Open Access Article
Open Access ArticleImproved charge carrier separation in barium tantalate composites investigated by laser flash photolysis
Jenny
Schneider
*a,
Konstantin
Nikitin
ab,
Michael
Wark
c,
Detlef W.
Bahnemann
ab and
Roland
Marschall
*d
aLeibniz University Hannover, Institute of Technical Chemistry, 30167 Hannover, Germany. E-mail: schneid@iftc.uni-hannover.de
bSaint-Petersburg State University, Laboratory “Photoactive Nanocomposite Materials”, Saint-Petersburg, 198504 Russia
cCarl von Ossietzky University Oldenburg, Institute of Chemistry, 26129 Oldenburg, Germany
dJustus-Liebig-University Giessen, Institute of Physical Chemistry, 35392 Giessen, Germany. E-mail: roland.marschall@phys.chemie.uni-giessen.de
First published on 15th December 2015
Abstract
Charge carrier dynamics in phase pure Ba5Ta4O15 and in a Ba5Ta4O15–Ba3Ta5O15 composite have been studied by means of diffuse reflectance laser flash photolysis spectroscopy in the presence and absence of an electron donor, in order to reveal the reason for the improved photocatalytic performance of the latter. For the first time the transient absorption of trapped electrons with a maximum at around 650 nm and of trapped holes with a transient absorption maximum at around 310 nm is reported for tantalates. The decay kinetics of the photogenerated charge carriers could be fitted by second order reaction kinetics, and the direct recombination of the trapped electrons with the trapped holes was proven. In the absence of an electron donor, no difference in the decay behavior between the phase pure material and the composite material is found. In the presence of methanol, for the pure phase Ba5Ta4O15 the recombination of the charge carriers could not be prevented and the trapped electrons also recombine with the ˙CH2OH radical formed via the methanol oxidation by the trapped holes. However, in the composite, the electron can be stored in the system, the ˙CH2OH radical injects an electron into the conduction band of the second component of the composite, i.e., Ba3Ta5O15. Thus, the electrons are available for an extended period to induce reduction reactions.
Introduction
Solar hydrogen (H2) is considered as an efficient solar fuel for future energy applications.1 Among the different strategies to generate H2, photocatalysis is one interesting approach, which has been investigated intensely in the last few decades.2,3 In fact, charge carrier generation upon light irradiation and their transfer in semiconductor materials to reduce and oxidise electrolytes have been studied in detail for many years.4 As a dream reaction, the photocatalytic overall water splitting into H2 and molecular oxygen (O2) would be ideal, however, O2 generation is severely hampered by the necessary four electron transfer needed for water oxidation.Thus, biomass derivatives such as methanol as hole reactants are often employed in photocatalytic H2 generation experiments.5 Methanol was proven to act as a sacrificial agent in such systems with no water splitting occurring and H2 evolving from water.6 The fast reaction of photogenerated holes with methanol is another advantage.7 Generally, more than 90% of the generated charge carriers recombine in picoseconds after generation.8 By a fast reaction of methanol with holes, less electron hole recombination preserves large H2 yields.
Another strategy to reduce charge carrier recombination is the formation of multiphase or multicomponent heterojunctions, the most famous example being Evonik Aeroxide P25 consisting of anatase and rutile TiO2.9 Many other different material composites have been investigated recently, including a Ba5Ta4O15–Ba3Ta5O15 composite system.10
Ba5Ta4O15 is a highly active water splitting photocatalyst upon UV illumination when modified with a co-catalyst.11 Its layered structure can be doped efficiently with nitrogen from the gas phase, leading to an extraordinary band gap reduction from 4.5 down to 1.8 eV.12 To further improve its activity, different sol–gel synthesis methods have been developed to enlarge the surface area of Ba5Ta4O15.10,13 By employing EDTA and citric acid as complexing agents, Marschall et al. also investigated the formation of the above mentioned composite system in situ.10 The composite showed higher photocatalytic activity both in terephthalic acid hydroxylation and in H2 generation without a co-catalyst, compared to phase-pure Ba5Ta4O15 prepared via a solid-state reaction or sol–gel preparation. The authors explained the higher activity by assuming reduced charge carrier recombination by enhanced electron lifetimes due to electron transfer being probable from Ba5Ta4O15 to Ba3Ta5O15. In order to prove these assumptions, we have now performed laser-flash photolysis experiments with both, pure Ba5Ta4O15 and the Ba5Ta4O15–Ba3Ta5O15 composite systems.
Laser flash photolysis is a well-known technique to investigate charge carrier trapping and lifetimes.14 In laser flash photolysis experiments a laser pulse initiates different chemical reactions, the kinetics of which are spectroscopically monitored by a flash lamp fired at a known delay after the laser pulse. The study presented in this paper has been performed on opaque samples, where the change in the level of diffusely reflected light after the laser excitation is monitored. A major advantage of the diffuse reflectance laser flash photolysis technique is that the obtained results can be directly correlated with the outcome of the photocatalytic tests, since in both systems powdered samples are applied.
The laser excitation of phase pure Ba5Ta4O15 and composite Ba5Ta4O15–Ba3Ta4O15 leads to the formation of electron–hole pairs:
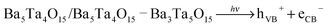 | (1) |
| eCB+(Ta5+) → etr−(Ta4+) | (2) |
| hVB+(Os2−/−OH) → htr+(Os˙−/˙OH) | (3) |
| etr− + htr+ → energy | (4) |
Since tantalates have been rarely investigated with time-resolved spectroscopic techniques a clear identification of the charge carriers formed is needed. Therefore, herein the transient absorption spectra of phase pure Ba5Ta4O15 and composite Ba5Ta4O15–Ba3Ta4O15 were recorded in the presence of methanol acting as an electron donor or rather a hole acceptor.6,17Reaction (5) presents the expected photo-induced process:
| CH3OH + htr+ → ˙CH2OH + H+ | (5) |
Experimental details
Samples for laser flash photolysis measurements were prepared according to the literature.10Fig. 1 illustrates the time-resolved diffuse reflection laser flash photolysis set-up used for this study. The excitation of the sample proceeds with an excimer laser (LPX 200) provided by Lambda Physik. The laser generates pulses of light with a wavelength of 248 nm and a duration of 20 ns. The laser energy per pulse is 30 mJ (determined with ferrioxalate actinometry). In the diffuse reflectance experiments the laser beam enters the sample at an oblique angle. The angle of the laser beam path can be adjusted by rotating the Pellin–Broca prism beam steering module. The change in the reflectance is monitored using a laser flash photolysis spectrometer LKS 80 from Applied Photophysics. The light absorption of the photo-generated transient species is analyzed using a 150 W xenon arc lamp. The pulsing of the xenon lamp for 1.5 ms by a capacitor discharge leads to a 50-fold increase of the analyzing light intensity. A diffuse reflectance accessory is employed to steer the incoming xenon light beam (via two plane folding mirrors) in relation to the main optical axis. A Spectrosil® lens focuses the optical beam onto the solid sample. The diffusely reflected light from the sample is collected by a second lens. The third folding mirror reflects the converging beam to the monochromator. Subsequently, the monochromator light falls into the photomultiplier detector (Hamamatsu R928 photomultiplier) where it produces a current. The photometric light level falling on the photomultiplier is kept at 100 mV for all measurements by applying the required high voltages of 550–800 V. The current output from the photomultiplier is terminated by the variable signal terminator (set to 100 Ω) inserted into the signal input socket of the digital oscilloscope. The transient decay is then recorded by the oscilloscope as a voltage change, and the data points are recalculated to the final signal ΔJ according to:
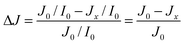 | (6) |
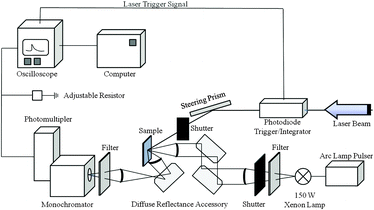 | ||
| Fig. 1 Schematic diagram of the nanosecond diffuse reflectance laser flash photolysis apparatus to produce and detect transient absorptions in light-scattering samples. | ||
It has been reported that the ΔJ value can be correlated with the transient absorption if ΔJ is less than 0.1,18 thus to describe the results obtained by the detection of the diffuse reflected light the term transient absorption will be employed.
A dry powder in a flat quartz cuvette has been used in all diffuse reflectance experiments. Herewith, the illumination area of the laser beam and of the analyzing light is 0.5 cm2 and 0.196 cm2, respectively. For measurements with a N2–methanol atmosphere, a few drops were added, and the powder was dried with N2.
Results and discussion
Irradiation of phase-pure Ba5Ta4O15
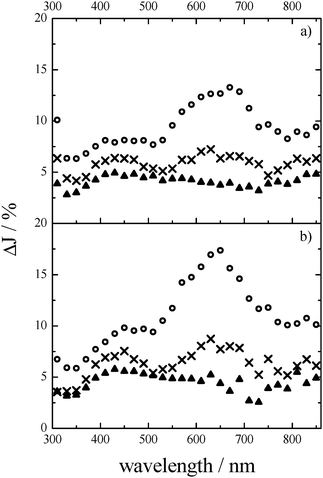
Ba5Ta4O15 has a very large band gap of 4.5 eV. Irradiation was therefore performed with a 248 nm laser pulse. Measuring the initial signal height immediately after the laser pulse, with the powder being kept under an inert N2 atmosphere, two very broad transient absorption maxima centred at around 650 nm and at around 430 nm can be observed, together with a third distinct absorption increase at 310 nm (Fig. 2a).In TiO2 nanomaterials, transient absorption signals observed in the range of 600 nm to 650 nm are attributed to trapped electrons.14,19 Kobayashi has investigated tantalates by means of dual-beam picosecond absorption spectroscopy and obtained for LiTaO3 a transient absorption maximum at around 650 nm.15 This transient absorption has been related to photo-induced colour complex centres.15,16 The rise in absorption at 310 nm is not comparable to TiO2, whereas the transient absorption observed between 400 nm and 530 nm is attributed to the trapped holes.14,19,20 Usually, the trapped holes are identified as O˙−/˙OH radicals. Free O˙−/˙OH radicals show a transient absorption at 266 nm.21 If the radicals are bound at the metal oxide surface its electrochemical environment is changed and thus a shift of the absorption is expected. In the case of TiO2 a red shift of the transient absorption by 100 nm has been reported.22 Regarding the large band gap of the investigated material Ba5Ta4O15, the transient absorption at around 310 nm might be attributed to holes trapped above the valence band (VB).
In addition, Fig. 2a shows the transient absorption spectra measured at different times after the laser pulse. Within the first μs the transient absorption spectra do not change, while 4.5 μs after the laser pulse the transient absorption maximum at around 650 nm disappears and a broad absorption spectrum with a slightly pronounced maximum at around 430 nm remains.
In order to relate the observed transient absorption signals to possible trapped charge carriers, we performed the same measurements in a N2–methanol environment, to investigate the changes in the transient spectra. In the presence of methanol the absorptions at 650 nm and 430 nm strongly increase, while the absorption at 310 nm deteriorates (see Fig. 2b). This indicates that the latter can actually be attributed to trapped holes, which react rapidly with methanol (see eqn (5)), resulting in a strongly decreased absorption signal after the laser pulse compared to the pure N2 atmosphere. For example, holes photogenerated in TiO2 are scavenged with methanol within 1 ns.23 Thus, since the holes have reacted with methanol, the probability of electron–hole recombination is reduced and the absorption signal for the electrons increases compared to the N2 atmosphere, in which the likelihood of electron–hole recombinations is considerably higher. Consequently, the signals at 650 nm and 430 nm can be attributed to trapped electrons in Ba5Ta4O15. From the time evolution of the transient spectra it can be concluded that the maximum at around 430 nm belongs to more deeply trapped electrons, since it remains even at 4.5 μs after the laser pulse, while the transient absorption maximum at around 650 nm disappears, similar to the transient absorption spectra obtained in a N2-atmosphere.
Fig. 3 shows the transient absorption kinetics at (a) 650 nm and (b) 310 nm in a N2-atmosphere (black curves) and in the presence of N2–methanol (red curves), respectively. As can be seen for both signals, a strong increase in absorption is apparent immediately after the laser pulse, which decreases rapidly in the first ∼250 ns, thereafter reaching a long-lasting, nearly constant absorption. The initial absorption J0 of the signal at 650 nm is higher than at 310 nm, while the decay kinetics of both signals are similar. The characteristic time after which J0 decays to J0/e (∼37%) is ∼0.7 ± 0.1 μs. This characteristic time has been determined for the recalculated decays, in which the constant absorption after 4.5 μs has been subtracted from the detected ΔJ. This allows more accurate comparison of the decay kinetics and has been applied for all further data analysis. Moreover, the decay kinetics have been analysed for all wavelengths in the range between 310 nm and 850 nm resulting in similar decay behaviour with a t1/e value of 0.7 ± 0.1 μs.
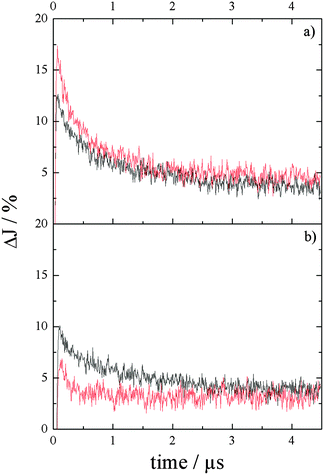 | ||
| Fig. 3 Transient absorption vs. time curves of phase-pure Ba5Ta4O15 in a N2 (black) and a N2–methanol (red) atmosphere, λex = 248 nm, at 650 nm (a) and 310 nm (b). | ||
Comparing the transient absorption kinetics at 650 nm and 310 nm in the presence of methanol (see Fig. 3a and b (red)), the decay at 650 nm shows a slightly longer life time following a strong decrease in absorption during the first 100 ns after the laser pulse. However, 37% (1/e) of the initial absorption is reached after 0.45 μs, while in N2 this value is reached after 0.7 μs (Fig. 3a). This means that in the presence of methanol the recombination of charge carriers could not be suppressed. It may be due to the fact that the methanol molecule reacts only with the surface trapped holes, while the bulk carriers remain in the system and recombine with trapped electrons. In general, a decrease rather than a complete disappearance of the transient absorption signal at 310 nm is observed. After 4 μs, in all atmospheres the observed transient absorption at 650 nm reaches a nearly constant plateau, but only in N2–methanol the absorption stays a little bit higher compared to the pure nitrogen system.
In contrast, the transient absorption at 310 nm decays fastest in N2–methanol and reaches J0/e already after 0.26 μs (Fig. 3b (red)), while it prevails longer in N2. This is in accordance with our assumption that this absorption can be attributed to trapped holes reacting with methanol (see eqn (5)), leading to a fast decay of the absorption.
Charge carriers in Ba5Ta4O15–Ba3Ta5O15 composites
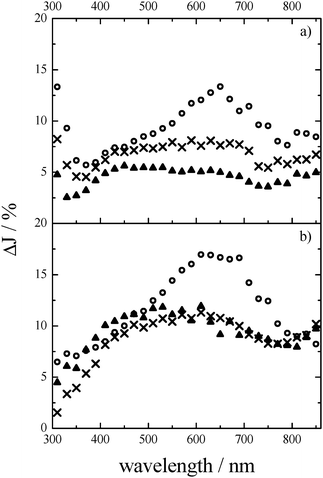
As already mentioned, Ba5Ta4O15–Ba3Ta5O15 composites exhibit improved photocatalytic performance for molecular H2 generation compared to phase-pure Ba5Ta4O15, both without co-catalysts. This fact was explained by the calculated band positions, indicating a probable electron transfer after excitation increasing the electron lifetimes in the composite.10A 248 nm laser pulse was again used to excite the composite powders, and to determine the wavelength-dependent transient absorption right after excitation in inert N2. Comparable to the phase pure Ba5Ta4O15 powder (Fig. 2a), the composite shows 80 ns after the laser pulse a broad transient absorption at around 650 nm, and an even stronger absorption at 310 nm, while in contrast to the pure material the maximum at around 430 nm is less pronounced (Fig. 4a). Moreover, the transient absorption spectrum changes with the decay time. Already 0.6 μs after the laser pulse a broad and featureless absorption in the wavelength range between 350 nm and 710 nm is observed, while the maximum at 310 nm remains. The broadness of the spectra can be explained by the fact that in the composite the charge carriers, mainly the electrons, are distributed at different trapping states within the band gap.
It is not surprising that similar transient absorption signals can be determined for the composite, since the formation of electron–hole pairs in the composite will generate the same kind of excited species, e.g., Ta4+, F+- and F-centers (see eqn (2) and (3)). Although the crystal structure of the two components is slightly different (hexagonal layered perovskite vs. tetragonal), tantalum is surrounded by 6 oxygen atoms in both structures, and the resulting TaO6 octahedra are corner-sharing. However, it is obvious that the transient absorption at 310 nm, which was attributed to holes in the phase-pure material and most likely also in the composite material investigated here, is nearly as high as the electron signal at 650 nm, in contrast to the phase pure material.
Fig. 4b shows the changes in transient absorption after the laser pulse when the composite powders were measured changing the atmosphere to N2–methanol. In the presence of methanol 80 ns after the laser pulse the transient absorption at 310 nm disappears again completely, indicating the oxidation of methanol via the trapped holes followed by the decrease of their absorption state. The transient absorption at around 650 nm increases strongly in a N2–methanol atmosphere, revealing the transient absorption of trapped electrons in the composite. The transient absorption spectra alter with the decay time, namely, a new transient absorption maximum at around 500 nm appears and remains even 4.5 μs after the laser pulse. This stability of the transient spectra differs from the transient spectra obtained for the phase-pure Ba5Ta4O15 thus conveying the ability of the composite to store the electrons, which can be subsequently used for reduction reactions, i.e., the generation of H2.
The transient absorption decay curves obtained at 650 nm and 310 nm in the absence of any electron acceptor or donor are shown in Fig. 5a and b (black curves). The initial absorption J0 and the t1/e values of the signals at 310 nm and at 650 nm are similar. J0 decays to J0/e within 0.7 ± 0.1 μs as has also been found for the pure phase. Nevertheless, the long-lasting, nearly constant transient absorption is higher in comparison to the pure phase tantalate indicating that the two absorbing species have longer lifetimes in the composite as compared with the phase-pure Ba5Ta4O15.
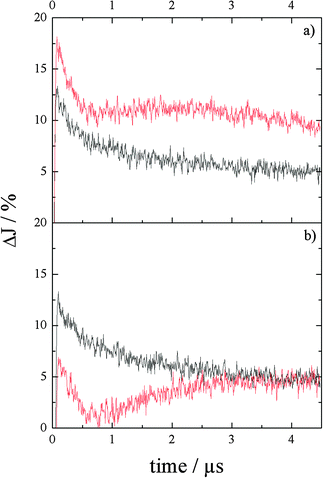 | ||
| Fig. 5 Transient absorption vs. time curves of Ba5Ta4O15–Ba3Ta5O15 composite powders in a N2 (black) and a N2–methanol (red) atmosphere, λex = 248 nm, at (a) 650 nm and (b) 310 nm. | ||
The red curves in Fig. 5a and b demonstrate the transient absorption decay of the two most pronounced absorption states in the composite powders in the presence of methanol. In a N2–methanol atmosphere the decay kinetics at 650 nm change drastically (Fig. 5a, red curve). Following the sharp rise in absorption immediately after the laser pulse and a rapid decay within the first 500 ns, the absorption rises again slightly reaching a maximum at around 2.2 μs, then levelling slowly off, however, being always much higher than the signal in pure N2. The rapid decay of the transient at 310 nm (Fig. 5b, red curve) within 500 ns evinces the reaction of the trapped hole with methanol. The following build-up indicates the formation of a new species exhibiting a transient absorption in the UV region, which may correspond to complex defects of the type: Ta4+:Ta4+xOvxTa4+, as it has been proposed by Antonov et al.16a for the absorption bands at around 450 and 550 nm.
The recombination of the charge carriers in the composite in the presence of methanol could be sufficiently reduced in contrast to the phase pure Ba5Ta4O15. Compared to Fig. 3a, in which the lifetime of electrons was slightly decreased, in the composite the transient absorption signal remains high even longer after the laser pulse, indicating an accumulation of the electrons in the system. Since both transients are very different, it is reasonable to argue charge carrier trapping in the composite after charge excitation gives rise to this behaviour. The main difference in the materials investigated is the second component Ba3Ta5O15 exhibiting a more anodic conduction band position (−0.5 V vs. NHE) than Ba5Ta4O15 (−1.2 V vs. NHE). Thus, it is likely that electrons are transferred onto this second component and are trapped there. This also explains the initial absorption decays, followed by a slow build-up of the transient absorption over time. The electrons on Ba5Ta4O15 can still recombine, as shown in Fig. 3a, but when they are transferred to Ba3Ta5O15, they accumulate and are trapped. To prove this behaviour, it would be useful to investigate phase pure Ba3Ta5O15, but preparation in pure form was not possible yet.
Charge carrier dynamics in the presence and absence of electron donors: pure Ba5Ta4O15vs. composite Ba5Ta4O15–Ba3Ta5O15
In many reports the transient absorption decay curves of the trapped charge carriers in TiO2 have been described employing second order reaction kinetics according to the bimolecular recombination of the electron–hole pairs coupled by Coulombic forces.24 Rothenberger et al. proposed for TiO2 a recombination process on the picosecond time scale involving trapped electrons and free valence band holes, while on the nanosecond time scale the participation of trapped holes in the recombination process was suggested.14dThe transient decay signals obtained for tantalates in a N2 atmosphere on the microsecond time scale could be successfully fitted by second order reaction kinetics:
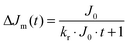 | (7) |
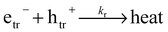 | (8) |
The observed long-lived component of the transient absorption follows more complicated non-second-order reaction kinetics thus for a better comparison of the initial decay behavior at different wavelengths, this plateau absorbance has been subtracted from the absorption decay. Moreover, the initial signal intensity has been set to t = 0 according to:
| ΔJm(t) = ΔJ(t + 80 ns) − ΔJ(t = 4.5 μs) | (9) |
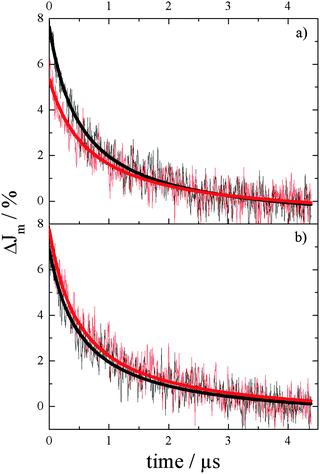 | ||
| Fig. 6 Modified transient absorption kinetics of phase-pure Ba5Ta4O15 powders (a) and Ba5Ta4O15–Ba3Ta5O15 composites (b) in a N2 atmosphere at 650 nm (black) and 310 nm (red). | ||
| Phase-pure Ba5Ta4O15 | Ba5Ta4O15–Ba3Ta5O15 composite | |||
|---|---|---|---|---|
| λ A [nm] | 650 | 310 | 650 | 310 |
| J 0 [%] | 8.7 ± 0.1 | 6.23 ± 0.09 | 7.6 ± 0.1 | 8.4 ± 0.1 |
| k r [s−1%] | (2.14 ± 0.09) × 105 | (2.4 ± 0.2) × 105 | (2.4 ± 0.1) × 105 | (2.3 ± 0.1) × 105 |
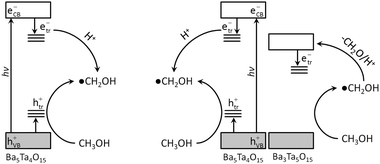
The decay kinetics of the transients for the phase pure material and for the composite in the presence of methanol differ from each other. As can be seen in Fig. 3, in the presence of methanol no build-up of the transient absorption as in the case of TiO2 is obtained,14b in contrast an accelerated decay at 650 nm occurs. Upon the reaction of the holes with methanol, ˙CH2OH radicals are formed. It has been reported that these radicals can also act as a recombination centers.20b,25 Since the conduction band of Ba5Ta4O15 is more negative (−1.2 V vs. NHE) than the reduction potential of the ˙CH2OH radical (−0.739 V vs. NHE),26 the latter is not able to inject an electron into the conduction band, i.e., a current-doubling process is energetically not possible here. In the absence of molecular O2 ˙CH2OH radicals will rather be reduced again by the trapped electrons effectively resulting in an overall e−/h+ recombination. In fact, the reaction of trapped electrons with ˙CH2OH radicals should be regarded as an electron coupled proton transfer yielding CH3OH rather than the pure electron transfer (see Fig. 7 left).27 However, in the case of a Ba5Ta4O15–Ba3Ta5O15 composite the α-hydroxy-methyl radical can inject an electron into the energetically favorable conduction band of Ba3Ta5O15 (−0.5 V vs. NHE). Hence, during the first 0.6 μs the reaction of the trapped electrons in Ba5Ta4O15 with ˙CH2OH is observed followed by the formation of more deeply trapped electrons in Ba3Ta5O15 (see Fig. 7 right). Consequently, in the transient absorption spectra (Fig. 4b) a new band is observed 0.6 μs after the laser pulse, related to trapped electrons in Ba3Ta5O15. In contrast to the phase pure powder the photogenerated electrons in the composite powder can thus be stored for reduction processes, which explains the higher photocatalytic performance for H2 generation under comparable conditions of the composite material, supporting the earlier drawn assumption of the concerted charge carrier transfer process in these composites.10
Conclusions
Two pronounced transient absorption maxima at 310 nm and 650 nm have been found for Ba5Ta4O15 and for a composite of Ba5Ta4O15–Ba3Ta5O15. The experiments performed in the presence of an electron donor such as methanol allowed for the first time to attribute these transient absorptions to trapped holes and electrons, respectively, with the former absorbing in the UV range while the latter absorbs in the visible light range. The transient absorption at 650 nm can be related to Ta4+, as it has been proposed earlier for LiTaO3.The analysis of the transient absorption signals revealed that the decay kinetics do not depend on the absorption wavelength. This means that the detected trapped electrons and holes react only with each other. They can reach one another through a hopping mechanism, similar to the one that has been observed for TiO2.
By comparison of the pure tantalate with the composite, it is obvious that the composite exhibits higher amounts of long lived charge carriers under a N2–methanol atmosphere. Moreover, the recombination of the charge carriers could be sufficiently reduced and an accumulation of the trapped electrons could be detected. A model explaining the different behaviours of the pure phase and the composite material was proposed, with either ˙CH2OH acting as a recombination center in phase-pure tantalate, or involving additional electron injection into the composite material (current doubling). This model explains the improved performance of the composite in photocatalytic hydrogen generation experiments, which has been reported previously.
Acknowledgements
We thank Julia Soldat, Ruhr-University Bochum, for providing the two samples. D. W. B. and R. M. acknowledge financial support from the BMBF (Bundesministerium für Bildung und Forschung), research project DuaSol (03SF0482C and 03SF0482D). R. M. gratefully acknowledges funding from the Emmy-Noether program (MA 5392/3-1) of the German Research Foundation DFG. M. W. acknowledges financial support from the DFG (WA 1116/28). The work carried out in Hannover (Germany) was supported by the Global Research Laboratory (GRL) Program (NRF-2014K1A1A2041044) funded by the Korea government (MSIP) through NSF. The present study was performed within the Project “Establishment of the Laboratory Photoactive Nanocomposite Materials” no. 14.Z50.31.0016 supported by a Mega-grant of the Government of the Russian Federation.Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919 CrossRef CAS PubMed.
- (a) T. A. Kandiel, R. Dillert, L. Robben and D. W. Bahnemann, Catal. Today, 2011, 161, 196 CrossRef CAS; (b) T. A. Kandiel, A. A. Ismail and D. W. Bahnemann, Phys. Chem. Chem. Phys., 2011, 13, 20155 RSC.
- T. A. Kandiel, I. Ivanova and D. W. Bahnemann, Energy Environ. Sci., 2014, 7, 1420 CAS.
- Y. Tamaki, A. Furube, M. Murai, K. Hara, R. Katoh and M. Tachiya, J. Am. Chem. Soc., 2006, 128, 416 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- (a) B. Ohtani, O. O. Prieto-Mahaney, D. Li and R. Abe, J. Photochem. Photobiol., A, 2010, 216, 179 CrossRef CAS; (b) R. Marschall, Adv. Funct. Mater., 2014, 24, 2421 CrossRef CAS.
- R. Marschall, J. Soldat, G. W. Busser and M. Wark, Photochem. Photobiol. Sci., 2013, 12, 671 CAS.
- H. Otsuka, K. Kim, A. Kouzu, I. Takimoto, H. Fujimori, Y. Sakata, H. Imamura, T. Matsumoto and K. Today, Chem. Lett., 2005, 34, 822 CrossRef CAS.
- A. Mukherji, C. Sun, S. C Smith, G. Q. Lu and L. Wang, J. Phys. Chem. C, 2011, 115, 15674 CAS.
- J.-J. Huang, Y.-J. Hsiao, T.-H. Fang and T.-H. Chen, J. Sol-Gel Sci. Technol., 2012, 62, 75 CrossRef CAS.
- (a) D. Duonghong, J. Ramsden and M. Grätzel, J. Am. Chem. Soc., 1982, 104, 2917 Search PubMed; (b) D. Bahnemann, A. Henglein, J. Lilie and L. Spanhel, J. Phys. Chem., 1984, 88, 709 CrossRef CAS; (c) D. Bahnemann, A. Henglein and L. Spanhel, Faraday Discuss. Chem. Soc., 1984, 78, 151 RSC; (d) G. Rothenberger, J. Moser, M. Grätzel, N. Serpone and D. K. Sharma, J. Am. Chem. Soc., 1985, 107, 8054 CrossRef CAS; (e) D. W. Bahnemann, M. Hilgendorff and R. Memming, J. Phys. Chem. B, 1997, 101, 4265 CrossRef CAS.
- T. Kobayashi, Solid State Commun., 1980, 33, 95 CrossRef CAS.
- (a) V. A. Antonov, P. A. Arsenev, I. G. Linda and V. L. Farstendiker, Phys. Status Solidi A, 1975, 28, 673 CrossRef CAS; (b) L. A. Kappers, K. L. Sweeney, L. E. Halliburton and J. H. W. Liaw, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 6792 CrossRef CAS.
- J. Schneider and D. W. Bahnemann, J. Phys. Chem. Lett., 2013, 4, 3479 CrossRef CAS.
- (a) T.-P. Lin and H. K. A. Kan, J. Opt. Soc. Am., 1970, 60, 1252 CrossRef CAS; (b) R. W. Kessler, G. Krabichler, S. Uhl, D. Oelkrug, W. P. Hagan, J. Hyslop and F. Wilkinson, Optica Acta: International Journal of Optics, 1983, 30, 1099 CrossRef CAS.
- Y. Murakami, J. Nishino, T. Mesaki and Y. Nosaka, Spectrosc. Lett., 2011, 44, 88 CrossRef CAS.
- (a) X. Wang, A. Kafizas, X. Li, S. J. A. Moniz, P. J. T. Reardon, J. Tang, I. P. Parkin and J. R. Durrant, J. Phys. Chem. C, 2015, 119, 10439 CrossRef CAS; (b) X. Yang and N. Tamai, Phys. Chem. Chem. Phys., 2001, 3, 3393 RSC; (c) T. Yoshihara, Y. Tamaki, A. Furube, M. Murai, K. Hara and R. Katoh, Chem. Phys. Lett., 2007, 438, 268 CrossRef CAS; (d) Y. Tamaki, A. Furube, M. Murai, K. Hara, R. Katoh and M. Tachiya, Phys. Chem. Chem. Phys., 2007, 9, 1453 RSC; (e) R. Katoh, M. Murai and A. Furube, Chem. Phys. Lett., 2010, 500, 309 CrossRef CAS; (f) J. Tang, A. J. Cowan, J. R. Durrant and D. R. Klug, J. Phys. Chem. C, 2011, 115, 3143 CrossRef CAS; (g) L. Jing, J. Zhou, J. R. Durrant, J. Tang, D. Liu and H. Fu, Energy Environ. Sci., 2012, 5, 6552 RSC.
- G. L. Hug, Optical Spectra of Nonmetallic Inorganic Transient Species in Aqueous Solution, NSRDS-NBS, 1981, 69.
- D. Lawless, N. Serpone and D. Meisel, J. Phys. Chem., 1991, 95, 5166 CrossRef CAS.
- Y. Tamaki, A. Furube, M. Murai, K. Hara, R. Katoh and M. Tachiya, J. Am. Chem. Soc., 2006, 128, 416 CrossRef CAS PubMed.
- (a) N. Serpone, D. Lawless, R. Khairutdinov and E. Pelizzetti, J. Phys. Chem., 1995, 99, 16655 CrossRef CAS; (b) D. P. Colombo and R. M. Bowman, J. Phys. Chem., 1996, 100, 18445 CrossRef CAS; (c) D. P. Colombo and R. M. Bowman, J. Phys. Chem., 1995, 99, 11752 CrossRef CAS; (d) D. E. Skinner, D. P. Colombo, J. J. Cavaleri and R. M. Bowman, J. Phys. Chem., 1995, 99, 7853 CrossRef CAS; (e) A. Furube, T. Asahi, H. Masuhara, H. Yamashita and M. Anpo, J. Phys. Chem. B, 1999, 103, 3120 CrossRef CAS.
- J. Rabani, K. Yamashita, K. Ushida, J. Stark and A. Kira, J. Phys. Chem. B, 1998, 102, 1689 CrossRef CAS.
- J. Lilie, G. Beck and A. Henglein, Ber. Bunsenges. Phys. Chem., 1971, 75, 458 CrossRef CAS.
- J. N. Schrauben, R. Hayoun, C. N. Valdez, M. Braten, L. Fridley and J. M. Mayer, Science, 2012, 336, 1298 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2016 |