. b Calculated as ([monomer]0/[CTA]0) × (Mw of monomer) × conversion + (Mw of CTA). c Determined by GPC in THF using polystyrene standards. | ||||||
| 1 | EVE | 50/1 | 93 | 3.6 | 3.2 | 1.20 |
| 2 | EVE | 100/1 | 94 | 6.9 | 6.3 | 1.16 |
| 3 | NPVE | 50/1 | 92 | 4.1 | 3.6 | 1.17 |
| 4 | NPVE | 100/1 | 94 | 8.3 | 7.2 | 1.19 |
| 5 | NBVE | 50/1 | >99 | 5.2 | 4.6 | 1.20 |
| 6 | NBVE | 100/1 | >99 | 10.2 | 9.8 | 1.30 |
| 7 | IPVE | 100/1 | >99 | 8.8 | 6.9 | 1.34 |
| 8 | TBVE | 100/1 | >99 | 10.2 | 9.0 | 1.41 |
| 9 | CyVE | 100/1 | >99 | 12.8 | 8.3 | 1.63 |
| 10 | DHF | 100/1 | 94 | 6.8 | 6.8 | 1.38 |
| 11 | Cl-EVE | 100/1 | 72 | 7.9 | 12.3 | 1.32 |
Next, the progress of this cationic DCT polymerization at a ratio of [IBVE]0/[CTA]0/[BPS] = 100/1/0.001 was tracked. Under the standard conditions, 50% conversion of monomer was observed within 15 min, reaching 97% conversion after 60 min (Fig. 2A). A linear relationship between ln([IBVE]0/[IBVE]t) and time was also observed (Fig. 2B). Importantly, linearly increased molecular weight with monomer conversion was achieved (Fig. 2C), which is in line with a living/controlled chain growth. The molecular weight distributions (Đ) decreased upon the polymer chain extended, and the GPC traces also clearly showed an increased molecular weight over conversion (Fig. 2D). All these data suggested that the good living/controlled characteristics of this BPS-mediated cationic DCT polymerization.
Therefore, to assess the capability of the current catalytic system on temporal control over the chain growth, light on–off experiments with short and long off periods were carried out. Using 10 ppm of bisphosphonium salt with blue LEDs, monomer conversion reached 31% after the first “ON” cycle. Then, the reaction was placed in dark, and notably no conversion was observed during this light-off period. When the reaction was re-exposed to the irradiation of blue LEDs, the polymerization rate could recover. This light on–off operation can be repeated several times, showing uniformly strict temporal control (Fig. 3A). Further, a long dark period at a high monomer conversion (>60%) was also examined. As shown in Fig. 3B, no conversion was observed with the long dark period up to 3 h. This strict temporal control by light indicated an efficient activation-deactivation mechanism of this BPS-mediated cationic DCT polymerization under visible light.
 | ||
| Fig. 3 (A) Time vs. monomer conversion and molecular weight (Mn) of the on/off experiment; (B) long dark period of the on/off experiment. | ||
The performance of this bisphosphonium salt in the cationic DCT polymerization was further examined in the one-pot chain extension and block copolymer synthesis with 10 ppm BPS as the catalyst (Fig. 4). The chain extension experiment was carried under the standard conditions, the pIBVE (4.3 kg mol−1) was first synthesized. Then, 40 equiv. of IBVE was added to the Schlenk tube, the pIBVE-b-pIBVE was obtained with 7.8 kg mol−1 and Đ 1.35 after 2 h (full conversion). A diblock copolymer pIBVE-b-pNBVE was also successfully synthesized with 10 ppm of BPS, with GPC traces showing a significant shift to high molecular weight region (7.3 kg mol−1vs. 4.5 kg mol−1).
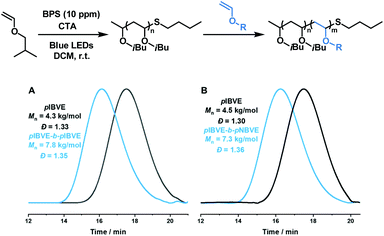 | ||
| Fig. 4 (A) Poly(isobutyl vinyl ether) chain extension; (B) synthesis of poly(isobutyl vinyl ether)-block-poly(butyl vinyl ether). | ||
Encouraged by the above chain extension experiments, we conceived this polymerization with high chain-end fidelity may be used to synthesize polymers with a chain-end functionalization (Fig. 5). Oxidation of the thioacetal by BPS* in the polymer chain-end generates carbocations, which can be thus trapped by nucleophilic substrates. To test this proposal, we first synthesized pIBVE ([M]0/[CTA] 0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by the cationic DCT polymerization. Then, 1.0 equiv of 2,6-di-tert-butylpyridine as the base and 3.0 equiv of alcohol as the nucleophilic substrates were added. After irradiation with 6 W Blue LEDs for 3 h at room temperature, the desired acetal polymers were obtained in high yields. The chemical structure of the chain-end groups in polymers were further confirmed by 1H NMR analysis (for details, please see Fig. S6 and S7†). Interestingly, the tetraphenylethylene functionalized poly(IBVE) showed strong aggregation-induced emission (AIE) behavior with a charming azure color.47–49
1) by the cationic DCT polymerization. Then, 1.0 equiv of 2,6-di-tert-butylpyridine as the base and 3.0 equiv of alcohol as the nucleophilic substrates were added. After irradiation with 6 W Blue LEDs for 3 h at room temperature, the desired acetal polymers were obtained in high yields. The chemical structure of the chain-end groups in polymers were further confirmed by 1H NMR analysis (for details, please see Fig. S6 and S7†). Interestingly, the tetraphenylethylene functionalized poly(IBVE) showed strong aggregation-induced emission (AIE) behavior with a charming azure color.47–49
Conclusions
In summary, the bisphosphonium salt (BPS) has been identified as effective organophotocatalyst for the cationic degenerate chain transfer (DCT) polymerization, which allowed for the development of the metal-free cationic DCT polymerization of vinyl ethers with strict temporal control under visible light. Through this DCT polymerization system, colourless poly(vinyl ether)s can be synthesized with good molecular weight control and high chain-end fidelity at a low ppm level of catalyst loading (<10 ppm). Further application of this polymerization system to the one-pot synthesis of block copolymers and polymer chain-end functionalization was also demonstrated.Conflicts of interest
There is no conflict of interest to report.Acknowledgements
We gratefully acknowledge the Recruitment Program of Global Experts of China, National Natural Science Foundation of China (21602028), Beijing National Laboratory for Molecular Sciences (BNLMS201913), 100-Talent program of Fujian, and Fuzhou University for the financial support.Notes and references
- R. B. Grubbs and R. H. Grubbs, Macromolecules, 2017, 50, 6979–6997 CrossRef CAS
.
- M. Kamigaito and M. Sawamoto, Macromolecules, 2020, 53, 6749–6753 CrossRef CAS
.
- F. A. Leibfarth, K. M. Mattson, B. P. Fors, H. A. Collins and C. Hawker, Angew. Chem., Int. Ed., 2013, 52, 199–210 CrossRef CAS
.
- X. Pan, M. Fantin, F. Yuan and K. Matyjaszewski, Chem. Soc. Rev., 2018, 47, 5457–5490 RSC
.
- Y. N. Zhou, J. J. Li, Y. Y. Wu and Z. H. Luo, Chem. Rev., 2020, 120, 2950–3048 CrossRef CAS
.
- S. Naumann and M. R. Buchmeiser, Macromol. Rapid Commun., 2014, 35, 682–701 CrossRef CAS
.
- S. Naumann and M. R. Buchmeiser, Catal. Sci. Technol., 2014, 4, 2466–2479 RSC
.
- H. J. Yoon, J. Kuwabara, J.-H. Kim and C. A. Mirkin, Science, 2010, 330, 66–69 CrossRef CAS PubMed
.
- C. K. Gregson, V. C. Gibson, N. J. Long, E. L. Marshall, P. J. Oxford and A. J. White, J. Am. Chem. Soc., 2006, 128, 7410–7411 CrossRef CAS PubMed
.
- E. M. Broderick, N. Guo, C. S. Vogel, C. Xu, J. R. Sutter, J. T. Miller, K. Meyer, P. Mehrkhodavandi and P. L. Diaconescu, J. Am. Chem. Soc., 2011, 133, 9278–9281 CrossRef CAS PubMed
.
- O. Coulembier, S. Moins, R. Todd and P. Dubois, Macromolecules, 2014, 47, 486–491 CrossRef CAS
.
- C. Lv, C. He and X. Pan, Angew. Chem., Int. Ed., 2018, 57, 9430–9433 CrossRef CAS PubMed
.
- N. Corrigan, S. Shanmugam, J. Xu and C. Boyer, Chem. Soc. Rev., 2016, 45, 6165–6212 RSC
.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Chem. Rev., 2016, 116, 10212–10275 CrossRef CAS PubMed
.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef CAS
.
- P. Xiao, J. Zhang, F. Dumur, M. A. Tehfe, F. Morlet-Savary, B. Graff, D. Gigmes, J. P. Fouassier and J. Lalevée, Prog. Polym. Sci., 2015, 41, 32–66 CrossRef CAS
.
- A. J. Magenau, N. C. Strandwitz, A. Gennaro and K. Matyjaszewski, Science, 2011, 332, 81–84 CrossRef CAS
.
- M. Fantin, A. A. Isse, A. Venzo, A. Gennaro and K. Matyjaszewski, J. Am. Chem. Soc., 2016, 138, 7216–7219 CrossRef CAS PubMed
.
- B. M. Peterson, S. Lin and B. P. Fors, J. Am. Chem. Soc., 2018, 140, 2076–2079 CrossRef CAS PubMed
.
- W. Song and Q. Yan, Angew. Chem., Int. Ed., 2018, 57, 4907–4911 CrossRef
.
- L. T. Strover, A. Cantalice, J. Y. L. Lam, A. Postma, O. E. Hutt, M. D. Horne and G. Moad, ACS Macro Lett., 2019, 8, 1316–1322 CrossRef CAS
.
- N. Corrigan, J. Yeow, P. Judzewitsch, J. Xu and C. Boyer, Angew. Chem., Int. Ed., 2019, 58, 5170–5189 CrossRef CAS
.
- B. P. Fors and C. J. Hawker, Angew. Chem., Int. Ed., 2012, 51, 8850–8853 CrossRef CAS PubMed
.
- J. Xu, K. Jung, A. Atme, S. Shanmugam and C. Boyer, J. Am. Chem. Soc., 2014, 136, 5508–5519 CrossRef CAS PubMed
.
- N. Corrigan, K. Jung, G. Moad, C. J. Hawker, K. Matyjaszewski and C. Boyer, Prog. Polym. Sci., 2020, 111, 101311 CrossRef CAS
.
- Q. Michaudel, V. Kottisch and B. P. Fors, Angew. Chem., Int. Ed., 2017, 56, 9670–9679 CrossRef CAS PubMed
.
- V. Kottisch, Q. Michaudel and B. P. Fors, J. Am. Chem. Soc., 2016, 138, 15535–15538 CrossRef CAS PubMed
.
- M. Ciftci, Y. Yoshikawa and Y. Yagci, Angew. Chem., Int. Ed., 2017, 56, 519–523 CrossRef CAS PubMed
.
- Q. Michaudel, T. Chauviré, V. Kottisch, M. J. Supej, K. J. Stawiasz, L. Shen, W. R. Zipfel, H. D. Abruña, J. H. Freed and B. P. Fors, J. Am. Chem. Soc., 2017, 139, 15530–15538 CrossRef CAS PubMed
.
- V. Kottisch, M. J. Supej and B. P. Fors, Angew. Chem., Int. Ed., 2018, 57, 8260–8264 CrossRef CAS PubMed
.
- J. Li, M. Zhang, X. Pan, Z. Zhang, S. Perrier, J. Zhu and X. Zhu, Chem. Commun., 2019, 55, 7045–7048 RSC
.
- J. Li, M. Chen, X. Lin, Q. Li, W. Zhang, G. Jin, X. Pan, J. Zhu and X. Zhu, ACS Macro Lett., 2020, 9, 1799–1805 CrossRef CAS
.
- A. J. Perkowski, W. You and D. A. Nicewicz, J. Am. Chem. Soc., 2015, 137, 7580–7583 CrossRef CAS
.
- R. J. Sifri, A. J. Kennedy and B. P. Fors, Polym. Chem., 2020, 11, 6499–6504 RSC
.
- K. A. Ogawa, A. E. Goetz and A. J. Boydston, J. Am. Chem. Soc., 2015, 137, 1400–1403 CrossRef CAS PubMed
.
- T. Krappitz, K. Jovic, F. Feist, H. Frisch, V. P. Rigoglioso, J. P. Blinco and A. J. Boydston, J. Am. Chem. Soc., 2019, 141, 16605–16609 CrossRef CAS PubMed
.
- V. K. Kensy, R. L. Tritt, F. M. Haque, L. M. Murphy, D. B. Knorr, S. M. Grayson and A. J. Boydston, Angew. Chem., Int. Ed., 2020, 59, 9074–9079 CrossRef CAS PubMed
.
- C. Fu, J. Xu and C. Boyer, Chem. Commun., 2016, 52, 7126–7129 RSC
.
- X. Zhang, Q. Ma, Y. Jiang, S. Hu, J. Li and S. Liao, Polym. Chem., 2021, 12, 885–892 RSC
.
- X. Zhang, S. Hu, Q. Ma and S. Liao, Polym. Chem., 2020, 11, 3709–3715 RSC
.
- M. Matsuda, M. Uchiyama, Y. Itabashi, K. Ohkubo and M. Kamigaito, Polym. Chem., 2022, 13, 1031–1039 RSC
.
- M. Uchiyama, K. Satoh and M. Kamigaito, Macromolecules, 2015, 48, 5533–5542 CrossRef CAS
.
- M. Uchiyama, K. Satoh and M. Kamigaito, Prog. Polym. Sci., 2022, 124, 101485 CrossRef CAS
.
- Q. Ma, J. Song, X. Zhang, J. Yu, L. Ji and S. Liao, Nat. Commun., 2021, 12, 429 CrossRef CAS PubMed
.
- J. Kreutzer, Nat. Rev. Chem., 2021, 5, 73 CrossRef CAS
.
- X. Zhang, Y. Jiang, Q. Ma, S. Hu and S. Liao, J. Am. Chem. Soc., 2021, 143, 6357–6362 CrossRef CAS PubMed
.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC
.
- R. Hu, A. Qin and B. Z. Tang, Prog. Polym. Sci., 2020, 100, 101176 CrossRef CAS
.
- L. Zhang, K. Jiang, X. Shen, Y. Gu, X. Lin and C. Mao, Macromolecules, 2020, 53, 1536–1542 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00134a |
| This journal is © The Royal Society of Chemistry 2022 |





