Palladium nanoparticles supported on a titanium dioxide cellulose composite (PdNPs@TiO2–Cell) for ligand-free carbon–carbon cross coupling reactions†
Sanjay Jadhava,
Ashutosh Jagdaleb,
Santosh Kamblec,
Arjun Kumbhar*b and
Rajshri Salunkhe*a
aDepartment of Chemistry, Shivaji University, Kolhapur, 416004, M.S., India
bDepartment of Chemistry, Padmabhushan Dr Vasantraodada Patil College, Tasgaon, (Sangli) Maharashtra 416312, India. E-mail: arjun2win@yahoo.co.in; Fax: +91 2346 250665; Tel: +91 2346 250575
cDepartment of Chemistry, Yashvantrao Chavan Institute of Science, Satara, Maharashtra 415001, India
First published on 11th December 2015
Abstract
Well-dispersed non-spherical PdNPs with a diameter of 39–45 nm supported on a TiO2–cellulose composite (PdNPs@TiO2–Cell) can be synthesized by a simple and clean route. The catalyst was well characterized by XRD, FE-SEM, EDS, and TEM techniques. The PdNPs have good dispersity on the TiO2–Cell support. This results in excellent catalytic activities for the synthesis of biphenyls, acrylates, acetylenes and prochiral ketones using low Pd loading (1 mol%) at comparatively low temperature. The effects of the nature and amount of bases, nature of solvents, amount of catalyst and the reaction temperature on the activity of PdNPs@TiO2–Cell were thoroughly investigated. The catalyst showed at least four times reusability without decrease in catalytic activity.
1. Introduction
Transition metal catalysis especially palladium catalyzed cross coupling reactions of aromatic halides in the presence of various nucleophiles is strategically important in organic synthesis. It has been widely used for the synthesis of a diverse array of biphenyls, acrylates, acetylenes and prochiral ketones by C–C cross coupling reactions. These compounds have profound importance in chemical, pharmaceutical and biochemical industries.1 Additionally, such compounds are also present in many natural as well as biologically active compounds,2 and are especially interesting in applications for organic light-emitting diodes and chemiluminescence detection systems.3 These compounds have been mostly synthesized by palladium catalyzed Suzuki–Miyaura,4 Mizoroki–Heck,5 Heck–Matsuda,6 Sonogashira–Hagihara,7 and carbonylative cross-coupling reactions.8 Recently, this area of research has attracted great interest because of its high compatibility to a wide variety of functional groups under mild reaction conditions.Though, most of these transformations have been extensively investigated by homogeneous palladium complexes in solution.9 The separation of metal catalysts from the reaction mixture and their reuse is highly desirable from economical and environmentally point of view.10 Additionally, the homogeneous Pd complexes also undergo deactivation due to the aggregation of Pd during the reactions. In this context, heterogeneous catalysts particularly, the PdNPs supported on suitable solid support has found immense importance for many cross coupling reactions.11 This strategy increases the catalytic activity of Pd and also reduces the amount of metal required for the reaction.12 Several oxides have been used as a support for PdNPs,13 because moderate to high dispersions was obtained on these oxides due to favorable metal–support interactions.14 Out of these oxides TiO2 based materials have found potential applications across many different areas.15 In recent years much like the noble metal nanoparticles, PdNPs supported TiO2 and Pd supported TiO2 core shell catalysts have seen an extensive amount of research in methanol reforming,16 hydrogenation,17 and photocatalysis.18
Biopolymers such as alginate, chitosan, starch, and cellulose has been developed as a most attractive support for immobilization of many Pd catalysts.19 The extensive number of –OH groups present in cellulose can facilitate the complexation of TiO2 to the molecular matrix, and play a significant role in guiding the organization of TiO2 among cellulose molecules. In addition to this, the use of cellulose has several key advantages, like no additional reducing agents are required.20 Cellulose also avoids the aggregations of PdNPs, as it acts as the protecting agent similar to other biopolymers.21 There is binding interaction between cellulose and the metal nanoparticles which provides a platform to PdNPs and helps to stabilize Pd as that of hydroxyl group containing solvents.22–24 As a result of this there is very less leaching of metal into solution. In addition it is possible to carry out reactions in aqueous medium due to the fine dispersibility of cellulose in water.25 Hybrid organic–inorganic composites have received much attention during recent years because of their unique properties.26 Due to these properties there is easy diffusion of reactants and reagents inside the pores of materials. They can be modified in the form of hybrid composites by easy processing with conventional techniques with good thermal and chemical stability.
Recently we showed that Pd supported on alumina cellulose composite (Pd@Al2O3–Cell catalyst) yields various biphenyls in good to excellent yields in aqueous medium.27 In continuation of our efforts to use natural feedstock such as carbon,28 agarose29 and chitosan30 for immobilization of Pd catalyst, herein we report an efficient method for the synthesis of PdNPs dispersed on TiO2 cellulose composite (PdNPs@TiO2–Cell). The prepared catalyst was well characterized by different techniques and applied for the synthesis of various biphenyls, acrylates, prochiral ketones and acetylenes using low Pd loading (1 mol%) at comparatively low temperatures.
2. Results and discussion
2.1 The preparation of PdNPs@TiO2–Cell catalyst
The preparation of PdNPs@TiO2–Cell catalyst is quite straightforward. TiO2–Cell composite31 obtained from cellulose and TiCl4 was stirred with Pd(OAc)2 in ethanol at 50 °C temperature as described in the experimental procedure affording the immobilized PdNPs on TiO2–Cell composite. The composite turned light gray after 4 h indicating the formation of PdNPs.2.2 The characterization of PdNPs@TiO2–Cell catalyst
The amount of Pd in the catalyst was 2.9 wt% as given by ICP-AES. The field emission scanning electron microscopy (FE-SEM) images of PdNPs@TiO2–Cell is shown in Fig. 1a and b. The SEM image shows the fibrous morphology of the catalyst. In EDS images (Fig. 1c and d) the bright points on the surface are due to the characteristic emission of the Ti. The EDS metal mapping shows that TiO2 was uniformly covered on the fibrous surface of the cellulose. EDS spectrum (Fig. 1e) also shows that palladium is exists in the composite along with titanium, oxygen. This also confirms the successful grafting of the Pd and TiO2 on cellulose.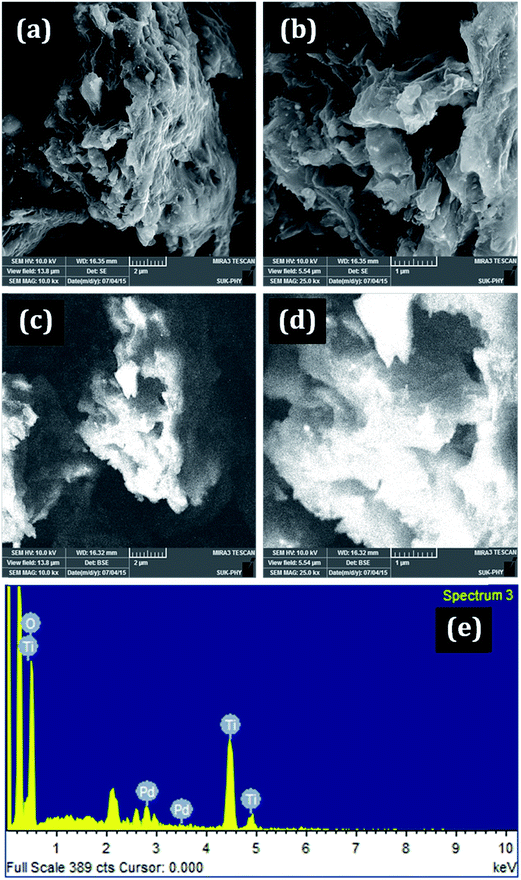 | ||
| Fig. 1 FE-SEM images of PdNPs@TiO2–Cell; SEM images (a and b), back scattering images (c and d), and EDS spectrum (e). | ||
The TEM micrographs of the catalyst are depicted in Fig. 2. It can be seen that non-spherical shaped PdNPs of 39–45 nm were homogeneously dispersed on composite. As shown in figure, the PdNPs are non-spherical and contain a large number of sharp corners, and edge sites. The surface atoms at these sites are physically unstable and are chemically active.
The XRD analysis of cellulose and catalyst (Fig. 3) shows that the coating of oxide is exclusively amorphous. This was indicated by no other diffraction peaks in XRD spectrum of the catalyst. The X-ray diffraction pattern also confirmed the formation of highly crystalline PdNPs.
2.3 The activity of PdNPs@TiO2–Cell catalyst for Suzuki–Miyaura coupling reaction
To test the activity of catalyst and effect of various parameters, we first carried out Suzuki–Miyaura cross-coupling reaction between 4-bromobenzophenone and phenylboronic acid (Scheme 1).To examine the effect of bases, series of bases were taken into consideration for the coupling between 4-bromobenzophenone and phenylboronic acid in 95% ethanol at 80 °C temperature (Table 1) using 1 mol% catalyst. The most common and inexpensive base K2CO3 was found to be most effective (Table 1, entry 7). Surprisingly, K3PO4, NaOAc, Na2CO3, and Cs2CO3 were found to be less effective (Table 1, entries 1–4). Though organic base Et3N showed good conversion (Table 1, entry 6), pyridine was completely failed to convert 4-bromobenzophenone into corresponding coupling product (Table 1, entry 5). We then studied the effect of quantity of base by carrying reactions using 0.5 to 3 equivalents of K2CO3 (Table 1, entries 7–10). When the reaction is carried out without K2CO3, no product was observed indicating that the base is known to activate32 phenylboronic acid (Table 1, entry 11). However, our results indicated that for this catalytic system base is not needed for the activation of phenylboronic acid, and its only role is to neutralize the boric acid33 as one equivalent base showed good conversion (90% yield) in 1 h (Table 1, entry 8). Thus, either a surface hydroxyl groups of TiO2 hydrolyzes the boronic acid to boric acid as reported for gold34 and/or to the cerium oxide.35
| Entry | Base (mmol) | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-bromobenzophenone (1.0 mmol), phenyl boronic acid (1.1 mmol), catalyst (1 mol%), base (0 to 3 mmol), 95% ethanol (5 mL), at 80 °C. Values in the parentheses are yields after 1 h.b Isolated yields after column chromatography. | |||
| 1 | K3PO4 (2) | 6 | 74 (55) |
| 2 | NaOAc (2) | 5 | 70 (50) |
| 3 | Na2CO3(2) | 5 | 82 (70) |
| 4 | Cs2CO3 (2) | 6 | 80 (65) |
| 5 | Pyridine (2) | 10 | Trace |
| 6 | Et3N (2) | 7 | 85 (75) |
| 7 | K2CO3 (2) | 5 | 90 (90) |
| 8 | K2CO3 (1) | 5 | 90 (90) |
| 9 | K2CO3 (0.5) | 6 | 50 (50) |
| 10 | K2CO3 (3) | 6 | 90 (90) |
| 11 | Base free | 12 | No reaction |
The screening of the solvents for model reaction showed that catalyst gave quantitative yield (90%) in 95% ethanol within 1 h (Table 2, entry 1). Unfortunately catalyst was less active in water (Table 2, entry 4), but showed moderate activity in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture (Table 2, entries 2 and 3). We then examined the activity of catalyst in DMF and DMF
H2O mixture (Table 2, entries 2 and 3). We then examined the activity of catalyst in DMF and DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture. The results showed that PdNPs@TiO2–Cell was less active as compared to our previously reported Pd@Al2O3–Cell catalyst27 in DMF and DMF
H2O mixture. The results showed that PdNPs@TiO2–Cell was less active as compared to our previously reported Pd@Al2O3–Cell catalyst27 in DMF and DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture. Use of 95% ethanol renders this protocol quite practical to large scale synthesis considering its economic and environmental point of view.
H2O mixture. Use of 95% ethanol renders this protocol quite practical to large scale synthesis considering its economic and environmental point of view.
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-bromobenzophenone (1.0 mmol), phenyl boronic acid (1.1 mmol), catalyst (1 mol%), K2CO3 (1.0 mmol), solvent (5 mL) at 80 °C.b Isolated yields after column chromatography. | |||
| 1 | 95% EtOH | 1 | 90 |
| 2 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (80 H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
6 | 70 |
| 3 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50 H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
6 | 40 |
| 4 | H2O | 6 | 25 |
| 5 | DMF | 6 | 80 |
| 6 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (80 H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
6 | 60 |
| 7 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50 H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
6 | 34 |
In order to evaluate the potential of the catalyst, the model reaction was run with changing the amount of catalyst. Lowering the palladium concentration might effectively suppress agglomeration of Pd as observed in ligand-free Suzuki–Miyaura coupling reaction by Pd supported zeolite.36 The results shown in Table 3 indicated that among the different Pd loadings 1 mol% proved to be the best and was chosen as the optimum loading for further study.
| Entry | Amount of catalyst (mol%) | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-bromobenzophenone (1.0 mmol), phenyl boronic acid (1.1 mmol), catalyst (0.25 to 2 mol%), K2CO3 (1.0 mmol), 95% ethanol (5 mL), room temperature to 80 °C.b Isolated yields after column chromatography.c Reaction using Pd@TiO2.d Reaction using Pd@cellulose. | ||||
| 1 | 0.25 | 80 | 1 | 10 |
| 2 | 0.50 | 80 | 1 | 70 |
| 3 | 0.75 | 80 | 1 | 70 |
| 4 | 1.00 | 80 | 1 | 90 |
| 5 | 1.25 | 80 | 1 | 90 |
| 6 | 1.50 | 80 | 1 | 90 |
| 7 | 2.00 | 80 | 1 | 90 |
| 8 | 1.00 | r.t. | 12 | 20 |
| 9 | 1.00 | 40 | 12 | 45 |
| 10 | 1.00 | 60 | 12 | 80 |
| 11 | 1.00 | 80 | 12 | 20c |
| 12 | 1.00 | 80 | 12 | 15d |
To study the effect of temperature on catalytic activity, the model reaction was carried out at different temperatures (Table 3, entries 8–10). The results indicated that the reaction temperature is crucial to the catalytic efficiency. Increasing the reaction temperature from room temperature to 60 °C, the yield of the product gradually increases from 20 to 80%. While, significant acceleration in rate was observed at 80 °C, affording a quantitative yield of the desired product in 1 h (Table 3, entry 4). It was observed that PdNPs@TiO2–Cell is more active as compared to Pd@TiO2 and Pd@Cellulose (Table 3, entries 11 and 12). This may be due to more diffusion of substrate and distribution of PdNPs over composite as compared to pure support.
To expand the substrate scope of the arylation, we performed a number of corresponding transformations using substituted aryl halides and aryl boronic acids using K2CO3 as base at 80 °C in 95% ethanol using 1 mol% catalyst. Several functional groups such as OCH3, COCH3, PhCO, and heterocyclic moieties were reacted smoothly giving the corresponding coupling products in good yields (Table 4). The position of substituents on the aromatic ring of aryl halides did not have any appreciable effect on progress of the reaction. We next turned our attention towards ortho substitution by coupling sterically hindered bromomesitylene with phenyl boronic acid. In this case good yield (72%) was achieved in 6 h (Table 4, entry 5). The C–X bond strength affects the activity towards the Suzuki–Miyaura reaction as the general cross coupling reactions follows reactivity order of C–I > C–Br > C–Cl. The main drawback of the many Pd mediated Suzuki–Miyaura cross-coupling reaction is that only aryl iodides and aryl bromides can be used efficiently. While aryl chlorides are either inert or gave very less conversion, because of the stronger C–Cl bond which is in fact responsible for the slower reaction rate of aryl chlorides.37 Unfortunately, coupling of chlorobenzene as well as 4-chlorobenzophenone with phenyl boronic acid was inefficient under optimized reaction conditions (Table 4, entries 14 and 15).
| Entry | Aryl halide | Arylboronic acid | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halides (1.0 mmol), arylboronic acids (1.1 mmol), catalyst (1 mol%), K2CO3 (1.0 mmol), 95% ethanol (5 mL) at 80 °C.b Isolated yields after column chromatography. | |||||
| 1 | 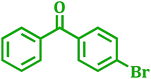 |
 |
 |
1 | 90 |
| 2 | 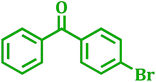 |
 |
 |
2 | 85 |
| 3 |  |
 |
 |
4 | 81 |
| 4 |  |
 |
 |
2.5 | 89 |
| 5 |  |
 |
 |
6 | 72 |
| 6 |  |
 |
 |
3.5 | 90 |
| 7 |  |
 |
 |
4 | 82 |
| 8 |  |
 |
 |
6 | 80 |
| 9 |  |
 |
 |
6 | 86 |
| 10 |  |
 |
 |
1 | 90 |
| 11 |  |
 |
 |
1 | 85 |
| 12 |  |
 |
 |
1 | 80 |
| 13 |  |
 |
 |
2 | 88 |
| 14 |  |
 |
 |
12 | 50 |
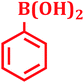
| 15 |  |
 |
 |
12 | 36 |
2.4 The activity of PdNPs@TiO2–Cell catalyst for Suzuki–Miyaura coupling reactions of arenediazonium salt
There are two main problems associated with use of aryl halides in many Pd catalyzed cross-coupling reactions. Firstly, most of aryl halides are insoluble in water hence it is very difficult to carry out reactions in aqueous medium. Secondly aryl halides undergo homocoupling38 many times hence separation and purification of products become tedious. One of the best way to overcome these drawbacks is the use of diazonium salts as substitute for aryl halides as they showed excellent reactivity towards cross coupling reactions.39 The diazonium salts are generally crystalline solids and show high solubility in water. They can be easily prepared from aromatic amines by diazotization in water.40 Furthermore arenediazonium salts undergo easy oxidative addition41 during the mechanistic cycle to generate active cationic aryl-Pd(II) intermediate. In addition the ligand-free palladium-catalyzed coupling reactions of diazonium salts do not undergo N2 elimination as observed in phosphine assisted cross-coupling reactions.42 In this connection we used arenediazonium salts for ligand-free Suzuki–Miyaura reactions using 1 mol% catalyst, K2CO3 as a base in water at room temperature (Table 5). Regardless of electron-rich or electron-poor substituents present on the phenyl rings, all the substrates could react smoothly to give the desired cross-coupling products with good to excellent yields (84–90%) in 2 to 5 h.| Entry | Arenediazonium salt | Aryl boronic acid | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: arenediazonium salts (1 mmol), aryl boronic acids (1.1 mmol), catalyst (1 mol%), K2CO3 (1.0 mmol), water (5 mL) at room temperature.b Isolated yields after column chromatography. | |||||
| 1 |  |
 |
 |
2 | 88 |
| 2 |  |
 |
 |
3 | 90 |
| 3 |  |
 |
 |
3.5 | 86 |
| 4 |  |
 |
 |
2.5 | 90 |
| 5 |  |
 |
 |
3 | 85 |
| 6 |  |
 |
 |
5 | 84 |
| 7 |  |
 |
 |
4 | 87 |
| 8 |  |
 |
 |
2 | 90 |
| 9 |  |
 |
 |
3.5 | 89 |
| 10 |  |
 |
 |
2.5 | 90 |
2.5 The activity of PdNPs@TiO2–Cell catalyst for Mizoroki–Heck and Heck–Matsuda coupling reactions
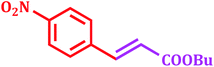
Encouraged by above satisfying results of Suzuki–Miyaura reaction, we studied the applicability of this catalytic system for Mizoroki–Heck and Heck–Matsuda cross-coupling reactions. Since, 1968 the Mizoroki–Heck coupling reaction43 has been emerged as an excellent catalytic tool for the formation of carbon–carbon bonds, as it has enormous efficiency to tolerate different functional groups.44 We explored this catalytic system for Mizoroki–Heck coupling reaction of various aryl bromides with different olefins in DMF using K2CO3 as a base at 100 °C. The results shown in Table 6 indicated that all substrates could smoothly react with different olefins in good to excellent yields (80–95%) in 3.5 to 9 h.| Entry | Aryl bromide | Olefin | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl bromides (1.0 mmol), olefins (1.1 mmol), catalyst (1 mol%), K2CO3 (1.0 mmol), DMF (5 mL) at 100 °C.b Isolated yields after column chromatography. | |||||
| 1 |  |
 |
 |
4 | 88 |
| 2 |  |
 |
 |
3.5 | 95 |
| 3 |  |
 |
 |
6 | 89 |
| 4 |  |
 |
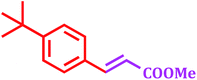 |
5 | 90 |
| 5 |  |
 |
 |
6 | 91 |
| 6 |  |
 |
 |
4.5 | 85 |
| 7 |  |
 |
 |
5 | 82 |
| 8 |  |
 |
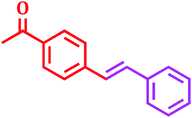 |
7 | 86 |
| 9 |  |
 |
 |
8 | 80 |
| 10 | 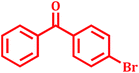 |
 |
 |
9 | 83 |
It has been known that Pd-catalyzed coupling reaction of arenediazonium salts by Heck–Matsuda reaction can easily carried out in water under base-free conditions.45 The first use of arenediazonium salt for Mizoroki–Heck reaction was reported by Matsuda46 and hence known as ‘Heck–Matsuda reaction’. This reaction has been emerged as an alternative to aryl halides for Pd catalyzed synthesis of olefinic compounds. Considering the possible use of water as universal solvent and excellent reactivity of diazonium salts as arylating agent herein we report use of PdNPs@TiO2–Cell catalyst for base-free Heck–Matsuda reaction in water at room temperature. The results shown in Table 7 clearly demonstrates the utility of this system in Heck–Matsuda reaction in which different arenediazonium salts were coupled with varies olefin acceptors under specified reaction conditions without base.
| Entry | Arenediazonium salt | Olefin | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: arenediazonium salts (1 mmol), olefins (1.1 mmol), catalyst (1 mol%), water (5 mL) at room temperature.b Isolated yields after column chromatography. | |||||
| 1 |  |
 |
 |
2 | 89 |
| 2 | 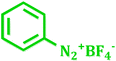 |
 |
 |
1 | 90 |
| 3 |  |
 |
 |
2.5 | 85 |
| 4 |  |
 |
 |
3 | 92 |
| 5 |  |
 |
 |
3.5 | 90 |
| 6 |  |
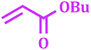 |
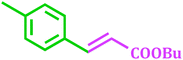 |
2 | 88 |
| 7 |  |
 |
 |
4 | 90 |
| 8 | 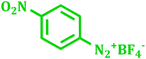 |
 |
 |
5 | 80 |
| 9 |  |
 |
 |
6 | 78 |
| 10 |  |
 |
 |
6 | 75 |
2.6 The activity of PdNPs@TiO2–Cell catalyst for carbonylative Suzuki–Miyaura coupling reaction
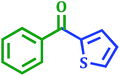
Carbonyl compounds including prochiral ketones are of considerable interest due to their existence in wide range of biologically active molecules.47 It also acts as a promising synthetic fragment for the construction of structural core of many pharmacologically active heterocycles.48 Palladium-catalyzed carbonylative cross-coupling of benzoyl chloride with aryl boronic acid is opened as an alternative way to the classical Friedel–Crafts reaction49 to synthesize carbonyl compounds. Thus using above catalyst the range of corresponding ketone derivatives were prepared from the different benzoyl chlorides in good to excellent yields using 1 mol% catalyst, K2CO3 as a base in 95% EtOH at room temperature (Table 8).| Entry | Benzoyl chloride | Aryl boronic acid | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzoyl chlorides (1 mmol), arylboronic acids (1.1 mmol), catalyst (1 mol%), K2CO3 (1 mmol), 95% EtOH (5 mL) at room temperature.b Isolated yields after column chromatography. | |||||
| 1 |  |
 |
 |
1 | 92 |
| 2 |  |
 |
 |
2 | 88 |
| 3 |  |
 |
 |
3 | 91 |
| 4 |  |
 |
 |
4 | 87 |
| 5 |  |
 |
 |
2.5 | 90 |
| 6 | 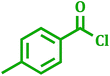 |
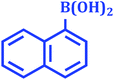 |
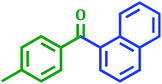 |
3.5 | 85 |
| 7 |  |
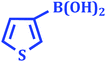 |
 |
1.5 | 91 |
| 8 |  |
 |
 |
2.5 | 90 |
2.7 The activity of PdNPs@TiO2–Cell catalyst for Sonogashira coupling reaction
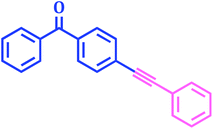
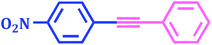
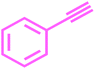
The compounds containing acetylene motifs are prevalent in many important natural products and have been prepared by Sonogashira reaction. Such compounds have several important biological as well as pharmaceutical properties.50 Beside these compounds, oligomers and polymers of acetylene have also been prepared via the Sonogashira reaction. Considering the importance of this reaction in synthesis of such important compounds,51 we next attempted to perform the Cu-free Sonogashira coupling52 of various aryl bromides with terminal alkynes using 1 mol% catalyst, K2CO3 as a base in DMF at 80 °C. Thus we are succeeded to carry out the reactions of terminal acetylenes with number of aryl bromides under Cu-free conditions (Table 9). The results showed that the nature of substituents did not exert significant effect on the yield and reaction time. The reactions were rapid giving good to excellent yields (85–95%) of the corresponding products in 1 to 7 h.| Entry | Aryl bromide | Phenyl acetylene | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl bromides (1.0 mmol), acetylenes (1.1 mmol), catalyst (1 mol%), K2CO3 (1 mmol), DMF (5 mL), at 80 °C.b Isolated yields after column chromatography. | |||||
| 1 |  |
 |
 |
1 | 85 |
| 2 |  |
 |
 |
4 | 94 |
| 3 |  |
 |
 |
3 | 95 |
| 4 |  |
 |
 |
6 | 90 |
| 5 |  |
 |
 |
5 | 88 |
| 6 |  |
 |
 |
6 | 85 |
| 7 |  |
 |
 |
2 | 94 |
| 8 |  |
 |
 |
2 | 92 |
| 9 |  |
 |
 |
6 | 90 |
| 10 |  |
 |
 |
7 | 89 |
2.8 Leaching and heterogeneity
To ascertain the heterogeneous nature of the catalyst we performed hot filtration and leaching test. This study was carried out by coupling 4-bromobenzophenone and phenyl boronic acid under optimized reaction condition of Suzuki–Miyaura reaction. After 30% conversion of the aryl halide (analyzed by HPLC), the catalyst was separated at the reaction temperature and the reaction mixture was continued to stirred at that temperature for another 2 h. There was no further increase in the product concentration, indicated heterogeneous nature of catalyst. This was further supported by Pd leaching test. Possible Pd leaching during the reaction was tested by analyzing Pd concentration in the reaction mixture by ICP-AES. It was observed that only 3 ppm of Pd was detected. The activity of the leached Pd was found to be negligible by carrying out an experiment in 3 ppm of Pd in the form of Pd(OAc)2 as catalyst and P(Ph3)3 ligand.2.9 Recyclability of catalyst
The feasibility of recycling the catalyst was also examined for coupling 4-bromobenzophenone with phenylboronic acid. After completion of reaction, the supported catalyst was separated by centrifugation washed with ethyl acetate, water, dried under vacuum and used directly for the next cycle without further treatment. The recycling results shown in Table 10 indicated that the catalyst was recycled for at least four times without decrease in product yield. TEM analysis of reused catalysts (Fig. 2b) showed particle size of PdNPs are 45 nm this indicated that TiO2–Cell composite avoids agglomeration of PdNPs.| Cycle | Yield (%) |
|---|---|
| Fresh catalyst | 90 |
| 1st cycle | 90 |
| 2nd cycle | 89 |
| 3rd cycle | 89 |
| 4th cycle | 89 |
3. Conclusions
In summary the Pd nanoparticles dispersed on the TiO2 particles supported on cellulose was prepared by a simple procedure. The immobilization of Pd and TiO2 on cellulose was confirmed by FE-SEM and EDS as well as XRD analyses. Furthermore the formation of PdNPs of 39–45 nm was confirmed by TEM. This methodology allows the synthesis of various substituted biphenyls and acrylates from aryl halides as well diazonium salts in environmentally benign solvents like 95% ethanol and water at low temperature in good to excellent yields. We have also demonstrated that the catalyst showed good activity for Sonogashira coupling of aryl bromides in DMF under Cu-free conditions. In almost all conversions 1 equivalent K2CO3 was required. Also, efficient cross-coupling could be accomplished with benzoyl chlorides for the synthesis of ketones at room temperature. The use of this catalytic system has many advantages such as easy separation, recyclability without decrease in yield, and the reaction does not require an inert atmosphere.Acknowledgements
This work was supported by the Science and Engineering Research Board, Department of Science and Technology (SERB-DST), Government of India, New Delhi, under the scheme of Start-Up research grants for Young Scientists (SB/FT/CS-153/2013).References
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- J. D. Sellars and P. G. Stee, Chem. Soc. Rev., 2011, 40, 5170–5180 RSC.
- S. Xu, E. H. Kim, A. Wei and E.-I. Negishi, Sci. Technol. Adv. Mater., 2014, 15, 044201 CrossRef.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- K. Mori, T. Mizoroki and A. Osaki, Bull. Chem. Soc. Jpn., 1973, 46, 1505–1508 CrossRef CAS.
- J. G. Taylor, A. V. Moro, C. Roque and D. Correia, Eur. J. Org. Chem., 2011, 1403–1428 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467–4470 CrossRef.
- Y. Li, D. Xue, C. Wang, Z.-T. Liu and J. Xiao, Chem. Commun., 2012, 48, 1320–1322 RSC.
- Q.-A. Chen, Z.-S. Ye, Y. Duan and Y.-G. Zhou, Chem. Soc. Rev., 2013, 42, 497–511 RSC.
- B. H. Lipshutz, A. R. Abela, Z. V. Boskovic, T. Nishikata, C. Duplais and A. Krasovskiy, Top. Catal., 2010, 53, 985–990 CrossRef CAS.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- C. Deraedt and D. Aatruc, Acc. Chem. Res., 2014, 47, 494–503 CrossRef CAS PubMed.
- L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS PubMed.
- L. M. Neal, D. Hernandez and H. E. Hagelin-Weaver, J. Mol. Catal. A: Chem., 2009, 307, 29–36 CrossRef CAS.
- M. Dahl, Y. Liu and Y. Yin, Chem. Rev., 2014, 114, 9853–9889 CrossRef CAS PubMed.
- L. S. Al-Mazroai, M. Bowker, P. Davies, A. Dickinson, J. Greaves, D. James and L. Millard, Catal. Today, 2007, 122, 46–50 CrossRef CAS.
- B. Tapin, F. Epron, C. Especel, B. K. Ly, C. Pinel and M. Besson, ACS Catal., 2013, 3, 2327–2335 CrossRef CAS.
- M. Maicu, M. C. Hidalgo, G. Colón and J. A. Navío, J. Photochem. Photobiol., A, 2011, 217, 275–283 CrossRef CAS.
- A. Kumbhar and R. Salunkhe, Curr. Org. Chem., 2015, 19, 2075–2121 CrossRef CAS.
- Y. Xu, L. Zhang and Y. Cui, J. Appl. Polym. Sci., 2008, 110, 2996–3000 CrossRef CAS.
- M. J. Gronnow, R. Luque, D. J. Macquarrie and J. H. Clark, Green Chem., 2005, 7, 552–557 RSC.
- B. Zhang, Y. Yuan, K. Philippot and N. Yan, Catal. Sci. Technol., 2015, 5, 1683–1692 CAS.
- E. E. Zvereva, S. Grimme, S. A. Katsyuba, V. V. Ermolaev, D. A. Arkhipova, N. Yan, V. A. Miluykov, O. G. Sinyashina and A. Aleksandrove, Phys. Chem. Chem. Phys., 2014, 16, 20672–20680 RSC.
- X. Yuan, G. Sun, H. Asakura, T. Tanaka, X. Chen, Y. Yuan, G. Laurenczy, Y. Kou, P. J. Dyson and N. Yan, Chem.–Eur. J., 2013, 19, 1227–1234 CrossRef CAS PubMed.
- F. Quignard and A. Choplin, Chem. Commun., 2001, 21–22 RSC.
- K. Kanamori and K. Nakanishi, Chem. Soc. Rev., 2011, 40, 754–770 RSC.
- A. Kumbhar, S. Jadhav, S. Kamble, G. Rashinkar and R. Salunkhe, Tetrahedron Lett., 2013, 54, 1331–1337 CrossRef CAS.
- S. Jadhav, A. Kumbhar, C. Rode and R. Salunkhe, Green Chem., 2015 10.1039/c5gc02314a.
- S. Jadhav, A. Kumbhar, S. Kamble, P. More and R. Salunkhe, C. R. Chim., 2013, 16, 957–961 CrossRef CAS.
- S. Jadhav, A. Kumbhar and R. Salunkhe, Appl. Organomet. Chem., 2015, 29, 339–345 CrossRef CAS.
- L.-Z. Meng, C.-Q. Du, Y.-Y. Chen and Y.-B. Ghe, J. Appl. Polym. Sci., 2002, 84, 61–66 CrossRef CAS.
- A. de Meijere and F. Diederich, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004, vol. 1–2 Search PubMed.
- S. Carrettin, J. Guzman and A. Corma, Angew. Chem., Int. Ed., 2005, 44, 2242–2245 CrossRef CAS.
- E. D. Park and J. S. Lee, J. Catal., 1999, 186, 1–11 CrossRef CAS.
- T. Tabakova, F. Boccuzzi, M. Manzoli and D. Andreeva, Appl. Catal., A, 2003, 252, 385–397 CrossRef CAS.
- A. Kumbhar, S. Kamble, A. Mane, R. Jha and R. Salunkhe, J. Organomet. Chem., 2013, 738, 29–34 CrossRef CAS.
- S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544–4568 CrossRef CAS.
- R. B. deVasher, L. R. Moore and K. H. Shaughnessy, J. Org. Chem., 2004, 69, 7919–7927 CrossRef CAS PubMed.
- A. Roglans, A. Pla-Quintana and M. Moreno-Manas, Chem. Rev., 2006, 106, 4622–4643 CrossRef CAS PubMed.
- B. S. Hannaford, A. J. Smith, P. W. G. Tatchell and A. R. Vogels, Textbook of Practical Organic Chemistry, 5th edn, Longman, Singapore, 1989 Search PubMed.
- N. Taccardi, R. Paolillo, V. Gallo, P. Mastrorilli, C. F. Nobile, M. Räisänen and T. Repo, Eur. J. Inorg. Chem., 2007, 4645–4652 CrossRef CAS.
- J. P. Knowlesa and A. Whiting, Org. Biomol. Chem., 2007, 5, 31–44 Search PubMed.
- R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518–5526 CrossRef CAS.
- A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2964 CrossRef CAS PubMed.
- F. Felpin, K. Miqueu, J. Sotiropoulos, E. Fouquet, O. Ibarguren and J. Laudien, Chem.–Eur. J., 2010, 16, 5191–5204 CrossRef CAS PubMed.
- K. Kikukawa, K. Nagira, F. Wada and T. Matsuda, Tetrahedron, 1980, 37, 31–36 CrossRef.
- J. Li, Y. Jiang and P.-F. Tu, J. Nat. Prod., 2005, 68, 1802–1804 CrossRef CAS PubMed.
- Y. Deng, Y. W. Chin, H. Chai, W. J. Keller and A. D. Kinghorn, J. Nat. Prod., 2007, 70, 2049–2052 CrossRef CAS PubMed.
- M. C. Wilkinson, Org. Lett., 2011, 13, 2232–2235 CrossRef CAS PubMed.
- D. Mujahidin and S. Doye, Eur. J. Org. Chem., 2005, 2689–2693 CrossRef CAS.
- R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC.
- F. Ngassa, E. Lindsey and B. Haines, Tetrahedron, 2009, 65, 4085–4091 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR data of representative compounds. See DOI: 10.1039/c5ra20680d |
| This journal is © The Royal Society of Chemistry 2016 |








