Boosting the catalytic performance of metalloporphyrin-based covalent organic frameworks via coordination engineering for CO2 and O2 reduction†
Abstract
Metalloporphyrin-based covalent organic frameworks (Por-COFs) are emerging as electrocatalysts; however, so far experiments have primarily focused on M–N4-coordinated Por-COFs. A wealth of coordination modified systems with potential catalytic capability remains to be explored. Herein, we report a proof-of-concept computational study on the coordination engineering of Por-COFs as electrocatalysts for reducing CO2 and O2. Systematic density functional theory calculations were performed on 15 types of heteroatomic Por-COFs, featuring M–NxOySz (M = Fe, Co, and Ni; x + y + z = 4) centers. Calculations predicted that the Co–N2O2-Por-COF is an optimal CO2-to-CO catalyst candidate (limiting potential 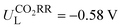 ) and the Ni–N2S2-Por-COF is an optimal O2-to-H2O catalyst candidate (overpotential ηORR = 0.46 V); both heteroatomic-Por-COFs display better catalytic activity and selectivity than their corresponding parent M–N4-Por-COFs. Electronic structure analysis attributes the enhanced catalytic performance to the additional stabilization, endowed by the heteroatoms, of critical reaction intermediates. Furthermore, feature importance analysis based on machine learning models confirmed that the interplay between the central metal and the coordinated atoms is crucial for catalytic performance. This work predicts new Por-COFs as electrocatalysts for CO2 and O2 reduction and showcases the great potential of coordination regulation strategies in designing high-performance COF-based electrocatalysts.
) and the Ni–N2S2-Por-COF is an optimal O2-to-H2O catalyst candidate (overpotential ηORR = 0.46 V); both heteroatomic-Por-COFs display better catalytic activity and selectivity than their corresponding parent M–N4-Por-COFs. Electronic structure analysis attributes the enhanced catalytic performance to the additional stabilization, endowed by the heteroatoms, of critical reaction intermediates. Furthermore, feature importance analysis based on machine learning models confirmed that the interplay between the central metal and the coordinated atoms is crucial for catalytic performance. This work predicts new Por-COFs as electrocatalysts for CO2 and O2 reduction and showcases the great potential of coordination regulation strategies in designing high-performance COF-based electrocatalysts.

- This article is part of the themed collection: FOCUS: Recent progress on electrocatalytic CO2 reduction


 Please wait while we load your content...
Please wait while we load your content...