Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hemicyanine based ratiometric fluorescence probe for mapping lysosomal pH during heat stroke in living cells†
Luling
Wu
ab,
Yang
Wang
a,
Tony D.
James
 *b,
Nengqin
Jia
*b,
Nengqin
Jia
 *a and
Chusen
Huang
*a
*a and
Chusen
Huang
*a
aThe Education Ministry Key Laboratory of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, and Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Department of Chemistry, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: nqjia@shnu.edu.cn; huangcs@shnu.edu.cn
bDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
First published on 11th April 2018
Abstract
Heat stroke is a lethal condition which can cause dysfunction in the central nervous system, multi-organ damage and even death. However, there is still limited knowledge of the detailed mechanism about the roles of lysosomes in heat stroke due to lack of effective tools. Herein, we introduce our previously developed hemicyanine with a large D–π–A structure as the key fluorophore to develop a new fluorescent probe (CPY) for ratiometric mapping of lysosomal pH changes in live cells under a heat shock stimulus.
Heat stroke (HS) is a heat-related pathology when the body temperature rises to 40 °C or higher. This hyperthermia is associated with central nervous system dysfunction and dry skin, which is a life-threatening condition.1 Heat stroke during annual heat waves affects immunocompromised individuals with weakened health status, as well as individuals on medications (or drug users) or certain genetic conditions, and environmental factors are also responsible for heat stroke. Additionally, long-term consequences of heat stroke increase the risk of multi-organ damage, long-term disability and even death.2–5 Particularly, systemic inflammatory response syndrome is considered to be closely related to heat stroke.4 However, compared to the emerging diseases that are thought to be due to heat stroke, the molecular and organelle mechanisms of this heat-related pathology are poorly understood. While, it has been found that heat shock proteins are involved in protecting heat-stroke-associated diseases,6,7 information concerning heat cytotoxicity, molecular and subcellular organelle behaviour during a heat stimulus in live cells is still limited.
The lysosome is a vital subcellular organelle with a quite distinct pH (3.5–6.0) from cytoplasm (pH 7.2).8–10 Due to the appropriate acid environment, a diversity of functions such as the digestion and degradation processes of hydrolytic enzymes are maintained,11 which is closely linked to other cell processes, including plasma membrane repair, energy metabolism, etc.12,13 A steady-state lysosomal acidic environment is maintained by the proton-pumping V-type ATPase, which pumps from the cytosol to the lysosomal lumen.14–16 Importantly, even a slight lysosomal pH fluctuation can have adverse effects on the cells’ status. For example, abnormal lysosomal pH is associated with aging,17 inflammation,18 lysosomal storage diseases,19,20 and tumours.20 Besides physiological processes, recent studies in SW480 cells unveiled that heat stress could activate the lysosomal–mitochondrial apoptotic pathway involved in the increase of lysosomal membrane permeability.21 And heat stroke might become another significant pathology responsible for aberrant lysosomal pH due to the increased lysosomal membrane permeability during a heat shock stimulus.22–24 Thus, further investigation into the interplay between lysosomal behaviour and heat stroke in live cells will allow for a better understanding of the detailed mechanism of heat stroke at the subcellular level.
Examples of exploring lysosomal pH changes under a heat shock stimulus are rare despite the fact that numerous fluorescence based probes have been developed for sensing lysosomal pH in live cells.25–28 To the best of our knowledge, only two examples have been previously reported for detecting lysosomal pH changes during a heat shock stimulus. Ma et al. used a ratiometric fluorescent probe to identify the lysosomal pH rise under heat stroke.29 While Yin et al. developed an “off–on” naphthalimide based probe to explain the relationship between the change of lysosomal pH values and heat stroke.30 However, the ratiometric fluorescent probe from Ma's group is based on the merged images of two channels to detect the pH changes in live cells and Yin's work is based on the “turn-on” fluorescence signal. Thus, a ratiometric map of lysosomal pH changes under heat stroke is still urgently needed because pH changes could then be directly observed with more accuracy and high resolution using the self-calibrated ratiometric fluorescence signal, which is of paramount importance in understanding the roles of lysosomes in heat stroke. Herein, to develop an effective ratiometric imaging tool for mapping lysosomal pH changes in live cells under a heat shock stimulus, we designed and synthesized a new ratiometric fluorescent probe for investigating the interplay between lysosomal behaviour and heat stroke in live cells.
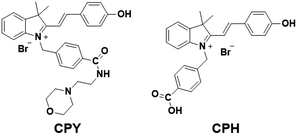
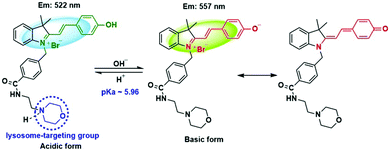
Our design was based on our previously developed ratiometric fluorescence based probe CPH that has a stable and large π-electron conjugated merocyanine system (D–π–A structure), and could be used for the ratiometric fluorescence sensing of intracellular pH in live cells.31 The relatively low pKa (6.44) value of CPH also makes it mainly localize in lysosomes. Given that a heat shock stimulus might increase the lysosomal membrane permeability, which likely causes the leakage of the lysosomal pH indicators into the cytoplasm matrix, we attached a lysosome-targeting group, morpholine,32 to CPH to provide a new ratiometric pH probe CPY (Scheme S1, ESI†) for specific labelling of lysosomes and ratiometric sensing of the lysosomal pH changes during the heat shock stimulus. Herein, the morpholine group could be used as the anchor for the localization of CPY in lysosomes, and the CPH part was taken for ratiometric sensing of pH changes (Fig. 1).
The spectroscopic properties of CPY were first assessed in ultrapure water with different pH values. The maximum absorption of CPY at 430 nm gradually shifted to 538 nm with pH values increasing from 3 to 11. And an isosbestic point was observed at 473 nm. Upon excitation at 473 nm (Fig. 3a), the maximum emission intensity at 522 nm decreased accompanied by an increase of fluorescence emission intensity at 557 nm when the pH value of the solution was increased (Fig. 3a and Scheme S1, ESI†), indicating the ratiometric fluorescence sensing of pH changes. Meanwhile, the pKa value of CPY was calculated by fitting the fluorescence intensity ratio changes (F522/F557) against the pH values. Interestingly, the pKa value of CPY is 5.96 (Fig. S3b, ESI†), which is slightly lower than that of CPH. These results suggested that CPY could be suitable for targeting lysosomes, where the pH value is in the range of 3.5–6. We then evaluated the reversibility of the probe CPY using NMR analysis (Fig. S4 and S5, ESI†). The fluorescence of CPY in water at pH 11.0 was measured and the fluorescence intensity ratio between the maximum emission at 522 and 557 nm (F522/F557) was recorded. Then the pH value of the CPY solution was adjusted to 3.0. The F522/F557 ratio increased from about 0.18 to nearly 1.2 when the pH value of the water solution decreased from 11 to 3. This sensing process could be repeated at least 5 times, indicating the high reversibility of CPY relative to pH changes due to protonation/deprotonation of the phenol group of CPY (Fig. 2).
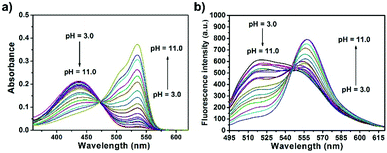 | ||
| Fig. 3 (a) Absorption and (b) fluorescence response of CPY (10 μM) towards different pH values in solutions containing 0.1% DMSO as a co-solvent (λex = 473 nm). | ||
Then, the stability of the fluorescence performance of CPY was evaluated towards different aqueous solutions with pH values of 4.5, 7.4, and 10.5, respectively (Fig. S1b, ESI†). No change of the fluorescence ratio F522/F557 was observed, even when the time increased to 110 min, indicating a stable pH sensing process with CPY. To explore if other biological reagents could interfere with the pH sensing of CPY, some commonly available biological species were evaluated as interferences (Fig. S1, ESI†). The ions and bioactive molecules had imperceptible influence on the fluorescence of CPY in both weak acid (pH = 4.5) and neutral (pH = 7.2) environments (Fig. S1c and d, ESI†). Finally, the influence of different temperatures (from 37 °C to 45 °C) on the fluorescence of CPY was also investigated. The fluorescence ratio F522/F557 of CPY solutions at pH values of 4.0, 6.0 and 9.0, respectively, remained stable when the temperature increased from 37 °C to 45 °C. All these results demonstrated the stable fluorescence performance of CPY in ratiometric sensing of pH changes (Fig. S6, ESI†).
The excellent photophysical performance of CPY prompted us to investigate its sensing performance towards intracellular pH in live cells. CCK-8 assays were initially conducted to evaluate the cytotoxicity of CPY. After the cells were treated with CPY for 24 h, no changes in cell viability were observed when the concentration of CPY is below 50 μM (Fig. S7, ESI†). However, it is interesting that some cytotoxicity for HeLa cells is observed when the concentration of CPY was increased to 70 μM, which was not observed for our previously reported probe CPH. We deduced that this phenomenon was a result of the alkaline morpholine moiety of CPY which could specifically interact with lysosomes and the long incubation (24 h) will lead to an increase of lysosomal pH and induce the apoptosis of the HeLa cells. Herein, 50 μM of CPY was used for the live cell imaging and no significant cytotoxicity was observed. Then, confocal laser scanning microscopy (CLSM) was used to perform ratiometric fluorescence imaging with CPY in live cells. A fluorescence signal in both channel 1 (green, 500–550 nm) and channel 2 (yellow, 570–620 nm) was observed, suggesting good cell membrane penetration of CPY and its potential application in intracellular labeling (Fig. S8, ESI†). In addition, the fluorescence signal from both channels was localized in a small and spherical zone, indicating lysosomal labeling. Considering the lysosomal pH range of 3.5–6.0,8 the pKa value of CPY (5.96) makes it localize in lysosomes and exist both in acidic and basic forms. It is notable that the relatively strong fluorescence signal from channel 1 compared to the fluorescence in channel 2 revealed that the acidic form of CPY is preferable to the basic form of CPY in the lysosomal environment (Fig. S8, ESI†).
To confirm whether CPY is a promising lysosome-targeting probe, colocalization experiments were conducted. The commercially available lysosome probe LysoTracker Deep Red was co-stained with CPY in live cells (Fig. S10a, b and S9, ESI†). The Pearson's correlation coefficient was calculated to be 0.898, which is higher than our previously reported probe CPH (Table S1, ESI†). These results indicated that the weakly basic amine – morpholine group – could improve the lysosome-targeting specificity. Furthermore, both the linear region of interest (ROI) and 3D surface plot analysis with the ImageJ software showed a large overlap between the CPY stained fluorescence signal and the commercial LysoTracker Deep Red stained signal (Fig. S10c–f, ESI†). Meanwhile, the CPY stained fluorescence signal collected from channel 1 also has the similar overlay results with the commercial LysoTracker Deep Red signal (Fig. S9 and S10, ESI†). All the results indicated that CPY was a lysosomal specific labelling probe and could be used for the ratiometric fluorescence sensing of pH changes of lysosomes in live cells.
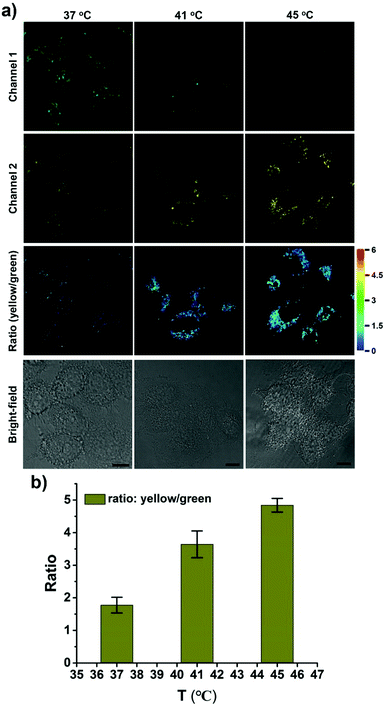
CPY was then used to investigate the fluctuation of lysosomal pH during heat stroke by ratiometric fluorescence imaging. Temperatures of 41 °C and 45 °C were used as model heat stroke temperatures, while 37 °C was used as the control (normal temperature). It has been shown that temperature could induce the pH increase of lysosomes due to increased membrane permeability.29,30 In order to confirm whether CPY could still accumulate well in lysosomes under heat stroke, colocalization experiments were conducted in live cells at 41 °C and 45 °C, respectively. Then the cells were cooled to normal temperature under incubation at 37 °C for another 20 min. The CPY signal collected at channel 2 (yellow) had a significant overlap with the commercial LysoTracker Deep Red signal, indicating the superior lysosome-targeting specificity of CPY under heat stress (Fig. 4). This phenomenon could be ascribed to the presence of the lysosome-targeting group morpholine, which was trapped in the lysosomes during the heat stress stimulus. Finally, the ratiometric sensing of pH changes in lysosomes under heat stroke was performed in live HeLa cells. Compared to the fluorescence images from the control group of HeLa cells stained with CPY at 37 °C for 40 minutes, the fluorescence images of CPY (50 μM) loaded HeLa cells at 41 °C or 45 °C exhibited a gradual decrease in green fluorescence (channel 1, 500–550 nm) and simultaneous enhancement in the yellow fluorescence (channel 2, 570–620 nm) as the temperature increased. Moreover, the ratiometric images were constructed from the fluorescence signals in the yellow channel (channel 2) relative to the green channel (channel 1) using the ImageJ software (Fig. 4a, ratio (yellow/green)). After the cells were exposed to an increased temperature, the pH increase in lysosomes was clearly mapped through the ratiometric signal from the fluorescence ratiometric images. The ratiometric images taken using CPY stained cells could provide clearly visible information about the pH distribution in lysosomes under heat stroke. Additionally, the fluorescence emission ratio values of average emission intensity for yellow images (channel 2) and green images (channel 1) were calculated using semi-quantitative calculations (Fig. 4b). Correspondingly, the average fluorescence emission ratio values increased from about 1.78 to 4.84 under heat stress.
Notably, all the heat shock experiments were conducted on cells that were firstly treated with heat stress at 41 and 45 °C, respectively, followed by cooling to normal temperature under incubation at 37 °C for another 20 min. Compared to the control cells that were incubated at 37 °C for another 40 min, the ratiometric fluorescence signal from the heat shock stimulus increased even when the cells were cooled to normal temperature under incubation at 37 °C for another 20 min after the heat stroke stimulus, which confirms the previous research result that the increase in lysosomal pH during heat shock is an irreversible process.29
An uneven pH distribution among lysosomes was also captured using enlarged ratiometric images (Fig. S13, ESI†), suggesting that not all lysosomes in one cell have the same response to heat stroke. Commonly several hundred lysosomes may be present in a single animal cell, which might be ascribed to the existence of different types of lysosomes with various functionalities in live cells.33,34 Thus, it is an interesting phenomenon that might point to differences in individual lysosomal behavior in a single cell during heat stress.
In summary, a new ratiometric fluorescent probe (CPY) was synthesized for the detection of pH changes in lysosomes under heat stroke. This was achieved by introducing a lysosomal targeting group morpholine into our previously developed ratiometric pH sensitive probe CPH; CPY contains both ratiometric pH sensing ability and lysosomal localization. Our results demonstrated that CPY has a high selectivity and stability in the sensing of pH fluctuation through ratiometric fluorescence signal readout. Live cell imaging suggested that CPY was mainly trapped in lysosomes and there is little leakage even during heat stroke. More importantly, unlike the previously reported fluorescence results constructed by merging images from both red and green channels,29 the ratiometric images were constructed from the CPY stained fluorescence signal from the yellow channel (channel 2) relative to the green channel (channel 1). The ratiometric images from the CPY stained cells clearly mapped the pH increase in lysosomes with increases in temperature during heat stroke and provided visible information about pH distribution in lysosomes through the discriminating pseudo-color relative to the different pH values.
Importantly, we observed an uneven pH distribution among lysosomes during heat stroke, which may indicate that not all lysosomes have the same response to a heat stimulus. In view of the compelling role of lysosomes in heat stroke22–24 and the increasing evidence for the lysosomal pH increase during heat stroke,29,30 our work provides an effective ratiometric imaging tool for mapping lysosomal pH changes in live cells under a heat shock stimulus as well as a potential chemical tool for unveiling the underlying mechanism of the behavior of lysosomes during heat stroke.
We thank the National Natural Science Foundation of China (Grants 21672150 and 21302125), Alexander von Humboldt Foundation (AvH), the Doctoral Fund of Ministry of Education of China (Grant No. 20133127120005), the Shanghai “Chenguang” Program (Grant 14CG42, supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation), and the Program for Changjiang Scholars and Innovative (IRT_16R49). LW wishes to thank China Scholarship Council and the University of Bath for supporting his PhD work in the UK. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Bouchama and J. P. Knochel, N. Engl. J. Med., 2002, 346, 1978–1988 CrossRef CAS PubMed.
- L. Argaud, T. Ferry and Q. Le, et al. , Arch. Intern. Med., 2007, 167, 2177–2183 CrossRef PubMed.
- R. F. Wallace, D. Kriebel, L. Punnett, D. H. Wegman and P. J. Amoroso, Environ. Res., 2007, 104, 290–295 CrossRef CAS PubMed.
- L. R. Leon and A. Bouchama, Comprehensive Physiology, John Wiley & Sons, Inc., 2011 Search PubMed.
- R. Carter III, S. N. Cheuvront, J. O. Williams, M. A. Kolka, L. A. Stephenson, M. N. Sawka and P. J. Amoroso, Med. Sci. Sports Exercise, 2005, 37, 1338–1344 CrossRef.
- M. Jäättelä and D. Wissing, J. Exp. Med., 1993, 177, 231–236 CrossRef.
- N. Kourtis, V. Nikoletopoulou and N. Tavernarakis, Nature, 2012, 490, 213 CrossRef CAS PubMed.
- J. R. Casey, S. Grinstein and J. Orlowski, Nat. Rev. Mol. Cell Biol., 2010, 11, 50–61 CrossRef CAS PubMed.
- M. H. Lee, J. H. Han, J. H. Lee, N. Park, R. Kumar, C. Kang and J. S. Kim, Angew. Chem., Int. Ed., 2013, 52, 6206–6209 CrossRef CAS PubMed.
- S. Ohkuma and B. Poole, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 3327–3331 CrossRef CAS.
- L. Wu, X. Li, Y. Ling, C. Huang and N. Jia, ACS Appl. Mater. Interfaces, 2017, 9, 28222–28232 CAS.
- C. Settembre, A. Fraldi, D. L. Medina and A. Ballabio, Nat. Rev. Mol. Cell Biol., 2013, 14, 283 CrossRef CAS PubMed.
- C. Settembre, R. Zoncu, D. L. Medina, F. Vetrini, S. Erdin, S. Erdin, T. Huynh, M. Ferron, G. Karsenty and M. C. Vellard, EMBO J., 2012, 31, 1095–1108 CrossRef CAS PubMed.
- J. Tian, L. Ding, H. Ju, Y. Yang, X. Li, Z. Shen, Z. Zhu, J. S. Yu and C. J. Yang, Angew. Chem., Int. Ed., 2014, 53, 9544–9549 CrossRef CAS PubMed.
- J. A. Mindell, Annu. Rev. Physiol., 2012, 74, 69–86 CrossRef CAS PubMed.
- Y. Ishida, S. Nayak, J. A. Mindell and M. Grabe, J. Gen. Physiol., 2013, 141, 705–720 CrossRef CAS PubMed.
- D. J. Colacurcio and R. A. Nixon, Ageing Res. Rev., 2016, 32, 75–88 CrossRef CAS PubMed.
- M. Yu, X. Wu, B. Lin, J. Han, L. Yang and S. Han, Anal. Chem., 2015, 87, 6688–6695 CrossRef CAS PubMed.
- A. Kogot-Levin, M. Zeigler, A. Ornoy and G. Bach, Pediatr. Res., 2009, 65, 686 CrossRef CAS PubMed.
- B. Winchester, A. Vellodi and E. Young, Biochem. Soc. Trans., 2000, 28, 150–154 CrossRef CAS PubMed.
- G. Yi, L. Li, M. Luo, X. He, Z. Zou, Z. Gu and L. Su, Oncotarget, 2017, 8, 40741 Search PubMed.
- C. Zhou and R. W. Byard, J. Forensic Leg. Med., 2011, 18, 6–9 CrossRef PubMed.
- A. K. Velichko, N. V. Petrova, S. V. Razin and O. L. Kantidze, Nucleic Acids Res., 2015, 43, 6309–6320 CrossRef CAS PubMed.
- M. Domenech, I. Marrero-Berrios, M. Torres-Lugo and C. Rinaldi, ACS Nano, 2013, 7, 5091–5101 CrossRef CAS PubMed.
- J.-T. Hou, W. X. Ren, K. Li, J. Seo, A. Sharma, X.-Q. Yu and J. S. Kim, Chem. Soc. Rev., 2017, 46, 2076–2090 RSC.
- J. Yin, Y. Hu and J. Yoon, Chem. Soc. Rev., 2015, 44, 4619–4644 RSC.
- B. Tang, F. Yu, P. Li, L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016–3023 CrossRef CAS PubMed.
- A.-M. Luo, Y. Shao, K.-J. Zhang, Y.-W. Wang and Y. Peng, Chin. Chem. Lett., 2017, 28, 2009–2013 CrossRef CAS.
- Q. Wan, S. Chen, W. Shi, L. Li and H. Ma, Angew. Chem., 2014, 126, 11096–11100 CrossRef.
- Y. Wen, W. Zhang, T. Liu, F. Huo and C. Yin, Anal. Chem., 2017, 89, 11869–11874 CrossRef CAS PubMed.
- L. Wu, X. Li, C. Huang and N. Jia, Anal. Chem., 2016, 88, 8332–8338 CrossRef CAS PubMed.
- L. Wang, Y. Xiao, W. Tian and L. Deng, J. Am. Chem. Soc., 2013, 135, 2903–2906 CrossRef CAS PubMed.
- G. M. Cooper, The Cell: A Molecular Approach, Sunderland (MA), Sinauer Associates, 2nd edn, 2000 Search PubMed.
- C. de Duve and R. Wattiaux, Annu. Rev. Physiol., 1966, 28, 435–492 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc02330a |
| This journal is © The Royal Society of Chemistry 2018 |