Origin of the 29Si NMR chemical shift in R3Si–X and relationship to the formation of silylium (R3Si+) ions†
Abstract
The origin in deshielding of 29Si NMR chemical shifts in R3Si–X, where X = H, OMe, Cl, OTf, [CH6B11X6], toluene, and OX (OX = surface oxygen), as well as iPr3Si+ and Mes3Si+ were studied using DFT methods. At the M06-L/6-31G(d,p) level of theory the geometry optimized structures agree well with those obtained experimentally. The trends in 29Si NMR chemical shift also reproduce experimental trends; iPr3Si–H has the most shielded 29Si NMR chemical shift and free iPr3Si+ or isolable Mes3Si+ have the most deshielded 29Si NMR chemical shift. Natural localized molecular orbital (NLMO) analysis of the contributions to paramagnetic shielding (σp) in these compounds shows that Si–R (R = alkyl, H) bonding orbitals are the major contributors to deshielding in this series. The Si–R bonding orbitals are coupled to the empty p-orbital in iPr3Si+ or Mes3Si+, or to the 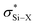 orbital in R3Si–X. This trend also applies to surface bound R3Si–OX. This model also explains chemical shift trends in recently isolated tBu2SiH2+, tBuSiH2+, and SiH3+ that show more shielded 29Si NMR signals than R3Si+ species. There is no correlation between isotropic 29Si NMR chemical shift and charge at silicon.
orbital in R3Si–X. This trend also applies to surface bound R3Si–OX. This model also explains chemical shift trends in recently isolated tBu2SiH2+, tBuSiH2+, and SiH3+ that show more shielded 29Si NMR signals than R3Si+ species. There is no correlation between isotropic 29Si NMR chemical shift and charge at silicon.

- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...
