DOI:
10.1039/C5RA19116E
(Paper)
RSC Adv., 2016,
6, 3735-3741
Recovery of solid sulfur from hydrogen sulfide gas by an electrochemical membrane cell†
Received
16th September 2015
, Accepted 30th November 2015
First published on 1st December 2015
Abstract
Sulfides are hazardous chemicals which endanger the lives of humans. Electrochemical oxidation of sulfides is a predominant method for sulfur removal from wastewater. A major limitation of the electrochemical method of sulfur removal is the deposition of sulfur on the anode surface which retards the electrochemical reaction. In the present study, sulfides were oxidized consequently in an electrochemical membrane process to generate elemental sulfur in a powdered form. Sulfide oxidation was achieved by employing flattened and standard titanium substrate insoluble anode (TSIA) meshes with low energy inputs (20 mA cm−2). The shape of the electrode (standard) consequently determines the electrochemical oxidation of the metal sulphide. The irreversible reaction of the sulfide oxidation reaction was explained by a cyclic voltammetry study. This investigation explains that the current distribution of the standard TSIA may be the reason for the sulfur recovery process by an electrochemical membrane reactor, and the recovered product was confirmed through X-ray diffraction and Raman spectroscopy. The spherical shape of the sulfur particles was observed by field emission scanning electron microscopes (FE-SEM and EDAX).
1. Introduction
Hydrogen sulfide is a toxic dangerous gas present in much industrial and domestic sewage water. It should be removed properly because of its corrosiveness, eco-toxicity and bad odor associated with the environment and human health. Sulfide is oxidized to form sulfur or sulfate by various methods such as chemical oxidation, biological oxidation and catalytic conversion, etc.,1–3 and biological reduction of sulfate has also been recognized as an efficient method4 where the foremost problem is the inhibition of bacterial growth by sulfide, which decreases the rate of sulfate reduction.5 Sulfide contaminated water undergoes an electro-oxidation reaction via two processes: (1) direct electro-oxidation (pollutant gets oxidized at the electrode surface); (2) indirect electro-oxidation (by an intermediate like HClO, H2S2O8 etc.).6 The direct electro-oxidation process of sulfide has been explained by many investigators, as have the products formed such as sulfur and sulfate.2
Sulfide is an electro-active compound removed by electrochemical processes such as fuel cell or electrolysis cell processes.7 Generation of energy through the removal of elemental sulfur by fuel cells was investigated by Zhao et al.8 Based on the experimental conditions, elemental sulfur, sulfite, sulfate and thiosulfate can be produced by sulfide oxidation. An effective method for the recovery of sulfur from domestic wastewater was reported by Pikaar et al.9 who compared various electrodes and concluded that a SnO2 electrode gave a better performance for sulfide oxidation and noticed sulfate formation in the anolyte. But the study did not attribute the sulfur deposition on the electrode surface. They concluded that Ta/Ir and Pt/Ir were the most suitable electrodes for sulfide oxidation since they have a low over potential for oxygen evolution. The deposition of elemental sulfur noticed by Dutta et al.3 is a spontaneous electrochemical sulfur removal process and they stated that the final predominant oxidation product was deposited on the graphite anode using Na2S as an electrolyte. The majority (>95%) of the oxidation product was deposited on the surface of the electrode. Dutta et al.10 also studied the electrochemical regeneration of a sulfur loaded carbon fiber electrode in a membrane process for the deposition of sulfur from Na2S·9H2O. The major down side in this electrochemical process was the deposition of elemental sulfur on the electrode surface as it increased the current density interrupting the electrochemical process where, in every alternative batch, the cathode was used as the anode and vice versa.10 In this experiment, sulfur was reduced to polysulfide and sulfide. Dutta et al.3 also opined that an efficient method to reactivate the electrode and to recover sulfur from the electrode surface needs to be developed. Electrolysis of hydrogen sulfide to its constituents in a solution containing equal molar concentrations of NaOH and NaHS has been carried out at 80 °C by Anani et al.11 for sulfur production.
The Claus process is a well-known gas desulfurizing high temperature process for recovering elemental sulfur from hydrogen sulfide present in natural gas.12 A modified version of the Claus process has been proposed to oxidize hydrogen sulfide from gas streams by ceramic or graphite electrodes at temperatures up to 900 °C to produce elemental sulfur.14 This multistep Claus process recovers sulfur from gaseous hydrogen sulfide derived from raw natural gas, the refining of crude oil and other industrial plant processes. The vast majority of sulfur produced worldwide is produced by this high temperature Claus process. Sulfur is used for manufacturing sulfuric acid, medicine, cosmetics, fertilizers, pesticides, rubber products etc.12 In the present study sulfur was recovered in a powdered form from H2S gas using electrochemical membrane technology. The present work is a single step process with an electrolyte preparation, and the reaction was carried out at room temperature. The thermal energy consumption and yield formation of sulfur was less than when compared to the multistep Claus process. Nowadays, both flattened and standard TSIA mesh electrodes are widely used in waste water treatment processes. The principal aim of this paper is to examine the effect of two different electrodes (flattened and standard) on sulfur recovery from H2S which is quantified in terms of yield efficiency. Surface modified electrodes were used in this process to account for the percentage of sulfur recovered. The electrochemical activity of the electrodes was studied to improve the efficiency of sulfur and alkali recovery by an electrochemical method.
2. Experimental
The electrochemical cell was designed with two chambers, i.e. an anode and a cathode chamber, fabricated using polypropylene materials. Flattened and standard titanium meshes were purchased from NISARG International Company, Mumbai. A metal mixed oxide (Ti/TiO2–RuO2–IrO2) coating was applied to the surface of both the flattened and standard titanium mesh electrodes with a thickness of 7 μm.13 The chemical composition of the standard and flattened meshes is shown in Fig. S1†, and the geometrical area was 10 cm × 5 cm to improve the corrosive resistance, stability and low oxygen overvoltage. A Nafion 430 (thickness 200 μm) cation exchange membrane was placed between the anode and cathode chambers. Metal mixed oxide coated flattened (system I) and standard (system II) mesh anodes were used to find the efficiency of sulfur recovery process. Titanium was used as the cathode for both the system. Hydrogen sulfide gas was produced by Kibb’s apparatus and used for anolyte preparation. 500 ml of 0.45 N sodium hydroxide solution was treated with hydrogen sulfide gas for 2 h and the resulting sodium sulfide used as an anolyte. 0.2 N sodium hydroxide was used as a catholyte. The potential of the anode was measured using a multimeter (Agilent U1232A). A saturated calomel electrode was used as a reference electrode. It was indirectly connected to the anolyte via an agar–agar salt bridge. The following reactions take place to form Na2S which was used as an anolyte.| | |
FeS + 2HCl → H2S↑ + FeCl2
| (1) |
| | |
H2S + 2NaOH → Na2S (anolyte) + 2H2O
| (2) |
 |
| | Fig. 1 Schematic overview of the electrochemical sulfide oxidizing membrane cell. (1) Anode compartment, (2) anode, (3) cathode, (4) cathode compartment, (5) cation exchange membrane, (6) power source, (7) catholyte inlet, (8) catholyte outlet, (9) anolyte inlet and (10) anolyte outlet. | |
The impact of the electrode surface on anodic sulfide oxidation was investigated using two types of experiments, system I (flattened) and system II (standard). A constant potential 4.7 V was applied to both systems and batch mode experiments were carried out at 28–30 °C. At the beginning of the experiment, the current density was 20 mA cm−2. The entire system was tightly packed to avoid the leakage of gaseous sulfide in the electrochemical cell shown in Fig. 1. During the process, sulfur was deposited at the anode surface in the flattened mesh system I and a powdered form of sulfur was collected in the standard mesh system II. The precipitated sulfur was collected from the anode compartment and recrystallized as follows. Powdered sulfur was added to a small amount of carbon disulfide solution which was sonicated for a few seconds and spread onto a watch glass. Pure fine yellow crystals of sulfur were formed and used for further analysis.
2.1. Estimation of sulfide and sodium hydroxide
The amount of sulfide was estimated volumetrically22 with the following procedure. 10 ml of the sample was dropped into a conical flask and shaken well with the addition of 15 ml of 0.025 N KIO3 and 10 ml of 10 M sodium hydroxide successively. The solution was boiled and allowed to cool, followed by the addition of 5 ml of KI which was titrated against a 0.1 N (Na2S2O3) thio solution until a pale yellow colour was obtained. About 2 ml of starch solution was added to get a blue colour and 20 ml of 4 M sulfuric acid was added, the titration was continued until the solution became colourless where the endpoint was noted. Sodium hydroxide and sulfate were estimated by volumetric titration and a gravimetric method, respectively, to find out the initial and final concentrations in the catholyte as well as the anolyte before and after the electrochemical process.
2.2. Confirmation of oxidation of sulfide by cyclic voltammetry
Cyclic voltammetry (CV) for the oxidation or anodic reaction of the Na2S solution was carried out in a three electrode system. Pt foil (1 cm2) was used as a working electrode and Pt mesh as a counter electrode. The two electrodes were immersed in 100 ml of sodium sulfide solution connected with the reference electrode by means of a salt bridge. The reference electrode used here was a saturated calomel electrode which was dipped into the solution of saturated KCl. Voltammograms were typically produced from a scan from −1.0 to +0.5 with a scan rate of 20 mV s−1.
2.3. Instrumentation
The images of the electrode surfaces were captured using a digital camera (Sony 12 Mega Pixel Cyber-shot) and the chemical composition of the electrode surface was quantified by micro-XRF analysis (model: bench top XGT-5200). The powder sample was collected in system II from the electrochemical cell and was gold sputtered (Jeol model JFC 1100) and observed under a field emission scanning electron microscope (FE-SEM – Carl Zeiss Supra 55VP/41/46). The nature of the powder sample was examined by energy dispersive X-ray absorption spectroscopy (EDAX-Bruker). The functional groups of the products were determined by Raman spectroscopy (Renishaw Invia Raman Microscope UK) with a wavelength of 500–1500 cm−1 with an exposure time of 25 s (100% intensity). The precipitated product was dried and crushed into a fine powder and used for X-ray diffraction analysis (XRD). A computer cone flattened XRD system, JOEL Model JDX-8030 was used to scan between 10° and 85°, 2θ with copper Kα radiation (Ni filter) at a rating of 40 kV, 20 mA. The peak search and match program of the built-in software (Syn master 793) was used to identify the peaks.
3. Results and discussion
The sulfide oxidation method was investigated using two types of experimental processes, system I and system II. During the course of electrolysis in both the systems, the colour of the anolyte gradually disappeared from deep yellow, indicating a decrease in the sodium ion concentration and the occurrence of sulfide oxidation in the anolyte chamber. The sulfide ions were oxidized and deposited as elemental sulfur at the anode surface in the flattened mesh of system I and the powdered form of sulfur was collected at the standard mesh of system II. In system I, 42% of the sulfur was recovered whereas in system II, 78% of the sulfur was recovered. In addition to this, 300 mg L−1 and 500 mg L−1 of sulfate were also noticed as a by-product in both systems I and II, respectively, and effort was not taken to minimise the production of sulfate ion in the anolyte.
The change in pH of the anolyte and catholyte at different time intervals in both the systems during the sulphide oxidation is depicted in Fig. 2. In system I, the anolyte pH range gradually decreases from 11.6 to 2 within 5 h which is due to the migration of sodium ions to the catholyte where water is oxidized to produce H+ ions. The initial colour of the anolyte (pH 11.6) was yellow and when electrolysis started the colour disappeared. At neutral pH, the yellow colour reappeared due to the formation of polysulfides where the sulfide was oxidized as sulfur.3 The yellow colour of the anolyte completely disappeared below neutral pH. The catholyte pH was slightly increased (11.8 to 14) which indirectly indicated the excess amount of sodium hydroxide formation. While using the standard mesh (system II), the anolyte pH tended to decrease (11.6 to 1.8) and the duration of the reaction was extended up to 7 h. In the case of system I, the sulfur deposited on the anode surface like a passive layer which retards the electrochemical activity of the electrode surface. In system II, relatively less sulfur was noticed. It was assumed that the electrochemical activity remained unaffected. Waterston et al.15 reported sulfide oxidation to sulfate at an acid pH with the help of hypochlorite. Chloride plays an important role in sulfate generation in 8 electron transfer reactions, where sulfur particles were not observed at the electrode surface. Dutta et al.3 also noticed elemental sulfur deposition on an anode while using Na2S as an anolyte with graphite granules as the anode and cathode in a fuel cell where 95% of the deposition of oxidation products was noticed at pH 6.75. They also observed that the limitation of the method over time is due to the deposition of sulphur on the electrode. Ateya et al. also noticed sulfur deposition over graphite and electrode passivation of the anode while using it in polluted brines and also observed sulfate formation in the electrochemical process.2,13 Pikaar et al. recovered 50% sulfur from waste water using Ta/Ir coated anode at a anolyte pH of 7.5.9 No elemental sulfur was visually observed on the electrode surface. The present investigation also supports the above observation regarding pH, where the formation of polysulfide enhances the conversion of sulfur at neutral pH. It may be assumed that sulfide is oxidized and adsorbs as sulfur on the anode surface during the electrochemical process without any interference of chloride.
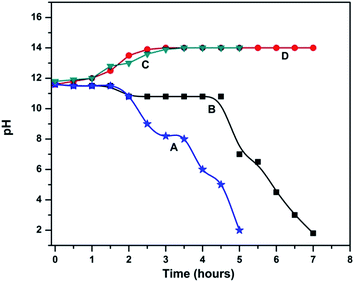 |
| | Fig. 2 pH changes in the anodic and cathodic compartments for system I and system II: (A) system I anolyte pH, (B) system II anolyte pH, (C) system I catholyte pH and (D) system II catholyte pH. | |
In the present study, the sodium ions move towards the cathode compartment via the cation exchange membrane and react with OH ions to increase the concentration of sodium hydroxide in the catholyte which results in the increase of the catholyte pH i.e. from 11.6 to 14. The sodium hydroxide concentration was about 6 g L−1 (0.3 N) and 9 g L−1 (0.45) in the cathode outlet after the electrolysis of system I and system II respectively. The change in pH due to electrolysis may be an important factor for sulfate formation in the electrochemical process. In system II, the maintenance of an alkaline pH for about 3 h and the duration of the electrolysis using a standard electrode are the causative factors for the formation of a higher quantity of sulfate in the anolyte (Table 1).
Table 1 Concentration of sodium hydroxide and sulphate before and after the electrochemical process
| S. No. |
System |
Catholyte – NaOH (g L−1) |
Anolyte – sulfate (g L−1) |
| Initial |
Final |
Initial |
Final |
| 1 |
I |
2 |
3 |
0 |
0.3 |
| 2 |
II |
2 |
4.5 |
0 |
0.5 |
Overall cell voltage and current density for systems I and II are shown in Fig. 3. The initial current density applied in both the systems was about 20 mA cm−2. In system I, the current density gradually decreased due to the deposition of elemental sulfur on the anode which in turn reduced the electrochemical activity of the electrode surface. But in system II, the decrease in current density was due to the deficiency of ions present in the anolyte. The relatively reduced quantity of sulfur present on the electrode surface did not affect the electrochemical activity of anode which may extend the duration of the electrochemical reaction. The same standard electrode was used to find out the stability of the electrode with the addition of five cycles. The initial current density was 20 mA cm−2 in experiments 1 and 2 and in the other experiments (experiments 3–5), the initial current density ranged between 13 and 15.8 mA cm−2. After 7 h, the final current density range was between 4.2 and 8 mA cm−2 for all the experiments which was due to the deficiency of ions in the anolyte (Fig. 2). The decreasing current density in experiments 3 to 5 is due to the adsorption of sulphur on the electrode. Fig. 4 shows the surface of the anode before and after the electrochemical process. Fig. 4a and c show the flattened and standard electrode surfaces, respectively, before the experiment. Fig. 4b shows the even distribution of the elemental sulfur layer of the flattened electrode, a relatively reduced sulfur layer was found at the standard anode surface (Fig. 4d). The following reactions occurred in the anode and cathode chambers during the electrochemical process in both the systems.
 |
| | Fig. 3 Overall current density and cell voltage differences in the system I (flattened electrode) and system II (standard electrode). | |
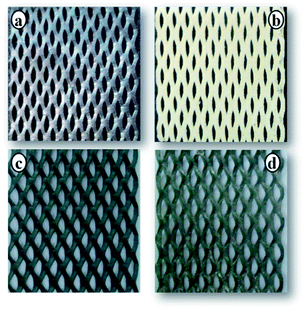 |
| | Fig. 4 System I (flattened TSIA mesh) anode surface before and after the electrochemical process: (a) before the experiment and (b) after the experiment (sulfur deposited at the electrode surface). System II (standard TSIA mesh) (c) before the experiment, and (d) after the experiment. | |
Anodic reaction
Cathodic reaction
In the above reactions, the sulfide ion donates its excess electrons to the anode and precipitates and deposits as sulfur powder, whereas at the cathode water is reduced to form OH− ions which in turn react with Na+ ions migrating from the anode chamber. Since the deposition of sulfur on the flattened electrode surface reduces the electrochemical activity and causes a negative impact by affecting consecutive reaction runs, this factor was considered a major limitation to the sulphide electro-oxidation process. It should be mentioned here that the sulfur deposit-rich flattened electrode cannot be cleaned using water and also cannot be reused. It can be concluded that concentration of sodium sulphide decrease due to the sodium ion mobility towards the cathode and oxidation of sulphide ion in the anolyte, so the current density was reduced in system II. In the present work, the use of two different electrodes may cause a change in the current density. While using the flattened (system I) anode mesh, the current density was evenly distributed throughout the electrode surface which may enhance the even deposition of sulfur on the anode. Besides, when using the standard or uneven surface anode mesh (system II), the current density will be uneven at the anode surface.19 It may decrease the sulfur deposition and does not affect the electrochemical activity resulting in the precipitation of sulfur. This system significantly enhances the precipitation to a greater extent than that of system I. The precipitated product was collected and characterized by FE-SEM Raman spectroscopy and XRD. The EDAX analysis and an FE-SEM image of the sulfur particles collected from the anolyte chamber (system II) are shown in Fig. 5. The size of the sulfur particles was less than 2.1 μm which supports the observation made by Su et al.17 The EDAX results revealed the presence of 100% of elemental sulfur in the recrystallized product. Fig. 6 shows the Raman spectrum of the electrochemically recovered product collected from system II. Peaks were attained at 217 cm−1 (S–S–S) and 472 cm−1 (S–S) along with less intense peaks at 246 cm−1 as well as 437 cm−1.16 This peak structure indicates the presence of orthorhombic sulfur or pure elemental sulfur.15 The inner image confirms the presence of sulfur in the precipitated product by the appearance of a yellow colour. The XRD pattern of elemental sulfur collected from the anolyte chamber in system-II is presented in Fig. 7. The three major peaks (222), (026) and (135) were identified with JCPDS-00-008-024 and confirmed the presence of orthorhombic sulfur in the collected precipitate from the anode chamber.17 The EDAX, Raman spectrum and XRD pattern analysis for sulfur in system I was similar to that of system II. There was no variation in the morphology of sulfur particles collected from system I and system II. Cyclic voltammetry for the oxidation or anodic reaction of the Na2S solution was carried out by employing a Pt electrode and the results are presented in Fig. 8. The Pt electrode was used as a standard to determine the sulphide oxidation potential which provides the supporting data for this sulphide oxidation process. A peak was obtained at −0.25 V vs. SCE.18 This peak indicates the oxidation of sulfide ions, which resulted in sulfur deposition on the electrode. The peak obtained is considered to be an adaptive stripping peak, which decreases gradually in the second cyclic process, because the electrode surface area is decreased by sulfur deposition. The cathodic reduction peak could not be observed in CV which revealed that the sulfide oxidation anodic reaction is an irreversible process. Pikaar9 suggested that sulfide can be oxidized to elemental sulfur, thiosulfate and sulfate, but it can be converted into sulfate using domestic wastewater (anolyte) with Ir/Ta MMO coated titanium at high current densities of 20 mA cm−2. In the present study, TSIA was used with an initial current density of 20 mA cm−2 and at a fixed cell voltage of 4.7 V. Generally the oxygen overvoltage of TSIA was almost above 0.640 V vs. SCE. The anode potential was in the range between 1.2 and 1.6 V vs. SCE. The interference of oxygen may be insignificant during the oxidation of sodium sulfate where the sulfur concentration was higher as compared to sulfate. Ateya et al.1 and Dutta et al.3 noticed sulfur deposition on carbon steel in a pollutant–brine solution, as well as on graphite in Na2S respectively, but there are no quantitative data available. In the present study, about 42% and 78% sulfur was recovered from system I and system II respectively using Na2S as the anolyte, and sulfur formation was noticed in a pH range between 10 and 7 where sulfate started to form below pH 7. Zhang et al.19 suggested that the active surface area of the mesh electrode is three times higher than that of the flat sheet electrode. It is assumed that the current distribution of the three dimensional mesh is somewhat different from the two dimensional mesh electrode. There are three types of current density distribution: primary, secondary and tertiary. Primary considers only the electric field, secondary considers the kinetics effect and electric field, whereas tertiary explains the concentration gradient, kinetics and current distribution. Uniform distribution of the electrode potential will determine the current efficiency or selectivity of the electrode.20 The non-uniform deposition reaction is due to the non-uniform current density involved in tertiary current distribution. The impact of the standard and flattened mesh electrodes on sulfur precipitation/deposition is the important factor in the sulphide oxidation process. The standard electrode mesh is three dimensional and the current distribution may be non-uniform when compared to flattened mesh (Fig. 3). Walsh et al.20 suggested that porous, three dimensional electrodes tend to produce a relatively non-uniform potential and current distribution, due to the anisotropy of the electrode matrix with respect to electrical conductivity. Hence, it can be claimed that the consecutive precipitation of sulfur from the anode is due to the geometry of the standard electrode.
 |
| | Fig. 5 FE-SEM and EDAX views of the elemental sulfur collected from the anolyte chamber (system II). (a) FE-SEM image of the elemental sulfur particles and (b) EDAX data for elemental sulfur. | |
 |
| | Fig. 6 Raman spectrum of the elemental sulfur collected from the anolyte chamber (inner image shows the yellow colour of elemental sulfur). | |
 |
| | Fig. 7 XRD spectrum of the sulphur collected from the anolyte chamber in system-II. | |
 |
| | Fig. 8 Cyclic voltammogram studies of the electro-oxidation of H2S treated with NaOH solution. | |
3.1. Energy consumption
The energy consumption of the sulfide oxidation process was analysed using the following equation.21| |
 | (7) |
Where Vs is the volume of the sample (ml) and V is the potential (V). The energy consumption of the electrochemical process was around 37.41 kW h m−3.
4. Conclusions
In the present study elemental sulfur was recovered from hydrogen sulphide gas by an electrochemical membrane process using flattened (yield of product 42%) and standard (yield of product 78%) TSIA meshes. Significant precipitation was obtained when the standard TSIA electrode was used in the process. The deposition of sulfur was found to be higher at the flattened mesh compared to at the standard mesh which interrupted the electrochemical activity. In this process, alkali (NaOH) was also collected at the catholyte. The energy consumption was 37.41 kW h m−3 for the feed solution to recover sulfur from the sulfide oxidation process. The surface of the standard mesh was uneven which may result in a non-uniform current distribution at the electrode. The non-uniform distribution of current density at the edges of the electrode may be the cause of the formation of sulfur powder instead of the deposition of sulfur on the standard electrode. This process creates an avenue to produce sulfur and sodium hydroxide from the dithionate production industry. This idea can be implemented in technology development for processes of sulfur recovery from industrial effluents. A bio-electrochemical process has also been started using a standard electrode for recovery of sulfur from industrial effluents.
Acknowledgements
CSIR-HRDG, New Delhi is gratefully acknowledged for the senior research fellowship of Hosimin Selvaraj. The authors thank the Council of Scientific and Industrial Research (CSIR), India for sponsoring this project under Sustainable Environmental Technology for Chemical Allied Industrial (SETCA) – CSC-0113.
References
- B. G. Ateya, F. M. AlKharafi and A. S. Al-Azab, Electrodeposition of sulfur from sulfide contaminated brines, Electrochem. Solid-State Lett., 2003, 6(9), C137–C140 CrossRef CAS.
- B. G. Ateya, F. M. AlKharafi, A. S. Alazab and A. M. Abdullai, Kinetics of the electrochemical deposition of sulfur from sulfide polluted brines, J. Appl. Electrochem., 2007, 37, 395–404 CrossRef CAS.
- P. K. Dutta, K. Rabaey, Z. Yuan and J. Keller, Spontaneous electrochemical removal of aqueous sulfide, Water Res., 2008, 42(20), 4965–4975 CrossRef CAS PubMed.
- R. Steudel, Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes, Ind. Eng. Chem. Res., 1996, 35, 1417–1423 CrossRef CAS.
- L. W. Hulshoff Pol, P. N. L. Lens, A. J. M. Stams and G. Lettinga, Anaerobic treatment of sulfate – rich waste water, Biodegradation, 1998, 9, 213–224 CrossRef CAS.
- A. Anglada, A. Uetiage and I. Ortiz, J. Chem. Technol. Biotechnol., 2009, 84, 1747–1755 CrossRef CAS.
- P. K. Dutta, R. A. Rozendal, Z. Yuan, K. Rabaey and J. Keller, Electrochemical regeneration of sulfur loaded electrodes, Electrochem. Commun., 2009, 11(7), 1437–1440 CrossRef CAS.
- Y. Zhao, Z. Liu, Z. Jia and X. Xing, Elemental Sulfur Recovery through H2 Regeneration of a SO2-Adsorbed CuO/Al2O3, Ind. Eng. Chem. Res., 2007, 46, 2661–2664 CrossRef CAS.
- I. Pikaar, R. A. Rozendal, Z. Yuan, J. Keller and K. Rabaey, Electrochemical sulfide oxidation from domestic wastewater using mixed metal-coated titanium electrodes, Water Res., 2011, 45, 5381–5388 CrossRef CAS PubMed.
- P. K. Dutta, R. A. Rozendal, Z. Yuan, K. Rabaey and J. Keller, Electrochemical regeneration of sulfur loaded electrodes, Electrochem. Commun., 2009, 11(7), 1437–1440 CrossRef CAS.
- A. A. Anani, Z. Mao, R. E. White, S. Srinivasan and A. J. Appleby, J. Electrochem. Soc., 1990, 137, 2703–2709 CrossRef CAS.
- R. J. Schoofs, Claus process improvement, US Pat., 4508699, 1985.
- P. Subbiah, S. Krishnamurthy, K. Asokan, K. Subramanian and V. Arumugam, Indian Pat., 178184, 1990.
- B. G. Ateya and F. M. Al-Kharafi, Anodic oxidation of sulfide ions from chloride brines, Electrochem. Commun., 2002, 4, 231–238 CrossRef CAS.
- K. Waterston, D. Bejan and J. Bunce, Electrochemical oxidation of sulfide ion at a boron-doped diamond anode, J. Appl. Electrochem., 2007, 37, 367–373 CrossRef CAS.
- R. W. Gómez, J. L. Pérez M, V. Marquina, R. Ridaura and M. L. Marquina, Polymerization of commercial Mexican sulfur, Rev. Mex. Fis., 2007, 53(1), 30–33 Search PubMed.
- Y. S. Su and A. Manthiram, A facile in situ sulfur deposition route to obtain carbon – wrapped sulfur composite cathodes for lithium–sulfur batteries, Electrochim. Acta, 2012, 77, 272–278 CrossRef CAS.
- P. Vanyesky, Handbook of chemistry and physics, CRC press LLC, 2000 Search PubMed.
- L. Zhang, P. de Schryver, B. de Gusseme, W. de Muynck, N. Boon and W. Verstraete, Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: a review, Water Res., 2008, 42, 1–12 CrossRef CAS.
- C. F. Walsh, A first course in Electrochemical Engineering, 1993.
- S. Annamalai, M. Santhanam, M. Sundaram and M. P. Curras, Electrokinetic remediation of inorganic and organic pollutant in textile effluent contaminated agriculture soil, Chemosphere, 2014, 117, 673–678 CrossRef CAS PubMed.
- I.
M. Kolthoff, E. B. Sandell, E. J. Meehan and S. Bruckenstein, Quantitative Chemical Analysis, 4th edn, 1969, Macmillan, New York, p. 857 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19116e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 








