Sergey Volkov, Lukáš Kumprecht, Miloš Buděšínský, Martin Lepšík, Michal Dušek and Tomáš Kraus
Org. Biomol. Chem., 2015,13, 2980-2985
DOI:
10.1039/C4OB02464H,
Paper
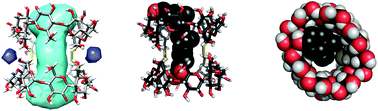
Per(2,3,6-tri-O-benzyl)-γ-cyclodextrin was debenzylated by DIBAL-H to produce a mixture of C6I,C6IV and C6I,C6V isomeric diols, which were separated and isolated. The C2-symmetrical C6I,C6V diol was transformed into dithiol and dimerized to produce a γ-cyclodextrin duplex structure. A crystal structure revealed tubular cavity whose peripheries are slightly elliptically distorted. The solvent accessible volume of the cavity of the γ-CD duplex is about 740 Å3. Due to this large inner space the duplex forms very stable inclusion complexes with steroids; bile acids examined in this study show binding affinities to the γ-cyclodextrin duplex in the range of 5.3 × 107 M−1–1.9 × 108 M−1.