Open Access Article
Open Access ArticlePalladium-catalyzed reductive coupling of phenols with anilines and amines: efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines†
Zhengwang
Chen
ab,
Huiying
Zeng
a,
Hang
Gong
a,
Haining
Wang
a and
Chao-Jun
Li
*a
aDepartment of Chemistry and FQRNT Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke St. W., Montreal, Quebec H3A 0B8, Canada. E-mail: cj.li@mcgill.ca
bSchool of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou 341000, PR China
First published on 30th April 2015
Abstract
Phenols, being readily available from naturally abundant lignins, are important future feedstocks for the renewable production of fuels, chemicals, and energy. Herein, a highly efficient Pd-catalyzed direct coupling of phenolic lignin model monomers and analogues with anilines to give cyclohexylamines using cheap and safe sodium formate as hydrogen donor is described. A variety of secondary and tertiary substituted cyclohexylamines can be synthesized under convenient conditions in moderate to excellent yields.
Lignocellulosic biomass represents the most abundant under-utilized organic matter and provides potential renewable feedstock for the production of fuels, chemicals, and energy in the future.1 However, lignin (the key component of lignocellulose) is a complicated three-dimensional amorphous polymer composed of substituted phenols.2 Owing to its irregularity and complexity, the lignin structure is often represented by model compounds such as phenol, guaiacol, 4-propylphenol, diphenylether, vanillin, guaiacylpropane, syringol, and syringylpropane, for chemical studies.3 Over the past decades, important progress has been made in catalytic conversions of lignin model compounds to valuable chemicals via oxidations,4 reductions,5 redox-neutral processes,6 and others.3,7
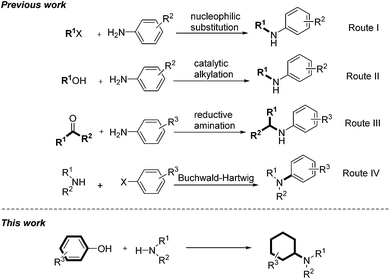
Amines are important structural motifs and intermediates for fine chemicals, pharmaceuticals, agrochemicals and natural products.8 Consequently, the development of general and efficient methods for the derivatization of amines is an active research topic in modern organic synthesis and medicinal chemistry.9 There are three general methods for direct amine alkylations: (1) via nucleophilic substitution of alkyl halides with amines (Scheme 1, route I);10 (2) via N-alkylation of amines with alcohols under catalytic conditions (Scheme 1, route II);11 and (3) via the reductive amination of carbonyl compounds and amines (Scheme 1, route III).12 Very recently, Beller's group presented a straightforward catalytic N-alkylation of amines using carboxylic acids and silanes as reducing agents.13 An alternative method for synthesis of alkylamines was achieved by Buchwald–Hartwig amination via N-arylation (Scheme 1, route IV).14 With an endeavor to develop novel methods to convert lignin into high-value chemicals, herein we report a highly efficient palladium-catalyzed reaction between anilines and phenolic lignin model monomers and analogues.15 It is noteworthy that the transformation represents a new catalytic technique to promote a C–O bond cleavage.16 Moreover, the direct use of phenolic compounds as building blocks in this transformation provides a new non-fossil-approach towards cyclohexylamine derivatives, widely explored structural units in the pharmaceutical industry.17
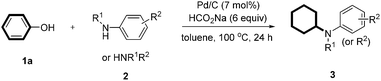
To begin our research, we chose the reaction between phenol (1a) with p-toluidine (2a) as the prototype. Our initial investigations were focused on attempts to achieve the transformation by using common metal catalysts. Many transition-metal complexes such as Rh, Ru, Pt, Ni, and Ir failed to produce any desired coupling products. To our delight, Pd/C can efficiently catalyze the reaction to produce 3a in 94% yield (Table 1, entry 1). The control experiment showed that the catalyst is essential for the reaction (Table 1, entry 2). Then, a variety of palladium catalysts were tested and Pd/C provided the best results (Table 1, entries 3–7). The reaction showed a strong dependence on the solvent, among which THF, ethanol, dioxane, and water were also effective besides toluene (Table 1, entries 8–13). The reaction was the most effective when conducted at 100 °C; whereas a higher or lower temperature resulted in a lower yield (Table 1, entries 14 and 15). An amount of 7 mol% of Pd/C appeared to be the optimal catalyst loading (Table 1, entries 16 and 17). It was found that the product was obtained in 66% yield when H2 was used as an alternative hydrogen source (Table 1, entry 18).
| Entry | Catalyst | Solvent | T/°C | Yieldb/% |
|---|---|---|---|---|
| a Reaction conditions: phenol (0.2 mmol), p-toluidine (0.2 mmol), catalyst (10 mol%), sodium formate (6 equiv.) and solvent (0.8 mL) under an argon atmosphere. b Yields were determined by GC analysis with mesitylene as internal standard; isolated yields in brackets. c Pd/C (7 mol%) was used. d Pd/C (5 mol%) was used. e H2 (1 atm) was used instead of sodium formate. | ||||
| 1 | Pd/C | Toluene | 100 | 94 |
| 2 | — | Toluene | 100 | n.r. |
| 3 | PdCl2 | Toluene | 100 | n.r. |
| 4 | Pd(PPh3)4 | Toluene | 100 | n.r. |
| 5 | Pd(dba)2 | Toluene | 100 | 34 |
| 6 | Pd(PPh3)2Cl2 | Toluene | 100 | n.r. |
| 7 | PdCl2(dtbpf) | Toluene | 100 | n.r. |
| 8 | Pd/C | THF | 100 | 89 |
| 9 | Pd/C | EtOH | 100 | 47 |
| 10 | Pd/C | Dioxane | 100 | 86 |
| 11 | Pd/C | H2O | 100 | 80 |
| 12 | Pd/C | MeCN | 100 | n.p. |
| 13 | Pd/C | DMF | 100 | n.r. |
| 14 | Pd/C | Toluene | 120 | 86 |
| 15 | Pd/C | Toluene | 80 | 73 |
| 16 | Pd/C | Toluene | 100 | 95(92) |
| 17d | Pd/C | Toluene | 100 | 83 |
| 18e | Pd/C | Toluene | 100 | 66 |
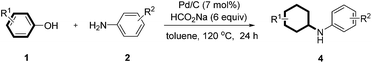
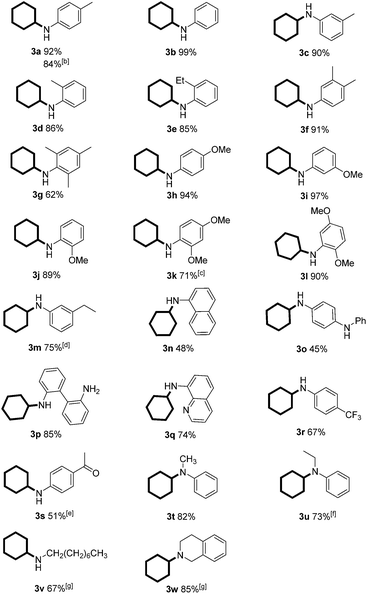
With the optimized reaction conditions in hand, we set out to test the generality of this reaction with regard to both reaction partners (Tables 2 and 3). We first investigated the scope of anilines by reacting with phenol under the optimized standard conditions. The reaction furnished the corresponding secondary or tertiary aniline derivatives in moderate to excellent yields (Table 2, 3a–u). Various anilines bearing electron-withdrawing and/or electron-donating groups were all effective for the transformation. In addition, the product yields were only slightly affected by the location of the substituents (on ortho-, meta-, or para-positions of the benzene ring) (Table 2, 3a–e and 3h–j). However, when both ortho positions were substituted, a moderate yield was obtained due to the increased steric hindrance (Table 2, 3g). Furthermore, both 2,4-dimethoxy substituted and 2,5-dimethoxy substituted anilines afforded the corresponding products efficiently, with the latter being slightly more effective (Table 2, 3k and 3l). For the aniline bearing a vinyl substituent, the product was formed in 75% yield in which the vinyl group was also reduced to an ethyl group (Table 2, 3m). Challenging substrates such as 2n (sterically hindered), 2o (diamine), 2p (free diamine, giving monocyclohexylation product selectively), 2q (quinoline), and 2r (an electron-withdrawing group) were all compatible with the standard conditions. It is noteworthy that the carbonyl-containing substrate gave the desired product in 51% yield in 16 h, in which the carbonyl survived and could be further reduced to an alkyl group with a prolonged reaction time (Table 2, 3s). Moreover, the standard conditions were compatible with secondary amines and afforded the tertiary amine products in good yields (Table 2, 3t and 3u). Aliphatic amines could also give the corresponding products in good yields (Table 2, 3v and 3w). Importantly, the product could be obtained in 84% yield when we scaled up the reaction to 11 mmol (1.03 g) (Table 2, 3a). As expected, the corresponding dehalogenated cyclohexyl amines were obtained when halo-substituted phenols or anilines were used as substrates.
| a Reaction conditions: phenol (0.2 mmol), arylamine (0.2 mmol), Pd/C (7 mol%), sodium formate (6 equiv.) and toluene (0.8 mL) at 100 °C for 24 h under an argon atmosphere; yields of isolated products are given. b The reaction was run at 11 mmol scale. c Reacted for 36 h. d 3-Vinylaniline was used as the substrate. e Reacted for 16 h. f Reacted for 36 h. g Reacted at 80 °C. |
|---|
|
| a Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), Pd/C (7 mol%), sodium formate (6 equiv.) and toluene (0.8 mL) at 120 °C for 24 h under an argon atmosphere; isolated yield and the ratio of cis/trans isomers was determined by crude 1H NMR analysis. b Naphthalen-1-ol was used as substrate. c Naphthalen-2-ol was used as substrate. d Catechol was used as substrate. e Hydroquinone was used as substrate with 2 equiv. aniline. |
|---|
|
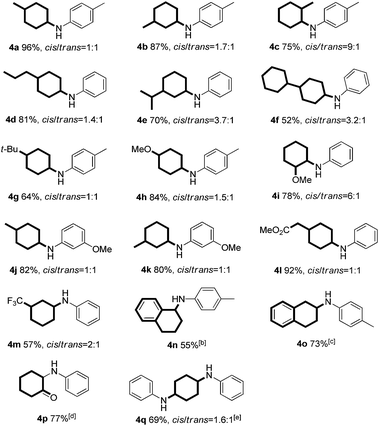
Continuing investigations of the reaction scope, various substituted phenols were explored (Table 3). With the presence of an additional substituent, diastereoisomers will be formed. Gratifyingly, in all cases, the reaction proceeded effectively at a slightly elevated temperature (120 °C), with the ratios of the cis/trans isomers ranging between 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 3, 4a–m and 4q). In general, both electron-rich and electron-deficient substituents on the phenol were compatible with the reaction (Table 3, 4a–m). For example, 4-propylphenol (as the most prevalent structural unit in lignin) gave the corresponding product in good yield (Table 3, 4d). The phenol bearing a bulky tert-butyl group afforded the product in a moderate yield (Table 3, 4g). Furthermore, both α- and β-naphthols also reacted smoothly with p-toluidine to give the corresponding products in moderate yields (Table 3, 4n and 4o). Finally, the dihydroxybenzenes, catechol and hydroquinone, could also react to provide the mono and di-substituted products in 77% and 69% yields, respectively (Table 3, 4p and 4q).
1 (Table 3, 4a–m and 4q). In general, both electron-rich and electron-deficient substituents on the phenol were compatible with the reaction (Table 3, 4a–m). For example, 4-propylphenol (as the most prevalent structural unit in lignin) gave the corresponding product in good yield (Table 3, 4d). The phenol bearing a bulky tert-butyl group afforded the product in a moderate yield (Table 3, 4g). Furthermore, both α- and β-naphthols also reacted smoothly with p-toluidine to give the corresponding products in moderate yields (Table 3, 4n and 4o). Finally, the dihydroxybenzenes, catechol and hydroquinone, could also react to provide the mono and di-substituted products in 77% and 69% yields, respectively (Table 3, 4p and 4q).
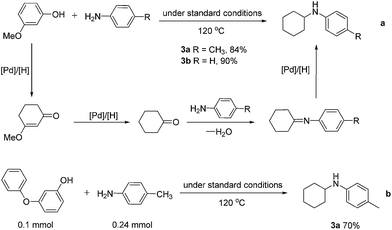
Interestingly, when 3-methoxyphenol was reacted with p-toluidine and aniline under the standard conditions at 120 °C, 3a and 3b were obtained in 84 and 90% yields, respectively, most likely resulting from the sequential reduction–elimination–reduction–imine formation–reduction processes (Scheme 2a). When 3-phenoxyphenol was used, similar transformation occurred and 3a was obtained in 70% yield (Scheme 2b), which shows promise of this work for direct lignin utilizations.
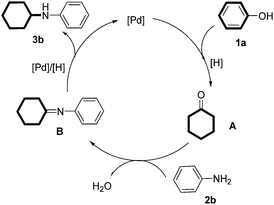
A tentative mechanism for the reaction was proposed in Scheme 3: firstly, phenol was reduced into cyclohexanone18 under the [Pd]/[H]-catalyzed reductive conditions.19 Then, the imine intermediate B was formed through the standard condensation between aniline and cyclohexanone. Finally, reduction of the imine intermediate under [Pd]/[H] generated the cyclohexylaniline derivative and regenerated the active palladium catalyst.20
Conclusions
In conclusion, we have developed a simple, efficient, and novel Pd-catalyzed direct reductive coupling of phenols with amines for the formation of secondary and tertiary cyclohexylamine derivatives. A wide range of substituted phenols and aniline derivatives could be coupled effectively by this method. Aliphatic amines are also effective by this method. In addition, the reaction employed cheap sodium formate as the hydrogen donor, avoiding the use of a potentially hazardous pressurized H2 atmosphere. Furthermore, the reaction of the phenolic lignin model compounds provides great opportunities for using lignin as a renewable feedstock for chemical synthesis. Further studies on the reaction mechanism and applications of this methodology are currently under way in our laboratory.Acknowledgements
We thank the CSC (China Scholarship Council) for a postdoctoral fellowship (to Z.W.C.) and NSERC, FQRNT, CFI, and the Canada Research Chair (to C.J.L.) for their support of our research.References
- (a) G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184–7201 CrossRef CAS PubMed; (b) J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200–9211 CrossRef PubMed.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- For selected examples, see: (a) S. K. Hanson, R. Wu and L. A. “Pete” Silks, Angew. Chem., Int. Ed., 2012, 51, 3410–3413 CrossRef CAS PubMed; (b) A. Rahimi, A. Ulbrich, J. J. Coon and S. S. Stahl, Nature, 2014, 515, 249–252 CrossRef CAS PubMed; (c) A. Rahimi, A. Azarpira, H. Kim, J. Ralph and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 6415–6418 CrossRef CAS PubMed; (d) S. H. Lim, K. Nahm, C. S. Ra, D. W. Cho, U. C. Yoon, J. A. Latham, D. Dunaway-Mariano and P. S. Mariano, J. Org. Chem., 2013, 78, 9431–9443 CrossRef CAS PubMed; (e) B. Biannic and J. J. Bozell, Org. Lett., 2013, 15, 2730–2733 CrossRef CAS PubMed; (f) K. Walsh, H. F. Sneddon and C. J. Moody, Org. Lett., 2014, 16, 5224–5227 CrossRef CAS PubMed.
- For selected examples, see: (a) A. G. Sergeev and J. F. Hartwig, Science, 2011, 332, 439–443 CrossRef CAS PubMed; (b) C. Zhao, Y. Kou, A. A. Lemonidou, X. Li and J. A. Lercher, Angew. Chem., Int. Ed., 2009, 48, 3987–3990 CrossRef CAS PubMed; (c) N. Yan, Y. Yuan, R. Dykeman, Y. Kou and P. J. Dyson, Angew. Chem., Int. Ed., 2010, 49, 5549–5553 CrossRef CAS PubMed; (d) J. He, C. Zhao and J. A. Lercher, J. Am. Chem. Soc., 2012, 134, 20768–20775 CrossRef CAS PubMed; (e) A. G. Sergeev, J. D. Webb and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 20226–20229 CrossRef CAS PubMed; (f) J. Y. Shin, D. J. Jung and S. Lee, ACS Catal., 2013, 3, 525–528 CrossRef CAS; (g) C. Zhao, W. Song and J. A. Lercher, ACS Catal., 2012, 2, 2714–2723 CrossRef CAS; (h) T. H. Parsell, B. C. Owen, I. Klein, T. M. Jarrell, C. L. Marcum, L. J. Haupert, L. M. Amundson, H. I. Kenttämaa, F. Ribeiro, J. T. Miller and M. M. Abu-Omar, Chem. Sci., 2013, 4, 806–813 RSC; (i) K. Mori, K. Furubayashi, S. Okada and H. Yamashita, Chem. Commun., 2012, 48, 8886–8888 RSC.
- For selected examples, see: (a) S. Son and F. D. Toste, Angew. Chem., Int. Ed., 2010, 49, 3791–3794 CrossRef CAS PubMed; (b) T. Kleine, J. Buendia and C. Bolm, Green Chem., 2013, 15, 160–166 RSC; (c) J. D. Nguyen, B. S. Matsuura and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 1218–1221 CrossRef CAS PubMed; (d) J. M. Nichols, L. M. Bishop, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2010, 132, 12554–12555 CrossRef CAS PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- (a) A. Ricci, Modern Amination Reactions, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) S. A. Lawrence, Amines: Synthesis Properties, and Applications, Cambridge University Press, Cambridge, UK, 2004 Search PubMed.
- For selective examples, see: (a) D. Crozet, M. Urrutigoity and P. Kalck, ChemCatChem, 2011, 3, 1102–1118 CrossRef CAS PubMed; (b) M. Stein and B. Breit, Angew. Chem., Int. Ed., 2013, 52, 2231–2234 CrossRef CAS PubMed; (c) T. J. Barker and E. R. Jarvo, Angew. Chem., Int. Ed., 2011, 50, 8325–8328 CrossRef CAS PubMed; (d) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed.
- R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785–7811 CrossRef CAS.
- For selected examples of catalytic alkylation of alcohols, see: (a) R. Kawahara, K. Fujita and R. Yamaguchi, Adv. Synth. Catal., 2011, 353, 1161–1168 CrossRef CAS PubMed; (b) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853–1864 CrossRef PubMed; (c) D. Weichmann, W. Frey and B. Plietker, Chem. - Eur. J., 2013, 19, 2741–2748 CrossRef PubMed; (d) X. Cui, X. Dai, Y. Deng and F. Shi, Chem. - Eur. J., 2013, 19, 3665–3675 CrossRef CAS PubMed; (e) Y. Du, S. Oishi and S. Saito, Chem. - Eur. J., 2011, 17, 12262–12267 CrossRef CAS PubMed; (f) L. He, X.-B. Lou, J. Ni, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, Chem. - Eur. J., 2010, 16, 13965–13969 CrossRef CAS PubMed; (g) F. Santoro, R. Psaro, N. Ravasio and F. Zaccheria, RSC Adv., 2014, 4, 2596–2600 RSC; (h) G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed.
- For selected examples of reductive amination of carbonyl compounds, see: (a) V. Kumar, U. Sharma, P. K. Verma, N. Kumar and B. Singh, Adv. Synth. Catal., 2012, 354, 870–878 CrossRef CAS PubMed; (b) Q. Lei, Y. Wei, D. Talwar, C. Wang, D. Xue and J. Xiao, Chem. - Eur. J., 2013, 19, 4021–4029 CrossRef CAS PubMed; (c) V. N. Wakchaure, J. Zhou, S. Hoffmann and B. List, Angew. Chem., Int. Ed., 2010, 49, 4612–4614 CrossRef CAS PubMed; (d) C. Wang, A. Pettman, J. Bacsa and J. Xiao, Angew. Chem., Int. Ed., 2010, 49, 7548–7552 CrossRef CAS PubMed; (e) P. D. Pham, P. Bertus and S. Legoupy, Chem. Commun., 2009, 6207–6209 RSC; (f) M. Zhang, H. Yang, Y. Zhang, C. Zhu, W. Li, Y. Cheng and H. Hu, Chem. Commun., 2011, 47, 6605–6607 RSC; (g) J. Deng, L.-P. Mo, F.-Y. Zhao, L.-L. Hou, L. Yang and Z.-H. Zhang, Green Chem., 2011, 13, 2576–2584 RSC; (h) S. Werkmeister, K. Junge and M. Beller, Green Chem., 2012, 14, 2371–2374 RSC; (i) V. Kumar, S. Sharma, U. Sharma, B. Singh and N. Kumar, Green Chem., 2012, 14, 3410–3414 RSC; (j) R. Apodaca and W. Xiao, Org. Lett., 2001, 3, 1745–1748 CrossRef CAS PubMed; (k) O.-Y. Lee, K.-L. Law and D. Yang, Org. Lett., 2009, 11, 3302–3305 CrossRef CAS PubMed.
- I. Sorribes, K. Junge and M. Beller, J. Am. Chem. Soc., 2014, 136, 14314–14319 CrossRef CAS PubMed.
- For selective reviews, see: (a) J. P. Wolfe, S. Wagaw, J.-F. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805–818 CrossRef CAS; (b) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS PubMed; (c) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27–50 RSC; (d) J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046–2067 CrossRef CAS; (e) J. F. Hartwig, Acc. Chem. Res., 1998, 31, 852–860 CrossRef CAS; (f) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS PubMed.
- A similar transformation between phenol and ammonia has been previous reported, see: H. Hamada, M. Yamamoto, Y. Kuwahara, T. Matsuzaki and K. Wakabayashi, Bull. Chem. Soc. Jpn., 1985, 58, 1551–1555 CrossRef CAS.
- For selective reviews of metal-catalyzed cross-coupling reactions of C–O electrophiles, see: (a) J. Cornella, C. Zarate and R. Martin, Chem. Soc. Rev., 2014, 43, 8081–8097 RSC; (b) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346–1416 CrossRef CAS PubMed; (c) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486–1495 CrossRef CAS PubMed.
- (a) R. Skouta, S. J. Dixon, J. Wang, D. E. Dunn, M. Orman, K. Shimada, P. A. Rosenberg, D. C. Lo, J. M. Weinberg and A. Linkermann, J. Am. Chem. Soc., 2014, 136, 4551–4556 CrossRef CAS PubMed; (b) J. Zhou and B. List, J. Am. Chem. Soc., 2007, 129, 7498–7499 CrossRef CAS PubMed.
- A control experiment of reduction of phenol was performed under the standard conditions in the absence of aniline, cyclohexanone could be formed in 85% yield.
- For selected examples of reduction of phenols, see: (a) H. Liu, T. Jiang, B. Han, S. Liang and Y. Zhou, Science, 2009, 326, 1250–1252 CrossRef CAS PubMed; (b) J.-F. Zhu, G.-H. Tao, H.-Y. Liu, L. He, Q.-H. Sun and H.-C. Liu, Green Chem., 2014, 16, 2664–2669 RSC; (c) Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362–2365 CrossRef CAS PubMed; (d) P. Makowski, R. D. Cakan, M. Antonietti, F. Goettmann and M.-M. Titirici, Chem. Commun., 2008, 999–1001 RSC; (e) Y. Li, X. Xu, P. Zhang, Y. Gong, H. Li and Y. Wang, RSC Adv., 2013, 3, 10973–10982 RSC.
- For reviews on the catalytic reduction of imines, see: (a) J.-H. Xie, S.-F. Zhu and Q.-L. Zhou, Chem. Rev., 2011, 111, 1713–1760 CrossRef CAS PubMed; (b) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069–1094 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc00941c |
| This journal is © The Royal Society of Chemistry 2015 |