Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural changes and mechanisms of reversible electrochemical lithium-ion cycling in (104) oriented LiNi1/3Mn1/3Co1/3O2 thin film cathodes prepared by pulsed laser deposition†
Blaž Jaklič *ab,
Jan Žuntarab,
Elena Tchernychovac,
Gregor Kapunc,
Martin Šala
*ab,
Jan Žuntarab,
Elena Tchernychovac,
Gregor Kapunc,
Martin Šala c,
Robert Dominko
c,
Robert Dominko cde and
Matjaž Spreitzer
cde and
Matjaž Spreitzer *a
*a
aAdvanced Materials Department, Jožef Stefan Institute, Jamova cesta 39, 1000 Ljubljana, Slovenia. E-mail: blaz.jaklic@ijs.si
bJožef Stefan International Postgraduate School, Jamova cesta 39, 1000 Ljubljana, Slovenia
cNational Institute of Chemistry, Hajdrihova ulica 19, 1000 Ljubljana, Slovenia
dFaculty of Chemistry and Chemical Technology, University of Ljubljana, Večna cesta 13, 1000 Ljubljana, Slovenia
eAlistore-European Research Institute, CNRS FR 3104, Hub de l'Energie, Rue Baudelocque, 80039, Amiens, France
First published on 7th April 2025
Abstract
This study examines the structural and electrochemical behavior of epitaxial (104) oriented LiNi1/3Mn1/3Co1/3O2 (NMC 111) thin film cathodes prepared by pulsed laser deposition, aiming to elucidate the underlying mechanisms of reversible lithium-ion cycling. The effect of growth parameters on film morphology and crystal structure is thoroughly studied. The surface analysis confirms the oxidation states of transition metal ions to be Ni2+, Mn4+ and Co3+. Microstructural analysis reveals twinned domains in the NMC 111 layered structure, which conforms with its 4-domain crystallographic orientation. After NMC 111 thin film is charged to 4.2 V, a change in the local electronic structure of nickel and oxygen ions is observed by electron energy loss spectroscopy as a consequence of nickel oxidation. By utilizing ex situ reciprocal space mapping after charging to 4.2 V, a negative unit cell volume change was observed, compensated by an increased mosaic spread of NMC 111 lattice planes. This structural adjustment is reversibly maintained upon discharging to 3.0 V. Based on defined epitaxial structures, the reversible mechanism of lithiation and delithiation in NMC 111 thin films is determined on a structural level, providing detailed insight into its functionality. To address structural instability in the charged state, the electrochemical performance was enhanced by cooling the NMC 111 thin films under high oxygen pressure.
1. Introduction
One of the promising cathode materials for lithium-ion batteries is LiNi1−x−yMnxCoyO2 (NMC) because of its high specific capacity, thermal stability and safety, as each transition metal in the structure has unique properties. Nickel ions contribute to high specific capacity, cobalt ions give high rate capabilities and manganese ions stabilize the structure because they are strictly in a 4+ oxidation state.1 However, NMC cathodes suffer from performance degradation that is linked to the instability of the material in the charged state. Structural instability of NMC at higher voltages can cause spontaneous reduction of Ni4+ ions to more stable Ni2+. The presence of Ni2+ in a charged cathode causes the migration of divalent nickel to lithium interstitials. The reason for cation disordering is a similarity in ionic radius of Ni2+ (0.69 Å) and Li+ (0.76 Å), so the cation mixing degree increases with nickel content, state of charge, and temperature.2–5 Consequently, NMC is exposed to structural changes and phase transitions from initial layered over spinel to NiO-like rock salt structure. Furthermore, structural instability is strongly related to oxygen non-stoichiometry, since oxygen vacancies initiate additional oxygen loss from the surface during the charge/discharge process and give rise to cation disorder on the surface of charged NMC cathode, so controlling the oxygen stoichiometry in NMC is crucial.6Thin film electrodes, prepared via pulsed laser deposition (PLD) can act as a model system for studying the intrinsic properties of battery materials and their interfaces, but it is necessary to carefully control synthesis parameters to prepare high-quality epitaxial thin films.7–9 Furthermore, oriented thin films can provide useful information on the anisotropic material properties since no binder and conductive additives are present in thin film electrodes. Several groups reported the growth of NMC thin films by PLD,10–18 of which using single crystal oxide substrates proved to be most successful in the preparation of epitaxial NMC thin films.10,15–17 Hirayama et al. studied the influence of crystal orientation of SrRuO3 (SRO)/Nb:SrTiO3 (Nb:STO) substrate on the growth of NMC 111 film. Films with preferred out-of-plane crystal orientations of (104),(1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 8), and (003) were deposited on SRO/Nb:STO substrates with out-of-plane crystal orientations of (100), (110), and (111), respectively. Electrochemical measurements of this study revealed the anisotropic properties of NMC 111 cathodes as (104) surface exhibited reversible behavior at deep cycling to 4.5 V, but the (1
8), and (003) were deposited on SRO/Nb:STO substrates with out-of-plane crystal orientations of (100), (110), and (111), respectively. Electrochemical measurements of this study revealed the anisotropic properties of NMC 111 cathodes as (104) surface exhibited reversible behavior at deep cycling to 4.5 V, but the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 8) and (003) surface planes showed fading of average discharge voltage and specific capacity.15 Moreover, a detailed analysis of the growth mechanism and the structural properties of epitaxial layered oxide thin films revealed a 4-domain crystallographic orientation of the films on (100) oriented STO substrates for both NMC15,16 and LiCoO2 (LCO).19–21 However, the microstructural evolution of epitaxial layered thin film cathodes during cycling was reported only for LCO (003), where TEM observations showed the relaxation of translational domain boundaries during cycling, while Electron Energy loss spectroscopy (EELS) suggested the reduction of cobalt ions on the surface, which impeded lithium ion insertion during discharging.22 With this in mind, the structural evolution of twinned domains and charge compensation mechanisms in NMC with (104) preferred orientation warrants further investigation, given the superior electrochemical properties demonstrated by such layered cathode thin films.23
8) and (003) surface planes showed fading of average discharge voltage and specific capacity.15 Moreover, a detailed analysis of the growth mechanism and the structural properties of epitaxial layered oxide thin films revealed a 4-domain crystallographic orientation of the films on (100) oriented STO substrates for both NMC15,16 and LiCoO2 (LCO).19–21 However, the microstructural evolution of epitaxial layered thin film cathodes during cycling was reported only for LCO (003), where TEM observations showed the relaxation of translational domain boundaries during cycling, while Electron Energy loss spectroscopy (EELS) suggested the reduction of cobalt ions on the surface, which impeded lithium ion insertion during discharging.22 With this in mind, the structural evolution of twinned domains and charge compensation mechanisms in NMC with (104) preferred orientation warrants further investigation, given the superior electrochemical properties demonstrated by such layered cathode thin films.23
In this study, we report the reversible mechanism of delithiation and lithiation on the structural level in (104) out-of-plane oriented NMC 111 thin films, as (104) surfaces of lithium layered oxides proved to be thermodynamically favorable and most stable electrochemically.24 The structural properties of NMC 111 thin films were studied in detail by HRXRD, proving the 4-domain in-plane crystallographic orientation and relaxation of the unit cell on SRO. Microstructural analysis revealed twinned domains and the presence of a rock-salt phase on the surface of the thin film while surface analysis revealed the sensitivity of NMC 111 thin films, exposed to ambient conditions. Ex situ HRXRD analysis showed how the structure of NMC 111 thin film evolves when lithium is removed from the structure, resulting in reversible negative volume change that is compensated by increased mosaicity in the thin film cathode at the charged state. The change in the local electronic structure of nickel and oxygen ions was observed by EELS, explaining the charge compensation mechanism by nickel oxidation. Thin films, prepared under different oxygen environments demonstrated that oxygen background pressure during growth and cooling plays an important role in the morphological and electrochemical properties of NMC 111. The overall electrochemical response is revealed by performing cyclic voltammetry and galvanostatic cycling at different current densities.
2. Experimental methods
2.1. Target and sample preparation
LiNi0.33Mn0.33Co0.33O2 target with 30% lithium excess was prepared in-house by conventional solid-state synthesis route. To obtain stoichiometric NMC 111 compound, Li2CO3 (Puratronic, 99.998%, 4% excess), NiO (Sigma-Aldrich, 99.99%), MnO2 (Thermo Fischer, 99.9%), and Co3O4 (Puratronic, 99.9985%) powders were mixed and dispersed in acetone. After the ball-milling (1 h, 250 rpm) and drying (30 min, 80 °C), powders were calcined two times at 800 °C for 12 h. Powders were uniaxially pressed to pellets (200 MPa) before firings. The as-prepared stoichiometric NMC 111 powder was mixed with a 15% molar excess of Li2CO3, to compensate for lithium loss during thin film synthesis. Finally, NMC powder with Li2CO3 excess was pressed with an isostatic press (700 MPa) and sintered at 850 °C for 12 h to prepare the PLD target. SrRuO3 (99.95%) target was purchased from Beijing Goodwill Metal Technology.NMC 111 thin films were synthesized using a PLD system from Twente Solid State Technology equipped with a 248 nm ultraviolet KrF excimer laser (Coherent COMPex 205) with a 20 ns pulse. Films were grown on 0,5%wt Nb-doped SrTiO3 (001) single crystals (CrysTec). Before NMC deposition, SrRuO3 film was grown epitaxially on Nb:STO (001). The spot size of the ablated area was fixed at 2.31 mm2 and the target-to-substrate distance was fixed at 55 mm for all depositions. For SrRuO3 deposition, the temperature of the resistive heater was set to 585 °C, the oxygen pressure in the chamber was 0.13 mbar and the laser fluence was fixed to 2.5 J cm−2. The ablation frequency and the number of laser pulses were 4 Hz and 3500 pulses, respectively. For NMC 111 thin film deposition the temperature of the resistive heater was set to 600 °C, the laser frequency was 5 Hz and the laser fluence was fixed to 1.5 J cm−2. The oxygen pressure in the PLD chamber during growth varied in ranges from 10−5 mbar to 0.25 mbar. Cooling oxygen pressure was the same as deposition pressure or 500 mbar for annealed samples. The heating and cooling rate was set to 10 °C min−1. Reflection high-energy electron diffraction (RHEED) was used to monitor surface structure changes and thin film growth during the deposition.
2.2. Structural and surface characterization
The θ − 2θ patterns, azimuthal φ patterns, reciprocal space maps (RSM), and X-ray reflectometry (XRR) measurements were collected with an X-ray diffractometer (Empyrean, Malvern PANalytical) with CuKα1 radiation (λ = 1.5406 Å). A double-bounce Ge (220) hybrid monochromator was used on the incident-beam side. A PIXcel3D detector operating in 1D mode captured and analyzed the diffracted beam. In azimuthal φ scans, χ was set to 45° and 55.15° to align STO (110) and NMC (003) planes with the X-ray beam, respectively. For RSMs, sample was aligned to Nb:STO planes before the measurement. The values were converted from angular units to reciprocal space coordinates Q (Qx for the in-plane component, Qy for the out-of-plane component) using equations Qx = R(cos
planes before the measurement. The values were converted from angular units to reciprocal space coordinates Q (Qx for the in-plane component, Qy for the out-of-plane component) using equations Qx = R(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ω − cos(2θ − ω)) and Qy = R(sin
ω − cos(2θ − ω)) and Qy = R(sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ω + sin(2θ − ω)), where R = 0.5. The results are presented in the form of contour plots of intensity versus Q (Qx and Qy) in the reciprocal lattice unit (r.l.u.). A parallel plate collimator was used at the diffracted side for X-ray reflectometry measurements. The thickness of the films was estimated from XRR measurements by the Fourier method.
ω + sin(2θ − ω)), where R = 0.5. The results are presented in the form of contour plots of intensity versus Q (Qx and Qy) in the reciprocal lattice unit (r.l.u.). A parallel plate collimator was used at the diffracted side for X-ray reflectometry measurements. The thickness of the films was estimated from XRR measurements by the Fourier method.
TEM lamella sample preparation was performed by using a FIB Helios Nanolab 650 (Thermo Fisher Scientific, The Netherlands). Sample and FIB lift-out grid was mounted on a vacuum transfer shuttle inside the Ar-filled glovebox and transferred directly to the FIB instrument without being exposed to air atmosphere. The sample surface was initially protected by 3 nm of carbon and 300 nm Pt layer by using electron beam-induced deposition (EBID, 2 kV @ 0.4 nA). Subsequently, an additional Pt layer was deposited by using Ga+ ion beam-induced deposition (IBID, 30 kV @ 0.23 nA) to achieve a protective layer with a final thickness of 1.5 μm. A rough lamella chunk with dimensions of 12 × 6 μm was milled perpendicular to [110] direction of Nb:STO substrate, thinned to 2 μm and transferred to the FIB grid using a micromanipulator. Due to sample sensitivity, lamella was first thinned to 250 nm thickness using FIB at 30 kV by sequential reducing ion beam currents from 780 pA to 80 pA. Subsequently, lamella was carefully thinned to 100 nm using FIB at 16 kV with 20 pA beam current. Afterward, lamella was sequentially polished on both sides using FIB at 5 kV @ 44 pA and 2 kV @ 25 pA until electron transparency (∼50 nm). As prepared lamella sample was transferred with a vacuum transfer system directly from the FIB chamber to the glovebox where it was mounted to the TEM vacuum transfer holder. The cross-sectional samples of interfaces were examined by a JEM-ARM200CF probe Cs-corrected scanning transmission electron microscope (STEM) equipped with a cold field emission electron source operated at 80 kV. EELS analysis was performed using a QuantumGIF imaging filter (GATAN, Plesanton, U.S.A.) attached to the JEM-ARM200CF probe Cs-corrected microscope. The 2D spectrum images with 0.75 eV energy resolution, 0.25 eV energy dispersion, 130 × 1 binning and 0.1s pixel time were recorded from the films' cross-sectional FIB lamellae. The analyzed EEL spectra were then extracted at the bulk of the film by summing up the pixels in lines parallel to the substrate, excluding the surface and the interface to compare pristine and charged EEL spectra of NMC 111 thin film. The changes in oxidation states of TMs were then determined by using a modified integral Mn, Ni and Co L3,2 white-line intensity ratios,25 calculated for each line sum spectrum. Simulated selected area diffraction patterns were obtained with the use of CrystalMaker Software.
X-ray photoelectron spectroscopy (XPS) was performed with the Versaprobe 3 AD (Phi, Chanhassen, US) using a monochromatic Al-Kα1 X-ray source. For each measurement, spectra were acquired on a 200 μm spot size with the charge neutralizer turned on, as the films/powders were put on a non-conductive double tape to prevent possible differential charging of lithium-containing material. High-resolution spectra were measured at 55 eV pass energy and step of 0.05 eV. Charge neutralization was used, so the energy scale of photoelectron spectra was corrected by shifting the C 1s peak of carbon to the binding energy of 284.8 eV. To clean the surface layer from carbon contamination, sputtering of the sample with an argon gas cluster ion beam (GCIB) was used. The film was cleaned with GCIB operating at 10 kV @ 30 nA over 2 mm × 2 mm area. After cleaning of the surface with GCIB, sputtering with argon ions was utilized operating at 3 kV over 2 mm × 2 mm area to remove lithium residuals. Since the shift of the spectra to C 1s after sputtering was not possible, spectra were shifted to O 1s peak of lattice oxygen in NMC 111 to the binding energy of 529.3 eV. XPS spectra were analyzed with PHI Multipak software. For the fits, the error in the binding energy scale for all peaks was limited to ±0.2 eV. Shirley background correction was used for all the spectra.
Atomic force microscopy (AFM) was performed with Veeco Dimension 3100 SPM to study the surface morphology of the films. AFM images were analyzed and edited with WSxM 5.0 software.26
Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used for the elemental analysis of bulk ceramic powders, while inductively coupled plasma-mass spectroscopy (ICP-MS) was used for the elemental analysis of thin films. All reagents were of analytical grade. For sample dilution and preparation of standards, ultrapure water (18.2 MΩ cm−1, Milli-Q, Millipore) and ultrapure acids (HNO3 and HCl, Merck-Suprapure) were used. Standards were prepared in-house by dilution of certified, traceable, inductively coupled plasma (ICP)-grade single-element standards (Merck CertiPUR). Before the ICP-OES analysis of bulk ceramics, each sample was weighed (approximately 10 mg) and digested by dissolving it in concentrated HCl (5 ml). Samples were then diluted with 2% vol. HNO3 until the concentration was within the desired concentration range. Before the ICP-MS analysis of thin films, the sample was digested in 2 ml of aqua regia at 100 °C for 30 minutes. The digested sample was cooled to room temperature and then diluted with 2% vol. HNO3 until the concentration was within the desired concentration range.
2.3. Electrochemical characterization
CR2032 type coin cells were assembled in the Ar-atmosphere glovebox with the inner stack consisting of 6 mm precycled Li4Ti5O12 (LTO) electrodes, Celgard™ 2320 separator (18 mm in diameter) and 10 × 10 × 0.5 mm thin-film NMC electrodes, with 15 μL of LP40 (1 M LiPF6 in EC/DEC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol., Sigma-Aldrich) being added as the electrolyte. The preparation of precycled LTO counter electrodes is described in the ESI file.† To improve charge transfer between the substrate and the stainless steel disk of the coin cell, gold was sputtered on the backside of the substrate before the assembly of the thin film battery. All electrochemical characterization was performed with a BioLogic VMP3 multichannel potentiostat. The coin cells were galvanostatically cycled in the range of 0.92–2.6 V vs. LTO voltage plateau (roughly corresponding to 2.5–4.2 V vs. Li/Li+) with a current density of 0.5 μA cm−2 (C/8 rate).
1 vol., Sigma-Aldrich) being added as the electrolyte. The preparation of precycled LTO counter electrodes is described in the ESI file.† To improve charge transfer between the substrate and the stainless steel disk of the coin cell, gold was sputtered on the backside of the substrate before the assembly of the thin film battery. All electrochemical characterization was performed with a BioLogic VMP3 multichannel potentiostat. The coin cells were galvanostatically cycled in the range of 0.92–2.6 V vs. LTO voltage plateau (roughly corresponding to 2.5–4.2 V vs. Li/Li+) with a current density of 0.5 μA cm−2 (C/8 rate).
To study the structural changes taking place in NMC 111 thin film electrodes with respect to the degree of lithiation of the material, NMC|Li liquid electrolyte pouch cells were assembled inside an Ar-atmosphere glove box and electrochemically analyzed. For the pouch cell assembly step, 12 mm disks were punched out of 110 μm thick lithium foil (FMC Corporation) and gently brushed with a plastic cylinder to obtain a fresh metal surface. Additionally, 18 mm disks were punched out of 260 μm thick glass fiber paper (Whatman, GF/A glass microfiber) and used as a separator. LP40 was used as the electrolyte, with 7 μL distributed on the Li foil and 75 μL on the GF/A separator, amounting to a total electrolyte volume of 82 μL. The pouch cell was made of polypropylene/polyethylene/polypropylene laminated Al foil, with thin metal foil strips serving as contacts (Al foil for the NMC electrode, and Cu foil for lithium). After each set of electrochemical measurements, the pouch cell was disassembled inside a glove box, where the delithiated NMC electrode was washed with diethyl carbonate (DEC, Sigma Aldrich) and stored for XRD analysis or FIB lamella preparation. The subsequent reassembly of the pouch cell with the analyzed NMC electrode was performed identically as described above.
3. Results and discussion
3.1. Target structure and stoichiometry
The X-ray diffraction (XRD) patterns of the calcined NMC 111 powder and NMC 111 target with lithium excess are shown in Fig. 1. All diffraction peaks can be indexed to polycrystalline reflections of layered R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group indicating a single crystalline phase in both powders, with minor NMC spinel impurity in the calcined powder. The stoichiometry and molar ratios between lithium, nickel, manganese and cobalt determined by ICP-OES were calculated to be Li0.98±0.01Ni0.33±0.01Mn0.35±0.01Co0.32±0.01O2−γ for calcined powder and Li1.21±0.01Ni0.33±0.01Mn0.35±0.01Co0.32±0.01O2−γ for NMC 111 target. Calcined NMC 111 powder was close to stoichiometric, while NMC 111 target with 30 mol% lithium excess added during synthesis exhibited 21 mol% lithium excess after sintering, indicating some lithium evaporated at elevated temperatures during the sintering of the target.
m space group indicating a single crystalline phase in both powders, with minor NMC spinel impurity in the calcined powder. The stoichiometry and molar ratios between lithium, nickel, manganese and cobalt determined by ICP-OES were calculated to be Li0.98±0.01Ni0.33±0.01Mn0.35±0.01Co0.32±0.01O2−γ for calcined powder and Li1.21±0.01Ni0.33±0.01Mn0.35±0.01Co0.32±0.01O2−γ for NMC 111 target. Calcined NMC 111 powder was close to stoichiometric, while NMC 111 target with 30 mol% lithium excess added during synthesis exhibited 21 mol% lithium excess after sintering, indicating some lithium evaporated at elevated temperatures during the sintering of the target.
 | ||
| Fig. 1 XRD pattern of (a) NMC 111 reference, (b) NMC 111 powder after 2nd calcination and (c) NMC 111 PLD target. * NMC spinel impurity peak. | ||
3.2. Structural analysis of LiNi1/3Mn1/3Co1/3O2 thin films
XRD pattern of NMC 111 thin film on the SRO/Nb:STO (001) is shown in Fig. 2a. The diffraction peaks can be attributed to the substrate, SRO and two NMC 111 peaks at about 44.5° and 98.4°, corresponding to (104) and (208) out-of-plane orientation of the film, respectively, indicating epitaxial growth of NMC 111 on SRO/Nb:STO (001) substrate. A comparison of XRD patterns for NMC 111 thin films, deposited at different oxygen pressures is shown in Fig. S1.† Deposition at different oxygen pressures influences the structure and that is reflected as a shift of (104) peak from the original position. The bulk value for d-spacing of (104) reflection was obtained at 0.01 mbar and annealed at 500 mbar oxygen pressure. Annealing of NMC 111 thin films in high oxygen pressure can initiate additional oxygen incorporation in the films, thus stabilizing the crystal structure of NMC 111. Since samples annealed in an oxygen-rich environment showed improved electrochemical performance of thin films, all structural properties were studied on those samples, respectively. Fig. 2c depicts an azimuthal φ scan on the (110) plane of the substrate and (003) plane of the NMC 111 film. This result proposed the pseudocubic growth of NMC 111 film on SRO/Nb:STO (001) substrate with 4-fold symmetry and 45° in-plane angle tilt to the substrate (Fig. 2d), which agrees with the literature reports on the growth of NMC on STO.15,16 RHEED patterns of Nb:STO substrate, as-deposited SRO and NMC 111 after annealing recorded in [100] azimuth direction indicate the epitaxial flat surface of as-deposited SRO and the slightly stepped surface of annealed NMC 111 (Fig. 2e). Additional RHEED pattern of NMC 111 thin film after annealing, recorded in [110] azimuth direction shows slightly modulated streaks, confirming multilevel stepped terraces on the surface.27 The morphology of pristine NMC 111 thin film, determined by atomic force microscope (Fig. 2b), showed a smooth flat surface with the RMS roughness value of 0.36 nm. A comparison of NMC 111 thin films, prepared in different oxygen environments, taken from the 2 μm × 2 μm area, is shown in Fig. S2.† It is evident that the films get porous at higher deposition pressures (0.25 mbar), while a morphology difference is observed when the films are annealed in high oxygen pressure after deposition, resulting in a smoother surface of dense films, so the NMC 111 thin films should be grown at lower pressures and cooled in high pressures to prepare compact and dense films with a flat surface.The thickness of the SRO bottom electrode and NMC 111 thin films was estimated by XRR using the Fourier method to be 35 nm ± 3 nm for SRO and 39 nm ± 2 nm, 45 nm ± 2 nm, 72 nm ± 3 nm and 79 nm ± 3 nm for NMC 111 deposited at 0.25 mbar, 0.1 mbar, 0.01 mbar and 10−5 mbar oxygen pressures, respectively (Fig. S3†). The growth rate of the film decreases with increasing oxygen pressure, since the kinetic energy of the incoming species in the plasma plume is slowed down by the background gas, so the film grows slower at high pressures. The stoichiometry of NMC 111 thin film, deposited at 0.01 mbar and subsequently annealed was estimated by ICP-MS to be Li1.00±0.05Ni0.32±0.01Mn0.28±0.01Co0.34±0.01O2−γ. Comparison between the stoichiometry of the PLD target and thin film confirmed some lithium loss during the deposition process, but since lithium excess was added to the target, the composition of the thin film was close to the stoichiometric.
A symmetrical RSM collected around the Nb:STO (002) reflection of a substrate is shown in Fig. S4a.† The broad reflection of the (104) peak indicates large mosaicity in the NMC 111 thin film due to 4-domain crystallographic orientations. To study the in-plane orientation relationship between SRO/Nb:STO (001) substrate and NMC 111 film, RSMs were collected around Nb:STO  reflection with the presence of NMC 111 (1 0 10) and (202) reflections (Fig. S4b†). The in-plane reciprocal direction Qx of the substrate and the film are far apart, indicating that the film is relaxed. Since SRO
reflection with the presence of NMC 111 (1 0 10) and (202) reflections (Fig. S4b†). The in-plane reciprocal direction Qx of the substrate and the film are far apart, indicating that the film is relaxed. Since SRO  is a forbidden reflection, additional RSM was performed around Nb:STO (0
is a forbidden reflection, additional RSM was performed around Nb:STO (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3) to confirm that SRO is coherently strained to the substrate (Fig. S4c†). The relaxation of NMC 111 is a consequence of a large lattice mismatch (>4%) between the film and strained SRO in the in-plane direction. The lattice parameters and volume of the hexagonal NMC 111 unit cell were calculated from RSMs, using equations described in the ESI file.† Calculated parameters from RSMs were 2.88 Å for a lattice parameter and 14.23 Å for c lattice parameter with a volume of 102.4 Å3. Similar lattice parameters were reported for polycrystalline NMC, calculated via Rietveld refinement from neutron diffraction data.28 Even though interfacial strain and slight differences in the stoichiometry of the film can influence lattice parameters and unit cell volume,29 our results are in good agreement with the reported values for bulk NMC ceramics, meaning that the proposed calculation of unit cell parameters from RSMs is an effective method for determining the crystal structure and unit cell volume of NMC epitaxial thin films.
3) to confirm that SRO is coherently strained to the substrate (Fig. S4c†). The relaxation of NMC 111 is a consequence of a large lattice mismatch (>4%) between the film and strained SRO in the in-plane direction. The lattice parameters and volume of the hexagonal NMC 111 unit cell were calculated from RSMs, using equations described in the ESI file.† Calculated parameters from RSMs were 2.88 Å for a lattice parameter and 14.23 Å for c lattice parameter with a volume of 102.4 Å3. Similar lattice parameters were reported for polycrystalline NMC, calculated via Rietveld refinement from neutron diffraction data.28 Even though interfacial strain and slight differences in the stoichiometry of the film can influence lattice parameters and unit cell volume,29 our results are in good agreement with the reported values for bulk NMC ceramics, meaning that the proposed calculation of unit cell parameters from RSMs is an effective method for determining the crystal structure and unit cell volume of NMC epitaxial thin films.
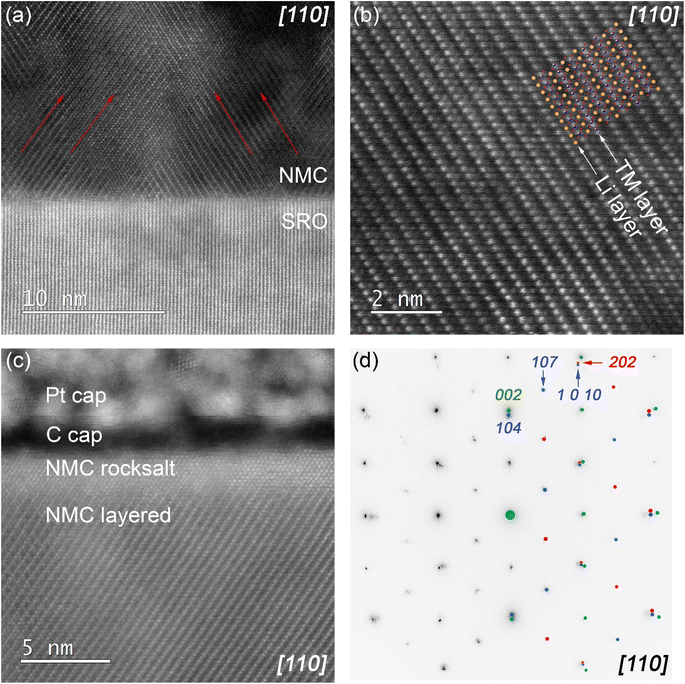
To understand more about the growth mechanism and microstructure, thin film lamella was cut in [110] azimuth direction of the Nb:STO (001) substrate to reveal the layered structure of NMC 111. STEM image of the NMC 111 – SRO interface (Fig. 3a) revealed twinned domains in NMC 111 thin film (indicated with red arrows, parallel to the a-axis of the NMC unit cell), due to the tilted growth in out-of-plane direction and since 4-fold symmetry was proved with HRXRD, twinned domains in the crystal are also expected in [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction. A high-resolution image of the NMC 111 layered structure (Fig. 3b), alternating between transition metal ions and lithium ions is consistent with the proposed structure determined with HRXRD (structural model of Nb:STO/SRO/NMC 111 thin film shown in Fig. S5†). However, STEM analysis of the thin film surface revealed the rock-salt structure of NMC 111 (Fig. 3c). The phase transition from layered to rock-salt structure might be initiated by exposure of the thin film to the ambient atmosphere or due to the sample preparation with FIB. The selected area diffraction patterns recorded in [110] azimuth direction (Fig. 3d) revealed diffraction spots of NMC 111, SRO and Nb:STO substrate. Simulated diffraction patterns for Nb:STO/SRO (green) and NMC 111 twins (red and blue) are consistent with the experimental patterns and clearly show twinning of the NMC 111 layers. Thus careful considerations must be taken before measuring asymmetric peaks with HRXRD to avoid overlapping of the crystal planes with the planes of the twin (e.g. (1 0 10) peak overlaps with the (202) peak from the twin), so (107) plane is chosen to measure lattice parameters accurately.
0] direction. A high-resolution image of the NMC 111 layered structure (Fig. 3b), alternating between transition metal ions and lithium ions is consistent with the proposed structure determined with HRXRD (structural model of Nb:STO/SRO/NMC 111 thin film shown in Fig. S5†). However, STEM analysis of the thin film surface revealed the rock-salt structure of NMC 111 (Fig. 3c). The phase transition from layered to rock-salt structure might be initiated by exposure of the thin film to the ambient atmosphere or due to the sample preparation with FIB. The selected area diffraction patterns recorded in [110] azimuth direction (Fig. 3d) revealed diffraction spots of NMC 111, SRO and Nb:STO substrate. Simulated diffraction patterns for Nb:STO/SRO (green) and NMC 111 twins (red and blue) are consistent with the experimental patterns and clearly show twinning of the NMC 111 layers. Thus careful considerations must be taken before measuring asymmetric peaks with HRXRD to avoid overlapping of the crystal planes with the planes of the twin (e.g. (1 0 10) peak overlaps with the (202) peak from the twin), so (107) plane is chosen to measure lattice parameters accurately.
3.3. Surface analysis of LiNi0.33Mn0.33Co0.33O2 thin films
The Li 1s, Ni 2p, Mn 2p, Co 2p and O 1s photoelectron spectra of NMC 111 calcined powder and NMC 111 thin film are shown in Fig. 4a–e. We use powder analysis as a reference for comparison with thin film photoelectron spectra. The lines in the graph are indicative binding energies of NMC 111 powder as reported elsewhere.30Due to spin–orbit coupling, the Co 2p spectrum is split into two parts, attributed to Co 2p3/2 (780 eV) and Co 2p1/2 (795 eV). The position of main lines and a satellite peak (789.9 eV), associated with photoemission metal–ligand charge transfer, are indicative of Co3+ ions in NMC 111 thin film.30 Ni 2p region consists of Ni 2p3/2 and Ni 2p1/2 doublet at 854.5 eV and 872.1 eV, respectively, and two satellite peaks located at 861.1 eV and 878 eV. The peak positions of Ni 2p peaks are consistent with values reported for NMC 111 powders, where Ni ions are predominantly in Ni2+ oxidation state,30,31 but we cannot exclude the presence of Ni3+ ions, because for the Ni 2p3/2 spectrum of the thin film there is a higher signal-to-noise ratio, so there may be some broadening of the main line due to small amount of Ni3+. Moreover, Ni 2p3/2 main peak overlaps with Mn LM1 Auger peak.32 Mn 2p spectrum depicts two components located at 642.3 eV and 653.8 eV, consistent with Mn 2p3/2 and Mn 2p1/2 values for Mn4+ ions, respectively.30 XPS analysis of transition metals spectra confirmed the presence of Co3+, Ni2+ and Mn4+, consistent with previous attributions.
The binding energy range of 45–80 eV (Fig. 4d) includes Mn 3p, Li 1s, Ni 3p and Co 3p spectra. Mn 3p spectrum consists of a main peak at 49.7 eV, Ni 3p spectrum has a main peak at 67.3 eV and a satellite peak at 73.1 eV, while Co 3p spectrum shows a main peak at 61 eV and a satellite peak at 71 eV. Description of Li 1s spectrum requires two contributions; one for Li+ in the oxide lithium layers at 54 eV, while the other can be attributed to lithium residuals (Li2CO3 and LiOH) present on the surface at 55 eV.30,32 In addition to this, the presence of lithium residuals on the surface can be confirmed from O 1s spectra. O 1s region exhibits two peaks; the peak at 529.3 eV can be attributed to the O2− framework in the NMC 111 and the one around 531.5 eV to the absorbed species and lithium residuals on the surface, as the sample was exposed to ambient atmosphere before XPS analysis.
Furthermore, NMC 111 thin film was aged for two months after XPS analysis to study the stability of the surface in the ambient atmosphere. The evolution of the NMC 111 surface after prolonged ambient exposure and a series of sputter cycles is shown on O 1s and Li 1s photoelectron spectra (Fig. 5). O 1s spectra were fitted with 3 peaks, attributed to lattice oxygen in NMC (529.3 eV), weakly absorbed species (531 eV) and lithium residuals (532 eV), while Li 1s spectra were fitted with two peaks, attributed to lithium in NMC layers (54 eV) and residual lithium on the surface (55 eV). After two months of ambient exposure, the contribution from lithium residuals on O 1s and Li 1s spectra starts to dominate in intensity, as moisture and CO2 in the ambient atmosphere enhance their formation. Afterward, the sample was cleaned with an argon cluster ion beam (GCIB) and argon ions to expose the underlying surface. A series of sputter cycles resulted in a clean surface of NMC 111, which was later exposed to ambient air for 10 minutes and measured again. Ambient atmosphere exposure resulted in the absorption of species that enhance the formation of lithium residuals, which suggests the high sensitivity of NMC 111 surfaces to moisture and CO2. Species absorbed in a short time of exposure were then quickly removed with argon ions.
 | ||
| Fig. 5 Evolution of O 1s and Li 1s photoelectron spectra of NMC 111 thin film after ambient atmosphere exposure and sputtering with argon ions. | ||
3.4. Structural changes in delithiated and lithiated LiNi1/3Mn1/3Co1/3O2 thin films
To understand how the structure of the NMC 111 changes after the delithiation and lithiation process, the thin film was charged to 4.2 V vs. Li/Li+ and discharged to 3.0 V vs. Li/Li+. The reversibility of lithium insertion in the thin film was analyzed by using the protocol described in the experimental section and presented in Fig. S6.† After the charge and discharge cycle, NMC 111 remains epitaxial and preserves the same orientation relationship to the substrate, but the shift and intensity of (104) reflection decrease in the charged state, which indicates a structural change in NMC 111 thin film (Fig. 6a). The volume change of NMC 111 at charged and discharged state was estimated from RSMs measured around (104) and (107) reflections of NMC 111 thin film (Fig. 6c–e), as discussed in the structural analysis chapter. Table 1 presents lattice parameters and respective volumes of pristine, charged, and discharged NMC 111 thin films. Results have confirmed that NMC 111 experiences a negative volume change with the delithiation process, related to shrinkage of a lattice parameter and elongation of c lattice parameter, as previously reported for polycrystalline NMC.28 Comparison of rocking curves on NMC 111 (104) peak (Fig. 6b) provides additional information about crystal structure at the charged state. We observed an increased broadening of the rocking curve in the delithiated NMC 111 compared to the pristine structure. The latter can be explained by the increase in mosaicity spread of NMC planes, which compensates for the negative volume change in the structure. Nevertheless, lithiation of the charged NMC 111 is reversible, as the positions of (104) and (107) reflections are comparable to a pristine thin film and the broadening of the NMC (104) peak reduces with lithiation. Based on these results, we can conclude that the delithiation induces negative volume change and broadening of crystal plane orientations in the epitaxial NMC 111 thin film.| a lattice parameter | c lattice parameter | Unit cell volume | |
|---|---|---|---|
| Pristine | 2.88 Å | 14.23 Å | 102.4 Å3 |
| Charged | 2.82 Å | 14.24 Å | 98.2 Å3 |
| Discharged | 2.88 Å | 14.22 Å | 102.1 Å3 |
3.5. Microstructural analysis of delithiated LiNi1/3Mn1/3Co1/3O2 thin films
To prove and correlate the structural changes that occur after delithiation, NMC 111 thin film was charged to 4.2 V vs. Li/Li+ identically as presented in Fig. S6,† followed by preparation of FIB lamella, cut in [110] azimuth direction of the Nb:STO (001) substrate, as described in the experimental section.Based on recorded diffraction patterns of pristine (Fig. 7a) and charged (Fig. 7b) NMC 111 thin film, corresponding with selected areas, presented in Fig. S7a and b,† no obvious change in the crystal symmetry is observed at the charged state and STEM analysis of NMC 111 thin film revealed twinned domains of the layered structure (Fig. S7c†), showing no deviation to pristine state. Nevertheless, the presence of the spinel phase in small amounts cannot be excluded.21 This result aligns closely with RSM data, which proved to be the more appropriate technique in terms of determining exact changes in distances between the planes. To understand the mechanism behind the delithiation process in NMC 111 thin films, EEL spectra were measured in the bulk of the pristine and charged thin film lamella, as shown in Fig. S7d and S7e,† respectively. Comparison of pristine and charged EEL spectra (Fig. 7c) showed the shift in O K-edge main peak position from 539.7 eV to 540.6 eV, which can be attributed to the change of local electronic structure of oxygen ions, thoroughly explained in the paper by Y. Koyama et al.33 The shift to higher energy is a consequence of nickel oxidation that increases the covalent bonding between nickel and oxygen, leading to the reduction of electron density at the oxygen ions. However, the shift in the peak position of the O K-edge pre-peak is not observed; this might be related to the beam sensitivity of the sample since the origin of the pre-peak can arise due to the radiation damage, observed in the complex oxides containing light alkali elements,34 impeding straight-forward explanation. Regarding the local electronic structure of transition metals at the charged state, there is no obvious shift in Mn L-edge and Co L-edge EEL spectra; in contrast, a broadening of Ni L-edge was observed. This change in Ni L-edge is related to the change in the nickel oxidation state; after lithium is extracted from the layered structure, nickel ions are oxidized to compensate for the charge and maintain charge neutrality in the delithiated structure, directly affecting the shape of Ni L-edge EEL spectrum, causing it to broaden towards higher energy values. Moreover, the change in intensity ratios between the L3 and L2 peaks of transition metals EEL spectra can indicate a change in the valence state.35,36 Determined L3/L2 intensity ratios in pristine and charged NMC thin film are presented in Table 2. Since the obvious change of L3/L2 intensity ratio occurs only on nickel EEL spectra, this further proves that the charge compensation mechanism in NMC is the oxidation of the nickel ions, which concurrently influences the local electronic structure of the oxygen ions.
| Mn L3/L2 ratio | Co L3/L2 ratio | Ni L3/L2 ratio | |
|---|---|---|---|
| Pristine | 1.92 | 2.03 | 3.58 |
| Charged | 1.92 | 2.05 | 3.17 |
3.6. Electrochemical properties of LiNi0.33Mn0.33Co0.33O2 thin films
Charge and discharge curves of non-annealed and annealed NMC 111 thin films vs. Li4Ti5O12 are shown in Fig. 8a and b. The capacity of NMC 111 thin film is presented in specific volumetric units, calculated from thicknesses measured with XRR, assuming theoretical density. Based on the observed low first cycle coulombic efficiency (57.6%) of the non-annealed thin film (Fig. 8e), we consider a certain degree of oxygen non-stoichiometry, as there is not enough oxygen to form stoichiometric NMC 111 film at lower pressures. This can induce structural instability at the charged state,6 resulting in irreversible structural changes that hinder electrochemical performance. Annealing of NMC 111 thin films after deposition improved the coulombic efficiency of the first cycle (76.2%) (Fig. 8e) and long-term cycling stability, which can be attributed to additional oxygen incorporation in the NMC 111 thin film during cooling in high oxygen pressure, thus stabilizing the crystal structure. Moreover, the instability of the oxygen-deficient film is reflected as a constant decline in specific charge and discharge capacities (Fig. 8c and d). On the other hand, stable performance was observed for the annealed NMC 111 thin film after the first formation cycle that reached a specific discharge capacity around 60 μA cm−2 μm−1, which corresponds to 125 mA h g−1, meaning that the practical specific capacity is comparable to the polycrystalline NMC 111 cycled vs. LTO anode.37 The correlation of oxygen non-stoichiometry to the structural instability and electrochemical performance of NMC is discussed in the work of Bi et al..38 They proved the dependence of oxygen content in the gaseous mixture during the annealing process on the degree of structural defects and electrochemical properties of NMC 811 powders. The sample prepared in pure oxygen exhibited the least amount of oxygen defects, leading to the highest discharge capacity, the best rate capability and the most stable cyclability. Even though this paper discusses NMC 811 composition, which is believed to be more susceptible to oxygen loss during high-temperature processing, the oxygen stoichiometry in NMC strongly depends on a processing technique. In another work, NMC 111 fibers are prepared via the electrospinning method, showing improved electrochemical properties of NMC 111 fibers, annealed in an oxygen atmosphere.39 In our case, oxygen pressure during PLD growth is moderate, which could produce oxygen-deficient films, so high-pressure annealing of NMC 111 thin films after deposition provides enough oxygen to be incorporated into the film, leading to improved electrochemical performance. Since there is a certain degree of capacity loss during the first cycle when comparing charge and discharge curves, we assume this is a result of kinetic limitations during discharge,40 irreversible structural changes at the charged state,40 or the formation of the solid electrolyte interphase layer on the surface of the cathode,41 which depends on the surface morphology and stoichiometry of the NMC 111 thin film.To further elucidate the overall electrochemical behavior of annealed NMC 111 thin film, cyclic voltammetry42 and galvanostatic cycling measurements43 are performed in the voltage range of 3.0–4.2 V vs. Li/Li+. A cyclic voltammogram, shown in Fig. 9a, displays a well-defined cathodic peak at 3.78 V and an anodic peak at 3.74 V. Those two features are characteristic of NMC 111 that undergoes the process of lithium-ion insertion/deinsertion,12 coupled to the redox reaction of nickel ions, which aligns well with the observed nickel oxidation in NMC 111 thin film, charged up to 4.2 V. Moreover, the appearance of only one redox peak in the voltage range of 3.0–4.2 V suggests the absence of phase transitions from hexagonal to monoclinic structure.44 The effect of applied current density on the specific capacity was tested via galvanostatic cycling of NMC 111 thin film (Fig. 9b). At a current density of 0.4 μA cm−2, corresponding to the rate of C/10, NMC 111 thin film achieved a specific capacity of 63.7 μA cm−2 μm−1 (133.5 mA h g−1), similar to the annealed NMC 111 thin film, cycled vs. LTO anode. The capacity gradually declined with increasing rates, reaching 57.6 μA cm−2 μm−1 (120.8 mA h g−1) at C/2, 53.2 μA cm−2 μm−1 (111.5 mA h g−1) at 1C, 47.5 μA cm−2 μm−1 (99.6 mA h g−1) at 2C, 41.9 μA cm−2 μm−1 (87.8 mA h g−1) at 4C and 38.0 μA cm−2 μm−1 (79.7 mA h g−1) at 6C. Gradual fading of the specific capacity at faster charge/discharge rates is caused by kinetic restrictions of active material since diffusivity coefficient of lithium ions is at least one order of magnitude lower than electronic conductivity in stoichiometric NMC 111, while even larger differences are observed when lithium is removed from the structure.45 Even though the diffusion length for lithium ion transport is short, the rate-controlling step for fast charge and discharge is the solid-state diffusion of lithium ions.
4. Conclusions
Epitaxial NMC 111 with (104) out-of-plane orientation deposited on SRO/Nb:STO (001) substrates by pulsed laser deposition proved to be fully relaxed with 4-fold symmetry and 45° tilt of the unit cell with respect to the substrate. Lattice parameters and the corresponding volume of NMC 111 unit cell are calculated from (104) and (107) reflections of the thin film, measured with HRXRD. Microstructure analysis revealed twinned domains in the layered structure of NMC 111 with the presence of a rock-salt phase on the surface. Surfaces of NMC 111 thin films are prone to absorb moisture and form lithium residuals on the surface when in contact with the ambient atmosphere, enhancing the formation of non-desirable phases. After delithiation of NMC 111 thin films, nickel ions are oxidized to maintain charge neutrality, while the crystal structure experienced negative volume change and increased mosaicity, which proved to be a reversible process. Poor electrochemical performance of oxygen-deficient NMC 111 thin films is a consequence of the structural instability in the charged state which can be improved by cooling the films in high oxygen pressure after deposition. Cyclic voltammetry displays well-defined cathodic and anodic peaks, while a decrease of specific capacity from 63.7 μA cm−2 μm−1 at C/10 to 38.0 μA cm−2 μm−1 at 6C revealed the kinetic limitations of NMC 111 at higher cycling rates.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and ESI file.† The data supporting the findings of this study were collected during the completion of a doctoral dissertation and are currently stored on a local computer. Due to data protection, they are not available in a public repository. However, the data may be accessed upon reasonable request. Contact Blaž Jaklič E-mail: blaz.jaklic@ijs.si.Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. Blaž Jaklič: writing – original draft, conceptualization, formal analysis, investigation, methodology, visualization. Jan Žuntar: investigation, formal analysis. Elena Tchernychova: investigation, formal analysis, validation, project administration. Gregor Kapun: investigation, formal analysis. Martin Šala: investigation, formal analysis. Robert Dominko: writing – review and editing, validation, resources, supervision, project administration, funding acquisition. Matjaž Spreitzer: writing – review and editing, validation, conceptualization, resources, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Medeja Gec for helping with TEM sample preparation and prof. Dr Janez Kovač for advising with XPS data interpretation. The authors would like to acknowledge funding from the Slovenian Research Agency (ARIS) under research programs P2-0091, P2-0423 and P1-0034 and research projects J2-3050, J7-4637 and HyBReED.References
- J.-M. Kim and H.-T. Chung, Electrochim. Acta, 2004, 49, 937–944 CrossRef CAS
.
- H. Maleki Kheimeh Sari and X. Li, Adv. Energy Mater., 2019, 9, 1901597 CrossRef CAS
.
- P. Teichert, G. G. Eshetu, H. Jahnke and E. Figgemeier, Batteries, 2020, 6, 8 CrossRef CAS
.
- J. Kim, H. Lee, H. Cha, M. Yoon, M. Park and J. Cho, Adv. Energy Mater., 2018, 8, 1702028 CrossRef
.
- T. Wang, K. Ren, W. Xiao, W. Dong, H. Qiao, A. Duan, H. Pan, Y. Yang and H. Wang, J. Phys. Chem. C, 2020, 124, 5600–5607 CrossRef CAS
.
- J. Zheng, J. Xiao and J.-G. Zhang, Nano Today, 2016, 11, 678–694 Search PubMed
.
- C. M. Julien and A. Mauger, Coatings, 2019, 9, 386 Search PubMed
.
- L. Indrizzi, N. Ohannessian, D. Pergolesi, T. Lippert and E. Gilardi, Helv. Chim. Acta, 2021, 104, 2 CrossRef
.
- M. Fenech and N. Sharma, Chem.–Asian J., 2020, 15, 1829–1847 CrossRef CAS
.
- M. Abe, K. Suzuki, H. Minamishima, K. Kim, S. Taminato, M. Hirayama and R. Kanno, J. Jpn. Soc. Powder Powder Metall., 2015, 62, 531–537 CrossRef CAS
.
- T. Kato, R. Yoshida, K. Yamamoto, T. Hirayama, M. Motoyama, W. C. West and Y. Iriyama, J. Power Sources, 2016, 325, 584–590 CrossRef CAS
.
- J. Deng, L. Xi, L. Wang, Z. Wang, C. Y. Chung, X. Han and H. Zhou, J. Power Sources, 2012, 217, 491–497 CrossRef CAS
.
- C. Jacob, T. Lynch, A. Chen, J. Jian and H. Wang, J. Power Sources, 2013, 241, 410–414 CrossRef CAS
.
- T. Ohashi, T. Hirano, K. Okazaki, T. Fukunaga and T. Abe, J. Electroanal. Chem., 2021, 899, 115675 Search PubMed
.
- M. Hirayama, M. Abe, S. Taminato, Y. Araki, K. Suzuki and R. Kanno, RSC Adv., 2016, 6, 78963–78969 RSC
.
- K. Nishio, N. Nakamura, K. Horiba, M. Kitamura, H. Kumigashira, R. Shimizu and T. Hitosugi, ACS Appl. Energy Mater., 2020, 3, 6416–6421 CrossRef CAS
.
- K. Nishio, N. Nakamura, K. Horiba, M. Kitamura, H. Kumigashira, R. Shimizu and T. Hitosugi, Appl. Phys. Lett., 2020, 116, 053901 CrossRef CAS
.
- M. Abe, H. Iba, K. Suzuki, H. Minamishima, M. Hirayama, K. Tamura, J. Mizuki, T. Saito, Y. Ikuhara and R. Kanno, J. Power Sources, 2017, 345, 108–119 CrossRef CAS
.
- K. Nishio, T. Ohnishi, K. Akatsuka and K. Takada, J. Power Sources, 2014, 247, 687–691 CrossRef CAS
.
- S. Takeuchi, H. Tan, K. K. Bharathi, G. R. Stafford, J. Shin, S. Yasui, I. Takeuchi and L. A. Bendersky, ACS Appl.
Mater. Interfaces, 2015, 7, 7901–7911 CrossRef CAS PubMed
.
- Z. Li, S. Yasui, S. Takeuchi, A. Creuziger, S. Maruyama, A. A. Herzing, I. Takeuchi and L. A. Bendersky, Thin Solid Films, 2016, 612, 472–482 CrossRef CAS PubMed
.
- H. Tan, S. Takeuchi, K. K. Bharathi, I. Takeuchi and L. A. Bendersky, ACS Appl. Mater. Interfaces, 2016, 8, 6727–6735 CrossRef CAS PubMed
.
- K. Kawashima, T. Ohnishi and K. Takada, ACS Appl. Energy Mater., 2020, 3, 11803–11810 Search PubMed
.
- J. C. Garcia, J. Bareño, J. Yan, G. Chen, A. Hauser, J. R. Croy and H. Iddir, J. Phys. Chem. C, 2017, 121, 8290–8299 CAS
.
- P. A. Van Aken and B. Liebscher, Phys. Chem. Miner., 2002, 29, 188–200 CAS
.
- I. Horcas, R. Fernández, J. M. Gómez-Rodríguez, J. Colchero, J. Gómez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 013705 CAS
.
- S. Hasegawa, in Reflection High-Energy Diffraction, Characterization of Materials, ed. E. N. Kaufmann, Wiley, 2nd edn, 2014, vol. 3, pp. 1925–1938 Search PubMed
.
- O. Dolotko, A. Senyshyn, M. J. Mühlbauer, K. Nikolowski and H. Ehrenberg, J. Power Sources, 2014, 255, 197–203 CrossRef CAS
.
- J. Hu, Q. Wang, B. Wu, S. Tan, Z. Shadike, Y. Bi, M. S. Whittingham, J. Xiao, X.-Q. Yang and E. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 2622–2629 CAS
.
- N. Andreu, D. Flahaut, R. Dedryvère, M. Minvielle, H. Martinez and D. Gonbeau, ACS Appl. Mater. Interfaces, 2015, 7, 6629–6636 CAS
.
- O. Bondarchuk, A. P. LaGrow, A. Kvasha, T. Thieu, E. Ayerbe and I. Urdampilleta, Appl. Surf. Sci., 2021, 535, 147699 CAS
.
- Z. Fu, J. Hu, W. Hu, S. Yang and Y. Luo, Appl. Surf. Sci., 2018, 441, 1048–1056 CrossRef CAS
.
- Y. Koyama, T. Mizoguchi, H. Ikeno and I. Tanaka, J. Phys. Chem. B, 2005, 109, 10749–10755 CrossRef CAS PubMed
.
- N. Jiang and J. C. H. Spence, Ultramicroscopy, 2006, 106, 215–219 Search PubMed
.
- X. Li, Z. Ren, M. Norouzi Banis, S. Deng, Y. Zhao, Q. Sun, C. Wang, X. Yang, W. Li, J. Liang, X. Li, Y. Sun, K. Adair, R. Li, Y. Hu, T.-K. Sham, H. Huang, L. Zhang, S. Lu, J. Luo and X. Sun, ACS Energy Lett., 2019, 4, 2480–2488 CrossRef CAS
.
- S. Hwang, S. M. Kim, S.-M. Bak, S. Y. Kim, B.-W. Cho, K. Y. Chung, J. Y. Lee, E. A. Stach and W. Chang, Chem. Mater., 2015, 27, 3927–3935 CrossRef CAS
.
- E. Björklund, D. Brandell, M. Hahlin, K. Edström and R. Younesi, J. Electrochem. Soc., 2017, 164, A3054–A3059 CrossRef
.
- Y. Bi, W. Yang, R. Du, J. Zhou, M. Liu, Y. Liu and D. Wang, J. Power Sources, 2015, 283, 211–218 CrossRef CAS
.
- C.-S. Kang and J.-T. Son, J. Electroceram., 2012, 29, 235–239 CrossRef CAS
.
- J. Kasnatscheew, M. Evertz, B. Streipert, R. Wagner, R. Klöpsch, B. Vortmann, H. Hahn, S. Nowak, M. Amereller, A.-C. Gentschev, P. Lamp and M. Winter, Phys. Chem. Chem. Phys., 2016, 18, 3956–3965 RSC
.
- V. S. K. Sungjemmenla, C. B. Soni, V. Kumar and Z. W. Seh, Energy Technol., 2022, 10, 2200421 CrossRef CAS
.
- T. Kim, W. Choi, H.-C. Shin, J.-Y. Choi, J. M. Kim, M.-S. Park, W.-S. Yoon and J. Electrochem, Sci. Technol., 2020, 11, 14–25 CAS
.
- P. Pang, Z. Wang, Y. Deng, J. Nan, Z. Xing and H. Li, ACS Appl. Mater. Interfaces, 2020, 12, 27339–27349 Search PubMed
.
- Y.-S. He, Z.-F. Ma, X.-Z. Liao and Y. Jiang, J. Power Sources, 2007, 163, 1053–1058 CrossRef CAS
.
- R. Amin and Y.-M. Chiang, J. Electrochem. Soc., 2016, 163, A1512–A1517 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08924c |
| This journal is © The Royal Society of Chemistry 2025 |