Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization of monosaccharides towards fructopyrazines in a new sustainable and efficient eutectic medium†
Svitlana
Filonenko
 *,
Antje
Voelkel
and
Markus
Antonietti
*,
Antje
Voelkel
and
Markus
Antonietti

Max Planck Institute of Colloids and Interfaces, Research Campus Golm, 14424 Potsdam, Germany. E-mail: svitlana.filonenko@mpikg.mpg.de
First published on 29th August 2019
Abstract
In this paper, we propose a new approach for valorization of monosaccharides derived from biomass into value added chemicals in a green and economically efficient manner by forming eutectic medium exclusively between reactants. Eutectic mixtures are emerging as an advantageous alternative to ionic liquids and have already found broad applications in biomass treatment; however they are preferably used only as low vapor pressure solvents. Increasing interest in eutectic solvents originates from their flexibility to adjust their properties to different biorefinery needs by simple variation of the compositions of eutectic mixtures. In this work, we made a first attempt to use a low molecular weight component, ammonium formate, to significantly change the physical properties of the eutectic mixtures and simultaneously increase their chemical reactivity. Using this approach, we valorised simple monosaccharides by turning them into fructopyrazines with high yields in their eutectic mixtures with ammonium formate. The research revealed a high sensitivity of the product yields to the temperature of the reaction and composition of the eutectic medium, as well as an important role of formate anions in product stabilization.
Introduction
The depletion of petroleum resources and the demand for a greener and more sustainable chemistry dictates economically efficient processes for the conversion of biomass into fine chemicals or liquid biofuels. A wide variety of products can be generated from biomass conversion.1,2 Particularly, cellulose, starch, sucrose and other carbohydrates are the most contributing components of biomass and thus are regarded as a promising renewable source for the production of chemicals.3,4 Simultaneously, the high complexity of carbohydrates requires complex and energy demanding synthetic techniques, more selective solvents and catalysts, best avoiding precious metals, for valorisation into value-added chemicals.5,6Important research in this area is aimed at expanding the solvent space for non-aqueous conversions, and includes utilization of deep eutectic solvents (DES) in different stages of biomass treatment.7,8 DES may have an ionic character and thus are considered to be an alternative to ionic liquids, but with much lower toxicity, easier preparation procedures, and lower costs.9 DES are mixtures of two or more components forming a liquid with melting point lower than those of their individual components. For practical utilization, DES with melting points below 100 °C are favoured. Urea as a hydrogen bond donor combined with choline chloride (hydrogen bond acceptor) constitutes the most common DES.10,11 Different hydrogen bond donors were tested to form eutectic mixtures with choline chloride or other quaternary ammonium or phosphonium salts. From this variety of combinations, a great advantage of DES as solvents arises, particularly that their physical properties (density, viscosity, hydrophobicity, and melting point) can be adjusted to fulfil desired requirements by changing the composition of DES in a wide range.9 For instance, in cellulose treatment, DES can act not only as the reaction medium, but also as the reagent and/or catalyst to drive the conversion.12,13 This can drastically increase the efficiency of biomass treatment processes and make it more selective towards the targeted products.
It is the aim of the present work to develop new eutectic media based on sugars derived from biomass hydrolysis and a secondary compound, having high reactivity towards the production of valuable products, as it is promising within the concept of sustainable and energy efficient processes. As such a sugar dissolving component, we decided to use ammonium formate, a low melting salt (melting point: 116 °C) composed of two of the cheapest mass chemicals, ammonia and formic acid. Due to the back reaction into the two components, this salt can even be removed by distillation, and due to its rather low molecular weight, its viscosity is low and mixing entropy is very high, thus driving the dissolution of many polar compounds in this molten salt. The salt can indeed be “distilled”, i.e. it has to be handled in autoclaves under autogenous pressure to explore its full potential.
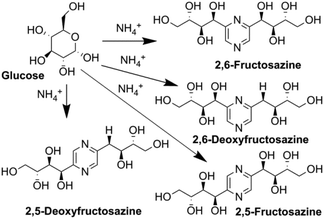
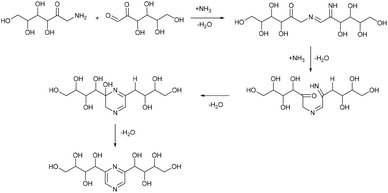
In analogy to DES, we used glucose as a hydrogen bond donor and extended it to another saccharide extracted from biomass or obtained from biomass hydrolysis. We aimed to synthesize nitrogen containing derivatives, namely the Maillard reaction products of glucose with ammonium compounds, which can be transformed by Amadori and Heyns rearrangements into several (polyhydroxyalkyl)pyrazines (Fig. 1).14–16
Due to their distinctive, appealing smell, deoxyfructosazine (DOF) and fructosazine (FZ) (Fig. 1) are used as flavouring agents, and thus can be commercially important for the food flavour industry.17–20 They have been identified in many food products and beverages including caramel, peanuts, soy sauce and carbonated soft drinks.21,22 An additional advantage for the food applications is the recently discovered antimicrobial activity of DOF and FZ to Gram-positive and Gram-negative bacteria.23,24 Special attention here is attracted to use these pyrazine derivatives against Escherichia coli (E. coli), a major contaminant and trouble maker in the meat industry. Several E. coli strains have developed high heat resistance and acquired specific virulence attributes, which can cause a broad spectrum of diseases and restrict the usual thermal treatment methods.23 Utilization of antimicrobial compounds is among the novel and alternative strategies that are available for food safety.24
Their low odour threshold has made these pyrazine derivatives also attractive for the tobacco industry, where they are used to increase or modify the flavour of tobacco smoke.25–27
Customarily, (polyhydroxyalkyl)pyrazines are used to treat and prevent diabetes.28 In addition, the interest in these compounds in the pharmaceutical industry has massively increased recently, as numerous studies have reported their high biological activity, especially an inhibitory effect on different types of tumours in both humans and animals.28 DOF also showed activity against immunological and anti-inflammatory diseases.29
These high-value heterocyclic compounds are usually formed in the reaction between reductive sugars and ammonia or ammonium salts in aqueous solutions under neutral or slightly acidic pH forming the so-called “ammonia caramel”.30,31 However, because of the complexity of the Maillard reaction, species with different molecular weights, such as furfural, as well as volatile and semi-volatile pyrazine derivatives are usually observed as by-products in such reactions when conducted in aqueous media.34,35 Usually, the products of these processes, which can be a mixture of DOF and FZ with other heterocyclic compounds, strongly depend on reaction conditions, particularly pH, which advocates the use of buffers or diluted solutions. The presence of water leads to rather low product yields and complex product mixtures, which are very difficult to separate. In the established manufacturing processes, the reaction with ammonium formate is conducted in dilute solutions with a glucose concentration of ca. 18 wt% and yields below 10%.32 Since the reaction requires elevated temperatures, use of water-free media gains a higher level of energy economy in accordance with the 12 principles of green engineering.33 Another pathway to obtain DOF is by self-condensation of aminosugars, fructosamine (1-amino-1-deoxyketose) or glucosamine (2-amino-2-deoxyaldose), in aqueous acidic reaction media, very often catalysed by metal cations.36
Alternatively, (polyhydroxyalkyl)pyrazines can be obtained from plant sources. They can be found in Eugenia jambolana Lamarck in sufficiently large amounts. Extracts isolated from grains of Eugenia jambolana Lamarck are used for the preparation of antidiabetic medicaments.37,38 However, preparation of the extracts requires tedious processes of seed drying, grinding, washing and extraction, involving large amounts of energy and solvents. Specifically, ethanol is used to remove the undesirable polyphenol and sterol complex from the plant raw material.
Regarding the low solubility of starting educts and (polyhydroxyalkyl)pyrazines in non-polar solvents, one of the possible solutions to increase the reaction yields is the use of DES as reaction media. Recently, Wu et al. developed a method to convert glucosamine into DOF and FZ in choline chloride–urea DES,39 showing that this reaction medium can be successfully used in an environmentally friendly process. The reaction in eutectic medium results in high substrate conversion with yields reaching up to 30% with amino acids used as catalysts. However, difficulties of separation of the polar products from the ionic components of the eutectic mixture can drastically decrease the commercial value of this process.
We will show here that our new ammonium formate–glucose system results in complete substrate conversion with higher yields and higher simplicity. In addition, the possibility of forming a reaction medium from reactants and products is in accordance with the atom economy principle of green chemistry,40,41 thus representing indeed the first use of this new, economical solvent medium for producing a high value industrial product in a more green and economical fashion which was not accessible before.
Results and discussion
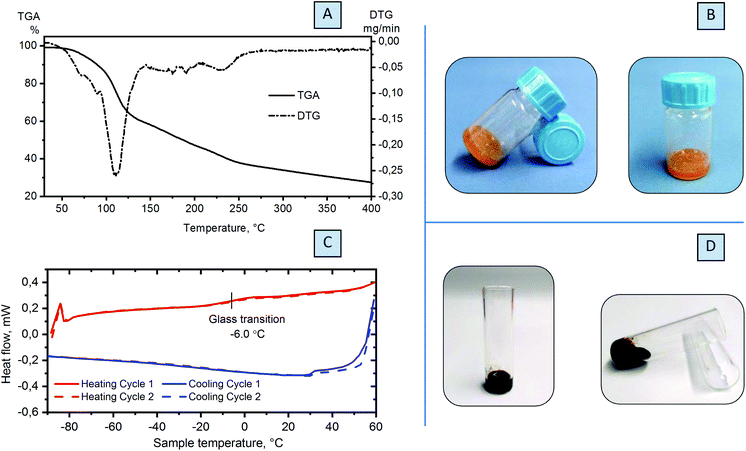
Eutectic mixtures or mixtures with low transition temperatures are rather common, but the nature of the interactions that take place depends on the type of the two components, and hydrogen bonds or even van der Waals forces interfere with the ability of the initial compounds to crystallize.42 The substantial lowering of the melting point observed for the DES is an attractive feature of these systems that allows their use as a liquid reaction medium with ionic character. Since melting temperature depletion is usually connected to the formation of hydrogen bonds between components,43,44 the compounds able to form a eutectic mixture are required to be hydrogen bond acceptors, such as chloride counterions in choline chloride, and hydrogen bond donors, such as urea and its derivatives, phenols, carboxylic acids or saccharides.Substitution of choline chloride for lower molecular weight ammonium formate, which is also active in hydrogen bond formation,45 and combining it with saccharides result in the formation of eutectic mixtures with unusual properties. That is, combining these two components leads to not only physical interactions and liquefaction of the mixture, but also chemical reaction evidenced by the gradual change of the colour from white to dark brown over time: slowly at room temperature (Fig. 2B and S3†) and in several hours at elevated temperatures (Fig. 2D). In both cases, DOF and FZ are observed as the products of this reaction (Fig. S4†).
In order to reveal the physical or chemical precedence of the interaction between ammonium formate and saccharides, we took glucose (EM-G, Table S1†) as a simple model and combined it with ammonium formate. For this mixture, we conducted thermal analysis coupled with mass spectrometry. According to the data from TGA, decomposition of the mixture occurs at a temperature considerably lower than that for both components (ca. 105 °C), forming one distinct maximum and evidencing formation of one phase (Fig. 2A). In the MS spectra of the mixture, ions with m/z = 45 are observed, similar to the thermal decomposition of ammonium formate (Fig. S9†). This peak is attributed to the formation of formamide. The masses m/z = 2 and 44, detected in the MS spectra of the sample, reveal the decomposition of HCOONH4 into hydrogen and carbon dioxide. However, both processes occur at lower temperatures (Fig. S7†).
Fast interaction of the components at high temperatures obstructs the phase transition measurements. For this reason, a specific procedure was developed for DSC investigation. The eutectic mixture was heated up to 60 °C to activate the interaction between components, and immediately cooled down to −90 °C and subsequently heated back to 60 °C. Heating and cooling cycles were repeated, and measurements were conducted without a pause in between the cycles in order to avoid the chemical reaction between glucose (fructose) and ammonium formate. Two cycles of heating and cooling were performed with reproducible glass transition at −6.0 °C (glucose, Fig. 2C) or at −25.3 °C (fructose, Fig. S10†). This confirmed the formation of the eutectic mixture prior to the chemical reaction.
From the chemical point of view, saccharides undergo the Maillard reaction with basic ammonia or ammonium salts in browning caramel solutions. Those reactions are important for the preparation of flavours and caramel colors.46 The Maillard reaction of sugars in this case pursues the following mechanism. The open-chain Amadori rearrangement products undergo numerous Heyns rearrangements, and can form α-amino carbonyls with different carbonyl group positions, and subsequently give pyrazines via self-condensation. When conducted in aqueous medium, the rearrangement reaction is sensitive to the presence of hydrogen or hydroxyl ions, and depending on the pH in aqueous solution, several pyrazine derivatives are found to be formed as products.31,47,48 Similarly, condensation of glucosamine has low selectivity, and despite the high substrate conversion sometimes, distribution of reaction products is influenced by different ammonium sources and the pH of the reaction mixture,49,50 and larger amounts of water can be identified as the source of product diversity.51 Conducting the reaction in eutectic media overcomes these factors and leads to a more single product directed process.31
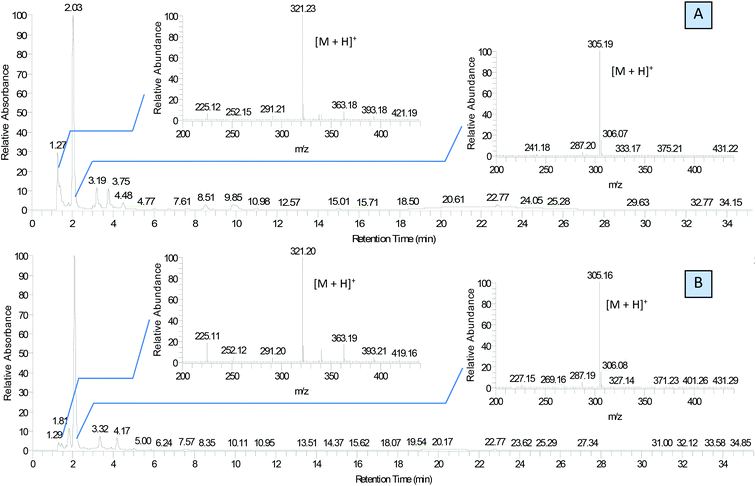
We used this advantage of water-free eutectic media to improve the selectivity of this process and developed a further step to use reagents as components of the eutectic media. To increase the reaction rate, we kept the eutectic mixture for 4 hours at 90 °C. The reaction was conducted in autoclaves to prevent the evaporation of components. Separation of the products was done using liquid chromatography, and their identification with MS/MS showed the predominant formation of molecular ions ([M + H]+) with m/z ratios 305 and 321 for EM-G and EM-F mixtures, respectively, (Fig. 3) or corresponding values for other saccharides (Fig. S6 and S7†). These compounds showed a maximum of absorption at 275.5 nm in UV-vis spectra (Fig. S11†), corresponding to the known transition in di-substituted pyrazines,31 and vibrational modes of the pyrazine ring in the FTIR spectra (Fig. S12†). The 1H NMR results show two sets of signals of aromatic compounds corresponding to disubstituted pyrazines with identical (FZ) and different (DOF) substituting groups (denoted with arrows in Fig. 4) in good agreement with the description from the literature.39 NMR signals of (polyhydroxyalkyl)pyrazines in the aliphatic region are difficult to analyse due to the overlap with numerous signals from –OH protons. The data of 13C NMR analysis indicated the formation of 2,6-DOF and 2,6-FZ as the main products of glucose transformation in the eutectic media with ammonium formate,35 while fructose under the same conditions forms 2,5-DOF and 2,5-FZ. This is in accordance with previous studies in water solutions of saccharides, showing that aldoses predominantly yield the 2,6-isomers while ketoses predominantly yield the 2,5-isomers.52
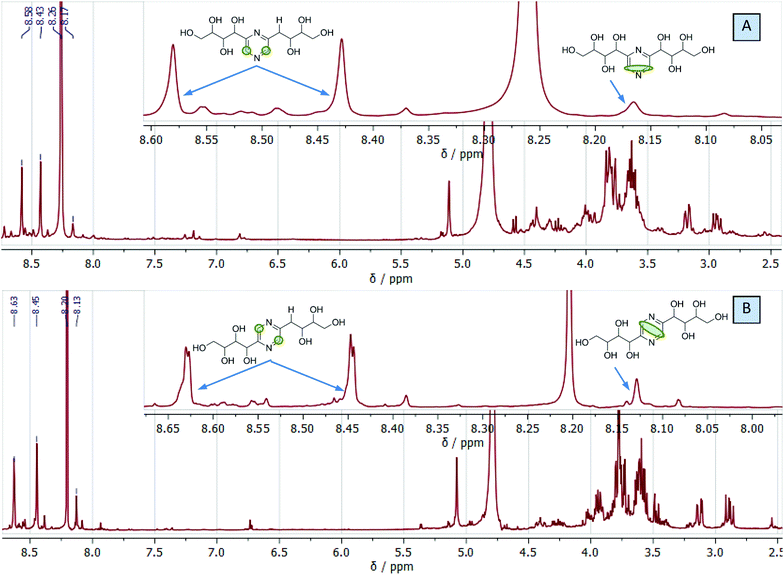 | ||
| Fig. 4 Representative 1H NMR spectra of eutectic medium composed of ammonium formate and glucose (A) or fructose (B) after the reaction showing the formation of DOF and FZ. | ||
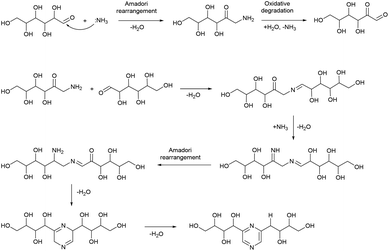
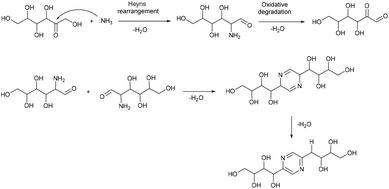
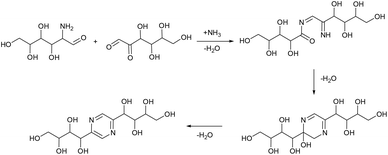
Based on this, we assumed that the mechanism of interactions in eutectic media is similar to that in water solutions. As the first step, a chain opening reaction of saccharides takes place. This reaction is catalysed by OH or H ions.53 Generation of these ions can be explained by the exchange interactions within the eutectic media. Most frequently, eutectics are formed by hydrogen bond interactions in the vicinity of hydrogen bond donors shielding the halide anions in quaternary ammonium salts, wherein our eutectic media formate anions play the role of hydrogen bond acceptors and can form a transition state with hydrogen atoms shared between formate and ammonium. This claim is supported by the presence of some amount of formic acid in eutectic media, detected by the TGA-MS analysis (Fig. S9†). Presumably, under the reaction conditions, formate anions form hydrogen bonds with glucose, simultaneously forming HCOOH and making ammonium cations more available. Open chain glucose molecules react with ammonia to form iso-glucosamine (Amadori rearrangement product), which can further undergo oxidative deamination to form D-glucosone.8 Furthermore, iso-glucosamine undergoes the Maillard reaction with glucose, and through the Amadori rearrangement cyclizes into 2,6-DOF according to Scheme 1.54 Formation of 2,6-FZ in EM-G eutectic medium occurs according to Scheme 2. Since it involves the interaction of iso-glucosamine with D-glucosone, the yields of this reaction are much lower compared to 2,6-DOF formation.
Aldoses react with ammonium species forming glucosamine, which undergoes dimerization into 2,5-DOF (Scheme 3) and 2,5-FZ (Scheme 4).
Other saccharides undergo similar browning reaction forming the products of corresponding pyrazine derivatives (Fig. S8†), namely hexoses, fructose, mannose and galactose form the products with m/z values 305 and 321, while pentoses result in the formation of two main products with m/z 245 and 261 detected using LC-MS (Fig. S6†). Mixing ammonium formate with disaccharides more obviously reveals the formation of the eutectic mixture, since chemical interactions in this case are much slower and colourless viscous liquids are formed when ammonium formate is heated with sucrose or maltose. However, after 4 hours at 90 °C, pyrazine derivatives are detected in these reaction mixtures as well. According to the LC-MS data, sucrose forms the pyrazine ring after breaking the glycosidic bonds, and the product with m/z = 305 is formed. Maltose under these conditions undergoes only partial breaking of the 1–4 bond, and products with m/z = 305, 467 and 629 are formed (Fig. S7†).
Under the reaction conditions, glucose undergoes numerous Maillard transformations similar to those in cooking processes. The main reaction under these conditions is the formation of glucosamine and its dimerization into the pyrazine derivatives. Additionally, there are side reactions of glucose and glucosamine degradation products which can produce a variety of more complex polymeric materials with higher molecular weights, different structures and elemental compositions.55 Higher temperatures lead to glucose oxidation to dicarbonyl compounds and inevitable degradation to heterocycles such as furans or pyranes, and their further inclusion into the polymerization processes.56 These polymers are usually referred to as caramel or melanoidin and can be identified by the brown colour and two absorption bands in UV-vis spectra at around 210 nm and in between 290 and 460 nm, depending on their molecular weight and composition.44,57 From the absorption bands at around 210 nm and a well distinguished shoulder at around 295 nm in the UV-vis spectra of the reaction mixtures, we identified melanoidin as a side product of this reaction (Fig. S11†).
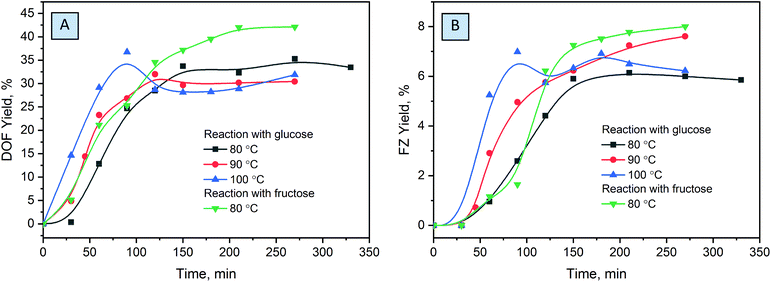
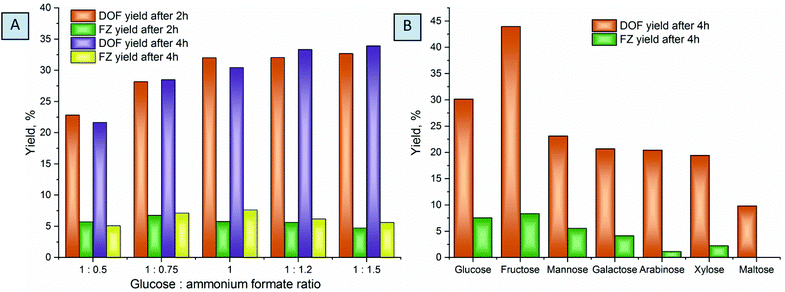
Optimization of the reaction conditions was conducted at different temperatures ranging from 80 to 100 °C, substrate conversion was monitored by UHPLC, and reaction yields were evaluated based on the NMR measurements (Fig. 5). The chosen temperatures used are common for molecule activation in DES formation processes.43,58 Complete glucose conversion is achieved after 5 hours at 80 °C, and after 4 hours at 90 °C and 100 °C. For the reactions without the solvent, higher temperatures are indispensable for liquefaction of the eutectic reaction medium in order to achieve homogeneous distribution of the reactants over the reaction mixture. However, the increase of temperature results in a decrease in the yields of DOF, which can be explained by the higher degree of melanoidin formation. When conducted at 100 °C, the reaction affords the maximum yield after ca. 1.5 hours while not reaching the complete conversion of glucose. The yields drastically drop at a longer reaction time, due to further polymerization or degradation of the products.
The ratio of the reactants plays an important role in the chemical transformations, and it is especially relevant for the eutectic media. Screening over the different compositions of reactive media showed that a decrease of the ammonium formate amount results in drastic lowering of the yields of products (Fig. 6A), probably related to the low concentration of formate anions in the reaction mixture, which, as we will discuss further, plays a crucial role in the stabilization of the products. An excess of ammonium formate slightly increases the yields of the main products, which is more pronounced at longer reaction times. We associate this with the fact that, unlike glucose, which can undergo a variety of side reactions and thus drastically decrease the yields of the main products when used in excess, ammonium formate is stable in this temperature range. However, ammonium formate is an alkali salt and increases the pH of the media when used in great excess. This is less desirable for the reaction, usually conducted in slightly acidic or neutral solutions.21,24,25
Comparing different saccharides shows that fructose affords considerably higher yields of DOF and FZ, while aldoses are much less active in the reaction. This can be related to the lower activity of the aldehyde groups of the saccharides to nucleophilic attack of ammonium in the Maillard reaction.
To gain deeper insight into the transformation of glucose in the ammonium formate eutectic medium, we performed in situ NMR studies under isobaric and isochoric conditions. Formation of DOF is observed after 30 min from the beginning of heating, significantly increasing its amount within the first hour of the reaction (Fig. 7). While moving along the reaction coordinates, the position of the formate group in the NMR spectrum gradually shifts from 8.41 ppm in the reaction mixture before the reaction to 8.25 after the reaction is completed. This clearly indicates the gradual change in the formate anion surroundings from the predominant ammonium cations before the reaction to the vicinity of the pyrazine derivative after the reaction has ended.
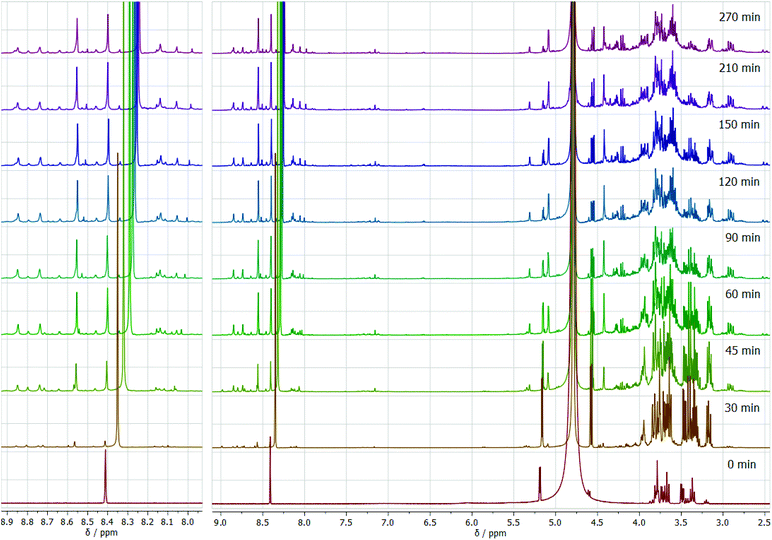 | ||
| Fig. 7 In situ 1H NMR spectra as a function of reaction time for the glucose transformation in the eutectic medium isochoric process at 90 °C. | ||
Under the isobaric conditions, the most notable difference is the possibility of the formic acid, obtained in the reaction, to evaporate. This can be clearly observed from the lowering of the intensity of the NMR signal over the reaction pathway in comparison with the autoclaved procedure (Fig. 8). As an advantage, this allowed us to reveal the role of formate anions in the synthesis process, since formally formate anions are not included in the reaction. It is worth mentioning here that pyrazine and its derivatives contain two nitrogen atoms able to form hydrogen bonds with acids and form salt-like compounds with strong ionic bonds. This explains the remaining amount of format anions ionically bonded to pyrazines after the end of the reaction despite evaporation under isobaric conditions. Similar to this, solutions of (polyhydroxyalkyl)pyrazines in pharmacies are prepared with acidic pH, preferably pH 4, used to stabilize positively charged pyrazines.59 Another relevant feature of these experiments is the formation of pyrazine in the reaction mixture over time. While the autoclaved conditions result in predominant formation of two aromatic heterocycles, DOF and FZ, in the experiment in an open vessel, we observe notable formation of unsubstituted pyrazine over the reaction, with an increase of its quantity over time. Since pyrazine formation in the experiment occurs with some delay, and its amount increases, while the products of the primary reaction fade away, we assume that degradation of DOF and FZ to pyrazine by elimination takes place due to the decrease of the formate anion content in the reaction mixture. Pyrazine is known to be one of the main products of the thermal decomposition of (polyhydroxyalkyl)pyrazines with different lengths of substituting groups, and its relative abundance in the products increases for the decomposition of pyrazines with C4 polyhydroxyalkyl chains.60,61 Similarly, pyrazine is found among the products of thermal decomposition of caramels or tobacco flavours, containing other polyhydroxyalkyl derivatives of pyrazine.62,63 Simultaneous depletion of DOF and FZ signals supports the assumption of their decomposition.
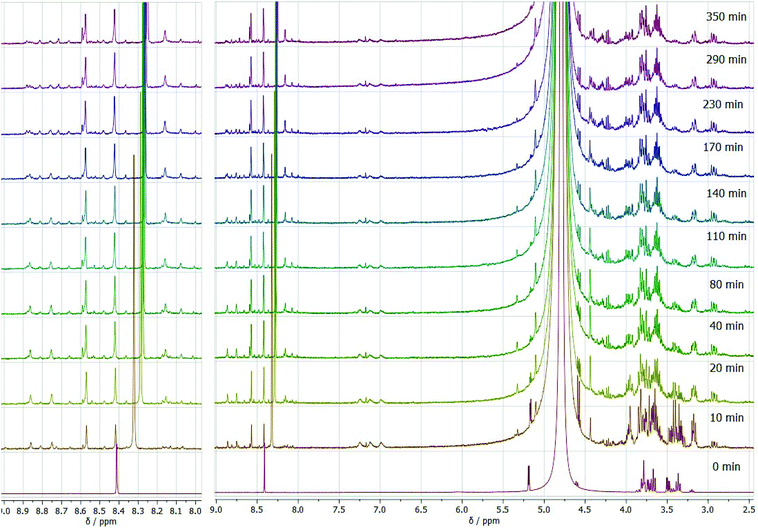 | ||
| Fig. 8 In situ 1H NMR spectra as a function of reaction time for the glucose transformation in the eutectic medium isobaric process at 90 °C. | ||
Another possible origin of pyrazine formation under these conditions is related to the cleavage of glucose to smaller bicarbonyl molecules, particularly to glyoxal, able to react with ammonium and form nitrogen containing heterocycles.64,65 This reaction pathway is used to explain the detection of pyrazine and a variety of its alkyl and hydroxyalkyl derivatives in sugar–ammonia systems.66,67 However, usually only low amounts of glyoxal are formed under glucose thermal decomposition.52,54 Thus, the idea of DOF thermal decomposition with time is more relevant. Both these approaches support the statement about the importance of the autoclaved conditions to remain as the optimal reaction medium and highlight the role of formate anions in stabilization of the products as more stable pyrazinium cations in the proposed eutectic reaction medium, an admittedly unplanned advantage which however explains the high yields.
Experimental
The samples were prepared by mixing different hexoses and pentoses, as well as disaccharides with ammonium formate, varying the molar ratios of components (Table 1). After heating to 60–80 °C, these formed a liquid mixture at room temperature. To conduct the reaction, two powdered components were mixed in a 10 mL Teflon-lined glass vessel and placed in a stainless steel Parr autoclave. The autoclave was kept under constant specified temperature (80 to 100 °C). After reacting for 4 hours, the autoclave was cooled to room temperature under a stream of water. The products after the reaction were freeze dried to obtain a brown solid with distinctive smell.| Entry | Sample | Saccharide | Molar ratio of saccharide/HCOONH4 | DOF yield, % | FZ yield, % |
|---|---|---|---|---|---|
| a Not detected by NMR. | |||||
| 1 | EM-G | Glucose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 8 |
| 2 | EM-F | Fructose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 | 8 |
| 3 | EM-X | Xylose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19 | 2 |
| 4 | EM-Man | Mannose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
23 | 6 |
| 5 | EM-Gal | Galactose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21 | 4 |
| 6 | EM-A | Arabinose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 1 |
| 7 | EM-Mal | Maltose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
10 | |
| 8 | EM-S | Sucrose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
For the thermal analysis coupled with mass spectrometry, a mixture of glucose and ammonium formate was heated from ambient (25 °C) temperature to 400 °C at a rate of 2.5 °C under a He atmosphere. Gas evolution occurring during the TGA measurements was recorded in the MS instrument. Intensities of the gas products were analysed using evolution curves obtained through a preliminary scan from 1 to 200 mass to charge (m/z) values.
DSC measurements were performed in aluminium pans as the reference. Glucose and ammonium formate were ground and mixed together, and then immediately transferred for the DSC measurements. The mixture was heated from ambient temperature to 60 °C to ensure eutectic formation and cooled to −90 °C at a heating/cooling rate of 10 °C min−1. Two more cycles of heating and cooling were conducted heating the sample up to 90 °C.
The components of the reaction mixture were separated using a Dionex UltiMate 3000 UHPLC system, which consisted of an UltiMate 3000 RS pump, an UltiMate 3000 RS autosampler, an UltiMate 3000 RS column compartment and an UltiMate 3000 RS variable wavelength detector (Dionex Sortfon GmbH, Germany). The mobile phase consisted of 0.1% formic acid in water (A) and methanol (B). The gradient program was as follows: 100% A (0–5 min), 100–95% A (5–15 min), 95–50% A (15–25 min), 50–95% A (25–35 min), 95–100% A (35–40 min), and 100% A (40–45 min). The flow rate was 0.5 mL min−1 and the injection volume was 5 μL. Elution was performed on a separation column (Thermo Scientific Accucore C18, 2.6 μm, 100 × 3) with a modified method reported by Hrynets et al.36,66 The components were detected using an LTQ Orbitrap XL (Thermo Scientific) linear ion trap quadrupole mass spectrometer equipped with an Ion Max electrospray ionization (ESI) source (Thermo Fisher Scientific) operated in the H-ESI mode with acquisition conditions similar to those for pyrazines.
A separate kinetic study of the reaction was conducted under isochoric and isobaric conditions at 90 °C in a glass vessel and Parr autoclave, respectively. At various time intervals, aliquots of the reaction mixture were stopped by dilution with cold distilled water. The diluted samples were subjected to 1H and 13C NMR analyses. The NMR spectra were recorded on an Agilent 400 MHz (at 400 MHz for 1H and 101 MHz for 13C) NMR spectrometer. The product yields were evaluated by 1H NMR spectroscopy.
Additional identification was made by UV-vis spectroscopy using a T70 + UV/VIS spectrometer from PG Instruments in a quartz cuvette using distilled water as a reference. The FTIR spectra were recorded on a Nicolet iS5 FTIR spectrometer equipped with an ATR/iD5 with a horizontal cell (Thermo Scientific®, EUA).
Conclusions
In this work, we presented a new ammonium formate based eutectic medium of unparalleled simplicity and cost level for purposeful conversion of biomass molecules, here monosaccharides, into valuable products in a green and economically efficient procedure. We showed the transformation of glucose into deoxyfructosazine and fructosazine with complete conversion of the substrate resulting in yields of up to 42% of DOF, considerably overcoming the existing methods of synthesis in water-based or DES-based solvents. This could be realized without any added catalysts. The proposed procedure fulfills the main requirements of green chemistry, since reactants and reaction products also form the reaction medium and ensure maximal atom utilization, while simplifying purification and separation procedures. Additionally, our experiments revealed a superior role of formate anions in the formation of eutectic media with high reactivity towards biomass valorization, and we also found product stabilization under the reaction conditions. The obtained results indicate the broader potential of ammonium formate based eutectic medium for biomass treatment. Current work relates to polyphenol valorization, with similar unexpected expansions of chemical space.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Max Planck Society and National Key Research. Open Access funding was provided by the Max Planck Society.References
- A. Demirbaş, Energy Convers. Manage., 2001, 42(11), 1357 CrossRef.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15(3), 550 RSC.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51(11), 2564–2601 CrossRef CAS PubMed.
- M. FitzPatrick, P. Champagne, M. F. Cunningham and R. A. Whitney, Bioresour. Technol., 2010, 101(23), 8915–8922 CrossRef CAS PubMed.
- F. Carvalheiro, L. C. Duarte and F. M. Gírio, J. Sci. Ind. Res., 2008, 849–864 CAS.
- J. P. Lange, Biofuels, Bioprod. Biorefin., 2007, 1(1), 39–48 CrossRef CAS.
- R. Wahlström, J. Hiltunen, M. P. D. S. N. Sirkka, S. Vuoti and K. Kruus, RSC Adv., 2016, 6(72), 68100–68110 RSC.
- Q. Xia, Y. Liu, J. Meng, W. Cheng, W. Chen, S. Liu, Y. Liu, J. Li and H. Yu, Green Chem., 2018, 20(12), 2711–2721 RSC.
- X. Ge, C. Gu, X. Wang and J. Tu, J. Mater. Chem. A, 2017, 5(18), 8209 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003,(1), 70 RSC.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9(11), 1155 RSC.
- J. A. Sirviö, M. Visanko and H. Liimatainen, Green Chem., 2015, 17(6), 3401 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126(29), 9142 CrossRef CAS PubMed.
- G. P. Ellis, The maillard reaction, in Advances in carbohydrate chemistry, Academic Press, 1959, vol. 14, pp. 63–134 Search PubMed.
- M. A. J. S. Van Boekel, Formation of flavour compounds in the Maillard reaction, Biotechnol. Adv., 2006, 24(2), 230–233 CrossRef CAS PubMed.
- F. Ledl and E. Schleicher, Angew. Chem., Int. Ed. Engl., 1990, 29(6), 565–594 CrossRef.
- H. E. Nursten, The Maillard reaction: chemistry, biochemistry, and implications, Royal Society of Chemistry, 2005 Search PubMed.
- F. Delgado-Vargas and O. Paredes-Lopez, Natural colorants for food and nutraceutical uses, CRC press, 2002 Search PubMed.
- Colour additives for foods and beverages, ed. M. J. Scotter, Elsevier, 2015 Search PubMed.
- R. Van Der Ark, P. Blokker, L. Bolshaw, E. R. Brouwer, P. S. Hughes, H. Kessels, F. Olierook and M. Van Veen, Heineken Supply Chain BV, U.S. Patent7989014, 2011 Search PubMed.
- R. L. Magaletta and C. T. Ho, J. Agric. Food Chem., 1996, 44(9), 2629 CrossRef CAS.
- H. Tsuchida, M. Komoto and S. Mizuno, Nippon Shokuhin Kogyo Gakkaishi, 1990, 37(2), 154 CrossRef CAS.
- M. A. Croxen, R. J. Law, R. Scholz, K. M. Keeney, M. Wlodarska and B. B. Finlay, Clin. Microbiol. Rev., 2013, 26(4), 822 CrossRef CAS PubMed.
- M. A. Del Nobile, A. Lucera, C. Costa and A. Conte, Front. Microbiol., 2012, 3, 287 Search PubMed.
- J. L. White and T. A. Perfetti, US Patent, 5121757, 1992 Search PubMed.
- J. H. Lauterbach, US Patent, 6131584, 2000 Search PubMed.
- W. M. Coleman III, T. A. Perfetti, R. L. Parks, M. F. Dube and L. M. Dominguez, US Patent, 6298858, 2001 Search PubMed.
- V. Chesnokov, B. Gong, C. Sun and K. Itakura, Cancer Cell Int., 2014, 14(1), 45 CrossRef PubMed.
- A. Zhu, J. B. Huang, A. Clark, R. Romero and H. R. Petty, Carbohydr. Res., 2007, 342(18), 2745 CrossRef CAS PubMed.
- H. Tsuchida, S. Tachibana, K. Kitamura and M. Komoto, Agric. Biol. Chem., 1976, 40(5), 921 CAS.
- H. Tsuchida, M. Komoto, H. Kato and M. Fujimaki, Agric. Biol. Chem., 1973, 37(11), 2571 CAS.
- G. Bashiardes, J.-C. Carry, M. Evers, B. Filoche and S. Mignani, Aventis Pharma, US20040209890, 2004 Search PubMed.
- S. Y. Tang, R. A. Bourne, R. L. Smith and M. Poliakoff, Green Chem., 2008, 10, 268–269 RSC.
- T. Shibamoto and R. A. Bernhard, Agric. Biol. Chem., 1977, 41(1), 143 CAS.
- S. Wu, H. Fan, Q. Zhang, Y. Cheng, Q. Wang, G. Yang and B. Han, Clean: Soil, Air, Water, 2011, 39(6), 572 CAS.
- Y. Hrynets, A. Bhattacherjee, M. Ndagijimana, D. J. Hincapie Martinez and M. Betti, J. Agric. Food Chem., 2016, 64(16), 3266 CrossRef CAS PubMed.
- A. R. Ratsimamanga, S. R. Ratsimamanga, P. Rasoanaivo, J. Leboul, J. Provost and D. Reisdorf, Institut Malgache de Recherches Appliquées, Rhône-Poulenc Rorer, WO1997028813, 1996.
- A. R. Ratsimamanga, S. R. Ratsimamanga, P. Rasoanaivo, J. Leboul, J. Provost and D. Reisdorf, Aventis Pharma SA and Institute Malgache de Recherches Appliquees, U.S. Patent, 6194412, 2001 Search PubMed.
- M. Wu, H. Ma, Z. Ma, Y. Jin, C. Chen, X. Guo, Y. Qiao, C. M. Pedersen, X. Hou and Y. Wang, ACS Sustainable Chem. Eng., 2018, 6(7), 9434 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, 1998, pp. 29–56 Search PubMed.
- R. Höfer, History of the sustainability concept–renaissance of renewable resources, in Sustainable Solutions for Modern Economies, 2009, vol. 4, pp. 1–11 Search PubMed.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52(11), 3074 CrossRef CAS PubMed.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41(21), 7108 RSC.
- C. R. Ashworth, R. P. Matthews, T. Welton and P. A. Hunt, Phys. Chem. Chem. Phys., 2016, 18(27), 18145 RSC.
- Y. J. Zheng and K. M. Merz Jr., J. Comput. Chem., 1992, 13(9), 1151 CrossRef CAS.
- L. Royle, J. M. Ames, L. Castle, H. E. Nursten and C. M. Radcliffe, J. Sci. Food Agric., 1998, 76(4), 579 CrossRef CAS.
- K. Agyei-Aye, M. X. Chian, J. H. Lauterbach and S. C. Moldoveanu, Carbohydr. Res., 2002, 337(21–23), 2273 CrossRef CAS PubMed.
- J. M. Wong and R. A. Bernhard, J. Agric. Food Chem., 1988, 36(1), 123 CrossRef CAS.
- J. Rohovec, J. Kotek, J. A. Peters and T. Maschmeyer, Eur. J. Org. Chem., 2001, 2001(20), 3899 CrossRef.
- S. Fujii, R. Kikuchi and H. Kushida, J. Org. Chem., 1966, 31(7), 2239 CrossRef CAS.
- L. Jia, Y. Wang, Y. Qiao, Y. Qi and X. Hou, RSC Adv., 2014, 4(83), 44253 RSC.
- M. X. Wang, Ph.D. Dissertation, University of Louisville, 1993.
- W. Pigman and H. S. Isbell, in Advances in Carbohydrate Chemistry, Academic Press, 1968, vol. 23, pp. 11–57 Search PubMed.
- T. M. Wrodnigg and B. Eder, Glycoscience, Springer, Berlin, Heidelberg, 2001, pp. 115–152 Search PubMed.
- V. A. Yaylayan and E. Kaminsky, Food Chem., 1998, 63(1), 25 CrossRef CAS.
- B. Cämmerer, W. Jalyschko and L. W. Kroh, J. Agric. Food Chem., 2002, 50(7), 2083 CrossRef PubMed.
- R. C. Borrelli, V. Fogliano, S. M. Monti and J. M. Ames, Eur. Food Res. Technol., 2002, 215(3), 210 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114(21), 11060 CrossRef CAS.
- R. Bastin, M. C. Hart and N. Hughes, Solution aqueuse stable de deoxyfructosazine, WO2001049261, 2000 (Aventis Pharma).
- R. Hardt and W. Baltes, J. Anal. Appl. Pyrolysis, 1988, 13(3), 191 CrossRef CAS.
- I. Ježo, Chem. Zvesti, 1963, 17, 126 Search PubMed.
- R. Hardt and W. Baltes, J. Anal. Appl. Pyrolysis, 1989, 15, 159 CrossRef.
- A. Droß and W. Baltes, Z. Lebensm.-Unters. Forsch., 1989, 188(6), 540 CrossRef.
- P. J. Thornalley, A. Langborg and H. S. Minhas, Biochem. J., 1999, 344(1), 109 CrossRef CAS.
- K. J. Wells-Knecht, D. V. Zyzak, J. E. Litchfield, S. R. Thorpe and J. W. Baynes, Biochemistry, 1995, 34(11), 3702 CrossRef CAS PubMed.
- Y. Hrynets, M. Ndagijimana and M. Betti, J. Agric. Food Chem., 2015, 63(27), 6249 CrossRef CAS.
- T. Shibamoto, et al. , J. Agric. Food Chem., 1977, 25(3), 609 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc02176k |
| This journal is © The Royal Society of Chemistry 2019 |