Asymmetric chlorination of A2–A1–D–A1–A2 type non-fullerene acceptors for high-voltage organic photovoltaics†
Peiqing
Cong‡
ab,
Xianda
Li‡
ac,
Ailing
Tang
 *ab,
Jiang
Wu
ac,
Jianhua
Chen
d,
Lie
Chen
*ab,
Jiang
Wu
ac,
Jianhua
Chen
d,
Lie
Chen
 e and
Erjun
Zhou
e and
Erjun
Zhou
 *ab
*ab
aCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: tangal@nanoctr.cn; zhouej@nanoctr.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
cHenan Institutes of Advanced Technology, Zhengzhou University, Zhengzhou 450003, China
dDepartment of Chemical Science and Technology, Yunnan University, Kunming 650091, China
eCollege of Chemistry, Nanchang University, 999 Xuefu Avenue, Nanchang, 330031, China
First published on 7th November 2022
Abstract
Herein, we synthesized an asymmetric A2–A1–D–A1–A2 type small molecule nonfullerene acceptor (NFA), HCl-BTA3, by chlorination on one side of A1. The synergistic effect of the asymmetric structure and chlorination endows HCl-BTA3 with a large dipole moment, close molecular packing, and high-efficiency charge transfer and transport. After being blended with a carboxylate-based polymer donor, TTC-Cl, HCl-BTA3 achieved a high open-circuit voltage (VOC) of 1.20 V and a satisfactory power conversion efficiency (PCE) of 11.2%, which are among the highest values for high-voltage carboxylate-based polymers.
As one of the renewable energy technologies, organic photovoltaics (OPVs) have received widespread attention due to their low cost, lightweight, and excellent solution-processability. Photovoltaic material design, an indispensable part of OPV development, continues to drive power conversion efficiency (PCE) to a higher value.1 Within a few years, non-fullerene acceptors (NFAs) have gradually replaced fullerenes as the primary acceptor material with the advantages of tunable energy levels, strong absorption, and ordered π–π stacking. As a result, the PCEs of single junction OPVs based on polymer donors and NFAs have surpassed 19%,2–5 showing excellent commercial potential.
The dominant small molecule NFAs mainly include three categories: low bandgap (LBG) A–DA′D–A type, middle bandgap (MBG) A–D–A type, and wide bandgap (WBG) A2–A1–D–A1–A2 type. Due to the multiple modifiable active sites, numerous symmetric molecular modification strategies have been developed, including ring extension, halogenation, alkyl substitution, etc., aiming to adjust absorption, energy levels, and molecular stacking, thus improving photovoltaic performance. On the other hand, the asymmetric design also attracts much attention because it can generate some unique properties. For example, it will induce a large dipole moment in the target molecules, reducing the exciton binding energy and increasing the intramolecular charge transfer ability.6,7 In addition, the increased dipole moment will enhance the intermolecular interaction, improving the molecular packing and order. The asymmetric design has been successfully applied to the central cores, side chains, and terminals in the A–D–A and A–DA′D–A structures and has promoted the rapid progress of OPVs.8
However, the asymmetric design is less applied to developing A2–A1–D–A1–A2 structured acceptors, though they have provided plentiful high-voltage OPVs with an open-circuit voltage (VOC) around 1.0–1.3 V.9,10 In addition, they are outstanding materials for application in indoor OPVs or ternary solar cells.11,12 For example, a classic A2–A1–D–A1–A2 type NFA, BTA3, composed of an IDT core, a benzotriazole (BTA) bridge, and 2-(1,1-dicyanomethylene)rhodanine terminals, has shown a promising PCE of 10.5% with a VOC of 1.24 V13 in 2019. When BTA3 is used as the third component, the corresponding ternary solar cells have attained over 18% efficiency.14 Moreover, indoor OPVs based on different material combinations containing BTA3 have produced high PCEs above 25%.15,16 These great potentials drive us to conduct more profound research for constructing highly efficient BTA-based A2–A1–D–A1–A2 type acceptors.
Herein, we present an asymmetric A2–A1–D–A1–A2 structure acceptor, HCl-BTA3, by introducing chlorinated and non-halogenated BTA as the asymmetric A1 units, respectively, as shown in Fig. 1. On the one hand, compared with symmetric BTA3, asymmetric HCl-BTA3 exhibits a large molecular dipole moment and ordered molecular packing, contributing to the charge transfer and transport.6,7 On the other hand, chlorination on a single side could provide a higher value of electroluminescence external quantum efficiency (EQEEL) than chlorination on both sides,17 generating a high VOC. Here, we use a carboxylate-containing polymer, TTC-Cl, as the donor material because of its suitable energy levels and low energy disorder.18 As a result, the TTC-Cl: HCl-BTA3 blend attains a good balance between short-circuit current (JSC) and VOC, yielding a high VOC of 1.20 V and a champion PCE of 11.2%. On the contrary, the BTA3-based device exhibits a high VOC of 1.26 V but a low PCE of 9.67%, while the Cl-BTA3-based device yields a low VOC of 1.15 V with a medium PCE of 11.05%. The results indicate that asymmetric chlorinated A1 engineering is an effective strategy to modulate molecular energy levels, enhance molecular packing, decrease voltage loss, and thus improve photovoltaic performance.
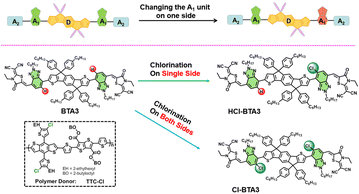 | ||
| Fig. 1 The asymmetric molecular design strategies in A2–A1–D–A1–A2 small molecules and the chemical structures of TTC-Cl, BTA3, HCl-BTA3, and Cl-BTA3 in this work. | ||
HCl-BTA3 is synthesized by two steps of direct arylation coupling and then Knoevenagel condensation reaction, and the detailed synthesis procedures and characterizations are described in the ESI.† These three acceptor materials show good solubility in chloroform (CF), chlorobenzene (CB), and o-dichlorobenzene (o-DCB). As shown in Fig. S1 (ESI†), HCl-BTA3 can maintain 95% weight at 365 °C and the corresponding temperatures for BTA3 and Cl-BTA3 are 395 °C and 339 °C,19 respectively, indicating all three acceptors have good thermal stability.
We utilize density functional theory (DFT) calculations to explore the effect of asymmetric chlorination on molecular structures. As shown in Fig. 2a, an asymmetric design and strong electron-withdrawing Cl atoms afford HCl-BTA3 a large molecular dipole moment of 2.11 Debye, whereas BTA3 and Cl-BTA3 with symmetric structures exhibit dipole moments of only 0.58 and 0.84 Debye, respectively. The larger dipole moments are thought to enhance the intermolecular interaction, improving the molecular packing and order. In addition, compared with BTA3, the introduction of Cl atoms distorts the conjugation backbone (Fig. S2, ESI†) because the large atomic radius of the Cl atom increases the dihedral angle between BTA and IDT units. Hence, chlorination may impede intermolecular packing to a certain extent.
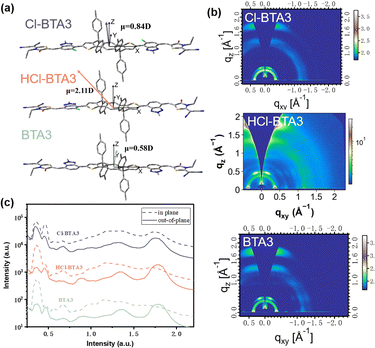 | ||
| Fig. 2 (a) The optimized molecular conformation with dipole moments; (b) 2D-GIWAXS patterns of pure films; and (c) IP and OOP line cuts of the 2D GIWAXS patterns. | ||
We further conducted two-dimensional grazing incidence wide-angle X-ray scattering (2D-GIWAXS) to explore the effect of dipole moment changes on the molecular stacking of the neat film. As shown in Fig. 2b and c, all three neat films exhibit (100) and (200) diffraction peaks at a similar location with qxy ≈ 0.36 Å−1 and 0.67 Å−1 in the in-plane direction. However, the unilateral chlorinated HCl-BTA3 and nonchlorinated BTA3 exhibit a stronger (010) diffraction peak in the out-of-plane direction, indicating that both predominantly adopt a face-on orientation. The (010) diffraction peak of the bilateral chlorinated Cl-BTA3 can be detected in both in-plane and out-of-plane directions, implying the coexistence of face-on and edge-on orientations. The π–π stacking distance of HCl-BTA3 (3.50 Å) is smaller than that of Cl-BTA3 (3.59 Å) and close to that of BTA3 (3.50 Å). Although the chlorination may hinder the molecular packing, the extra intermolecular dipole–dipole interaction in HCl-BTA3 can counteract this effect and form close molecular stacking.
The normalized absorption spectra of the four materials in the neat films are shown in Fig. 3a, and the data are summarized in Table S1 (ESI†). HCl-BTA3 shows red-shifted absorptions of about 50 nm in the neat films compared to those in the CB solution, implying the existence of molecular aggregation. Compared with BTA3 (λmax = 654 nm), the maximum absorption peaks of HCl-BTA3 and Cl-BTA3 slightly red-shift, which may be related to the strong electron-accepting capability of the Cl atom. The optical bandgaps (Eoptg) of BTA3, HCl-BTA3, and Cl-BTA3 are 1.76, 1.72, and 1.72 eV, respectively, calculated from the absorption onsets of the films.
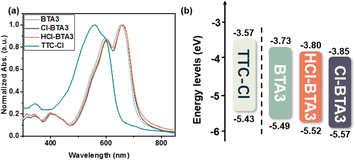 | ||
| Fig. 3 (a) UV-vis absorption spectra in films of TTC-Cl, BTA3, HCl-BTA3 and Cl-BTA3, and (b) the energy level diagram of the four compounds. | ||
The frontier molecular orbital energy levels are tested by cyclic voltammetry. As shown in Fig. S3 (ESI†), the highest occupied molecular orbital (HOMO) energy levels of BTA3, HCl-BTA3, and Cl-BTA3 are calculated to be −5.49, −5.52, and −5.57 eV, respectively. The lowest unoccupied molecular orbital (LUMO) energy levels, deduced from the equation ELUMO = EHOMO + Eoptg, are −3.73, −3.80, and −3.85 eV, respectively. The schematic energy-level diagrams of the four compounds are displayed in Fig. 3b. The density functional theory (DFT) calculations (Fig. S2, ESI†) revealed the same variation trend in the energy levels. With the gradual increase of Cl atoms on BTA3, the energy levels are reduced. The increased energy offsets between the donor and acceptor may further promote charge transfer.
To investigate the influence of asymmetric halogenated BTA on photovoltaic properties, we fabricated a traditional device structure of ITO/PEDOT:PSS/active layer/PFNBr/Al with a carboxylate-containing polymer, TTC-Cl, as the electron donor. The detailed optimization processes, including the weight ratio of the donor/acceptor (D/A), thermal annealing temperature, and solvent additive, are shown in Tables S2–S4 (ESI†). The current density–voltage (J–V) curves are displayed in Fig. 4a, and the detailed photovoltaic parameters are collected in Table 1. With the stepwise chlorination on BTA units, VOC gradually declines from 1.26 V (BTA3) to 1.20 V (HCl-BTA3) and 1.15 V (Cl-BTA3). However, the JSC of the corresponding devices exhibits opposite trends, increasing from 11.30 to 13.42 and 13.81 mA cm−2, related to the efficient exciton dissociation and charge transport. The devices based on TTC-Cl: HCl-BTA3 and TTC-Cl: Cl-BTA3 show the same FF of 0.696, slightly higher than that of the TTC-Cl: BTA3 (0.679) device. It is worth noting that the device based on TTC-Cl: HCl-BTA3 exhibits the highest PCE of 11.20% due to the excellent balance between VOC and JSC, which is among the champion PCEs for high-VOC carboxylate-containing polymer systems. The external quantum efficiency (EQE) curves in Fig. 4b indicate that all the devices show a similar photoresponse range of 300–750 nm, but the values of EQEmax gradually increase. The mismatch in JSC between the value calculated from EQE curves and that measured from J–V curves is less than 5%.
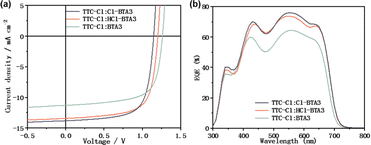 | ||
| Fig. 4 (a) J–V curves of the optimized photovoltaic devices; (b) EQE spectra of the optimized solar cells. | ||
| Blends | V OC (V) | J SC (Jcalc.b) (mA/cm2) | FF | PCE | |
|---|---|---|---|---|---|
| Donor | Acceptor | ||||
| a The values in parentheses are the average values with standard deviation obtained from 10 devices. b The values in parentheses are the integrated current density values calculated from EQE spectra. | |||||
| TTC-Cl | BTA3 | 1.26 | 11.30 (10.89) | 0.679 | 9.67% (9.48 ± 0.15)a |
| HCl-BTA3 | 1.20 | 13.42 (12.84) | 0.696 | 11.20% (11.04 ± 0.11%) | |
| Cl-BTA3 | 1.15 | 13.81 (13.11) | 0.696 | 11.05% (10.96 ± 0.09%) | |
We first perform photoluminescence (PL) quenching measurements to estimate the exciton dissociation efficiency in the hole transfer process with the excitation wavelength at 650 nm. Fig. S4 (ESI†) shows the calculated PL quenching efficiencies of 89.2%, 97.9%, and 98.8% for TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 blends, respectively, implying chlorination could promote the hole transfer. To explain these phenomena, we estimate the driving force for exciton dissociation in CT states. As shown in Fig. S5 (ESI†), the charge transfer energy (ECT) can be deduced from the EL and highly sensitive Fourier-transform photocurrent EQE spectroscopy (FTPS). The ECT of TTC-Cl: HCl-BTA3 (1.70 eV) is lower than those of TTC-Cl: BTA3 (1.73 eV) and TTC-Cl: Cl-BTA3 (1.71 eV). Accordingly, the three devices exhibit a similar charge transfer driving force (ΔECT = Eg − ECT) around 0.1 eV. Hence, in the three systems of TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3, the different PL quenching efficiencies may be attributed to the molecular structure and molecular arrangement.
We further use atomic force microscopy (AFM) to investigate the influence of surface morphology on photocurrent, and the height images are shown in Fig. S6 (ESI†). The root-mean-square (RMS) roughness values of TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 blends are 1.33, 1.12, and 0.91 nm, respectively. As the degree of chlorination increases, the blend film has smoother surfaces due to the weaker crystallinity of HCl-BTA3 and Cl-BTA3. The relatively rough surface of the TTC-Cl: BTA3 film may exhibit a bulky domain size, also leading to a decrease in JSC and FF. The GIWAXS data (Fig. S7, ESI†) reveal that, when blended with the donor, all three blend films show similar (100) diffraction peaks in the in-plane direction at qxy = 0.33 Å−1. The (010) diffraction peaks of the BTA3-, HCl-BTA3- and Cl-BTA3-based blend films are located at 1.75 Å−1, 1.73 Å−1, and 1.72 Å−1, respectively, in the out-of-plane direction. The π–π stacking distances in the blend films are 3.59, 3.63, and 3.65 Å for the TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3, respectively, larger than that of their respective neat film. The smallest π–π stacking distance of the BTA3-based blend film demonstrates the strongest and close molecular packing, consistent with the largest RMS value in AFM data.
The exciton dissociation probabilities (Pdiss) were calculated from the JSC/Jsat ratio (Fig. S8, ESI†), and the Pdiss values for BTA3, HCl-BTA3 and Cl-BTA3 are calculated to be 94.9%, 95.2%, and 97.5%, respectively. Compared with the TTC-Cl: BTA3-based device, chlorination will improve the Pdiss values. Furthermore, charge recombination was investigated by measuring the values of JSC and VOC at different light intensities (Fig. S9a and b, ESI†). According to the equation: JSC ∝ Pα, the devices exhibit weak bimolecular recombination when α is close to 1. In our work, all the devices show similar bimolecular recombination (α = 0.97). The trap-assisted recombination was evaluated by the slope of the formula: VOC ∝ nkT/q![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(P). The slopes of TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 are 1.38, 1.25, and 1.23 kT/q, respectively, which indicate that chlorination is an effective strategy for reducing trap-assisted recombination. In addition, we used the space charge limited current (SCLC) method to measure the charge carrier mobility. As shown in Fig. S9c and d (ESI†), the values of hole mobility for TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 are 3.02 × 10−5, 8.18 × 10−5, and 9.02 × 10−5 cm2 V−1 s−1, respectively. Moreover, the corresponding electron mobility values are 1.74× 10−4, 2.70 × 10−4, and 3.81 × 10−4 cm2 V−1 s−1, respectively. With chlorination on BTA units, TTC-Cl: HCl-BTA3 and TTC-Cl: Cl-BTA3 blend films exhibit increased and balanced carrier mobility, contributing to the improvement of JSC and FF.
ln(P). The slopes of TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 are 1.38, 1.25, and 1.23 kT/q, respectively, which indicate that chlorination is an effective strategy for reducing trap-assisted recombination. In addition, we used the space charge limited current (SCLC) method to measure the charge carrier mobility. As shown in Fig. S9c and d (ESI†), the values of hole mobility for TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3 are 3.02 × 10−5, 8.18 × 10−5, and 9.02 × 10−5 cm2 V−1 s−1, respectively. Moreover, the corresponding electron mobility values are 1.74× 10−4, 2.70 × 10−4, and 3.81 × 10−4 cm2 V−1 s−1, respectively. With chlorination on BTA units, TTC-Cl: HCl-BTA3 and TTC-Cl: Cl-BTA3 blend films exhibit increased and balanced carrier mobility, contributing to the improvement of JSC and FF.
To get a deep insight into the high VOC for these three blends, we further investigate the energy loss of these devices (Table S5 and Fig. S10a, ESI†). The radiative recombination voltage losses above the bandgap (ΔV1) are around 0.286–0.287 V in the three devices. The radiative recombination voltage losses below the bandgap (ΔV2) are 0.097, 0.093, and 0.095 eV for TTC-Cl: BTA3, TTC-Cl: HCl-BTA3, and TTC-Cl: Cl-BTA3, respectively. The somewhat smaller ΔV2 for the asymmetric acceptor may be attributed to the minor energetic disorder with a smaller Urbach energy (Eu in Table S5, ESI†). ΔV3 is the non-radiative recombination voltage loss and depends on EQEEL. As shown in Fig. S10b (ESI†), the values of EQEEL obviously decrease from BTA3 through HCl-BTA3 to Cl-BTA3, indicating that chlorination on the BTA unit will increase the non-radiative recombination voltage loss. As a result, the TTC-Cl: BTA3 blend affords the smallest ΔV3 of 0.18 V and TTC-Cl: Cl-BTA3 yields the highest ΔV3 of 0.27 V. The ΔV3 of asymmetric HCl-BTA3 falls between these two (0.23 V). Compared with symmetric Cl-BTA3, the decreased ΔV3 and unchanged Eg afford HCl-BTA3 a higher VOC of 1.20 V.
In summary, we design a new asymmetric A2–A1–D–A1–A2 type acceptor, HCl-BTA3, by introducing a chlorine atom on one side of the A1 unit. Compared with symmetric BTA3, asymmetric HCl-BTA3 showed a larger dipole moment and stronger intermolecular arrangement, leading to efficient hole transfer and transport. Furthermore, compared with Cl-BTA3, unilateral chlorine substitution on BTA affords HCl-BTA3 a higher value of EQEEL, acting on the higher VOC. As a result, the synergistic effect of the asymmetric structure and chlorination endows HCl-BTA3 with a higher PCE of 11.2% with a VOC of 1.20 V. Our work demonstrates that building an asymmetric A2–A1–D–A1–A2 structure is also an effective approach to construct efficient photovoltaic materials.
The authors acknowledge the support of the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB36000000) and the National Natural Science Foundation of China (NSFC, No. 21875052, 51873044 and 52073067).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef.
- Y. Cui, Y. Xu, H. Yao, P. Bi, L. Hong, J. Zhang, Y. Zu, T. Zhang, J. Qin, J. Ren, Z. Chen, C. He, X. Hao, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2102420 CrossRef PubMed.
- L. Zhu, M. Zhang, J. Xu, C. Li, J. Yan, G. Zhou, W. Zhong, T. Hao, J. Song, X. Xue, Z. Zhou, R. Zeng, H. Zhu, C. C. Chen, R. C. I. MacKenzie, Y. Zou, J. Nelson, Y. Zhang, Y. Sun and F. Liu, Nat. Mater., 2022, 21, 656–663 CrossRef PubMed.
- C. He, Y. Pan, Y. Ouyang, Q. Shen, Y. Gao, K. Yan, J. Fang, Y. Chen, C.-Q. Ma, J. Min, C. Zhang, L. Zuo and H. Chen, Energy Environ. Sci., 2022, 15, 2537–2544 RSC.
- K. Chong, X. Xu, H. Meng, J. Xue, L. Yu, W. Ma and Q. Peng, Adv. Mater., 2022, 34, 2109516 CrossRef CAS PubMed.
- W. Gao, M. Zhang, T. Liu, R. Ming, Q. An, K. Wu, D. Xie, Z. Luo, C. Zhong, F. Liu, F. Zhang, H. Yan and C. Yang, Adv. Mater., 2018, 30, 1800052 CrossRef PubMed.
- M. Li, Y. Zhou, J. Zhang, J. Song and Z. Bo, J. Mater. Chem. A, 2019, 7, 8889–8896 RSC.
- Y. Zhang, Y. Ji, Y. Zhang, W. Zhang, H. Bai, M. Du, H. Wu, Q. Guo and E. Zhou, Adv. Funct. Mater., 2022, 32, 2205115 CrossRef CAS.
- B. Xiao, A. Tang, J. Zhang, A. Mahmood, Z. Wei and E. Zhou, Adv. Energy Mater., 2017, 7, 1602229 Search PubMed.
- A. Tang, B. Xiao, Y. Wang, F. Gao, K. Tajima, H. Bin, Z.-G. Zhang, Y. Li, Z. Wei and E. Zhou, Adv. Funct. Mater., 2018, 28, 1704507 CrossRef.
- Y. Xu, Y. Cui, H. Yao, T. Zhang, J. Zhang, L. Ma, J. Wang, Z. Wei and J. Hou, Adv. Mater., 2021, 33, e2101090 CrossRef PubMed.
- Z. Wang, A. Tang, H. Wang, Q. Guo, Q. Guo, X. Sun, Z. Xiao, L. Ding and E. Zhou, Chem. Eng. J., 2023, 451, 139080 CrossRef CAS.
- A. Tang, W. Song, B. Xiao, J. Guo, J. Min, Z. Ge, J. Zhang, Z. Wei and E. Zhou, Chem. Mater., 2019, 31, 3941–3947 CrossRef CAS.
- A. Lan, Y. Lv, J. Zhu, H. Lu, H. Do, Z.-K. Chen, J. Zhou, H. Wang, F. Chen and E. Zhou, ACS Energy Lett., 2022, 7, 2845–2855 CrossRef CAS.
- P. Bi, J. Ren, S. Zhang, J. Wang, Z. Chen, M. Gao, Y. Cui, T. Zhang, J. Qin, Z. Zheng, L. Ye, X. Hao and J. Hou, Nano Energy, 2022, 100, 107463 CrossRef CAS.
- Z. Chen, T. Wang, Z. Wen, P. Lu, W. Qin, H. Yin and X.-T. Hao, ACS Energy Lett., 2021, 6, 3203–3211 CrossRef CAS.
- J. Qin, Z. Chen, P. Bi, Y. Yang, J. Zhang, Z. Huang, Z. Wei, C. An, H. Yao, X. Hao, T. Zhang, Y. Cui, L. Hong, C. Liu, Y. Zu, C. He and J. Hou, Energy Environ. Sci., 2021, 14, 5903–5910 RSC.
- X. Li, A. Tang, Q. Guo, X. Guo, J. Chen, Q. Guo, M. Ji, Y. Meng, X. Li and E. Zhou, ACS Appl. Mater. Interfaces, 2022, 14, 32308–32318 CrossRef CAS.
- T. Dai, Q. Nie, P. Lei, B. Zhang, J. Zhou, A. Tang, H. Wang, Q. Zeng and E. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 58994–59005 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05631c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |
