NiMoO4@Co(OH)2 core/shell structure nanowire arrays supported on Ni foam for high-performance supercapacitors†
Weiji Renab,
Di Guoa,
Ming Zhuoa,
Bingkun Guana,
Dan Zhangc and
Qiuhong Li*ab
aKey Laboratory for Micro-/Nano-Optoelectronic Devices of Ministry of Education, School of Physics and Electronics, Hunan University, Changsha 410082, China. E-mail: liqiuhong@xmu.edu.cn; Tel: +86-592-2187198
bPen-Tung Sah Institute of Micro-Nano Science and Technology of Xiamen University, Xiamen, 361005, China
cDepartment of Electronic Engineering, School of Information Science and Engineering, Xiamen University, Xiamen 361005, China
First published on 6th February 2015
Abstract
NiMoO4@Co(OH)2 core/shell structure nanowire arrays (NWAs) supported on Ni foam were successfully fabricated via a facile hydrothermal growth and electrochemical deposition route, and applied in supercapacitors (SCs). The smart combination of Co(OH)2 and NiMoO4 nanostructures in nanowire arrays exhibited greatly enhanced electrochemical performance. Co(OH)2 nanoflakes were uniformly wrapped on the surface of each NiMoO4 nanowire, which increased the capacitance of NiMoO4 NWAs to a high areal capacitance of 2.335 F cm−2 at 5 mA cm−2 and 0.909 F cm−2 at 50 mA cm−2. The electrode also exhibited good cycling ability; 83% of the initial capacity was retained after 5000 cycles at a current density of 20 mA cm−2. These results indicate that the NiMoO4@Co(OH)2 NWAs could be a promising electrode material for high-performance electrochemical capacitors.
1. Introduction
As one of the important electrochemical energy storage devices, supercapacitors (SCs) have attracted extensive research interest due to their excellent performance. Supercapacitors are superior due to their high power density, fast rechargeability, and long cycle life.1,2 As we know the excellent characteristics of supercapacitor are due to the nanostructured electrode materials,3 which have brought about great advancement of new supercapacitor technologies owing to their high surface area and short electron and ion transport pathways.4 In recent years, great efforts have been made to increase the capacitance and energy density of electrode materials. In this regard, many research groups have focused on the rational design of advanced core/shell heterostructures with fascinating synergetic properties.5–15Pseudocapacitive-type electrode materials, especially transition metal oxides5–12 or hydroxides,9,26–34,37 such as ruthenium oxide (RuO2), manganese oxide (MnO2), and nickel oxide (NiO), can provide high energy density relative to that delivered by electrical double layer capacitors using carbon-based active materials. Thus, transition metal oxides have been widely investigated as candidates for the use in supercapacitors in view of their multiple oxidation states for pseudocapacitance generation. However, the experimentally obtainable capacitance values are often considerably lower than the theoretical expectations because of the inadequate use of entire pseudocapacitive materials and limited electrical conductivity of metal oxides at high rates. To overcome the abovementioned shortcomings, metal molybdates, such as CoMoO4, NiMoO4, MnMoO4, have been recently demonstrated to have vastly improved electrochemical performance compared to single component oxides. In particular, rate capability and durability have been significantly enhanced in nickel molybdate (NiMoO4) supercapacitors16–25 because of their high chemical stability and low cost.18 For example, Liu et al.19 demonstrated a NiMoO4·xH2O nanorod supercapacitor with a maximum specific capacitance of 1136 F g−1 at 5 mA cm−2. To date, most of the reported NiMoO4 materials usually show low open space and do not exhibit short diffusion distance, and only the surface part of electroactive materials can be effectively used, which leads to a less satisfactory areal-specific-capacitance (ASC).35 Therefore, it is urgent to find a way to boost the electrochemical utilization and aggrandize ASC pseudocapacitive materials. A smart and rational concept is to directly grow integrated array architectures with the combination of two types of materials and/or nanostructures on conducting substrates as binder-free electrodes for supercapacitors.12,26,34–45 To date, there have been some reported works about 3D core/shell structure for supercapacitors. Despite these achievements, choosing novel suitable electrode materials and their assemblies in appropriate architecture to achieve better performance still remains a challenge due to the complicated synthesis processes.
In this work, we chose NiMoO4 NWAs with high chemical stability as “core” due to their good electron conductivity to provide a direct path for the electron transport, and to create channels for the effective transport of electrolyte, ultrathin Co(OH)2 nanosheet with high theoretical capacity (3000 F g−1) have been chosen as “shell” because of their enlarged surface area to shorten ion diffusion path and provide more efficient contacts between the electrolyte ions and active materials for faradaic energy storage.36 Therefore, such rational design of NiMoO4@Co(OH)2 core/shell structure with fascinating synergistic properties would provide high ASC capacitance, and the possibility of the efficient transport of electrons and ions.
We developed a cost-effective and simple strategy to fabricate NiMoO4@Co(OH)2 core/shell NWAs on Ni foam as a SCs electrode, for the first time, with the synthesis of uniform NiMoO4 nanowires as the “core” via a hydrothermal method and that of ultrathin Co(OH)2 nanoflakes as the “shell” by electrodeposition.26–34 The electrode possessed several advantages such as: (I) NiMoO4 NWAs with high capacitance and stability directly grown on conductive Ni foam serve as both backbone and electron “superhighway” for both charge storage and delivery; (II) both the core NiMoO4 and shell Co(OH)2 materials are good pseudocapacitive materials exhibiting redox reactions in the electrolyte, which contribute to the total energy storage;35 (III) the NiMoO4@Co(OH)2 NWAs are well separated and strongly supported on Ni foam, avoiding the use of polymer binder/conductive additives, which decreases the ohmic polarization. In addition, the Ni foam as substrate with 3D porous structure could provide numerous express electron-transport pathways. More importantly, the micropore of Ni foam could facilitate kinetic ion diffusion and provide more efficient contacts between the electrolyte ions and the active materials for faradaic energy storage;36–45 (IV) the unique NiMoO4@Co(OH)2 core/shell NWAs can provide additional interfaces, where the ultrathin Co(OH)2 nanoflakes are wrapped on the surface of NiMoO4 nanowires to increase high specific surface area and greatly shorten the diffusion distance of electrolytes during the charging/discharging process. In the electrode design, not only “core” and “shell” materials are effectively integrated but also an improvement in the electrochemical performance was realized. In particular, compared with pure NiMoO4 NWAs, the NiMoO4@Co(OH)2 core/shell NWAs exhibited larger ASC (2.335 F cm−2 at 5 mA cm−2), better rate performance, and more stable cycling ability (83% of its initial capacity was retained after 5000 cycles).
2. Experimental section
2.1 Materials synthesis
All the reagents used were of analytical grade and purchased from Tianjin Chemical Reagent Co. and used without any purification process. NiMoO4 NWAs were synthesized by a simple hydrothermal method. Prior to the synthesis, Ni foam substrate (length × diameter × thickness = 3 × 2 × 0.1 cm3) was rinsed with ethanol and distilled water for 30 min.2.2 Characterization of the NiMoO4 and NiMoO4@Co(OH)2 core/shell NWAs
The crystal structure of the samples was characterized by X-ray diffraction (XRD, Cu Kα irradiation) with a SIEMENS D5000 X-ray diffractometer. The morphology and microstructure of the synthesized sample were characterized by scanning electron microscopy (SEM, Hitachi S4800 equipped with an EDS) and transmission electron microscopy (TEM; JEOL-2010 with an accelerating voltage of 200 kV).2.3 Electrochemical measurements of NiMoO4@Co(OH)2 core/shell NWAs
All the electrochemical experiments were performed on a CHI660e electrochemical workstation (Chenhua, Shanghai) containing 2 M KOH aqueous solution as the electrolyte. The NWAs/Ni foam was applied as the working electrode, a standard calomel electrode (SCE) was used as the reference electrode, and a Pt foil was used as the counter-electrode. CV measurements were carried out at a scanning rate of 5–100 mV s−1 between −0.2 and 0.8 V at 25 °C. The specific capacitance was calculated according to the following equation:38,39
 | (1) |
3. Results and discussion
The direct fabrication of NiMoO4@Co(OH)2 core/shell NWAs on Ni foam substrate involves two key steps, as shown in Scheme 1. First, NiMoO4 NWAs were grown on a Ni foam substrate via a hydrothermal reaction. Subsequently, Co(OH)2 ultrathin layers were coated on the surface of NiMoO4 NWAs by electrochemical deposition and NiMoO4@Co(OH)2 core/shell nanowire arrays were obtained. | ||
| Scheme 1 The representative synthetic procedure and structure details of the NiMoO4@Co(OH)2 core/shell NWAs on Ni foam. | ||
Morphologies of the NiMoO4 and NiMoO4@Co(OH)2 NWAs were examined by SEM. The NiMoO4 NWAs uniformly covered the substrate (Fig. S1 in the, ESI†). The low-magnification SEM image, presented in Fig. 1a, shows the products on the skeletons of the Ni foam, which were uniformly distributed. Fig. 1b and c display that the ultrathin Co(OH)2 nanoflakes were wrapped on the NiMoO4 NWAs. Fig. 1d shows that these ultrathin nanoflakes with a thickness of about 10 nm to 20 nm connected with each other to form a stable structure. In addition, to investigate the height of the NWA on Ni foam, the cross-sectional SEM image of the NiMoO4@Co(OH)2 NWAs was obtained, which is shown in Fig. S2.† Obviously, the height of the NWA was about 2.2 μm.
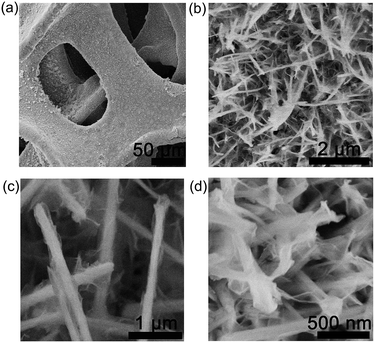 | ||
| Fig. 1 Low-magnification (a) and high-magnification (b–d) SEM images of the NiMoO4@Co(OH)2 NWAs on Ni foam. | ||
Fig. 2a shows the XRD patterns of the NiMoO4@Co(OH)2 NWAs on Ni foam (XRD patterns for NiMoO4 NWAs are shown in Fig. S3†). XRD pattern indicates that the diffraction peaks can be indexed to NiMoO4, which is in agreement with the reported values of JCPDS no. 86-0361. Moreover, the characteristic (100) peak at 32.5° and (011) peak at 38.0° are the typical peaks of Co(OH)2 (JCPDS card no. 74-1057), indicating the successful formation of Co(OH)2. The energy dispersive X-ray spectroscopy (EDS) analysis, as shown in Fig. S4,† demonstrates the existence of Co and Mo elements in the obtained material. Transmission electron microscopy (TEM) observations were carried out to further investigate the structure of the NiMoO4@Co(OH)2 NWAs. Fig. 2b and c depict the low-magnification TEM image of the NiMoO4@Co(OH)2 NWAs after it was subjected to a strong ultrasonic vibration in ethanol, which is consistent with the SEM observation. The high-resolution TEM (HRTEM) image, shown in Fig. 2d, reveals the well-resolved lattice planes of the NiMoO4@Co(OH)2 NWAs, and the lattice fringes show an inter-planar spacing of 0.35 nm corresponding to the (−112) crystal planes of NiMoO4. The lattice fringes also show an inter-planar spacing of 0.27 nm corresponding to the (100) crystal plane of Co(OH)2. The SAED pattern (inset in Fig. 2d) further shows a well-defined lattice, confirming the polycrystalline characteristics.
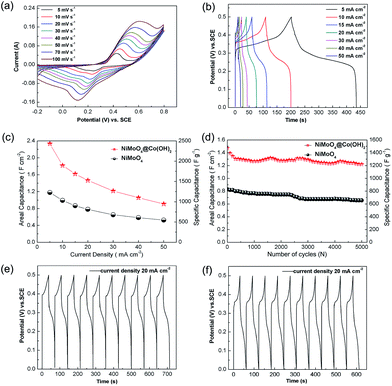
The electrochemical measurements were performed in a three-electrode configuration with 2 M KOH as the electrolyte. Typical CV curves of the NiMoO4@Co(OH)2 NWAs under various scan rates are shown in Fig. 3a. The CV curves of NiMoO4 are also shown in Fig. S5a.† A pair of strong redox peaks can be found in each CV curve, indicating that the capacitance characteristic was mainly governed by faradaic redox reactions. The two primary redox couples mainly governed by redox mechanism can be expressed as follows:19,24,28,34
| Ni2+ ⇔ Ni3+ + e−; | (2) |
| Co(OH)2 + OH− ⇔ CoOOH + H2O + e−; | (3) |
| CoOOH + OH− ⇔ CoO2 + H2O + e−. | (4) |
Fig. 3b shows the galvanostatic charge/discharge profiles of the NiMoO4@Co(OH)2 NWAs at different current densities ranging from 5 to 50 mA cm−2 (Fig. S5c† for NiMoO4 NWAs). For comparison, the CV curves and charging/discharging voltage profiles of pristine NiMoO4 NWs are also shown in the Fig. S5b and 5d in ESI.† The voltage plateaus can be seen at approximately 0.2 to 0.3 V, which matched well with the peaks observed in the CV curve. On the basis of eqn (1), the specific capacitances of the NiMoO4@Co(OH)2 NWAs were calculated to be 2.335, 1.820, 1.620, 1.472, 1.215, 1.056 and 0.909 F cm−2 (2122.7, 1654.5, 1472.7, 1338.2, 1104.5 and 960, 826.4 F g−1) at the current densities of 5, 10, 15, 20, 30, 40 and 50 mA cm−2 (4.5, 9.1, 13.6, 18.2, 27.3, 36.4 and 45.4 A g−1), respectively. These results indicated that the NiMoO4@Co(OH)2 NWAs exhibited high capacitance and good rate capability. The areal specific capacitance (Csp) based on the mass of all active materials versus current density plots are also shown in Fig. 3c. At 5 mA cm−2, the NiMoO4@Co(OH)2 NWAs can achieve a specific capacitance of 2.335 F cm−2; moreover, even at a current density of 50 mA cm−2, they can deliver a Csp of 0.909 F cm−2. However, the NiMoO4 NWAs can achieve a specific capacitance of 1.229 F cm−2 and 0.549 F cm−2 at the current densities of 5 mA cm−2 and 50 mA cm−2, respectively. Through the comparative analysis of Fig. S5b and d† it can be inferred that the core/shell structure has a better electrochemical performance. This was mainly attributed to the great contribution of ultrathin Co(OH)2 nanoflakes, which enhanced electrochemical redox reaction to boost the charge storage capability.40 In addition, the long-term cycle stability of the NiMoO4 and NiMoO4@Co(OH)2 NWAs were also investigated by repeating the chronopotentiometry (CP) tests at a current density of 20 mA cm−2 for 5000 cycles, and the results are presented in Fig. 3d. The areal capacitance of NiMoO4@Co(OH)2 NWAs electrode reached 1.472 F cm−2 in the first cycle and it gradually decreased to 1.221 F cm−2 after 5000 cycles, resulting in an overall capacitance loss of only 17%. In contrast, the areal capacitance of NiMoO4 NWAs electrode reached 0.8192 F cm−2 in the first cycle and it gradually decreased to 0.6553 F cm−2 after 5000 cycles, resulting in an overall capacitance loss of about 20%. Obviously, the NiMoO4@Co(OH)2 NWAs electrode displayed higher areal capacitance and better cycling stability than the NiMoO4 NWAs electrode.
Fig. 3e and f illustrate the first and last ten cycles of representative voltage profiles of the NiMoO4@Co(OH)2 NWAs electrode from galvanostatic charge/discharge measurements, respectively. Although, there was attenuation of Csp from the NiMoO4@Co(OH)2 NWAs, the final Csp (1.2208 F cm−2) was still better than that of pure NiMoO4 NWAs (0.8192 F cm−2). The capacitance loss occurred mainly during the first several hundred cycles and then remained stable after this stage. The decay of the capacitance was generally explained by the collapse of the mechanical arrays and the dissolution of some electrode material during the cycling.41,42 The corresponding coulombic efficiencies were obtained from the first cycles to 5000 cycles at 20 mA cm−2 during the discharge/charge processes. Most of the values were over 99%, as shown in Fig. S6.† Undoubtedly, such good performance of the NiMoO4@Co(OH)2 NWAs can be attributed to the Co(OH)2 decorated on NiMoO4 NWAs, which increased the surface to volume ratio of the electrode. Their high surface areas and open spaces can not only provide more sites for the adsorption of ions, but also facilitate the fast intercalation and de-intercalation of the active species.43 Multiple valence states of transition metal cobalt provides more advantages for redox reaction, and cobalt salts exhibit good electrochemical performance in pseudocapacitors. The capacitance value of NiMoO4@Co(OH)2 NWAs electrode was greatly enhanced compared with previously reported values for NiMoO4 and its composite electrode materials, as shown in Table S1,† such as, NiMoO4·H2O nanoclusters (680 F g−1 at 1 A g−1), CoMoO4–NiMoO4 nanobundles (1039 F g−1 at 2.5 mA cm−2), nano β-NiMoO4–CoMoO4·xH2O composites (1472 F g−1 at 5 mA cm−2), and GO-1D NiMoO4·nH2O nanorods (367 F g−1 at 5 A g−1). This result further proved the great advantages of the NiMoO4@Co(OH)2 core/shell NWAs. In addition, the Ni foam substrate with micro-meter scale pores can improve the pseudocapacitive performance of electrodes.46,47 Electro-active materials loaded on the concave geometry Ni foam substrate can suppress collapse and aggregation in contrast to the directly generated nanomaterials. The performance of the NiMoO4@Co(OH)2 core/shell NWAs and NiMoO4 NWAs as SCs have been summarized by a radar plot in Fig. 4; we can comprehensively evaluate the metrics of the as-prepared supercapacitor electrodes, including cycle life, internal resistance, and capacitance.38 All these results reveal the better performance of the NiMoO4@Co(OH)2 NWAs electrode.
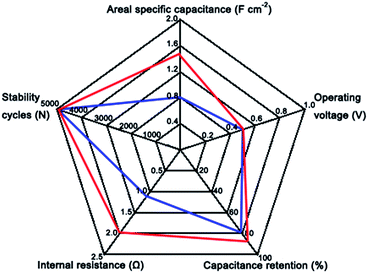 | ||
| Fig. 4 Radar plots to compare the supercapacitor performance of NiMoO4@Co(OH)2 core/shell NWAs (red curves) and NiMoO4 NWAs (blue curves) on Ni foam. | ||
Fig. 5 is the schematic illustration of electron path about the NiMoO4@Co(OH)2 NWAs on Ni foam, which provides considerably shorter paths for electron transport and a solid structure to improve their capacitive performance. In practice, the NiMoO4@Co(OH)2 NWAs produce more ion migration channels to promote the electrolyte ion transport in three-dimensional space, and not just at the edge of the NiMoO4 NWAs. To further investigate the detailed electrochemical characteristics of the supercapacitor electrodes, electrochemical impedance spectroscopy (EIS) measurements were performed in the frequency range of 0.01 to 100 kHz, and the corresponding impedance Nyquist plots are shown in Fig. 6 with a well-fitted equivalent circuit showing the components of the whole impedance (Rb is the bulk solution resistance, Rct is the faradaic interfacial charge-transfer resistance, and W is the Warburg impedance).43,48 On comparing A1 with B1, it can be observed that the Rb increased from 1.13 Ω to 2.0 Ω at the high-frequency intercept of the real axis (from the inset), suggesting that the NiMoO4@Co(OH)2 NWAs electrode showed a bigger Rb than the NiMoO4 NWAs electrode. B1 displayed lower charge-transfer resistance and Warburg impedance than A1. Therefore, the NiMoO4@Co(OH)2 NWAs improved the electron transfer and the occurrence of redox reaction, thus increasing the capacitance.43 On Comparing B1 with B2, it can be observed that the internal resistance (Rb) increased from 2.0 Ω to 2.65 Ω at the high-frequency intercept of the real axis after 5000 cycles, which is not an obvious change. The Warburg impedance was almost the same after the cycle test, indicating the stability of the electrode. The low Rct suggested that the electrode has a large electroactive surface area.48 The large electroactive surface area was owing to the large specific surface area and high electrical conductivity of the ultrathin Co(OH)2 nanoflakes. The slight increase in the Rct after 5000 cycles was probably due to the loss of adhesion of some active material with the current collector, and the corrosion of the current collector caused by the dissolved oxygen in the electrolytes during the charge/discharge cycling or matching problems.49 Compared with NiMoO4 NWAs electrode material, the superior electrochemical performances of the NiMoO4@Co(OH)2 NWAs were mainly attributed to the assembly of ultrathin Co(OH)2 nanoflakes. These interconnected ultrathin nanoflakes have the following advantages: first, high conductivity and high specific capacitance are advantageous for fast electron transport; second, the ultrathin nanoflakes structure could provide efficient ion and electron transport, facilitating faster kinetics and resulting in high charge/discharge capacities even at high current densities; moreover, the ultrathin nanoflakes characteristics of the nanostructure resulted in a large surface area, providing more electroactive sites for faradaic energy storage. In addition, the morphological analysis of the electrodes tested after many cycles could provide interesting structural and electrochemical information. As shown in Fig. S7,† by SEM characterization, it can be observed that the nanostructures were well maintained with a little structural deformation after 5000 cycles. Co(OH)2 nanoflakes were still coated on the surface of the NiMoO4 nanowires with little structural collapse during the oxidation–reduction reaction. NiMoO4@Co(OH)2 NWAs electrode synthesized by electrochemical deposition method did not easily collapse and fell after thousands of cycles, demonstrating the good cycling ability of the arrays. Together with the electrochemical measurements, these results confirm the superiority of our rational design of the NiMoO4@Co(OH)2 core/shell NWAs nanostructure.
 | ||
| Fig. 5 Proposed schematic illustration of the high-performance of the NiMoO4@Co(OH)2 NWAs electrode. | ||
4. Conclusions
We have demonstrated a facile and scalable strategy for the direct growth of NiMoO4@Co(OH)2 nanowire arrays on Ni foams via hydrothermal reaction and electrochemical deposition, and its electrochemical properties were investigated. Compared with the pure NiMoO4 NWAs, the NiMoO4@Co(OH)2 core/shell NWAs exhibited higher electrical capacity and reliability. Ultrathin Co(OH)2 nanoflakes were assembled on NiMoO4 nanowire arrays, resulting in a high specific surface area, rich accessible electroactive sites, short ion transport pathways, and superior electron collection efficiency. Taking advantages of the synergetic effects, the NiMoO4@Co(OH)2 core/shell NWAs exhibited high ASC, desirable cycle life and rate performance. Our results indicate that the NiMoO4@Co(OH)2 NWAs are promising for high-performance SCs. We believe that our work can contribute to the rational design concept of electrode materials for extensive application.Acknowledgements
This work was partly supported from the National Natural Science Foundation of China (Grant no. 61376073, 61107023) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120161110016, 20110121120020).Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- Y. Wang, Z. Hong, M. Wei and Y. Xia, Adv. Funct. Mater., 2012, 22, 5185 CrossRef CAS.
- A. Arico, P. Bruce, B. Scrosati, J. Tarascon and W. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- G. Zhang, H. Wu, H. Hoster, M. Chan-Park and X. Lou, Energy Environ. Sci., 2012, 5, 9453 CAS.
- X. Lu, T. Zhai, X. Zhang, Y. Shen, L. Yuan, B. Hu, L. Gong, J. Chen, Y. Gao, J. Zhou, Y. Tong and Z. Wang, Adv. Mater., 2012, 24, 938 CrossRef CAS.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao and H. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- J. Liu, J. Jiang, M. Bosmanc and H. Fan, J. Mater. Chem., 2012, 22, 2419 RSC.
- H. Jiang, C. Li, T. Sun and J. Ma, Chem. Commun., 2012, 48, 2606 RSC.
- G. Li, Z. Wang, F. Zheng, Y. Ou and Y. Tong, J. Mater. Chem., 2011, 21, 4217 RSC.
- J. Kim, K. Zhu, Y. Yan, C. Perkins and A. Frank, Nano Lett., 2010, 10, 4099 CrossRef CAS PubMed.
- Q. Yang, Z. Lu, T. Li, X. Sun and J. Liu, Nano Energy, 2014, 7, 170 CrossRef CAS PubMed.
- L. Mai, F. Yang, Y. Zhao, X. Xu, L. Xu and Y. Luo, Nat. Commun., 2011, 2, 2041 Search PubMed.
- J. Yan, E. Khoo, A. Sumboja and P. Lee, ACS Nano, 2010, 4, 4247 CrossRef CAS PubMed.
- L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215 CrossRef CAS PubMed.
- D. Cai, D. Wang, B. Liu, Y. Wang, Y. Liu, L. Wang, H. Li, H. Huang, Q. Li and T. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12905 CAS.
- M. Liu, L. Kong, C. Lu, X. Ma, X. Li, Y. Luo and L. Kang, J. Mater. Chem. A, 2013, 1, 1380 CAS.
- B. Senthilkumar, D. Meyrick, Y. Lee and R. Selvan, RSC Adv., 2013, 3, 16542 RSC.
- M. Liu, L. Kang, L. Kong, C. Lu, X. Ma, X. Li and Y. Luo, RSC Adv., 2013, 3, 6472 RSC.
- B. Senthilkumar, K. Sankar, R. Selvan, M. Danielle and M. Manickam, RSC Adv., 2013, 3, 352 RSC.
- D. Cai, B. Liu, D. Wang, Y. Liu, L. Wang, H. Li, Y. Wang, C. Wang, Q. Li and T. Wang, Electrochim. Acta, 2014, 115, 358 CrossRef CAS PubMed.
- H. Wan, J. Jiang, X. Ji, L. Miao, L. Zhang, K. Xu, H. Chen and Y. Ruan, Mater. Lett., 2013, 108, 164 CrossRef CAS PubMed.
- D. Ghosh, S. Giri and C. Das, Nanoscale, 2013, 5, 10428 RSC.
- D. Guo, P. Zhang, H. Zhang, X. Yu, J. Zhu, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 9024 CAS.
- D. Cai, B. Liu, D. Wang, Y. Liu, L. Wang, H. Li, Y. Wang, C. Wang, Q. Li and T. Wang, Electrochim. Acta, 2014, 125, 294 CrossRef CAS PubMed.
- X. Xia, J. Tu, Y. Zhang, J. Chen, X. Wang, C. Gu, C. Guan, J. Luo and H. Fan, Chem. Mater., 2012, 24, 3793 CrossRef CAS.
- J. Chang, C. Wu and I. Sun, J. Mater. Chem., 2010, 20, 3729 RSC.
- L. Kong, M. Liu, J. Lang, M. Liu, Y. Luo and L. Kang, J. Solid State Electrochem., 2011, 15, 571 CrossRef CAS.
- Z. Yu, Y. Dai and W. Chen, J. Chin. Chem. Soc., 2010, 57, 423 CAS.
- W. Zhou, D. Zhao, M. Xu and H. Li, Electrochim. Acta, 2008, 53, 7210 CrossRef CAS PubMed.
- A. Jagadale, V. Kumbhar, D. Dhawale and C. Lokhande, Electrochim. Acta, 2013, 98, 32 CrossRef CAS PubMed.
- S. Kandalkar, H. Lee, H. Chae and C. Kim, Mater. Res. Bull., 2011, 46, 48 CrossRef CAS PubMed.
- V. Gupta, T. Kusahara, H. Toyama, S. Gupta and N. Miura, Electrochem. Commun., 2007, 9, 2315 CrossRef CAS PubMed.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. Wang and M. Liu, Nano Lett., 2013, 13, 3135 CrossRef CAS PubMed.
- L. Yu, G. Zhang, C. Yuan and X. Lou, Chem. Commun., 2013, 49, 137 RSC.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078 CrossRef CAS PubMed.
- H. Chen, L. Hu, Y. Yan, R. Che, M. Chen and L. Wu, Adv. Energy Mater., 2013, 3, 1636 CrossRef CAS.
- G. Xiong, C. Meng, R. Reifenberger, P. Irazoqui and T. Fisher, Electroanalysis, 2014, 26, 30 CrossRef CAS.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905 CrossRef CAS PubMed.
- G. Zhang, T. Wang, X. Yu, H. Zhang, H. Duan and B. Lu, Nano Energy, 2013, 2, 586 CrossRef CAS PubMed.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li and T. Wang, Nano Energy, 2014, 3, 36 CrossRef CAS PubMed.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- H. Zhang, Y. Chen, W. Wang, G. Zhang, M. Zhuo, H. Zhang, T. Yang, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 8593 CAS.
- G. Xiong, C. Meng, R. Reifenberger, P. Irazoqui and T. Fisher, Adv. Energy Mater., 2014, 4, 1300515 Search PubMed.
- G. Xiong, K. Hembram, R. Reifenberger and T. Fisher, J. Power Sources, 2013, 227, 254 CrossRef CAS PubMed.
- G. Yang, C. Xu and H. Li, Chem. Commun., 2008, 6537 RSC.
- W. Zhou, M. Xu, D. Zhao, C. Xu and H. Li, Microporous Mesoporous Mater., 2009, 117, 55 CrossRef CAS PubMed.
- M. Wu and H. Hsieh, Electrochim. Acta, 2008, 5, 3427 CrossRef PubMed.
- Z. Fan, J. Yan, T. Wei, L. Zhi, G. Ning, T. Li and F. Wei, Adv. Funct. Mater., 2011, 21, 2366 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The XRD patterns and SEM images of NiMoO4 NWs; the cross section image of the NiMoO4@Co(OH)2 NWAs; the EDS patterns of NiMoO4@Co(OH)2 NWAs; CV curves and galvanostatic discharge curves of NiMoO4 NWs and comparison of the NiMoO4@Co(OH)2 and NiMoO4 NWAs electrode; coulombic efficiency of the NiMoO4@Co(OH)2 NWAs electrode. See DOI: 10.1039/c5ra01604e |
| This journal is © The Royal Society of Chemistry 2015 |