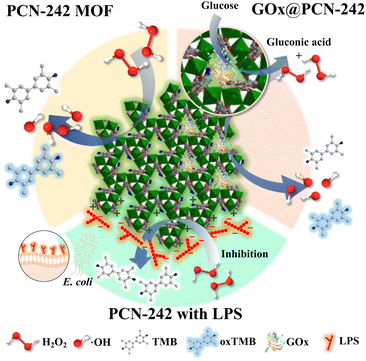
Dual-functional PCN-242 (Fe2Co) MOF for sensitive bacterial endotoxin detection†
Sivasankar
Kulandaivel
 a,
Yung-Kang
Lu
a,
Chia-Her
Lin
a,
Yung-Kang
Lu
a,
Chia-Her
Lin
 *ab and
Yi-Chun
Yeh
*ab and
Yi-Chun
Yeh
 *a
*a
aDepartment of Chemistry, National Taiwan Normal University, Taipei 11677, Taiwan. E-mail: chiaher@mx.nthu.edu.tw; yichuny@ntnu.edu.tw
bDepartment of Chemistry, National Tsing Hua University, Hsinchu 300044, Taiwan
First published on 12th November 2024
Abstract
Endotoxin detection is paramount for monitoring bacterial contamination in food, pharmaceuticals, and clinical diagnostics. The limulus amebocyte lysate (LAL) test, which relies on horseshoe crab blood, has long been the gold standard for endotoxin detection. However, the widespread adoption of this method is constrained by ethical concerns and the high costs associated with harvesting endangered species. Although nanozyme-based colorimetric methods present a more cost-effective and straightforward alternative, their application is limited by suboptimal selectivity and sensitivity. In this study, we report the synthesis and rigorous characterization of the bimetallic PCN-242 (Fe2Co) metal–organic framework (MOF), synthesized using 2-amino terephthalic acid and a pre-synthesized [Fe2Co(μ3-O)(CH3COO)6] cluster. Steady-state kinetic analyses revealed that PCN-242 (Fe2Co) MOF exhibits a significantly higher affinity for hydrogen peroxide (H2O2) compared to horseradish peroxidase (HRP) and other iron-based MOFs. The development of a PCN-242 (Fe2Co)-based colorimetric sensor demonstrated a low limit of detection (LOD) of 1.36 μg mL−1 for endotoxins, with excellent selectivity and reproducibility, thereby enabling effective detection of bacterial endotoxins. Recognizing the potential of the PCN-242 (Fe2Co) MOF beyond endotoxin detection, we explored its utility in glucose biosensing. Moreover, incorporating glucose oxidase (GOx) into the PCN-242 (Fe2Co) MOF framework further enhanced its peroxidase-like catalytic activity. This integration enabled sensitive glucose detection, achieving LODs of 4.24 μM for glucose and 2.2 μM for H2O2 within a linear range of 1 to 150 μM. The dual functionality of PCN-242 (Fe2Co) MOF as a peroxidase mimic and biosensor platform highlights its potential for advanced catalytic and diagnostic applications, offering a versatile and ethical alternative to conventional methods.
Introduction
Metal–organic frameworks (MOFs), porous coordination polymers, consist of inorganic metal nodes and organic linkers.1 They have garnered significant interest across various fields such as gas storage, separation, catalysis, sensing, and biomedicine due to their vast surface area, diverse architectures, and customizable functions.2,3 An infinite number of framework structures can be generated using metal cations or clusters as building blocks. MOFs featuring trinuclear [M3(μ3-O)] (M = Fe3+, Cr3+, Al3+, Sc3+, In3+, etc.) clusters are particularly appealing due to their exceptional chemical stability and structural tunability.4–6 In these structures, each M3+ atom coordinates with four carboxylate O, a (μ3-O), and a terminal ligand, with the remaining M3+ coordinated to a counterion and solvent molecules.7 Notable examples include the MIL series (MIL-100, MIL-101, and MIL-88) and the PCN series (PCN-333, PCN-250, PCN-888), widely applied in gas storage, separation, water adsorption, catalysis, and drug delivery.8–13 Heterometals can be substituted for one of the Fe3+ atoms to further adjust the characteristics of [Fe3(μ3-O)]-based MOFs.14[Fe2M(μ3-O)(CH3COO)6] has long been recognized as a fundamental essential carboxylate.15 Their remarkable solubility hints at their potential as building blocks in various applications. Additionally, the robust electrostatic interactions between Fe3+ and μ3-O2− make these clusters inherently stable while allowing for carboxylate substitution under solvothermal conditions.16 Using [Fe2M(μ3-O)(CH3COO)6] as a precursor allows for the direct synthesis of bimetallic MOFs through a simple ligand substitution process, with framework growth following conventional stepwise reactions.17 Previous studies have shown that ligand substitution and dissociation can be finely controlled by adjusting concentrations and introducing competitive chemicals.14 The D3h cluster's configuration, with six carboxylate arms in a trigonal prismatic arrangement, facilitates the formation of three-dimensional frameworks easily.18
The surge of interest in nanozymes arises from their impressive physicochemical properties as nanomaterials and remarkable catalytic activity.19–22 They are easy to fabricate and exhibit broad applicability, tunable catalytic activity, and cost-effective production compared to natural enzymes.23,24 Following the pioneering discovery of Fe3O4 nanoparticles demonstrating peroxidase (POD) activity a decade ago, researchers have uncovered numerous synthetic nanozymes.25,26 Particularly, MOFs have garnered attention for their capacity to self-assemble organic ligands around metal nodes, forming crystalline structures with unique microstructures characterized by cavities, adjustable porosity, and increased surface area.27 Among MOFs, Fe-MOFs have emerged as frontrunners due to their superior peroxidase catalytic activity, prompting significant exploration into the potential of equivalent secondary building units (SBUs) in Fe-MOF platforms.28
Endotoxins, or lipopolysaccharides (LPS), are integral components of the outer membrane of Gram-negative bacteria and represent a significant health hazard due to their potent pyrogenic effects in humans.29–31 Comprising a hydrophobic domain known as lipid A (or endotoxin), a nonrepeating oligosaccharide, and a distal polysaccharide, LPS are structurally complex molecules. They are a leading cause of sepsis, a life-threatening condition with a mortality rate of 42%, contributing to one in five deaths in intensive care units.32,33 Given the severe implications of endotoxin exposure, stringent monitoring of endotoxin levels is mandated across various industrial sectors, including food and pharmaceuticals.
Endotoxins are ubiquitous in environmental matrices such as air and water.34 Although low concentrations may not elicit a substantial immune response, elevated levels in contaminated environments pose severe risks to animal and human health.35 Consequently, precise and reliable detection of endotoxins is of paramount importance. Traditional methods for endotoxin detection include the rabbit pyrogen test (RPT) and the limulus amebocyte lysate (LAL) test.36 The RPT, which assesses febrile responses following intravenous endotoxin administration in rabbits, presents significant ethical issues and is prone to outcome variability.37 The LAL test, which detects endotoxins through a gel-clot reaction involving horseshoe crab blood, offers greater accuracy but faces ethical and sustainability challenges due to its reliance on harvesting horseshoe crabs.38 Given these limitations, there is a pressing need for innovative, ethical, and cost-effective alternatives for endotoxin detection.
We developed a novel sensor based on PCN-242 (Fe2Co) MOF in response to these limitations, leveraging its peroxidase-mimicking activity for endotoxin detection. Since endotoxins maintain a stable negative charge in solution, they can form a protective layer on the MOF surface, inhibiting peroxidase-like reactions (Scheme 1). This inhibition forms the basis for a colorimetric detection method, where absorbance changes correlate linearly with the endotoxin concentration, offering a simplified and effective alternative to traditional assays.
Additionally, inspired by the cascade catalytic processes of natural enzymes, we explored glucose detection using a glucose oxidase (GOx)-modified PCN-242 MOF. GOx catalyzes glucose oxidation to produce H2O2, which is further decomposed by the MOF's peroxidase-like activity, facilitating a colorimetric response (Scheme 1). While glucose detection is crucial for managing diabetes, existing enzyme-based methods often struggle with the complexity of physiological fluids due to enzyme instability. The integration of GOx with the PCN-242 MOF addresses these challenges, offering enhanced stability and sensitivity.
The bimetallic PCN-242 (Fe2Co) MOF, synthesized using pre-formed clusters, exhibits a well-defined crystalline structure and uniform particle size, optimizing its enzymatic activity. By incorporating both peroxidase and GOx activities, this MOF-based nanozyme is a versatile platform for endotoxin and glucose detection (Scheme 1). The developed colorimetric sensors are simple and cost-effective and demonstrate significant potential for applications in clinical diagnostics, food safety, and pharmaceutical quality control, surpassing the limitations of traditional methods such as the LAL test.
Experimental
Chemicals and materials
All chemicals were procured from commercial vendors and used without further purification.Synthesis of PCN-242 (Fe2Co) MOF
The method for preparing Fe2Co(μ3-O)(CH3COO)6—referred to as the Fe2Co cluster in MOF synthesis—involves the following steps:14 A solution comprising iron(III) nitrate nonahydrate (8 g, 0.02 mol) and cobalt nitrate hexahydrate (29 g, 0.1 mol) in water (70 ml) was filtered and stirred. This solution was combined with another solution containing sodium acetate (25.3 g, 0.308 mol) in water (70 ml). After stirring the mixture for 12 hours at room temperature, the resulting brown precipitate was filtered and left to dry in the open air at 70 °C.The synthesis of PCN-242 (Fe2Co) involved ultrasonically dissolving 50 mg of NH2BDC, 50 mg of Fe2Co cluster, and 0.1 ml of CH3COOH in 5 ml of DMF in a Pyrex vial. The mixture was then heated to 150 °C. The target temperature was reached in 2 hours, maintaining 150 °C for 12 hours and cooling down for 15 hours. After cooling, centrifugation was carried out at 4000 rpm for 10 minutes, with subsequent washing three times with DMF and methanol, each followed by centrifugation. Finally, the product was oven-dried at 75 °C for one day. The resulting brown MOF powder was stored in a sample vial for future use.
Peroxidase-like activity of PCN-242 (Fe2Co) MOF
The following method was used to detect H2O2 and determine the activity of the peroxidase nanozyme: a 1.6 mL microcentrifuge tube was filled with a reaction mixture created by adding 200 μL of 1 mg mL−1 PCN-242 MOF in buffer 10 mM NaAc–HAc (pH 3.6), 200 μL of 1 mM TMB, and various quantities of H2O2 solution in that order.28 After 20 minutes of incubation at 37 °C, the mixture was centrifuged for two minutes at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The resultant solution was then examined at room temperature using UV-vis spectroscopy in the 300–700 nm range. Measurements of the absorbance at 652 nm were made for quantitative analysis. By measuring the absorbance of the reaction product at 652 nm using an extinction coefficient of 16
000 rpm. The resultant solution was then examined at room temperature using UV-vis spectroscopy in the 300–700 nm range. Measurements of the absorbance at 652 nm were made for quantitative analysis. By measuring the absorbance of the reaction product at 652 nm using an extinction coefficient of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1, the activity of the nanozyme was verified.27 The peroxidase reaction kinetics were determined using the Michaelis–Menten equation.
300 M−1 cm−1, the activity of the nanozyme was verified.27 The peroxidase reaction kinetics were determined using the Michaelis–Menten equation.| V = Vmax × [S]/(([S] + Km)) |
Experiment for endotoxin inhibition
The endotoxins (lipopolysaccharide/LPS) enzymatic inhibition activity on PCN-242 MOF was optimized. 200 μL of PCN-242 MOF acetic acid buffer solution (0.25 mg mL−1, pH 3.6) was mixed with 200 μL of various concentrations of endotoxins and incubated at 37 °C for approximately 10 minutes. Subsequently, 200 μL of 1 mM H2O2 and 1 mM TMB were sequentially added, and the reaction proceeded for 20 minutes. After the reaction, the solution was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 minutes, and 250 μL of the supernatant was transferred to a 96-well plate for absorbance measurement at 652 nm. The absorbance values were compared to the blank to calculate the limit of detection (LOD). The same method evaluated the inhibitory effects of various substances, including endotoxins, chitosan, glucose, sucrose, glycine, serine, lysine, and aspartic acid, on PCN-242 MOF peroxidase activity. Except for chitosan (10 μg mL−1), all substances were tested at a 100 μg mL−1 concentration. Only endotoxins demonstrated a measurable response with PCN-242 MOF.
000 rpm for 2 minutes, and 250 μL of the supernatant was transferred to a 96-well plate for absorbance measurement at 652 nm. The absorbance values were compared to the blank to calculate the limit of detection (LOD). The same method evaluated the inhibitory effects of various substances, including endotoxins, chitosan, glucose, sucrose, glycine, serine, lysine, and aspartic acid, on PCN-242 MOF peroxidase activity. Except for chitosan (10 μg mL−1), all substances were tested at a 100 μg mL−1 concentration. Only endotoxins demonstrated a measurable response with PCN-242 MOF.
Endotoxin testing of real samples
Escherichia coli K12 MG1655 wild-type strain was selected for real-sample testing. The strain was cultured in LB broth for 16–18 hours and diluted to the desired concentration range based on OD600 measurements to estimate CFU per mL. After centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, the bacterial culture was diluted with water to remove interfering components, resulting in the mother solution of the real sample. The E. coli mother solution was diluted to various concentrations in CFU per mL. The 200 μL diluted concentration of the E. coli sample was mixed with 200 μL of PCN-242 MOF solution (0.25 mg mL−1, pH 3.6) and incubated at 37 °C for approximately 10 minutes. Subsequently, 200 μL of 1 mM H2O2 and 1 mM TMB were added, and the colorimetric reaction proceeded for 20 minutes. Finally, the absorbance of the 250 μL supernatant was measured and compared to the blank solution to calculate the LOD.
000 rpm, the bacterial culture was diluted with water to remove interfering components, resulting in the mother solution of the real sample. The E. coli mother solution was diluted to various concentrations in CFU per mL. The 200 μL diluted concentration of the E. coli sample was mixed with 200 μL of PCN-242 MOF solution (0.25 mg mL−1, pH 3.6) and incubated at 37 °C for approximately 10 minutes. Subsequently, 200 μL of 1 mM H2O2 and 1 mM TMB were added, and the colorimetric reaction proceeded for 20 minutes. Finally, the absorbance of the 250 μL supernatant was measured and compared to the blank solution to calculate the LOD.
Synthesis of the GOx@PCN-242 Fe2Co MOF composite
In the synthesis of the GOx@PCN-242 Fe2Co MOF, GOx was attached to the MOF via glutaraldehyde crosslinking chemistry, a method modified from the previous literature.39 Initially, 10 mg of PCN-242 Fe2Co MOF was dispersed in 5 mL of 10 mM NaAc-HAc buffer (pH 3.6), generating a homogeneous solution. The mixture was subjected to vortexing for five minutes and sonication for ten minutes. Subsequently, 25 μL of a 20% glutaraldehyde solution was added, and the mixture was agitated for two hours at 4 °C. Afterward, the glucose oxidase enzyme and the stock solution (1 mg mL−1) were introduced into the reaction mixture, and stirring was continued for an additional two hours at room temperature. Upon completion of the reaction, the product was retrieved by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for three minutes, followed by triple rinsing with an acidic buffer. Finally, the obtained GOx@PCN-242 Fe2Co MOF composite underwent freeze-drying in liquid nitrogen for 24 hours to facilitate further characterization.
000 rpm for three minutes, followed by triple rinsing with an acidic buffer. Finally, the obtained GOx@PCN-242 Fe2Co MOF composite underwent freeze-drying in liquid nitrogen for 24 hours to facilitate further characterization.
Detection of glucose using the GOx@PCN-242 Fe2Co MOF
Cascade experiments were conducted to assess the catalytic performance of the GOx@PCN-242 Fe2Co MOF composite in glucose detection. Following a similar procedure to the peroxidase-like activity experiments, glucose was added instead of H2O2. A reaction mixture was prepared in a 1.6 mL microcentrifuge tube by sequentially adding 200 μL of 1 mg mL−1 GOx@PCN-242 Fe2Co MOF in a buffer of 10 mM NaAc–HAc (pH 3.6), 200 μL of 1 mM TMB, and varying concentrations of glucose solution. The mixture was then incubated at 37 °C for 20 minutes and centrifuged for two minutes at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The resulting solution was analyzed at room temperature using UV-vis spectroscopy across the 300–700 nm range.
000 rpm. The resulting solution was analyzed at room temperature using UV-vis spectroscopy across the 300–700 nm range.
After the initial GOx@PCN-242 Fe2Co MOF peroxidase reaction, the composite was recovered by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes, facilitating the separation of the solid catalyst from the reaction mixture. The supernatant, containing oxidized TMB (oxTMB), was carefully discarded. To thoroughly clean the solid GOx@PCN-242(Fe2Co) composite, it was washed multiple times with 10 mM NaAc–HAc buffer, ensuring the removal of residual TMB, oxTMB, glucose, gluconic acid and H2O2 from the MOF. Subsequently, the regenerated GOx@PCN-242(Fe2Co) composite was reintroduced into fresh reaction mixtures for subsequent cycles of the colorimetric assay.
000 rpm for 3 minutes, facilitating the separation of the solid catalyst from the reaction mixture. The supernatant, containing oxidized TMB (oxTMB), was carefully discarded. To thoroughly clean the solid GOx@PCN-242(Fe2Co) composite, it was washed multiple times with 10 mM NaAc–HAc buffer, ensuring the removal of residual TMB, oxTMB, glucose, gluconic acid and H2O2 from the MOF. Subsequently, the regenerated GOx@PCN-242(Fe2Co) composite was reintroduced into fresh reaction mixtures for subsequent cycles of the colorimetric assay.
Characterization
The MOF was characterized using various techniques to thoroughly investigate their crystal structure, morphology, elemental composition, surface area, and pore volume. Powder X-ray diffraction (PXRD) with CuKα radiation (λ = 1.54178) was performed on a Bruker D8 Advance Eco instrument to analyze the crystal structure. Morphological studies were carried out using field emission scanning electron microscopy (FE-SEM), and elemental analysis was done using an FE-SEM combined with energy dispersive X-ray spectroscopy (EDS) on a JEOL JSM-7600F instrument equipped with an Oxford X-Max 80 detector. Transmission electron microscopy (TEM) images were recorded on a JEOL-JEM-2100-TEM with an accelerating electron source voltage of 200 kV. The Fe and Co elemental analysis studies were conducted using an inductively coupled plasma-mass spectrometer (ICP-MS); the equipment model was Thermo Fisher Scientific iCAP TQ from Germany (NTHU, instrumentation center). X-ray photoelectron spectroscopy (XPS) was used to analyze the elemental composition, utilizing Thermofisher Scientific, Waltham, MA, USA (NTU, instrumentation center) equipment. Surface area and pore volume were determined using the Brunauer–Emmett–Teller (BET) method, where nitrogen adsorption/desorption isotherms were measured with a Micromeritics 3Flex Physisorption surface area analyzer. Before the analysis, samples were degassed for 12 hours under a high vacuum at 120 °C. BET surface areas were calculated from N2 adsorption isotherms at 77 K, and micropore volume and pore size distribution were estimated using non-linear density functional theory (NLDFT) fitting of the measured adsorption isotherms. The zeta potential of samples was measured using an ELSZ-2000 (Otsuka Electronics, Osaka, Japan). Fourier-transform infrared (FTIR) analyses were performed using a Bruker Tensor 27 FT-IR spectrometer. The infrared (IR) spectra were collected using attenuated total reflectance (ATR) in the 4000–400 cm−1 range with a resolution of 4.0 cm−1. UV-visible spectra were recorded with a BioTek Synergy HT microplate reader using a transparent 96-well plate.Results and discussion
Synthesis and characterization of PCN-242 (Fe2Co) MOF
The preparation of PCN-242 (Fe2Co) MOF is facilitated through the utilization of a premade [Fe2Co(μ3-O) (CH3COO)6] cluster (Fig. S1, ESI†). In our efforts to construct the PCN-242 (Fe2Co) MOF powder via the solvothermal process, we used the cluster [Fe2Co(μ3-O) (CH3COO)6] as the starting material, employing 2-amino terephthalic acid (NH2BDC) ligand and acetic acid (acetate after deprotonation) as a competitive reagent and the reactions were conducted at 150 °C 12 hours. After washing the MOF with the solvent, activated at 75 °C in the oven, the resulting brown color powder was obtained upon activation. The ICP-MS was used to validate the Fe and Co wt% in the [Fe2Co(μ3-O) (CH3COO)6] cluster and PCN-242 (Fe2Co) MOF, and shows that the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios are 1.81
Co ratios are 1.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.55
1 and 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively. The PCN-242 (Fe2Co) MOF crystallizes in the P
1 respectively. The PCN-242 (Fe2Co) MOF crystallizes in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group within the triclinic system with the asymmetric unit containing two Co ions, four Fe ions, two μ3-O ions, six NH2BDC ligands, and six coordinated H2O molecules.40 Each NH2BDC ligand is connected to two Fe2Co clusters, with the six CH3COO− groups in each cluster entirely replaced by carboxyl groups from the NH2BDC.
space group within the triclinic system with the asymmetric unit containing two Co ions, four Fe ions, two μ3-O ions, six NH2BDC ligands, and six coordinated H2O molecules.40 Each NH2BDC ligand is connected to two Fe2Co clusters, with the six CH3COO− groups in each cluster entirely replaced by carboxyl groups from the NH2BDC.
The analysis of MOFs using powder X-ray diffraction (PXRD) was conducted to verify their crystallinity, as shown in Fig. 1a, where X-ray diffraction patterns are compared with simulated PXRD (CCDC number: 1485504).40 Notably, in the PCN-242 Fe2Co structure, reflections at 2θ = 9.29 (002), 10.04 (100), and 11.2 (111) were observed, with a shift in 2θ from higher to lower angles, approximately 0.5. The structural flexibility of MIL-88B leads to minor variations in reflection intensity and 2θ position due to the movement induced by guest species within the pore channels.41 The controllable synthesis of Fe2Co cluster-based MOFs significantly contributes to their flexible and dry form, causing PXRD peak shifts to lower angles. The nitrogen adsorption–desorption isotherm of PCN-242 (Fe2Co) reveals limited pore accessibility, observed in the MIL-88B(Fe)-NH2 case (Fig. S2, ESI†). This closed structure arises due to the constrained steric effects of the amino (–NH2) functional groups in the organic linkers, which restrict pore opening. The non-local density functional theory (NLDFT) estimation of the pore size likely reflects structural defects rather than the true porosity. The BET surface area of 20.0557 m2 g−1 is consistent with other MIL-88B variants, where similar pore sizes (∼3.8 Å) restrict nitrogen adsorption, limiting gas penetration and adsorption capacity in analogous frameworks like MIL-88B(4F) and MIL-88B(NO2).40
The FTIR spectra of the materials are depicted in Fig. 1b. Characteristic bands at 1578 and 1424 cm−1 were observed, corresponding to symmetric and anti-symmetric stretching of C–O bonds. Additionally, a peak at 1254 cm−1 in the as-prepared MOFs indicated C–N stretching. The absence of a band at around at 1678 cm−1 suggested the lack of unreacted NH2-BDC. Notably, the planar Fe3(μ3-O) cluster with D3h symmetry displayed an asymmetric in-plane vibration at around 576 to 600 cm−1 for the central oxygen. However, this band separated into two components in the case of a mixed-metal cluster Fe2Co(μ3-O) due to the loss of symmetry from D3h to C2v. Consequently, the Fe2Co(μ3-O) cluster formation in the MOFs should be characterized by two bands at 702 and 515 cm−1, with a weak band observed at around 600 cm−1.35
X-ray photoelectron spectroscopy (XPS) investigations confirmed the presence of trivalent iron ions (Fe3+) and divalent cobalt ions (Co2+) in the PCN-242 (Fe2Co) MOF (Fig. 2a). The XPS survey spectrum revealed elevated carbon and oxygen levels, indicating a high ligand content in the MOF. The distinct binding energy peaks were observed at 284.04 eV and 287.67 eV for C 1s, 399.06 eV for N 1s, 531.02 eV for O 1s, 711.01 eV and 724.45 eV for Fe 2p, and 780.68 eV and 796.31 eV for Co 2p. The Fe 2p spectra (Fig. 2b) were deconvoluted into four peaks: Fe 2p3/2 and Fe 2p1/2 peaks at 711.01 eV and 724.45 eV, with satellite peaks at 715.33 eV and 727.81 eV, indicative of Fe3+. The Co 2p spectra showed peaks at 780.68 eV and 796.31 eV for Co 2p3/2 and Co 2p1/2, respectively, and satellite peaks at 785.39 eV and 801.68 eV, confirming the presence of Co2+ species in the framework (Fig. 2c). The N 1s spectrum peaked at 399.06 eV, indicating primary amine (NH2) functional groups (Fig. 2d). The high-resolution XPS C 1s spectrum showed peaks at 284.04 eV and 287.67 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–C/C
O and C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively (Fig. 2e). The O 1s spectrum displayed a peak at 531.02 eV, corresponding to Fe–O–Fe/Fe–O–Co and C–O–Fe/C–O–Co (Fig. 2f).
C bonds, respectively (Fig. 2e). The O 1s spectrum displayed a peak at 531.02 eV, corresponding to Fe–O–Fe/Fe–O–Co and C–O–Fe/C–O–Co (Fig. 2f).
The high-resolution transmission electron microscopy (HR-TEM) image demonstrated exceptional dispersibility of the PCN-242 (Fe2Co) MOF, revealing particles with a uniform bipyramidal hexagonal prism morphology nm size scale (Fig. 3). The uniformity in size and morphology of the MOF particles was consistently achieved. We also measured the FE-SEM image of MOF, showing bipyramidal hexagonal prism morphology (Fig. S3, ESI†). Previous studies employed non-ionic triblock copolymer F127 as a surfactant and acetic acid as a deprotonation agent to synthesize size-controlled MOF Fe-MIL-88B-NH2.42 In contrast, our approach utilized pre-formed clusters reacting with ligands to form the MOF, with acetic acid serving as a competing agent to direct nucleation. Crucially, the presence of iron and cobalt within each cluster was unattainable without using pre-cluster materials. Direct use of metal salts in the bimetallic MOF synthesis resulted in poor and unequal metal distribution.39,43 PCN-242 (Fe2Co) MOF powder was soaked in NaAc–HAc pH 3.6 at room temperature to assess chemical stability. The PXRD peaks after 1 day indicate that the MOF retained their crystalline structure, demonstrating good chemical stability under acidic conditions suitable for potential application in acidic peroxidase reactions (Fig. S4, ESI†). The exceptional chemical stability of Fe2Co-MOFs is attributed to solid coordination bonds formed by high-valence Fe3+ at cluster nodes (HSAB principle), facilitating the creation of rigid frameworks with improved stability.
 | ||
| Fig. 3 HR-TEM shows the as synthesized PCN-242 (Fe2Co) MOF particle morphology at different magnification scales. (a) 1 μm, (b) 0.5 μm, (c) 200 nm, and (d) 100 nm. | ||
Peroxidase-like activity of PCN-242 (Fe2Co) nanozymes
Following the practical application of synthesized Fe2Co cluster-based PCN-242 MOF nanozyme, we used TMB as a chromogenic substrate in the presence of H2O2 to assess the nanozyme peroxidase activity. The PCN-242 MOF nanozyme's catalytic mechanism was similar to the one described in our previous research.28 It represents the Fe3(μ3-O) cluster based MOF in an acidic medium, the Fe3+ and Fe2+ facilitates the dissociation of H2O2 into two ˙OH radicals. Subsequently, TMB oxidation is started by these extremely oxidizing ˙OH radicals. Experimental validation supports our findings, which show PCN-242 has catalytic activity resembling that of peroxidase (POD-like). The production of superoxide radicals via H2O2 breakdown and electron transfer gives cobalt its synergistic impact. This accelerates the oxidation of TMB to oxTMB. PCN-242 is an efficient catalyst for the oxidation of TMB, as shown in Fig. 4a. This leads to the creation of chromogenic oxTMB, which has a maximum absorbance of 652 nm in the visible wavelength range. On the other hand, no appreciable absorption was seen in the reaction system when PCN-242 or H2O2 was absent (Fig. S5, ESI†).Furthermore, it investigated how the concentrations of PCN-242 and TMB affected the reaction rate. The UV-vis absorbance at 652 nm significantly increased with rising TMB concentrations (Fig. S6, ESI†). This elevation, observed under constant H2O2 conditions, indicates the generation of highly reactive hydroxyl radicals (˙OH), which facilitate the oxidation of TMB. The reaction reached saturation at a TMB concentration of 5 mM within the fixed reaction time, suggesting that the TMB oxidation rate plateaued beyond this concentration due to the limited availability of additional reactive sites or radicals under the experimental conditions. Similarly, the absorbance increased progressively as the quantity of PCN-242 increased from 0.02 to 1 mg mL−1 (Fig. S7, ESI†). Higher PCN-242 nanozyme concentrations led to faster reaction rates and more reaction product formation. The remarkable catalytic activity of PCN-242 nanozymes was highlighted by the maximum catalytic efficiency obtained at a concentration of 0.5 mg mL−1. Further concentration hikes produced comparable absorbances.
Furthermore, there is ample evidence about environmental variables' impact on the catalytic activity of naturally occurring enzymes, including pH and temperature. The enzymatic activity of PCN-242 nanozymes was assessed in this work at different pH levels. The catalytic activity of the nanozymes varied within the pH range of 3.6 to 5.6, as shown in Fig. 4b; pH = 3.6 was the ideal pH for PCN-242's relative enzyme activity. The impact of temperature on PCN-242's catalytic activity was then investigated. The relative enzyme activity of PCN-242 was determined from 4 to 60 °C, and activity decreased with temperature significantly above 42 °C (Fig. S8, ESI†).
Furthermore, we conducted peroxidase-like catalytic tests and made comparisons with other Fe-based MOFs featuring similar secondary building units (SBUs), including PCN-333(Fe), MIL-100(Fe), MOF-919(Fe-Cu), MIL-88B(Fe) (1,4-NDC), and MIL-88B(Fe) (1,4-BDC). The results demonstrate that PCN-242 (Fe2Co) exhibited markedly superior peroxidase activity compared to these analogs, highlighting the enhanced catalytic performance of the bimetallic Fe2Co system (Fig. S9, ESI†).
A steady-state kinetic experiment was conducted to investigate the kinetics of the PCN-242 Fe2Co MOF nanozyme. Fig. 4c shows the Michaelis–Menten curves for H2O2 across a specific range of substrate concentrations. The Michaelis–Menten constant (Km) and maximum starting velocity (Vmax) were calculated using the relevant Michaelis–Menten equation. The H2O2 substrate had a Vmax value of 0.07 μmol s−1, while the Km value for PCN-242 was determined to be 607 μM. Despite having a lower Km value than HRP, NH2-MIL-88B, Fe/Co-MIL-88 (NH2), and Fe/Co-TPY-MIL-88(NH2), PCN-242 (Fe2Co) demonstrated better overall catalytic activity (Table S2, ESI†).39,43 The analytical performance of PCN-242 Fe2Co MOF was examined at low H2O2 concentrations. Fig. 4d illustrates that the absorption intensity increased proportionally across H2O2 concentrations from 0.5 to 150 μM. The detection limit (LOD) was determined to be 2.1 μM based on 3σ/S.
Peroxidase-like activity of the PCN-242 (Fe2Co) nanozyme mechanism
The synergistic effect of Fe and Co in Fe/Co-MIL-88(NH2) and Fe and Ni in NiFe2 MIL-101 MOFs is crucial for their enhanced peroxidase-like activity.43,44 The potential application of these MOFs, especially when integrated with glucose oxidase (GOx), for inducing cell death and electron transfer from Ni2+ to Fe3+ triggers a Fenton-like reaction, generating hydroxyl radicals (˙OH). Note that the conversion rate of Fe3+ to Fe2+ is considerably slower than the generation of hydroxyl radicals by Fe2+ reacting with H2O2, thereby limiting its catalytic efficacy. During the catalytic process of the nanozyme, the Fe2Co MOF acted as an electron pump, extracting electrons from H2O2 and transferring them to the Co2+ ions in the Fe2Co MOF in an acidic solution. This results in the Co2+ oxidizing to Co3+, while Fe3+ is reduced to Fe2+.45 The robust oxidation of Co3+ and subsequent regeneration of Co2+ is an important step. This oxidation process can facilitate the breakdown of H2O2 into oxygen (O2).46 It's important to note that Co3+ is usually unstable and can be reduced back to Co2+, thereby completing the catalytic cycle. Concurrently, in an acidic medium, H2O2 readily accepts electrons from Fe2+, generating ˙OH via the Fenton-like reaction and the regeneration of Fe3+.47 This cyclic process ensured the circulation of Co2+/Co3+ throughout the catalytic cycle.Sensing capabilities of the PCN-242 (Fe2Co) nanozyme sensor for endotoxin detection
In solution, PCN-242 MOF exhibits a positively charged surface, facilitating the electrostatic attraction and subsequent adsorption of lipopolysaccharide (LPS) molecules when introduced into the medium. This adsorption process forms a protective LPS layer on the MOF surface, effectively isolating the MOF from further molecular interactions. To validate the interaction between the endotoxins and the PCN-242 MOF, which forms a protective barrier inhibiting the peroxidase (POD) catalytic reaction, changes in the zeta potential and particle size of the MOF suspension were analyzed using a zeta potential and particle size analyzer pre- and post-LPS addition. The data revealed a significant increase in the hydrodynamic diameter of the MOF particles from 654.5 nm to 2398.0 nm, coupled with a decrease in the zeta potential from +7.07 mV to −3.33 mV, confirming that LPS effectively coats the MOF surface (Fig. S10, ESI†).The reaction medium influences the catalytic performance of PCN-242 MOF. The peroxidase reaction system, consisting of MOF in acetic buffer, TMB, and H2O2, was optimized to determine the ideal conditions for LPS or E. coli addition. The LB medium inhibits MOF activation with a neutral pH of 7. However, adjusting the pH of the LB medium to 3.6–3.8, similar to acetic acid buffer pH, successfully enables TMB oxidation. Conversely, the high phosphate concentration in the M9 medium can deactivate the MOF peroxidase activity, leading to ineffective reactions (Fig. S11, ESI†). Notably, using ddH2O did not interfere with the peroxidase activity. For experimental quantification, PCN-242 MOF in acetate buffer was mixed with an LPS standard solution (prepared in ddH2O) and incubated at 37 °C for 10 minutes. An equal volume of 1 mM H2O2 and 1 mM TMB were added after incubation for subsequent colorimetric analysis. Note that oxidized TMB (oxTMB) exhibits maximum absorbance at 652 nm without endotoxin in the reaction. Higher endotoxin concentrations result in reduced ˙OH generation from H2O2, leading to decreased oxTMB formation and a corresponding drop in absorption, observable by a color change. The change in absorbance (ΔAbsorbance) was plotted against the increasing endotoxin concentration, confirming a detection limit of 1.396 μg mL−1 (3σ/S) (Fig. 5a).
To extend the applicability of this inhibition method for detecting Gram-negative bacteria, explicitly targeting E. coli in complex matrices, we performed interference studies using chitosan, glucose, sucrose, and various biosynthetic amino acids. The results indicated that these substances did not significantly interfere with detection (Fig. S12, ESI†). For real-sample detection, cultures of wild-type E. coli MG1655 K12, at various concentrations, were incubated with PCN-242 MOF after removing the LB medium. The ensuing colorimetric assay demonstrated a detection limit of 1.8 × 105 CFU per mL (Fig. 5b).
Glucose detection by the GOx@PCN-242 (Fe2Co) nanozyme
PCN-242 (Fe2Co) was modified by post-synthetic modification (PSM) of the MOF with glutaraldehyde and glucose oxidase (GOx). PXRD was employed to assess the stability of the modified framework following PSM of the MOF with glutaraldehyde and GOx. The PXRD data revealed that the characteristic diffraction peaks of the MOF remained unchanged after modification, confirming that the crystalline structure was preserved (Fig. 6a). The lack of significant alterations in peak intensity suggests that the incorporation of glutaraldehyde and GOx did not compromise the overall integrity of the framework. FT-IR spectra of PCN-242(Fe2Co) MOF before and after PSM with GOx (Fig. S13, ESI†). The characteristic peak at 1676 cm−1, corresponding to the amide I bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration, confirms the successful incorporation of GOx, in line with the protein-specific amide bonds reported in the literature.48 Additionally, peaks at 1004 cm−1 and 1024 cm−1 indicate interactions between GOx and the MOF framework, potentially reflecting changes similar to those observed in related GOx-modified MOFs.49
O) stretching vibration, confirms the successful incorporation of GOx, in line with the protein-specific amide bonds reported in the literature.48 Additionally, peaks at 1004 cm−1 and 1024 cm−1 indicate interactions between GOx and the MOF framework, potentially reflecting changes similar to those observed in related GOx-modified MOFs.49
Additionally, tests were conducted to verify the activity of GOx under acidic conditions, which is known to affect many oxidase enzymes adversely. GOx retained its activity under acidic conditions, which was the rationale for selecting GOx for modification in our experiments (Fig. 6b). Various concentrations of the GOx stock solution (0.1 mg mL−1, 0.25 mg mL−1, 0.5 mg mL−1, and 1 mg mL−1) were tested, and it was observed that enzyme activity increased with higher concentrations (Fig. 6c). Consequently, a concentration of 1 mg mL−1 was chosen for subsequent experiments to analyze the linear range and calculate the limit of detection (LOD).
The glucose detection capability of PCN-242 Fe2Co MOF was assessed across a range of standard glucose solution concentrations (1–150 μM; Fig. 6d), demonstrating varying UV absorbance with glucose concentration (Fig. 6d). Notably, a robust linear relationship was observed at glucose concentrations ranging from 1 to 150 μM, with a detection limit of 4.24 μM. This approach demonstrates a significantly improved glucose detection limit compared to recently reported methods.50–52 The enzyme-MOF composite demonstrated several notable advantages, including reusability and consistent catalytic activity under optimal conditions. Up to the fifth cycle, the catalytic activity remained stable (Fig. S14, ESI†). Our study employed the GOx@PCN-242 Fe2Co MOF cascade reaction to analyze real-world samples, specifically diluted orange juice and sports drinks (diluted 200 times with ddH2O). As shown in Fig. S15 (ESI†), glucose was successfully detected in these complex matrices, confirming the sensitivity and efficiency of our GOx@PCN-242 Fe2Co MOF system. The PCN-242 Fe2Co MOF demonstrated a bifunctional catalytic feature that allowed it to identify endotoxins as well as glucose, demonstrating how versatile it is for practical analytical uses.
Conclusions
In conclusion, we successfully synthesized and characterized the PCN-242 (Fe2Co) MOF, demonstrating its superior catalytic properties and versatility in various applications. First, the MOF's peroxidase-like activity was effectively utilized for the optical detection of H2O2, achieving a low detection limit of 2.2 μM. Second, the PCN-242 (Fe2Co) MOF demonstrated a novel application in endotoxin detection, where its peroxidase activity was inhibited by LPS, leading to a colorimetric detection method with a detection limit of 1.36 μg mL−1. This approach was extended to detect E. coli, providing both qualitative and quantitative analysis of bacterial contamination. Third, by incorporating GOx into the MOF, we developed a sensitive glucose detection system with detection limits of 4.24 μM for glucose within a linear range of 1 to 150 μM. Overall, the study highlights the potential of PCN-242 (Fe2Co) MOF as a multifunctional platform for advanced catalytic and biosensing applications, offering high sensitivity, selectivity, and structural robustness.Author contributions
Sivasankar Kulandaivel: conceptualization, investigation, methodology, data treatment, and writing – original draft; Yung-Kang Lu: investigation, methodology, and data treatment; Chia-Her Lin: conceptualization, supervision, and funding acquisition; Yi-Chun Yeh: conceptualization, supervision, project organization, funding acquisition, and writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Taiwan's Ministry of Science and Technology supported this research through projects 112-2113-M-003-015. Dr Kulandaivel Sivasankar thanks the Taiwan Ministry of Science and Technology for the postdoctoral fellowship 113-2811-M-003-033. Furthermore, the authors gratefully acknowledge financial support from the Taiwan Ministry of Education (MOE) under the Higher Education Sprout Project framework, facilitated by the National Taiwan Normal University (NTNU).References
- S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang and T. Gentle Iii, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- T. Steenhaut, Y. Filinchuk and S. Hermans, J. Mater. Chem. A, 2021, 9, 21483–21509 RSC.
- M. Y. Masoomi, A. Morsali, A. Dhakshinamoorthy and H. Garcia, Angew. Chem., 2019, 131, 15330–15347 CrossRef.
- P. Horcajada, S. Surblé, C. Serre, D.-Y. Hong, Y.-K. Seo, J.-S. Chang, J.-M. Grenèche, I. Margiolaki and G. Férey, Chem. Commun., 2007, 2820–2822 RSC.
- W. Chen, Z. Wang, Q. Wang, K. El-Yanboui, K. Tan, H. M. Barkholtz, D.-J. Liu, P. Cai, L. Feng, Y. Li, J.-S. Qin, S. Yuan, D. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2023, 145, 4736–4745 CrossRef CAS.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- S. Surblé, C. Serre, C. Mellot-Draznieks, F. Millange and G. Férey, Chem. Commun., 2006, 284–286 RSC.
- D. Feng, T.-F. Liu, J. Su, M. Bosch, Z. Wei, W. Wan, D. Yuan, Y.-P. Chen, X. Wang and K. Wang, Nat. Commun., 2015, 6, 5979 CrossRef PubMed.
- Y. Chen, Z. Qiao, H. Wu, D. Lv, R. Shi, Q. Xia, J. Zhou and Z. Li, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS.
- X. Lian, Y.-P. Chen, T.-F. Liu and H.-C. Zhou, Chem. Sci., 2016, 7, 6969–6973 RSC.
- D. Feng, K. Wang, Z. Wei, Y.-P. Chen, C. M. Simon, R. K. Arvapally, R. L. Martin, M. Bosch, T.-F. Liu, S. Fordham, D. Yuan, M. A. Omary, M. Haranczyk, B. Smit and H.-C. Zhou, Nat. Commun., 2014, 5, 5723 CrossRef CAS PubMed.
- Y. Lv, S. W. Ke, Y. Gu, B. Tian, L. Tang, P. Ran, Y. Zhao, J. Ma, J. L. Zuo and M. Ding, Angew. Chem., 2023, 135, e202305246 CrossRef.
- L. Z. Dong, L. Zhang, J. Liu, Q. Huang, M. Lu, W. X. Ji and Y. Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 2659–2663 CrossRef CAS PubMed.
- M. Gu, S.-C. Wang, C. Chen, D. Xiong and F.-Y. Yi, Inorg. Chem., 2020, 59, 6078–6086 CrossRef CAS PubMed.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang and P. Zhang, Adv. Mater., 2018, 30, 1704303 Search PubMed.
- S. Kulandaivel, W.-C. Lo, C.-H. Lin and Y.-C. Yeh, Anal. Chim. Acta, 2022, 1227, 340335 CrossRef CAS PubMed.
- S. Kulandaivel, C.-H. Lin and Y.-C. Yeh, Talanta, 2023, 255, 124206 CrossRef CAS PubMed.
- F. Shi, G. Li, H. Zhu, L. Li, M. Chen, J. Li, H. Shen, H. Zeng, L. Min and Z. Yang, Chin. Chem. Lett., 2024, 110333, DOI:10.1016/j.cclet.2024.110333.
- Y. Xia, F. Shi, R. Liu, H. Zhu, K. Liu, C. Ren, J. Li and Z. Yang, Anal. Chem., 2024, 96, 1345–1353 CrossRef CAS PubMed.
- N. Dhiman, S. Ghosh, Y. K. Mishra and K. M. Tripathi, Mater. Adv., 2022, 3, 3101–3122 RSC.
- Z. Zhang and K. Fan, Nanoscale, 2023, 15, 41–62 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- H. Wei and E. Wang, Anal. Chem., 2008, 80, 2250–2254 CrossRef CAS PubMed.
- S. Kulandaivel, C.-H. Lin and Y.-C. Yeh, Chem. Commun., 2022, 58, 569–572 RSC.
- S. Kulandaivel, H.-T. Chen, C.-H. Lin and Y.-C. Yeh, J. Mater. Chem. B, 2023, 11, 10362–10368 RSC.
- B. Bertani and N. Ruiz, EcoSal Plus, 2018, 8, 10–1128 CrossRef PubMed.
- I. Stewart, P. J. Schluter and G. R. Shaw, Environ. Health, 2006, 5, 1–23 CrossRef PubMed.
- F. Di Lorenzo, K. A. Duda, R. Lanzetta, A. Silipo, C. De Castro and A. Molinaro, Chem. Rev., 2021, 122, 15767–15821 CrossRef PubMed.
- K. Chriscaden, World Health Organization. https://www.who.int/news/item/08-09-2020-who-calls-for-global-action-on-sepsis—cause-of-1-in-5-deaths-worldwide, 2020.
- P. Zhu, V. A. Papadimitriou, J. E. van Dongen, J. Cordeiro, Y. Neeleman, A. Santoso, S. Chen, J. C. T. Eijkel, H. Peng and L. I. Segerink, Sci. Adv., 2023, 9, eadf5509 CrossRef CAS PubMed.
- H. R. Shamsollahi, M. Ghoochani, J. Jaafari, A. Moosavi, M. Sillanpää and M. Alimohammadi, Ecotoxicol. Environ. Saf., 2019, 174, 236–244 CrossRef CAS.
- L. Rasuli, M. H. Dehghani, M. Aghaei, A. H. Mahvi, N. M. Mubarak and R. R. Karri, Chemosphere, 2022, 303, 135089 CrossRef CAS PubMed.
- M. Schneier, S. Razdan, A. M. Miller, M. E. Briceno and S. Barua, Biotechnol. Bioeng., 2020, 117, 2588–2609 CrossRef CAS PubMed.
- W. Su and X. Ding, J. Lab. Autom., 2015, 20, 354–364 CrossRef CAS PubMed.
- J. L. Ding and B. Ho, Endotoxins: structure, function and recognition, 2010, pp. 187–208 Search PubMed.
- Q. Jiang, Y. Xiao, A. N. Hong, Z. Gao, Y. Shen, Q. Fan, P. Feng and W. Zhong, ACS Appl. Mater. Interfaces, 2022, 14, 41800–41808 CrossRef CAS PubMed.
- P. Horcajada, F. Salles, S. Wuttke, T. Devic, D. Heurtaux, G. Maurin, A. Vimont, M. Daturi, O. David, E. Magnier, N. Stock, Y. Filinchuk, D. Popov, C. Riekel, G. Férey and C. Serre, J. Am. Chem. Soc., 2011, 133, 17839–17847 CrossRef CAS PubMed.
- C. Serre, C. Mellot-Draznieks, S. Surblé, N. Audebrand, Y. Filinchuk and G. Férey, Science, 2007, 315, 1828–1831 CrossRef CAS PubMed.
- M.-H. Pham, G.-T. Vuong, A.-T. Vu and T.-O. Do, Langmuir, 2011, 27, 15261–15267 CrossRef CAS PubMed.
- Q. Jiang, Y. Xiao, A. N. Hong, Y. Shen, Z. Li, P. Feng and W. Zhong, ACS Sens., 2023, 8, 1658–1666 CrossRef CAS PubMed.
- X. Zhao, N. Zhang, T. Yang, D. Liu, X. Jing, D. Wang, Z. Yang, Y. Xie and L. Meng, ACS Appl. Mater. Interfaces, 2021, 13, 36106–36116 CrossRef CAS PubMed.
- X. Lin, J. Li, J. Wu, K. Guo, N. Duan, Z. Wang and S. Wu, ACS Appl. Mater. Interfaces, 2024, 16, 11809–11820 CrossRef CAS PubMed.
- Y. Fang, Q. Liu, Y. Song, F. Jia, Y. Yang and H. Li, Chem. Eng. J., 2023, 470, 144386 CrossRef CAS.
- Q. Liu, Q. Chen, X. Deng, Y. Zhu, Z. Shi, R. Miao, M. Ma, N. Ran, C. Li and H. Chen, Chem. Eng. J., 2024, 493, 152753 CrossRef CAS.
- B. S. Goud, G. Shin, S. V. P. Vattikuti, N. Mameda, H. Kim, G. Koyyada and J. H. Kim, Biochem. Eng. J., 2022, 188, 108669 CrossRef CAS.
- J. Zhao, L. Liu, L. Gu, Z. Li, Y. Li, Z. Wu, B. Sun, X. Wang and T. Sun, Mater. Today Adv., 2023, 17, 100329 CrossRef CAS.
- J. Cao, F. Shi, L. Chen, X. Xu, Z. Chen, Z. Yang and X. Jiang, New J. Chem., 2023, 47, 5773–5779 RSC.
- L. Tian, B. Zhao, J. Zhang, X. Luo and F. Wu, Colloids Surf., A, 2023, 666, 131309 CrossRef CAS.
- P. Koley, R. Jakku, T. Hosseinnejad, S. Periasamy and S. K. Bhargava, Nanoscale, 2024, 16, 5561–5573 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01944j |
| This journal is © The Royal Society of Chemistry 2025 |