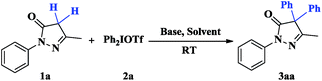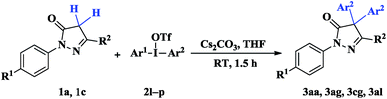Metal-free one-pot sequential direct diarylation of pyrazolin-5-ones with diaryliodonium salts†
Tao Huang,
Xinfei Ji,
Wei Wu,
Fang Liang and
Song Cao*
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology (ECUST), Shanghai 200237, China. E-mail: scao@ecust.edu.cn; Fax: +86-21-64252603; Tel: +86-21-64253452
First published on 30th July 2015
Abstract
A novel and efficient one-pot sequential C4-diarylation of pyrazolin-5-ones with diaryliodonium salts in the absence of a metal catalyst was reported. A variety of C4-diarylated pyrazolin-5-one derivatives were obtained in good to high yields under mild conditions.
Diaryliodonium salts (Ar2IX) are versatile electrophilic arylating agents and have been broadly used in organic synthesis due to their high reactivity, stable nature, easiness to handle and ready availability.1 The metal-catalyzed or metal-free C-arylation of arenes and heteroarenes,2 N-arylation of secondary anilines and amides,3 O-arylation of aliphatic alcohols, phenols and carboxylic acids4 with diaryliodonium salts have become powerful methods for the rapid construction of C–C bonds, C–N bonds and C–O bonds, respectively.
The direct activation and functionalization of the inert aromatic and heteroaromatic C–H bond without the prefunctionalization of substrates have been considered as one of the most challenging goals in organic synthesis.5 Diaryliodonium salts exhibited extraordinary reactivity toward less reactive Csp2–H bond in heterocycles both in the absence and presence of metal transition catalyst.6 For example, in 2012, Zhang and Yu developed a novel transition metal-free direct C-2 arylation of pyrrole with diaryliodonium salts.7 Ackermann reported an unprecedented metal-free C-2 arylation of artificial indoles with diaryliodonium salts.8 Direct C–H arylation of quinones and naphthoquinones was also achieved with diaryliodonium salts under mild and metal-free conditions.9 More recently, Wang has successfully developed the highly efficient metal-free C4-arylation of 4-substituted-pyrazolin-5-ones with diaryliodonium salts using 4-dimethylaminopyridine (DMAP) as base and toluene as solvent.10
Pyrazolinones, the five-membered-ring lactams, are often found as structural subunits in medicinal chemistry and agrochemical chemistry (Fig. 1).11 They also serve as versatile nucleophiles in organic synthesis for the preparation of complex molecules because they have two nucleophile sites, one is carbon anion and another is oxygen anion.12 Recently, selective functionalization of pyrazolin-5-ones at the C-4 position has attracted much attention.13 However, the introduction of two bulky substituents such as aryl group at C-4 position of pyrazolin-5-ones in a one-pot process has rarely been explored.14 In this communication, we report a one-pot protocol for direct C–H bond diarylation of pyrazolin-5-ones with diaryliodonium salts under the assistance of Cs2CO3 at room temperature without any additional metal catalyst (Scheme 1).
In our initial study, the reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 1a with diphenyliodonium triflate 2a was conducted to screen the reaction conditions (Table 1). The results indicated that most solvents could provide excellent yields of diphenylated product 3aa (entries 1–8), except for water (entry 9). Considering THF as a readily available, economical, and environmentally friendly solvent, we selected it as solvent to perform this one-pot sequential diarylation reaction. To our delight, the reaction was finished within 1.5 h to give excellent yield of 3aa (99%, entry 11). Further screening of bases revealed that the transformation could also proceed efficiently in presence of different bases such as K2CO3, tBuOK, and NaOH (entries 14–16), and Cs2CO3 is the optimal base (entry 11). No reaction was observed in the absence of base (entry 13). Subsequently, the effect of the anion in iodonium salts was evaluated. Both diphenyliodonium tetrafluoroborate (Ph2IBF4) and diphenyliodonium p-toluenesulfonate (Ph2IOTs) were also reacted under the optimal conditions (entry 11) to afford the desired product 3aa in excellent (entries 17 and 18). It is very interesting that no monophenylated byproduct was detected in all cases even when decreasing the amount of 2a to 1.0 equiv. or reaction temperature to −20 °C, respectively.
| Entry | Base | Solvent | Reaction time (h) | Yield of 3aab (%) |
|---|---|---|---|---|
| a Reagents and conditions: 1a (0.25 mmol), 2a (0.525 mmol, 2.1 equiv.), base (0.525 mmol, 2.1 equiv.), solvent (2 mL), room temperature.b Yields determined by GC analysis and based on 1a.c Ph2IOTs was used.d Ph2IBF4 was used. | ||||
| 1 | Cs2CO3 | DMSO | 4.0 | 98 |
| 2 | Cs2CO3 | DMF | 4.0 | 96 |
| 3 | Cs2CO3 | THF | 4.0 | 99 |
| 4 | Cs2CO3 | CH3CN | 4.0 | 93 |
| 5 | Cs2CO3 | NMP | 4.0 | 97 |
| 6 | Cs2CO3 | Toluene | 4.0 | 94 |
| 7 | Cs2CO3 | Dioxane | 4.0 | 99 |
| 8 | Cs2CO3 | CH2Cl2 | 4.0 | 97 |
| 9 | Cs2CO3 | H2O | 4.0 | 0 |
| 10 | Cs2CO3 | THF | 2.0 | 99 |
| 11 | Cs2CO3 | THF | 1.5 | 99 |
| 12 | Cs2CO3 | THF | 1.0 | 91 |
| 13 | None | THF | 1.5 | 0 |
| 14 | K2CO3 | THF | 1.5 | 70 |
| 15 | tBuOK | THF | 1.5 | 85 |
| 16 | NaOH | THF | 1.5 | 95 |
| 17c | Cs2CO3 | THF | 1.5 | 98 |
| 18d | Cs2CO3 | THF | 1.5 | 95 |
With the optimized reaction conditions in hand (Table 1, entry 11), we next investigated the performance of various diaryliodonium triflates for the direct diarylation of pyrazolinones (Tables 2 and 3). As shown in Table 2, most symmetrical diaryliodonium salts with electron-withdrawing groups (F, Cl, Br, CF3) (for example entries 2–4, 7) or with electron-neutral group (entry 1) could afford the diarylated products in good to excellent yields and no monoarylated byproduct was observed. Although diaryliodonium salt with electron-donating substituents (CH3, CH3O) also underwent diarylation in good yields (entries 5, 6, 11), a small or trace amount of monoarylated byproduct was detected. The position of the substituent on the diaryliodonium salts played a key role in this reaction. Generally, diaryliodonium salts possessing substituent in the para-position on the benzene ring proceeded well. However, when ortho- or meta-substituted diaryliodonium salts were used as the aryl sources, the diarylation reaction did not proceed efficiently and only small or trace amounts of the desired products were observed (entries 15–17) due to the steric effect of the substituent attached at the ortho- or meta-position. Unfortunately, almost no reaction took place with [(2-thienyl)2I]OTf as the coupling partner (entry 18). In addition, the results indicated that the substituents (R1 and R2) in pyrazolinones 1a–c had no obvious influence on the yields of the products (for example 3aa versus 3ca).
| Entry | R1 | R2 | Ar | 3 | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a–c (0.5 mmol), symmetrical diaryliodonium salts 2a–k (1.05 mmol, 2.1 equiv.), Cs2CO3 (1.05 mmol, 2.1 equiv.), THF (4 mL).b Isolated yield.c (4-MeOC6H4)2IOTs was used.d (4-CF3C6H4)2IBF4 was used.e GC-MS analysis. | |||||
| 1 | H | Me | Ph | 3aa | 90 |
| 2 | H | Me | 4-FC6H4 | 3ab | 92 |
| 3 | H | Me | 4-ClC6H4 | 3ac | 83 |
| 4 | H | Me | 4-BrC6H4 | 3ad | 80 |
| 5 | H | Me | 4-MeC6H4 | 3ae | 86 |
| 6 | H | Me | 4-MeOC6H4 | 3af | 80c |
| 7 | H | Me | 4-CF3C6H4 | 3ag | 91d |
| 9 | F | Me | Ph | 3ba | 82 |
| 10 | F | Me | 4-BrC6H4 | 3bd | 77 |
| 11 | F | Me | 4-MeOC6H4 | 3bf | 75 |
| 12 | H | Ph | Ph | 3ca | 90 |
| 13 | H | Ph | 4-FC6H4 | 3cb | 91 |
| 14 | H | Ph | 4-MeOC6H4 | 3cf | 81 |
| 15 | H | Me | 3-MeC6H4 | 3ah | 28e |
| 16 | H | Me | 2,5-Me2C6H3 | 3ai | Trace |
| 17 | H | Me | Mesityl | 3aj | Trace |
| 18 | H | Me | 2-Thienyl | 3ak | Trace |
The reactions of unsymmetrical diaryliodonium salts with pyrazolinones were also investigated (Table 3). It was found that with unsymmetrical diaryliodonium salts (2l, entry 1 and 2m, entries 2–3), the more electron-poor aryl moiety was selectively transferred to the products. In case of arylmesityl iodonium salts, only 4-trifluoromethylphenyl group in 2n (entry 4) or phenyl group in 2o (entry 5) was introduced into the arylated products due to the steric hindrance of the mesityl group. When [(Ph)I(2-thienyl)]OTf (2p, entry 6) was used as the coupling partner, transfer of the phenyl group was favored and the diphenylated product 3aa was obtained as major product.
| Entry | R1 | R2 | Ar1 | Ar2 | Product 3 | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a, 1c (0.5 mmol, 1.0 equiv.), unsymmetrical diaryliodonium salts 2l–p (1.05 mmol, 2.1 equiv.), Cs2CO3 (1.05 mmol, 2.1 equiv.), THF (4 mL).b Isolated yield. | ||||||
| 1 | H | Me | 4-OMeC6H4 | 4-NO2C6H4 | 3al | 87 |
| 2 | H | Me | 4-OMeC6H4 | 4-CF3C6H4 | 3ag | 85 |
| 3 | H | Ph | 4-OMeC6H4 | 4-CF3C6H4 | 3cg | 83 |
| 4 | H | Me | Mesityl | 4-CF3C6H4 | 3ag | 78 |
| 5 | H | Me | Mesityl | Ph | 3aa | 73 |
| 6 | H | Me | 2-Thienyl | Ph | 3aa | 89 |
Generally, diaryliodonium salts react with nucleophiles via either a polar or radical pathway.3c,15 Furthermore, the α-arylation of carbonyl compounds may follow an ionic or radical mechanism depending on the substrates and reaction conditions.2b,9,16 To gain some mechanistic insight into the diarylation of pyrazolin-5-ones with diaryliodonium salts, two comparative experiments were performed using the model reaction under the standard reaction conditions. When the reaction of 1a with 2a was performed in the presence of one equivalent of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), a well-known radical scavenger, or one equivalent of the free neutral radical galvinoxyl, the reactions proceeded smoothly and provided the desired product 3aa in high yields (95% and 85%, respectively). Based on these results, a radical-type mechanism for the diarylation reaction can be ruled out. Therefore, we suggest that the mechanism of this diarylation probably includes the initial formation of the O–I bond or C–I bond, followed by reductive elimination of PhI to afford product 3aa via [2,3]- or [1,2]-rearrangement, respectively (Scheme 2, with 1a as the example).2b,3c,16a,16b It should be noted that attempts to isolate the monoarylated products were unsuccessful because only small or trace amount of them were formed.
In summary, we have developed an efficient and straightforward synthetic protocol for C4-diarylation of pyrazolin-5-ones with diaryliodonium salts. The reaction proceeded efficiently under metal-free conditions at room temperature and afforded the corresponding diarylated products in high yields. The use of environmentally friendly solvent (THF versus toluene) and economical base (Cs2CO3 versus DMAP) makes the process more useful and practical. Based on preliminary mechanistic studies, we could rule out a radical mechanism and suggest a reductive elimination pathway for the diarylation reaction.
Acknowledgements
We are grateful for financial supports from the National Natural Science Foundation of China (Grant No. 21472043, 21272070).Notes and references
- (a) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (c) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS PubMed; (d) F. Liu, H. Yang, X. Hu and G. Jiang, Org. Lett., 2014, 16, 6408 CrossRef CAS PubMed.
- (a) R. J. Phipps, N. P. Grimster and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 8172 CrossRef CAS PubMed; (b) Z. Chai, B. Wang, J.-N. Chen and G. Yang, Adv. Synth. Catal., 2014, 356, 2714 CrossRef CAS PubMed; (c) A. Monastyrskyi, N. K. Namelikonda and R. Manetsch, J. Org. Chem., 2015, 80, 2513 CrossRef CAS PubMed; (d) S. Castro, J. J. Fernández, R. Vicente, F. J. Fañanás and F. Rodríguez, Chem. Commun., 2012, 48, 9089 RSC; (e) F. Pan, P.-X. Shen, L.-S. Zhang, X. Wang and Z.-J. Shi, Org. Lett., 2013, 15, 4758 CrossRef CAS PubMed.
- (a) Y. Yang, X. Wu, J. Han, S. Mao, X. Qian and L. Wang, Eur. J. Org. Chem., 2014, 6854 CrossRef CAS PubMed; (b) F. Guo, L. Wang, P. Wang, J. Yu and J. Han, Asian J. Org. Chem., 2012, 1, 218 CrossRef CAS PubMed; (c) F. Tinnis, E. Stridfeldt, H. Lundberg, H. Adolfsson and B. Olofsson, Org. Lett., 2015, 17, 2688 CrossRef CAS PubMed; (d) J. Li and L. Liu, RSC Adv., 2012, 2, 10485 RSC; (e) M. A. Carroll and R. A. Wood, Tetrahedron, 2007, 63, 11349 CrossRef CAS PubMed.
- (a) L. Chan, A. McNally, Q. Y. Toh, A. Mendoza and M. J. Gaunt, Chem. Sci., 2015, 6, 1277 RSC; (b) B. Xiong, X. Feng, L. Zhu, T. Chen, Y. Zhou, C.-T. Au and S.-F. Yin, ACS Catal., 2015, 5, 537 CrossRef CAS; (c) H. Gao, Q.-L. Xu, C. Keene and L. Kürti, Chem.–Eur. J., 2014, 20, 8883 CAS; (d) R. Ghosh, E. Stridfeldt and B. Olofsson, Chem.–Eur. J., 2014, 20, 8888 CrossRef CAS PubMed; (e) N. Jalalian, T. B. Petersen and B. Olofsson, Chem.–Eur. J., 2012, 18, 14140 CrossRef CAS PubMed.
- (a) B. G. Hashiguchi, S. M. Bischof, M. M. Konnick and R. A. Periana, Acc. Chem. Res., 2012, 45, 885 CrossRef CAS PubMed; (b) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (c) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369 CrossRef CAS PubMed; (d) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (e) F. Vallée, J. J. Mousseau and A. B. Charette, J. Am. Chem. Soc., 2010, 132, 1514 CrossRef PubMed; (f) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed.
- (a) R. J. Phipps and M. J. Gaunt, Science, 2009, 323, 1593 CrossRef CAS PubMed; (b) S. G. Modha and M. F. Greaney, J. Am. Chem. Soc., 2015, 137, 1416 CrossRef CAS PubMed; (c) J. Malmgren, A. Nagendiran, C.-W. Tai, J.-E. Bäckvall and B. Olofsson, Chem.–Eur. J., 2014, 20, 13531 CrossRef CAS PubMed; (d) D. Kumar, M. Pilania, V. Arun and S. Pooniya, Org. Biomol. Chem., 2014, 12, 6340 RSC; (e) L. Ackermann, M. Dell'Acqua, S. Fenner, R. Vicente and R. Sandmann, Org. Lett., 2011, 13, 2358 CrossRef CAS PubMed; (f) A. M. Wagner, A. J. Hickman and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 15710 CrossRef CAS PubMed; (g) J. S. Ho, L. C. M. Castro, Y. Aihara, M. Tobisu and N. Chatani, Asian J. Org. Chem., 2014, 3, 48 CrossRef CAS PubMed.
- J. Wen, R.-Y. Zhang, S.-Y. Chen, J. Zhang and X.-Q. Yu, J. Org. Chem., 2012, 77, 766 CrossRef CAS PubMed.
- Y. Zhu, M. Bauer, J. Ploog and L. Ackermann, Chem.–Eur. J., 2014, 20, 13099 CrossRef CAS PubMed.
- D. Wang, B. Ge, L. Li, J. Shan and Y. Ding, J. Org. Chem., 2014, 79, 8607 CrossRef CAS PubMed.
- S. Mao, X. Geng, Y. Yang, X. Qian, S. Wu, J. Han and L. Wang, RSC Adv., 2015, 5, 36390 RSC.
- (a) J. A. Pfefferkorn, C. Choi, T. Winters, R. Kennedy, L. Chi, L. A. Perrin, G. Lu, Y.-W. Ping, T. McClanahan, R. Schroeder, M. T. Leininger, A. Geyer, S. Schefzick and J. Atherton, Bioorg. Med. Chem. Lett., 2008, 18, 3338 CrossRef CAS PubMed; (b) M. A. Metwally, M. E. Khalifa and F. A. Amer, Dyes Pigm., 2008, 76, 379 CrossRef CAS PubMed; (c) Y. Li, S. Zhang, J. Yang, S. Jiang and Q. Li, Dyes Pigm., 2008, 76, 508 CrossRef CAS PubMed; (d) H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and K.-I. Saito, J. Pharm. Sci., 1997, 281, 921 CAS; (e) F. Lehmann, M. Holm and S. Laufer, J. Comb. Chem., 2008, 10, 364 CrossRef CAS PubMed; (f) G. Zech, G. Hessler, A. Evers, T. Weiss, P. Florian, M. Just, J. Czech, W. Czechtizky, J. Görlitzer, S. Ruf, M. Kohlmann and M. Nazaré, J. Med. Chem., 2012, 55, 8615 CrossRef CAS PubMed; (g) J.-J. Xiao, M. Liao, M.-J. Chu, Z.-L. Ren, X. Zhang, X.-H. Lv and H.-Q. Cao, Molecules, 2015, 20, 807 CrossRef PubMed.
- (a) S. Muramulla and C.-G. Zhao, Tetrahedron Lett., 2011, 52, 3905 CrossRef CAS PubMed; (b) X. Tang, J. Chang, C. Liu and B. Zhang, Tetrahedron Lett., 2014, 55, 6534 CrossRef CAS PubMed; (c) S. Gogoi and C.-G. Zhao, Tetrahedron Lett., 2009, 50, 2252 CrossRef CAS PubMed.
- (a) A. Mazzanti, T. Calbet, M. Font-Bardia, A. Moyanoc and R. Rios, Org. Biomol. Chem., 2012, 10, 1645 RSC; (b) F. Li, L. Sun, Y. Teng, P. Yu, J. C.-G. Zhao and J.-A. Ma, Chem.–Eur. J., 2012, 18, 14255 CrossRef CAS PubMed; (c) Z. Yang, Z. Wang, S. Bai, X. Liu, L. Lin and X. Feng, Org. Lett., 2011, 13, 596 CrossRef CAS PubMed; (d) M. Šimek, M. Remeš, J. Veselý and R. Rios, Asian J. Org. Chem., 2013, 2, 64 CrossRef PubMed; (e) Z. Wang, Z. Yang, D. Chen, X. Liu, L. Lin and X. Feng, Angew. Chem., 2011, 123, 5030 CrossRef PubMed; (f) J.-W. Wu, F. Li, Y. Zheng and J. Nie, Tetrahedron Lett., 2012, 53, 4828 CrossRef CAS PubMed.
- X.-G. Gao, C.-W. Yang, Z.-Z. Zhang, C.-C. Zeng, X.-Q. Song, L.-M. Hua, R.-G. Zhong and Y.-B. She, Tetrahedron, 2010, 66, 9880 CrossRef CAS PubMed.
- N. Yamaoka, K. Sumida, I. Itani, H. Kubo, Y. Ohnishi, S. Sekiguchi, T. Dohi and Y. Kita, Chem.–Eur. J., 2013, 19, 15004 CrossRef CAS PubMed.
- (a) C. H. Oh, J. S. Kim and H. H. Jung, J. Org. Chem., 1999, 64, 1338 CrossRef CAS; (b) P.-O. Norrby, T. B. Petersen, M. Bielawski and B. Olofsson, Chem.–Eur. J., 2010, 16, 8251 CrossRef CAS PubMed; (c) K. Chen and G. F. Koser, J. Org. Chem., 1991, 56, 5764 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedure for synthesis, characterization data, and 1H, 13C, 19F NMR, IR and HRMS spectra of compounds 3. See DOI: 10.1039/c5ra14368c |
| This journal is © The Royal Society of Chemistry 2015 |