Macromolecular crowders and osmolytes modulate the structural and catalytic properties of alkaline molten globular 5-aminolevulinate synthase†
Bosko M. Stojanovski‡
*a,
Leonid Breydoab,
Vladimir N. Uverskyab and
Gloria C. Ferreira *ac
*ac
aDepartment of Molecular Medicine, Morsani College of Medicine, University of South Florida, MDC 7, Tampa, FL 33612-4799, USA. E-mail: stojanovskibm@slu.edu; gferreir@health.usf.edu; Fax: +1-813-974-0504; Tel: +1-314-977-9249 Tel: +1-813-974-5797
bUSF Health Byrd Alzheimer's Research Institute, Morsani College of Medicine, University of South Florida, Tampa, FL 33612-4799, USA
cDepartment of Chemistry, University of South Florida, Tampa, FL 33612-4799, USA
First published on 28th November 2016
Abstract
The molten globule state is a dynamic ensemble of conformational subsets, where proteins lack well-defined tertiary structure, but retain native-like content of secondary structure and a relatively compact fold. Using various spectroscopic techniques, we characterized the effects of macromolecular crowders and osmolytes on the structural and catalytic properties of the alkaline molten globule state of murine erythroid 5-aminolevulinate synthase (mALAS2alk defined at pH 9.5/37 °C). The tertiary structure rigidity of mALAS2alk, as discerned from near-UV circular dichroic (CD) measurements, increased in the presence of the osmolytes N-trimethylamine oxide (TMAO) and glycerol. In contrast, the macromolecular crowders Dextran 200 and Ficoll 400, even at concentrations of 20% (w/v), were far less effective in rigidifying the tertiary structure. At this concentration of Dextran 200, the far-UV ellipticity of mALAS2alk intensified, implicating stabilization of secondary structural elements in the crowded environment. Furthermore, in the presence of Dextran, the solubility of mALAS2alk strongly depended on the molar concentration of the AMPSO buffer, suggesting changes in the surface hydration of the enzyme. Through ligand-induced enhancement of the tertiary structure rigidity, the solubility of mALAS2alk increased under crowded conditions that otherwise favored precipitation of the holoenzyme. The kcat value of mALAS2alk (pH 9.5/37 °C) increased significantly upon the addition of cosolvents, although the rates remained lower than the kcat determined under physiological conditions (pH 7.5/37 °C). Our data suggest that the molecular properties of at least some molten globular proteins might be modulated within the highly crowded and osmolyte enriched intracellular environment.
Introduction
The molten globule state represents one of the best characterized partially folded intermediates that has been detected under equilibrium and non-equilibrium conditions during the induced denaturation of various globular proteins.1 Because of largely disrupted close packing of the side chain groups in the protein core, molten globular proteins lack a rigid tertiary structure and exist as highly dynamic ensembles of conformational subsets.1 However, their level of secondary structure content is native-like, and the protein fold remains relatively compact, indicating overall topology that resembles that of the native state.1 For globular proteins, the equilibrium molten globule state is often stabilized at mildly denaturing conditions, usually through variations in pH or by perturbing the system with moderate concentrations of chemical denaturants, and among the various well documented examples, its distinct structural properties have been described for numerous proteins including: α-lactalbumin,2,3 cytochrome c,4,5 apomyoglobin,6,7 carbonic anhydrase,8 lysozyme,9 apoflavodoxin,10–12 β-lactamase,13 and many others.1An important question pertinent to partially folded protein conformations, such as the molten globule, relates to the level of structural disorder that can persist within crowded cellular environments where the volume available to proteins is fairly restricted in comparison to that available under dilute conditions. In fact, since the intracellular environment is highly crowded, with macromolecules present at concentrations up to 400 mg mL−1,14 it is obvious that there would be severe limitations in the volume available to be occupied by a particular protein in vivo.15–17 Consequently, this limitation in the available volume that is imposed by the crowding of macromolecules within the cell, can potentially modulate the structural properties of a given protein and shift the equilibrium of conformational subsets toward more compact states.15–17 In fact, when macromolecular crowders, such as large polymers (e.g., Dextran and Ficoll) and inert proteins, were used to mimic the crowded cellular environment in vitro, changes in the structural properties of some ordered and disordered proteins, alike, were reported.17 These changes that are induced in the presence of macromolecular crowders include and are not limited to: increased thermal stability, folding of proteins into more compact conformational subsets, and association into higher oligomeric states (systemized in ref. 17).
The underlying phenomenon that facilitates these processes is believed to be the propensity of proteins to minimize their hydrodynamic volume in the crowded system.16,17 Because restriction in the available volume under crowded conditions reduces the randomness of particle distribution, which results in reduction in the overall entropy and an increase in the free energy of the solute, proteins minimize the extent of volume exclusion in order to increase the overall entropy in the system.17 Thus, the structural properties of some proteins are expected to be modulated when populating crowded (i.e., in vivo-like) as opposed to dilute environments, and this is primarily reflected in their ability to fold into more compact conformations or to oligomerize.
Apart from the large restriction in the available volume due to the presence of large quantities of macromolecules, the intracellular environment is also populated by various small organic osmolytes that regulate the osmotic pressure of cells and stabilize proteins against stress-induced denaturation.18,19 Polyols (e.g., glycerol), various sugars, methylamines [e.g., N-trimethylamine oxide (TMAO)] and amino acids are among the different classes of stabilizing osmolytes that are present in various organisms, including higher eukaryotes.18 As a result of the stabilizing effects that these osmolytes exert on the native state of proteins, it is reasonable to expect that some induced disordered protein states that can be detected in the absence of osmolytes might not be inducible in their presence. Regarding the mechanism of stabilization, it was originally proposed that osmolytes exert their stabilizing effect by being preferentially excluded from the protein surface because of unfavorable interactions with the protein which raises its chemical potential.20 This unfavorable association, postulated to originate from an interaction between the osmolyte and the polypeptide main chain,19,21 induces folding of the protein into more compact conformational subsets in order to minimize the accessible protein surface area that can come into contact with the osmolyte.19,20 In contrast to this model, other studies have implicated that osmolytes stabilize proteins against denaturation by modulating the structure of water,22–26 which in turn restricts its ability to solvate the denatured state of the protein.
Based on the aforementioned considerations, we set to examine how the structural properties intrinsic to molten globular proteins are affected in the presence of macromolecular crowders and osmolytes. We used the alkaline-induced molten globule state of murine erythroid-specific 5-aminolevulinate synthase (mALAS2) as a model protein.27 This homodimeric, pyridoxal 5′-phosphate (PLP)-dependent enzyme (MW = 112 kDa), catalyzes the initial step of heme biosynthesis in non-plant eukaryotes and certain bacteria: the formation of 5-aminolevulinate (ALA) from glycine and succinyl-CoA.28,29 We have previously demonstrated that in the absence of cosolvents, mALAS2 populates the alkaline molten globule state (henceforth abbreviated as mALAS2alk) upon an increase in the alkalinity of the solution to pH 9.5 at 37 °C.27 Under these conditions, the rigidity of the tertiary structure of mALAS2alk is perturbed, but the enzyme retains native-like content of secondary structure and relatively compact fold. Our study also revealed that ALAS retains catalytic activity, albeit reduced, even under conditions where the holoenzyme populates the alkaline molten globule state, and that the conformational fluctuations of mALAS2alk are reduced upon association with the ligand, ALA.27 To examine how the structural and catalytic properties of mALAS2alk are affected in the presence of crowders and osmolytes, in the present study, we conducted detailed biophysical and kinetic studies using the flexible hydrophilic polymers Dextran 200 (MW ∼ 200 kDa) and Ficoll 400 (MW ∼ 400 kDa) as macromolecular crowders, while as osmolytes, we chose glycerol (MW ∼ 92.1 g mol−1) and TMAO (MW ∼ 75.1 g mol−1). Our choice of crowders was influenced by the fact that the volume-excluding effect becomes most pronounced when the size of the crowder is comparable to that of the protein of interest,17 and since the size of homodimeric mALAS2 is 112 kDa, the aforementioned crowders were selected due to similar molecular masses. We also tried to characterize the dichroic properties of mALAS2alk in the presence of the smaller polymer, Dextran 100 (MW ∼ 100 KDa), but the commercially available batch (Sigma-Aldrich #BCBP1501V) did not give reliable absorbance results, which precluded us from collecting the near-UV CD spectra in its presence due to high tension voltage readings.
To the best of our knowledge, our previous work27 was the first reported case of a catalytically active alkaline molten globular enzyme. Together with ALAS, only five enzymes30–33 have been reported to retain catalytic activity under conditions in which the holoenzymes populate the molten globule state. Importantly, information is lacking on how the structural and catalytic properties of these molten globular enzymes might be affected by crowded and osmolyte-enriched conditions that resemble the intracellular environment. Here, we demonstrate that osmolytes and macromolecular crowders modulate the catalytic and structural properties of mALAS2alk. The rigidity of the mALAS2alk tertiary structure is particularly sensitive to the presence of osmolytes, while the macromolecular crowders that were examined (Dextran 200 and Ficoll 400) are less effective in stabilizing fluctuations of tertiary contacts, although an additional increase in the secondary structure content was observed in the presence of Dextran 200. Furthermore, all examined cosolvents enhanced the catalytic properties of mALAS2alk.
Experimental
Reagents
The following reagents were purchased from Fisher Scientific: glycine, potassium phosphate monobasic, and glycerol. Pyridoxal 5′-phosphate, Dextran 200 (#BCBN1147V), TMAO, and succinyl-CoA were from Sigma-Aldrich. AMPSO was from MP Biomedicals, 5-aminolevulinate hydrochloride and trichloroacetic acid (TCA) from Acros Organic, and Ficoll 400 (#10166878) from Alfa Aesar.Protein overproduction and purification
The wild-type mALAS2 cDNA was subcloned into the pGF22 vector,34 and its expression was under control of the alkaline phosphate promoter as previously described.34 The generated recombinant wild-type mALAS2 was overproduced and purified as previously described.34 Protein purity was assessed by SDS-PAGE, while the concentration was determined spectroscopically.35 The concentrated enzyme (∼8 mg mL−1) was dialyzed against 2 L of buffer composed of 50 mM phosphate pH 7.5 (36 mM K2HPO4/14 mM KH2PO4), and 40 μM PLP, and stored into small aliquots at −80 °C for further use.Circular dichroism measurements
CD measurements were conducted on a JASCO J-815 spectropolarimeter at a scan speed of 20 nm min−1 under constant nitrogen purging. The temperature was regulated at 37 °C using a thermostatically controlled cell holder. Protein concentration of 1 mg mL−1 (from a mALAS2 stock concentrated to ∼8 mg mL−1) and a cell with a path length of 1 cm were used for the measurements in the near-UV region (260–310 nm). Spectra of mALAS2 in the absence or presence of 10% or 20% (w/v) TMAO, glycerol, Dextran 200, and Ficoll 400 were collected after equilibration of the reaction mixture for 3 minutes at 37 °C. The pH of the solution was maintained at 9.5 using an AMPSO concentration of 50 mM for the measurements conducted in the presence of Dextran 200 and Ficoll 400, and an AMPSO concentration of 250 mM for those conducted in the presence of TMAO and glycerol. To ensure that the pH of the reaction was not modified by the addition of the cosolvents, stock solutions of 30% glycerol (v/v), and 300 mg mL−1 Ficoll 400 or Dextran 200 were prepared in 50 mM AMPSO, pH 9.5. Stock solution of TMAO at a concentration of 5 M was prepared in deionized H2O. Of note, we verified experimentally using an Accumet AR15 pH meter that the pH of the 250 mM AMPSO buffer remained 9.5 upon addition of 10 or 20% TMAO from the above prepared stock. Each reported near-UV measurement represents the average of three accumulated spectra. All experimental conditions (including buffer and cosolvent compositions, temperature, protein concentration, and cell path length) for the CD measurements in the 310–490 nm range were identical as those described above for the near-UV region (260–310 nm) experiments, except that each measurement represents the average of two accumulated spectra.CD spectra in the near-UV region (260–310 nm) were also collected in the presence of 4 mM ALA at 37 °C and pH 9.5 following equilibration for 3 minutes. The measurements were conducted in a cell with a 1 cm path length using 1 mg mL−1 of enzyme in 250 mM AMPSO, pH 9.5, with or without 20% (w/v) TMAO, glycerol, Ficoll 400, or Dextran 200. The stock solutions of the cosolvents were as described above. Prior to its mixing with the protein sample, ALA (from a 0.5 M stock) was diluted in equal volume of 1 M NaOH. This was necessary in order to ensure that the pH of the buffer was not modified by the addition of ALA, which is commercially available in a hydrochloric form. Each measurement is the average of three accumulated spectra.
Enzyme concentration of 1 mg mL−1 and a cell with 0.1 mm path length were used for the CD measurements in the far-UV region (200–260 nm), which were conducted at 37 °C and pH 9.5. Prior to spectra collection, wild-type mALAS2 was pre-incubated for 3 minutes at 37 °C in 20 mM AMPSO, pH 9.5, buffer with or without 10% or 20% (w/v) of TMAO, glycerol, Ficoll 400, or Dextran 200. Because the AMPSO buffer produced high tension voltage readings at low wavelengths (195–210 nm), which were further amplified with the addition of cosolvents, we had to lower the molar concentration of AMPSO to 20 mM and use a cell with a path length of 0.1 mm. Moreover, stock solutions of 30% glycerol (v/v), and 300 mg mL−1 of Dextran 200 or Ficoll 400 were prepared in 20 mM AMPSO, pH 9.5, whereas a stock solution of 5 M TMAO was prepared in deionized H2O. In preliminary studies, we verified that the pH of the buffer was ∼9.5 upon the addition of 20% TMAO. Each reported measurement is the average of three accumulated spectra.
Steady-state kinetics
A discontinuous colorimetric assay36 was used to measure the steady-state kinetic activities of wild-type mALAS2 at pH 9.5 and 37 °C. All measurements were conducted in the presence of 20% (w/v) cosolvent. In contrast to previous studies that were conducted in the absence cosolvent,27 where we validated that the addition of 200 mM glycine to the 250 mM AMPSO, pH 9.5, buffer did not modify the pH, here, in preliminary studies, we observed that the addition of glycine to a final concentration of 200 mM in a buffering system consisting of 250 mM AMPSO pH 9.5 and 20% cosolvent (also prepared in 250 mM AMPSO, pH 9.5) lowered the pH of the buffer by about 0.5 units. To overcome this, we had to raise the pH of the 250 mM AMPSO buffer to 10, which upon the addition of 200 mM glycine, was lowered to about 9.5. Therefore, 5 M TMAO [37.5% (w/v)], 30% glycerol (v/v), 300 mg mL−1 Ficoll 400, and 300 mg mL−1 Dextran 200 were individually prepared in 250 mM AMPSO, pH 10. The final volume of each reaction (0.3 mL), was adjusted with 250 mM AMPSO, pH 10.The reaction components and respective final concentrations were: 250 mM AMPSO, pH 10, cosolvent (prepared in 250 mM AMPSO, pH 10 as described above) at a final concentration of 20% (w/v) and 200 mM glycine (from a 2 M stock prepared in deionized H2O). The addition of glycine to the mixture lowered the pH to ∼9.5 in all cases. Succinyl-CoA (from a 3 mM stock prepared in deionized H2O) was the variable substrate, whose concentration in the reaction assays ranged from 10–400 μM. The reactions were started by adding 4 μM wild-type mALAS2 (final concentration in the reaction assays), and, after immediate vortexing, were readily transferred to a water-bath at 37 °C. We note that no enzyme precipitation was detected during the incubation period. All enzymatic reactions conducted in the presence of 20% TMAO (w/v) or 20% glycerol (v/v) were quenched after 5 minutes incubation at 37 °C by adding half-reaction volume of 50% trichloroacetic acid (TCA) and placed on ice, while the reactions in the presence of 20% Ficoll 400 (w/v) or 20% Dextran 200 (w/v) were quenched with TCA after 2 minutes incubation. In all cases, we verified that the velocity measurements were quenched during the linear kinetic phase of the reaction. The details of the colorimetric assay procedure had been described elsewhere.27 From the absorbance maximum at 552 nm, the concentration of ALA was determined using an extinction coefficient of 45 (mmol/L)−1 cm−1, and the initial reaction velocities expressed in units of concentration per minute were plotted as a function of succinyl-CoA concentration. The steady-state kinetic parameters were then obtained by fitting the data to the Michaelis–Menten equation using non-linear regression analyses (Sigma-Plot). All reactions were done in duplicates.
Turbidity measurements
Turbidity measurements were conducted using a Shimadzu UV-2401 PC spectrophotometer by continuously monitoring the absorbance at 600 nm over a time period of 0.5 h. The reactions were conducted at 37 °C in a cell with a path length of 1 cm, using mALAS2 concentration of 1 mg mL−1 (from a stock of protein concentrated to ∼8 mg mL−1). Stock solutions of 300 mg mL−1 Dextran 200 were prepared in either in 50 mM K2HPO4, pH 7.5 or in 50 mM AMPSO, pH 9.5. These stock solutions were then diluted to a final concentration of 20% (w/v) Dextran 200 for the respective measurements at pH 7.5 and pH 9.5. The final volume of the reaction was adjusted with either 250 mM K2HPO4, pH 7.5, or with AMPSO, pH 9.5 (see Results section for specific concentration of the AMPSO buffer). The turbidity measurements with ALA were also conducted in the presence of 20% Dextran 200 (diluted from the above described stock solutions of Dextran) using 250 mM K2HPO4, pH 7.5 or 250 mM AMPSO, pH 9.5 as buffers. Prior to the initiation of the measurements, ALA (from 0.5 M stock) was diluted in equal volume of 1 M NaOH in order to neutralize its acidity, which is commercially available in hydrochloric form.Results
Effects of crowders and osmolytes on the secondary structure of mALAS2alk
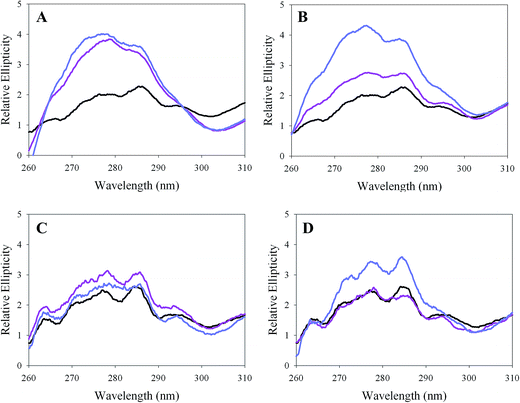
The effects of macromolecular crowders and osmolytes on the secondary structure of mALAS2alk were characterized by the analyses of circular dischrosim (CD) spectra in the far-UV region (200–260 nm) at pH 9.5 and 37 °C. The addition of TMAO or glycerol up to respective concentrations of 20% (w/v) did not significantly perturb the secondary structure content of mALAS2alk (Fig. 1A and B). In contrast, subtle, but nonetheless detectable, changes in the far-UV spectrum of mALAS2alk were observed in the presence of macromolecular crowders, although their effects on the secondary structure content differed (Fig. 1C and D). In particular, while the addition of Ficoll 400 [up to concentrations of 20% (w/v)] resulted in a subtle reduction in the intensity of the far-UV elliptical signal (Fig. 1C), Dextran 200, in a concentration-dependent manner, moderately increased the intensity of this spectrum when its concentration reached 20% (w/v) (Fig. 1D). We note that this Dextran-induced intensification of the far-UV CD signal is not an artifact of the short pathlength of the cuvette that was used in the present measurements (0.1 mm) because we observed similar increase in the elliptical intensity when the spectra collections were done in a cell with a pathlength of 1 mm (260–205 nm region; data not shown).Effects of crowders and osmolytes on the tertiary structure of mALAS2alk
CD spectra in the near-UV region (260–310 nm) were collected to examine the effects of cosolvents on the tertiary structure of mALAS2alk (pH 9.5 and 37 °C). The increased ellipticity in the near-UV region indicates that the osmolytes enhance the rigidity of the tertiary structure of mALAS2alk (Fig. 2A and B). TMAO, at concentrations of either 10 or 20% (w/v), was equally effective at stabilizing the tertiary structure of mALAS2alk against alkaline-induced denaturation (Fig. 2A and 3). In contrast, the ellipticity intensified when the concentration of glycerol was increased from 10 to 20% [(v/v) Fig. 2B and 3]. Our results also suggest that TMAO is more efficient than glycerol at rigidifying the tertiary structure of mALAS2alk, considering that the spectra acquired in the presence of either 20% TMAO or 20% glycerol are comparable (in terms of signal intensity), while at a concentration of 10%, the elliptical signal is significantly more pronounced in the presence of TMAO (Fig. 2A, B and 3). We further note that the intensity of the elliptical signal at alkaline conditions in the presence of 20% osmolyte (either TMAO or glycerol) is comparable to that of the native state of mALAS2 at pH 7.5 and 37 °C (Fig. S1†). | ||
| Fig. 3 Graphical representation of the near-UV elliptical signal intensities measured in the presence of various cosolvents. The black bars represent the difference in ellipticity at 278 nm constructed by subtracting the signal intensity measured in the presence of 10% (w/v) cosolvent from that measured in the absence of cosolvents. The gray bars represent the difference in ellipticity at 278 nm constructed by subtracting the signal measured in the presence of 20% (w/v) cosolvent from that without cosolvent. To account for the subtle differences in the PLP microenvironment that were induced by the inclusion of certain cosolvents (see Fig. 2 and 6), all spectra were initially normalized by subtracting the elliptical difference at 278 nm from that at 301 nm. | ||
Within the range of concentrations used in this study, the macromolecular crowders were considerably weaker than the osmolytes at minimizing the alkaline-induced distortion of the tertiary structure of mALAS2 (Fig. 2 and 3). In fact, only a subtle increase in the near-UV elliptical signal was discerned upon increasing the concentration of Ficoll 400 to 10% (w/v), while at 20%, the spectrum intensity became comparable to that observed in the absence of crowder (Fig. 2C and 3). In contrast, Dextran 200 was most effective in enhancing the intensity of the near-UV spectrum when its concentration reached 20% [(w/v) Fig. 2D and 3].
Effects of buffer concentration, pH, and ligands on the solubility of mALAS2
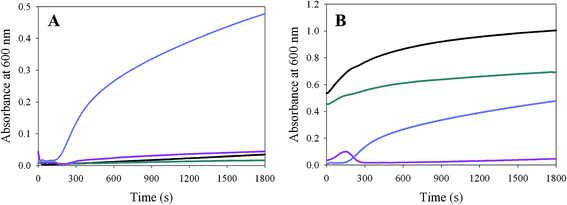
In preliminary studies, we observed that under crowded conditions, the solubility of mALAS2 (at concentration of 1 mg mL−1 and at pH 9.5 and 37 °C) was influenced by the molar concentration of the 3-([1,1-dimethyl-2-hydroxyethyl]amino)-2-hydroxypropanesulfonic acid (AMPSO) buffer. Protein precipitation was noticeable in the presence of 10 or 20% (w/v) Dextran 200 [or 20% (w/v) Ficoll 400], when we used the AMPSO buffer (pH 9.5) at a concentration of 250 mM. The molar concentration of the buffer was originally selected for conducting steady-state kinetic measurements in the presence of saturating concentrations of the fixed substrate glycine, whose concentration was maintained at 200 mM (see Materials and Methods for details). We note that in the absence of crowders, up to at least 250 mM AMPSO, pH 9.5, the buffer concentration does not significantly affect the structural properties of mALAS2alk, as discerned from fluorescence spectroscopy (data not shown). Since we did not detect protein precipitation during the far-UV measurements, when the AMPSO concentration was 20 mM, we set to examine more closely the dependence of protein solubility on the molar concentration of the buffer.To this end, we conducted turbidity measurements by following the changes in absorbance at 600 nm over a time period of 30 minutes (at pH 9.5 and 37 °C). Our analysis revealed that in a solution of 20% (w/v) Dextran 200, the solubility of mALAS2alk was strongly dependent on the molar AMPSO concentration. In fact, when the molar concentration of the AMPSO buffer was 20 or 50 mM (pH 9.5), the turbidity signal remained near baseline during the examined time interval, implicating that the enzyme remains soluble (Fig. 4A). In contrast, when the concentration of AMPSO in the crowded solution was increased to 250 mM, we noted a pronounced increase in the turbidity signal, which is indicative of protein precipitation (Fig. 4A). Importantly, the elimination of the macromolecular crowder from the solution rendered mALAS2alk soluble even at AMPSO concentrations of 250 mM (Fig. 4A).
As discerned from the turbidity measurements (Fig. 4B), the addition of 8 mM ALA, which binds to the active site of ALAS through the formation of an imine linkage,37 increased the solubility of mALAS2alk under conditions that otherwise favored precipitation of the holoenzyme (i.e., 1 mg mL−1 protein in a solution of 20% Dextran 200 and 250 mM AMPSO, pH 9.5 and at 37 °C). Furthermore, our data also indicate that mALAS2, in the presence of 20% Dextran 200, is even less soluble at pH 7.5 (250 mM K2HPO4) than at pH 9.5. In fact, at physiological pH and crowded conditions, mALAS2 precipitated out of solution immediately upon mixing. The absence of a lag phase in the turbidity progress curve and higher absorbance signal reflect the increased rate of protein precipitation at pH 7.5 (Fig. 4B). The addition of ALA to the crowded solution was not able to fully reverse the precipitation of the protein at pH 7.5, although the absorbance signal was decreased relative to that in the absence of ligand (Fig. 4B). This finding suggests that ligand binding improved the solubility of mALAS2, albeit subtly, even at physiological pH.
Effects of crowders and osmolytes on the tertiary structure of ligand-bound mALAS2alk
By monitoring the CD signal in the near-UV range (260–310 nm) we also examined the extent to which ALA binding is coupled to the folding of the tertiary structure of mALAS2alk in the presence of different cosolvents [pH 9.5 and 37 °C (Fig. 4A–F)]. In agreement with previous results27 (which were conducted in 50 mM AMPSO, pH 9.5 as opposed to the use of 250 mM AMPSO, pH 9.5 in the present study), we discerned that in the absence of cosolvents, the binding of ALA to the active site of mALAS2alk increases the level of ellipticity in the near-UV region (Fig. 5A). This indicates that ALA binding enhances the rigidity of the tertiary structure of mALAS2alk. When the spectra were recorded in the presence of 20% TMAO or 20% glycerol, we observed that the association of ALA with the enzyme did not substantially change the elliptical content in the near-UV region (Fig. 5B and C). Because the sole inclusion of osmolytes enhances the rigidity of the tertiary structure of mALAS2alk, our results suggest that the binding of ALA does not induce additional and significant augmentation in the close packing of tertiary contacts, as was the case for the loosely packed molten globule state when osmolytes were excluded from the media.In the preceding section we have shown that the solubility of the enzyme in the presence of crowders depends on the molar concentration of the AMPSO buffer (Fig. 4). The insoluble enzyme under crowded conditions produces near-UV spectra that are characterized by a negative elliptical signal (Fig. 5D and E). However, when the enzyme was equilibrated with 4 mM ALA in the presence of 20% crowder (either Dextran 200 or Ficoll 400 in 250 mM AMPSO, pH 9.5, and at 37 °C), we observed that the enzyme remained soluble, and that the near-UV CD spectra of the ALA-associated proteins became positive (Fig. 5D and E) and similar to the spectrum of the holoenzyme at pH 7.5 (Fig. S1†), whose tertiary structure is fairly ordered. These data reveal that the binding of ALA enhances the tertiary structure rigidity of the protein within the crowded media. Furthermore, because the concentration of ALA is not sufficiently high to significantly modulate the properties of the solvent in the system, our results suggest that the increased solubility of the enzyme primarily results from restrictions in conformational fluctuations that are imposed by the binding of ALA, which, in its absence, probably increase the exposure of structural regions that promote self-association into amorphous aggregates.
Effects of crowders and osmolytes on the active site PLP microenvironment
As a result of the positioning of the internal aldimine adduct (PLP-Lys313 in mALAS2) in a chiral environment, the near-UV/visible CD spectrum (490–310 nm) of mALAS2 under physiological conditions (pH 7.5 and 37 °C) is characterized by two positive dichroic bands with maxima at approximately 430 nm and 325 nm (Fig. 6). Increase in the alkalinity of the solution to pH 9.5 modifies the equilibrium distribution of the PLP-associated maxima, resulting in a profound reduction in the intensity of the 430 nm band, together with intensification and a blue-shift of the 325 nm maximum to 317 nm (Fig. 6). To probe how the PLP microenvironment of the enzyme is affected by cosolvents, we also collected CD spectra in the 310–490 nm region for mALAS2alk that was equilibrated with osmolytes or macromolecular crowders (pH 9.5 and 37 °C). Relative to the spectrum acquired under alkaline conditions and in the absence of cosolvents, the most pronounced spectral changes were seen in the presence of 20% TMAO (w/v), characterized by a red-shift of the 317 nm maximum to 325 nm, together with intensification in the ellipticity of the 430 nm band (Fig. 6A). The 317 nm maximum was also red-shifted to 321 and 330 nm upon the respective additions of 20% glycerol and Dextran 200, but without intensifying the ∼430 nm band (Fig. 6A and B). In contrast, we did not observe any significant changes in the PLP-associated CD spectrum of mALAS2alk when 20% Ficoll 400 (w/v) was included in the medium (Fig. 6B). It is possible that the cosolvent-induced modifications of the PLP chiral environment originate from structural changes that propagate to the enzymatic active site upon rigidification of the tertiary structure, while in the case of the osmolytes, direct binding to the active site might also be a contributing factor. | ||
| Fig. 6 Effects of cosolvents on the chiral environment of the internal aldimine adduct of mALAS2. Near UV-visible CD spectra collected in the presence of TMAO (purple) and glycerol (pink) are shown in (A), while in (B) are those with Ficoll 400 (pink) and Dextran 200 (purple). The concentration of all cosolvents was 20% (w/v). In both (A) and (B), the spectra measured in the absence of cosolvents at pH 7.5 (gray) and pH 9.5 (black) were originally reported in ref. 27. | ||
Effects of crowders and osmolytes on the steady-state kinetic activities of mALAS2alk
Previously, we reported that the steady-state kinetic parameters of mALAS2 were adversely affected upon an increase in the alkalinity of the buffer, with respective 40- and 250-fold reductions in the kcat and kcat/KSCoAm values measured at alkaline (pH 9.5 and 37 °C) relative to those at physiological conditions (pH 7.5 and 37 °C).27 As a continuation to these studies, here we measured the steady-state kinetic parameters of mALAS2alk (pH 9.5 and 37 °C) in the presence of various cosolvents maintained at a final concentration of 20% (Fig. S2†). In comparison to the kcat determined at pH 9.5 and in the absence of cosolvents, the addition of 20% TMAO, glycerol, Dextran 200, or Ficoll 400 resulted in respective 2.3-, 7-, 5.4-, or 5.7-fold increases in the kcat value (Table 1). Furthermore, while all cosolvents improved the specificity constant, kcat/KSCoAm, the most pronounced increase was detected in the presence of Dextran 200, mainly due to greater reduction in the KSCoAm value (Table 1). We also note that even though the inclusion of cosolvents improved the catalytic properties of mALAS2 under alkaline conditions, the kcat and kcat/KSCoAm values were still significantly reduced relative to the same constants determined at pH 7.5 and in the absence of cosolvents (Table 1).| Buffer components | kcat (min−1) | KSCoAm (μM) | kcat/KSCoAm (min−1 μM−1) |
|---|---|---|---|
| a Values determined in the absence of cosolvents originally reported in ref. 27. | |||
| Phosphate, pH 7.5a | 2.80 ± 0.10 | 16 ± 4 | 175 × 10−3 ± 50 × 10−3 |
| AMPSO, pH 9.5a | 0.07 ± 0.01 | 103 ± 32 | 0.68 × 10−3 ± 0.2 × 10−3 |
| 20% TMAO, pH 9.5 | 0.16 ± 0.02 | 52 ± 5 | 3.1 × 10−3 ± 0.3 × 10−3 |
| 20% glycerol, pH 9.5 | 0.49 ± 0.02 | 103 ± 18 | 4.8 × 10−3 ± 1 × 10−3 |
| 20% Dextran 200, pH 9.5 | 0.38 ± 0.04 | 23 ± 8 | 17 × 10−3 ± 7 × 10−3 |
| 20% Ficoll 400, pH 9.5 | 0.4 ± 0.01 | 35 ± 5 | 11 × 10−3 ± 2 × 10−3 |
Discussion
In the present study we characterized how the structural and catalytic properties of the alkaline molten globule state of mALAS2 are modulated by macromolecular crowders and osmolytes. Our data revealed that the rigidity of the tertiary structure of mALAS2alk becomes significantly enhanced in the presence of osmolytes, as discerned from the increased intensities of the near-UV CD spectra (Fig. 2A, B and 3). Because the intensity of these spectra depends on the positioning of the aromatic side chains in a chiral and rigid environment,1,38 our results suggest that the osmolytes reduce the tertiary structure fluctuations in the alkaline molten globule state and shift the conformational ensemble toward more native-like conformations. These observations are in line with several previous studies, where the effectiveness of osmolytes in reducing the conformational fluctuations of proteins was detailed. A notable example represents the implementation of NMR spin-relaxation measurements by Doan-Nguyen and Loria,39 who reported that TMAO effectively restricted the conformational motions of guanidine hydrochloride-destabilized ribonuclease A (RNase A). Furthermore, hydrogen exchange experiments had shown that TMAO can efficiently increase the protection of amide protons against solvent exchange in urea-exposed RNase A40 and cold shock protein A,41 suggesting restrictions in their conformational fluctuations. Finally, we are also aware of several studies which explicitly demonstrated TMAO to promote the ordering of the tertiary structure in different molten globular proteins.42–45The CD measurements also revealed differences between the osmolytes in their effectiveness to counter the alkaline-induced denaturation of the tertiary structure of mALAS2. In fact, among the two osmolytes that we characterized, TMAO was more effective than glycerol at stabilizing the tertiary structure of mALAS2alk, given that the intensity of the near-UV CD signal was far more pronounced in the presence of 10% TMAO (w/v) relative to the signal intensity at the same concentration of glycerol (Fig. 2A, B and 3). These results are in good agreement with previous studies which validated the increased potency of TMAO over other osmolytes, including glycerol and glycine betaine, in countering the denaturing effects of destabilizing stressors.46–49 Lastly, while in the present study, we demonstrated that the addition of glycerol and TMAO favored rigidification of the tertiary structure of the alkaline molten globule state of mALAS2 and its shift toward more native-like conformations, we note that in certain cases, the use of osmolytes had failed to favor induction of molten globular proteins into their respective native states. For example, Cremades and Sancho50 reported that the addition of sucrose at concentrations ranging from 0–1.5 M did not stabilize the native over the molten globule state of Helicobacter pylori apoflavodoxin. Hence, we speculate that the addition of osmolytes is likely to produce a dissimilar effect on the structural properties of different molten globular proteins.
In comparison to the osmolytes, the two macromolecular crowders that we examined were far less effective in their ability to rigidify the tertiary structure of mALAS2alk (Fig. 2C, D and 3). Subtle gain in near-UV elliptical signal was noted when the concentration of Ficoll 400 was increased to 10% (w/v), but upon further increase in its concentration to 20%, the spectrum intensity became comparable to that measured in the absence of cosolvent. Relative to Ficoll 400, greater increase in the near-UV elliptical signal was discerned when the concentration of the smaller macromolecular crowder Dextran 200 reached 20% (w/v). It appears that Dextran 200, which is expected to be the more effective crowder of the two because its molecular mass is closer to that of homodimeric mALAS2 (112 kDa), affects the conformational properties of the enzyme, at least partly, through volume exclusion in the system. We inferred this from the moderate intensification of the near-UV and far-UV CD spectra of mALAS2alk (Fig. 1–3) that were noted when the concentration of Dextran 200 was raised from 10 to 20%. This crowder-induced folding of secondary and tertiary elements, which proceeded in a Dextran concentration-dependent manner, is expected to be observed when crowders exert their effects on the protein structure through volume exclusion.17 In contrast to Dextran, it seems unlikely that the subtle effects of Ficoll 400 on the structural properties of mALAS2 are predominantly effected through volume exclusion. Given the unusual changes in the near-UV CD spectrum of mALAS2 that were detected in the presence of Ficoll 400, where the elliptical signal subtly increased in the presence of 10% Ficoll, but when the concentration of Ficoll was raised to 20%, the gain in elliptical intensity diminished, we believe that it seems improbable that the moderate changes in the near-UV spectrum of ALAS are predominantly effected through volume exclusion. For if they were, one would expect to observe intensification of the near-UV elliptical signal upon increases in the concentration of Ficoll 400. Since non-specific interactions between crowders and proteins are known to occur at high concentrations of proteins,17 it is plausible that at the higher concentration of Ficoll used in our study [20% (w/v)], some non-specific interactions between the crowder and ALAS occur, and these interactions are potentially destabilizing given the reduction in the near-UV ellipticity that was detected upon the increase in the concentration of Ficoll from 10 to 20%. As documented experimentally in various studies, the interactions between crowder and proteins can indeed have an adverse effect on the structural properties of proteins.51–55
Interestingly, we also observed that the addition of Dextran 200 moderately increased the intensity of the far-UV spectrum of mALAS2alk in a concentration dependent manner (Fig. 1D). These results implicate that Dextran 200 induces the folding of secondary structural elements which are only transiently present or altogether absent from the conformational ensemble under dilute conditions. Since intrinsic disorder algorithms have identified several regions within the primary sequence of mALAS2 that are predicted to be disordered,56 it is possible that some of these structure-less regions fold into stable secondary elements upon encountering limitation in the available volume when the in vitro environment becomes crowded. In line with this hypothesis, several studies had reported crowding to induce the folding of secondary structural elements in intrinsically disordered proteins with various degrees of disorder.57–59 Also, macromolecular crowders are known to restore the secondary structure content to native-like levels in proteins with induced-disorder, as it is the case in the acid-denatured states of apomyoglobin60 and cytochrome c.61 Lastly, even the secondary structure contents of predominantly ordered proteins were shown to increase when encountering limitations in the available volume as a result of crowding.62–64 All these studies indicate that the secondary structure content of predominantly ordered and disordered proteins can be modulated by the inclusion of crowders.
Even though ALAS retains catalytic activity under conditions where the holoenzyme populates the alkaline molten globule state, the increase in alkalinity adversely affects the steady-state kinetic parameters, resulting in 40- and 250-fold reductions in the kcat and kcat/KSCoAm values in comparison to those determined at physiological pH (Table 1). Our present data indicate that the inclusion of cosolvents in the media partly counters the alkali-mediated impairment of catalysis (Table 1). Relative to the values determined at pH 9.5 and 37 °C and in the absence of cosolvents, the highest increase in the kcat was measured in the presence of 20% glycerol (7-fold increase), while Dextran 200 was most effective in enhancing the specificity constant, kcat/KSCoAm (25-fold increase). In contrast, TMAO was the least effective, with respective increases of only ∼2.3- and ∼4.5-fold in the kcat and kcat/KSCoAm values.
Furthermore, in comparison to the kcat measured at pH 7.5 and 37 °C, we still observed reductions of 82% and 94% in the kcat values determined under alkaline conditions (pH 9.5 and 37 °C) and in the presence of 20% glycerol or 20% TMAO, respectively (Table 1). Even if we simplistically assume that the entire increase in the turnover rate stems from the ability of the osmolytes to enhance the rigidity of the tertiary structure and reduce the level of conformational fluctuations in the alkaline molten globule state, the kcat values are still significantly lower than the turnover rate determined at physiological pH. These findings strongly implicate that the diminished catalytic prowess of the enzyme cannot be solely attributed to the lack of ordered tertiary structure, and that the change in the ionization state of important catalytic centers also adversely affects catalysis. In fact, during the ALAS-catalyzed reaction cycle, general acid–base chemistry is extensively implemented in the formation of various reaction intermediates,29 and any perturbation in the ionization state of the reaction groups that participate in these reactions is likely to attenuate the catalytic effect of the enzyme. For example, Hunter and Ferreira65 have identified mALAS2 Lys313 as the general base catalyst that catalyzes the removal of the pro-R proton of glycine upon the formation of the initial quinonoid intermediate, and this same residue was implicated as the acid catalyst during the conversion of an enol intermediate into the ALA-external aldimine complex.66 Furthermore, based on structural analyses and homology modeling, it was inferred that His207 acts as a general acid catalyst during the decarboxylation of the 2-amino-3-ketoadipate intermediate into the quinonoid intermediate II.66 Active site residues with ionizable side groups are also involved in the coordination of PLP and in the enhancement of its electron-withdrawing properties. Among these, Asp279 and His282, whose side chains respectively interact with the PLP pyridine nitrogen (in order to increase its pKa above physiological values)67 and with the phenolic oxygen (through a hydrogen bonds which is important in maintaining the protonation of the imine group),68 were shown to be indispensable for catalysis. Finally, the steady-state kinetic parameters,69 as well as the pre-steady-state rates for the formation and decay of the second quinonoid intermediate66 are adversely affected upon increases in alkalinity. Based on these previous results together with the data presented here, we conclude that the reduction in the turnover rate that is observed in the alkaline molten globule state does not solely originate from disruption in the rigidity of the tertiary structure, and that the change in the ionization state of catalytically important residues is also a significant adverse factor.
We note that the inclusion of macromolecular crowders in the buffer also led to enhanced kcat values (Table 1), even though their ability to stabilize the tertiary fluctuations of mALAS2alk was far less pronounced in comparison to the osmolytes (Fig. 2 and 3). While presently we cannot confidently predict the molecular details through which the crowders modulate the catalytic turnover of mALAS2, we believe that future studies should be directed toward examining the connection between ALAS solvation and catalysis. As inferred from the propensity of the enzyme to precipitate out of solution upon increases in the concentration of AMPSO within the crowded solution (see Fig. 4 and 5), it is obvious that the macromolecular crowders profoundly affect the solvation of ALAS. In fact, macromolecular crowders such as PEG and inert proteins are known to affect protein hydration and hydration dynamics.70,71 Because at physiological pH the rate limiting step of the reaction is dominated by segmental motions that guide the release of ALA from the active site,66 and because protein dynamics can be strongly coupled with the dynamics of water,72 it is plausible that the changes in the hydration of ALAS that occur in the presence of crowders can influence the rates of conformational motions that are closely linked to catalysis. Furthermore, it is clear from crystallographic73 and substrate protection studies74 that the association of succinyl-CoA with the enzyme minimizes the exposure of the active site interior to the solvent, partly due to stabilization of the closed conformation and occupancy of the narrow channel by the pantetheine moiety. Hence, the accessibility of the solvent to the active site is severely impeded during the catalytic cycle. If there is a large energetic penalty associated with the desolvation of the active site upon initiation of catalysis and if under alkaline conditions, the chemical transformation of substrates into products is at least partly rate limiting, then the changes in the levels of hydration that ALAS undergoes upon crowding might lower this potentially unfavorable desolvation penalty and influence the rate of catalysis.
Our results also indicate that the solubility of mALAS2alk under crowded conditions is strongly dependent on the AMPSO molar concentration (Fig. 4). In particular, mALAS2alk was rendered insoluble when in the presence of 20% Dextran 200 (w/v), the molar concentration of AMPSO was increased to 250 mM (at pH 9.5 and 37 °C). Since the solubility of proteins depends on the availability of water molecules to hydrate their solvent accessible surfaces,75 we postulate that the insolubility of mALAS2alk under the above described conditions results from changes in the levels of hydration of the enzyme. In other words, because of the increased concentration of cosolvents, there is a reduction in the water molecules available to hydrate the surface of mALAS2, leading to the self-association of the enzyme into amorphous aggregates.
Furthermore, when the pH of the crowded media was lowered to 7.5 (20% Dextran 200 (w/v) and 250 mM K2HPO4, pH 7.5 and at 37 °C), the apparent lag phase in the turbidity measurement curve disappeared, indicating that the enzyme is even less soluble at physiological than at alkaline pH (Fig. 4). This reduced solubility of mALAS2 at physiological pH can be rationalized if we consider that proteins are least soluble near their pI values,75,76 where due to the reduction in their net charge, self-association between protein molecules is more likely to occur. Because the theoretical pI value of mALAS2 is 6.62, the enzyme is expected to be less soluble at pH 7.5 than at pH 9.5. Our present findings corroborate the validity of this prediction.
Interestingly, we observed that the binding of ALA rendered mALAS2 soluble under crowded and alkaline conditions, which otherwise promoted precipitation of the holoenzyme (Fig. 4). Since the binding of ALA enhances the rigidity of the tertiary structure and minimizes the extent of conformational fluctuations in the alkaline molten globule state (Fig. 5), the increased solubility is likely to be mediated by reduction in the exposure of structural region that promote self-association of the enzyme into amorphous aggregates. However, we also noted that at pH 7.5 (20% Dextran 200 and 250 mM K2HPO4), ALA binding did not fully reverse the precipitation of the enzyme out of solution, albeit the extent of solubility was increased relative to that of the holoenzyme, as discerned from the turbidity measurements (Fig. 4).
While it is premature to make any general conclusions, we believe that it is worth examining if protein solubility (particularly that of enzymes) can be increased by introducing natural ligands that modulate the conformational properties of the protein. If so, then this might serve as another strategy that can be used to improve protein solubility in addition to those that implement substitution of surface exposed hydrophobic residues with hydrophilic ones75 and enrich the solution with the addition of amino acids cosolvents.77
Conclusions
In the present study, we have shown that the rigidity of the tertiary structure of the alkaline molten globule state of mALAS2 becomes enhanced in the presence of osmolytes. The crowders, in contrast, were far less effective in rigidifying the tertiary structure, although, Dextran 200, in a concentration-dependent manner, induced folding of secondary structural elements that are either transiently present or altogether absent from the conformational ensemble under dilute conditions. We also validated that, through ligand induced enhancement of the tertiary structure rigidity, the solubility of mALAS2alk increased under conditions that favored precipitation of the holoenzyme. Lastly, even though all cosolvents increased the kcat value of mALAS2alk, the turnover rate remained lower than that under physiological conditions, implicating that the change in the ionization state of important catalytic centers adversely affects the ALAS-catalyzed reaction. We conclude that, within the intracellular environment, the crowding of macromolecules and the accumulation of osmolytes can modulate the structural properties of at least some molten globular proteins.Abbreviations
| ALAS | 5-Aminolevulinate synthase |
| ALAS2 | Erythroid-specific isoform of 5-aminolevulinate synthase |
| AMPSO | 3-([1,1-Dimethyl-2-hydroxyethyl]amino)-2-hydroxypropanesulfonic acid |
| mALAS2 | Murine erythroid-specific isoform of 5-aminolevulinate synthase |
| mALAS2alk | Alkaline molten globule state of mALAS2 as defined at pH 9.5 and 37 °C |
| PLP | Pyridoxal 5′-phosphate |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| CD | Circular dichroism |
| TCA | Trichloroacetic acid |
| TMAO | N-Trimethylamine oxide |
Acknowledgements
This work was supported by a grant from the American Heart Association (#13GRNT16970019).References
- O. B. Ptitsyn, Adv. Protein Chem., 1995, 47, 83–229 CrossRef CAS PubMed
.
- D. A. Dolgikh, R. I. Gilmanshin, E. V. Brazhnikov, V. E. Bychkova, G. V. Semisotnov, S. Venyaminov and O. B. Ptitsyn, FEBS Lett., 1981, 136, 311–315 CrossRef CAS PubMed
.
- K. Kuwajima, FASEB J., 1996, 10, 102–109 CAS
.
- M. Ohgushi and A. Wada, FEBS Lett., 1983, 164, 21–24 CrossRef CAS PubMed
.
- Y. Goto and S. Nishikiori, J. Mol. Biol., 1991, 222, 679–686 CrossRef CAS PubMed
.
- Y. Luo and R. L. Baldwin, Biochemistry, 2001, 40, 5283–5289 CrossRef CAS PubMed
.
- P. A. Jennings and P. E. Wright, Science, 1993, 262, 892–896 CAS
.
- D. A. Dolgikh, A. P. Kolomiets, I. A. Bolotina and O. B. Ptitsyn, FEBS Lett., 1984, 165, 88–92 CrossRef CAS PubMed
.
- L. A. Morozova, D. T. Haynie, C. Arico-Muendel, H. Van Dael and C. M. Dobson, Nat. Struct. Biol., 1995, 2, 871–875 CrossRef CAS PubMed
.
- S. Maldonado, M. A. Jimenez, G. M. Langdon and J. Sancho, Biochemistry, 1998, 37, 10589–10596 CrossRef CAS PubMed
.
- M. P. Irun, M. M. Garcia-Mira, J. M. Sanchez-Ruiz and J. Sancho, J. Mol. Biol., 2001, 306, 877–888 CrossRef CAS PubMed
.
- S. M. Nabuurs, A. H. Westphal and C. P. van Mierlo, J. Am. Chem. Soc., 2009, 131, 2739–2746 CrossRef CAS PubMed
.
- Y. Goto and A. L. Fink, Biochemistry, 1989, 28, 945–952 CrossRef CAS PubMed
.
- S. B. Zimmerman and S. O. Trach, J. Mol. Biol., 1991, 222, 599–620 CrossRef CAS PubMed
.
- R. J. Ellis, Trends Biochem. Sci., 2001, 26, 597–604 CrossRef CAS PubMed
.
- A. P. Minton, J. Biol. Chem., 2001, 276, 10577–10580 CrossRef CAS PubMed
.
- I. M. Kuznetsova, K. K. Turoverov and V. N. Uversky, Int. J. Mol. Sci., 2014, 15, 23090–23140 CrossRef PubMed
.
- P. H. Yancey, J. Exp. Biol., 2005, 208, 2819–2830 CrossRef CAS PubMed
.
- D. W. Bolen and G. D. Rose, Annu. Rev. Biochem., 2008, 77, 339–362 CrossRef CAS PubMed
.
- K. Gekko and S. N. Timasheff, Biochemistry, 1981, 20, 4667–4676 CrossRef CAS PubMed
.
- Y. Liu and D. W. Bolen, Biochemistry, 1995, 34, 12884–12891 CrossRef CAS PubMed
.
- B. J. Bennion and V. Daggett, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 6433–6438 CrossRef CAS PubMed
.
- P. Bruzdziak, A. Panuszko and J. Stangret, J. Phys. Chem. B, 2013, 117, 11502–11508 CrossRef CAS PubMed
.
- L. Breydo, A. E. Sales, L. Ferreira, O. Fedotoff, M. P. Shevelyova, S. E. Permyakov, K. G. Kroeck, E. A. Permyakov, B. Y. Zaslavsky and V. N. Uversky, Arch. Biochem. Biophys., 2015, 570, 66–74 CrossRef CAS PubMed
.
- S. Paul and G. N. Patey, J. Am. Chem. Soc., 2007, 129, 4476–4482 CrossRef CAS PubMed
.
- L. Larini and J. E. Shea, J. Phys. Chem. B, 2013, 117, 13268–13277 CrossRef CAS PubMed
.
- B. M. Stojanovski, L. Breydo, G. A. Hunter, V. N. Uversky and G. C. Ferreira, Biochim. Biophys. Acta, 2014, 1844, 2145–2154 CrossRef CAS PubMed
.
- E. J. Fratz, B. M. Stojanovski and G. C. Ferreira, in Handbook of Porphyrin Science, ed. G. C. Ferreira, K. M. Kadish, K. M. Smith and R. Guilard, World Scientific Publishing Co., Singapore, 2013, vol. 26, pp. 1–78 Search PubMed
.
- G. A. Hunter and G. C. Ferreira, Biochim. Biophys. Acta, 2011, 1814, 1467–1473 CrossRef CAS PubMed
.
- Y. Li and G. Jing, J. Biochem., 2000, 128, 739–744 CrossRef CAS PubMed
.
- V. N. Uversky, V. P. Kutyshenko, N. Protasova, V. V. Rogov, K. S. Vassilenko and A. T. Gudkov, Protein Sci., 1996, 5, 1844–1851 CrossRef CAS PubMed
.
- K. Vamvaca, B. Vogeli, P. Kast, K. Pervushin and D. Hilvert, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12860–12864 CrossRef CAS PubMed
.
- B. Zambelli, M. Stola, F. Musiani, K. De Vriendt, B. Samyn, B. Devreese, J. Van Beeumen, P. Turano, A. Dikiy, D. A. Bryant and S. Ciurli, J. Biol. Chem., 2005, 280, 4684–4695 CrossRef CAS PubMed
.
- G. C. Ferreira and H. A. Dailey, J. Biol. Chem., 1993, 268, 584–590 CAS
.
- B. M. Stojanovski, G. A. Hunter, M. Jahn, D. Jahn and G. C. Ferreira, J. Biol. Chem., 2014, 289, 22915–22925 CrossRef CAS PubMed
.
- L. F. Lien and D. S. Beattie, Enzyme, 1982, 28, 120–132 CAS
.
- B. M. Stojanovski and G. C. Ferreira, J. Biol. Chem., 2015, 290, 30750–30761 CrossRef CAS PubMed
.
- S. M. Kelly, T. J. Jess and N. C. Price, Biochim. Biophys. Acta, 2005, 1751, 119–139 CrossRef CAS PubMed
.
- V. Doan-Nguyen and J. P. Loria, Protein Sci., 2007, 16, 20–29 CrossRef CAS PubMed
.
- Y. Qu and D. W. Bolen, Biochemistry, 2003, 42, 5837–5849 CrossRef CAS PubMed
.
- V. A. Jaravine, K. Rathgeb-Szabo and A. T. Alexandrescu, Protein Sci., 2000, 9, 290–301 CrossRef CAS PubMed
.
- R. L. Kingston, L. S. Gay, W. S. Baase and B. W. Matthews, J. Mol. Biol., 2008, 379, 719–731 CrossRef CAS PubMed
.
- F. Georgescauld, I. Mocan, M. L. Lacombe and I. Lascu, FEBS Lett., 2009, 583, 820–824 CrossRef CAS PubMed
.
- M. Kjaergaard, K. Teilum and F. M. Poulsen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12535–12540 CrossRef CAS PubMed
.
- C. B. Millard, V. L. Shnyrov, S. Newstead, I. Shin, E. Roth, I. Silman and L. Weiner, Protein Sci., 2003, 12, 2337–2347 CrossRef CAS PubMed
.
- S. Ortiz-Costa, M. M. Sorenson and M. Sola-Penna, Arch. Biochem. Biophys., 2002, 408, 272–278 CrossRef CAS PubMed
.
- A. T. Russo, J. Rosgen and D. W. Bolen, J. Mol. Biol., 2003, 330, 851–866 CrossRef CAS PubMed
.
- P. H. Yancey, M. D. Rhea, K. M. Kemp and D. M. Bailey, Cell. Mol. Biol., 2004, 50, 371–376 CAS
.
- R. Kumar, J. M. Serrette and E. B. Thompson, Arch. Biochem. Biophys., 2005, 436, 78–82 CrossRef CAS PubMed
.
- N. Cremades and J. Sancho, Biophys. J., 2008, 95, 1913–1927 CrossRef CAS PubMed
.
- D. L. Zhang, L. J. Wu, J. Chen and Y. Liang, Acta Biochim. Biophys. Sin., 2012, 44, 703–711 CrossRef CAS PubMed
.
- A. P. Schlesinger, Y. Wang, X. Tadeo, O. Millet and G. J. Pielak, J. Am. Chem. Soc., 2011, 133, 8082–8085 CrossRef CAS PubMed
.
- Y. Wang, M. Sarkar, A. E. Smith, A. S. Krois and G. J. Pielak, J. Am. Chem. Soc., 2012, 134, 16614–16618 CrossRef CAS PubMed
.
- M. Sarkar, A. E. Smith and G. J. Pielak, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 19342–19347 CrossRef CAS PubMed
.
- W. B. Monteith and G. J. Pielak, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 11335–11340 CrossRef CAS PubMed
.
- B. M. Stojanovski, L. Breydo, V. N. Uversky and G. C. Ferreira, Biochim. Biophys. Acta, 2016, 1864, 441–452 CrossRef CAS PubMed
.
- J. Aden and P. Wittung-Stafshede, Biochemistry, 2014, 53, 2271–2277 CrossRef PubMed
.
- M. M. Dedmon, C. N. Patel, G. B. Young and G. J. Pielak, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12681–12684 CrossRef CAS PubMed
.
- A. Roque, I. Ponte and P. Suau, Biophys. J., 2007, 93, 2170–2177 CrossRef CAS PubMed
.
- P. McPhie, Y. S. Ni and A. P. Minton, J. Mol. Biol., 2006, 361, 7–10 CrossRef CAS PubMed
.
- K. Sasahara, P. McPhie and A. P. Minton, J. Mol. Biol., 2003, 326, 1227–1237 CrossRef CAS PubMed
.
- L. Stagg, S. Q. Zhang, M. S. Cheung and P. Wittung-Stafshede, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 18976–18981 CrossRef CAS PubMed
.
- D. Homouz, M. Perham, A. Samiotakis, M. S. Cheung and P. Wittung-Stafshede, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11754–11759 CrossRef CAS PubMed
.
- M. Perham, L. Stagg and P. Wittung-Stafshede, FEBS Lett., 2007, 581, 5065–5069 CrossRef CAS PubMed
.
- G. A. Hunter and G. C. Ferreira, Biochemistry, 1999, 38, 3711–3718 CrossRef CAS PubMed
.
- G. A. Hunter, J. Zhang and G. C. Ferreira, J. Biol. Chem., 2007, 282, 23025–23035 CrossRef CAS PubMed
.
- J. Gong, G. A. Hunter and G. C. Ferreira, Biochemistry, 1998, 37, 3509–3517 CrossRef CAS PubMed
.
- T. D. Turbeville, J. Zhang, G. A. Hunter and G. C. Ferreira, Biochemistry, 2007, 46, 5972–5981 CrossRef CAS PubMed
.
- J. Zhang, A. V. Cheltsov and G. C. Ferreira, Protein Sci., 2005, 14, 1190–1200 CrossRef CAS PubMed
.
- C. Reid and R. P. Rand, Biophys. J., 1997, 72, 1022–1030 CrossRef CAS PubMed
.
- J. T. King, E. J. Arthur, C. L. Brooks III and K. J. Kubarych, J. Am. Chem. Soc., 2014, 136, 188–194 CrossRef CAS PubMed
.
- M. C. Bellissent-Funel, A. Hassanali, M. Havenith, R. Henchman, P. Pohl, F. Sterpone, D. van der Spoel, Y. Xu and A. E. Garcia, Chem. Rev., 2016, 116, 7673–7697 CrossRef CAS PubMed
.
- I. Astner, J. O. Schulze, J. van den Heuvel, D. Jahn, W. D. Schubert and D. W. Heinz, EMBO J., 2005, 24, 3166–3177 CrossRef CAS PubMed
.
- B. M. Stojanovski and G. C. Ferreira, FEBS Open Bio, 2015, 5, 824–831 CrossRef CAS PubMed
.
- S. R. Trevino, J. M. Scholtz and C. N. Pace, J. Pharm. Sci., 2008, 97, 4155–4166 CrossRef CAS PubMed
.
- K. L. Shaw, G. R. Grimsley, G. I. Yakovlev, A. A. Makarov and C. N. Pace, Protein Sci., 2001, 10, 1206–1215 CrossRef CAS PubMed
.
- A. P. Golovanov, G. M. Hautbergue, S. A. Wilson and L. Y. Lian, J. Am. Chem. Soc., 2004, 126, 8933–8939 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22533k |
| ‡ Current address: Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, St Louis, MO, 63104, USA. |
| This journal is © The Royal Society of Chemistry 2016 |