Exploring the compositional space of a metal–organic framework with ionic liquids to develop porous ionic conductors for enhanced signal and selectivity in VOC capacitive sensors†
Bruna F.
Gonçalves
 *a,
Eduardo
Fernández
a,
Ainara
Valverde
a,
Mattia
Gaboardi
b,
Hugo
Salazar
*a,
Eduardo
Fernández
a,
Ainara
Valverde
a,
Mattia
Gaboardi
b,
Hugo
Salazar
 a,
Viktor
Petrenko
a,
Viktor
Petrenko
 ac,
José María
Porro
ac,
José María
Porro
 ac,
Leide P.
Cavalcanti
d,
Karmele
Urtiaga
e,
José M. S. S.
Esperança
ac,
Leide P.
Cavalcanti
d,
Karmele
Urtiaga
e,
José M. S. S.
Esperança
 f,
Daniela M.
Correia
f,
Daniela M.
Correia
 g,
Felix
Fernandez-Alonso
g,
Felix
Fernandez-Alonso
 bch,
Senentxu
Lanceros-Mendez
bch,
Senentxu
Lanceros-Mendez
 ac and
Roberto
Fernández de Luis
ac and
Roberto
Fernández de Luis
 a
a
aBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940 Leioa, Spain. E-mail: bruna.ferreira@bcmaterials.net
bMaterials Physics Center, CSIC-UPV/EHU, Paseo Manuel de Lardizabal 5, 20018, Donostia – San Sebastian, Spain
cIKERBASQUE, Basque Foundation for Science, 48009 Bilbao, Spain
dISIS Neutron and Muon Source, Science and Technology Facilities Council, Rutherford Appleton Laboratory, Didcot OX11 0QX, UK
eGeology Department, University of the Basque Country (UPV/EHU), Barrio Sarriena s/n, 48940, Leioa, Spain
fLAQV, REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516, Lisboa, Portugal
gChemistry Centre of Minho and Porto Universities (CF-UM-UP), University of Minho, 4710-057, Braga, Portugal
hDonostia International Physics Center (DIPC), Paseo Manuel de Lardizabal 4, 20018 Donostia San Sebastian, Spain
First published on 20th May 2024
Abstract
The monitoring of atmospheric pollutants, especially non-methane-based volatile organic compounds (NMVOCs), is an important paradigm towards secure air quality surroundings. However, existing gas sensing technologies face challenges in selectively and sensitively detecting individual NMVOCs due to their low concentration in comparison to the main atmospheric components. In this research, the compositional space between a Metal–Organic Framework (MOF) and Ionic Liquids (ILs) is explored to fine tune the signal and selectivity of a capacitive gas sensing layer. Firstly, by tuning the weight ratio of the MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL components, ionic conductive materials ranging from solid porous to partially porous inks are produced. Secondly, by combining the sensitivity arising from the IL's dielectric characteristics with the selectivity endowed by the MOF's porosity, varied capacitive responses are obtained. Finally, the sensing responses of thirteen sensors towards the detection of water vapor, ethanol, acetone and isopropanol demonstrate that the hybridization of the MOF/IL offers a suitable avenue to balance the porosity, magnitude of response, and partial selectivity. In addition, when the responses of multiple MOF/IL sensors are evaluated, cross-selectivity detection of individual NMVOCs is reached. This approach contributes to fine-tuning the MOF/IL sensor performance not just by expanding the MOF/IL combinations, but optimizing sensor processing by advanced printing and electronics design.
IL components, ionic conductive materials ranging from solid porous to partially porous inks are produced. Secondly, by combining the sensitivity arising from the IL's dielectric characteristics with the selectivity endowed by the MOF's porosity, varied capacitive responses are obtained. Finally, the sensing responses of thirteen sensors towards the detection of water vapor, ethanol, acetone and isopropanol demonstrate that the hybridization of the MOF/IL offers a suitable avenue to balance the porosity, magnitude of response, and partial selectivity. In addition, when the responses of multiple MOF/IL sensors are evaluated, cross-selectivity detection of individual NMVOCs is reached. This approach contributes to fine-tuning the MOF/IL sensor performance not just by expanding the MOF/IL combinations, but optimizing sensor processing by advanced printing and electronics design.
Introduction
Non-methane-based volatile organic compounds (NMVOCs) have garnered significant attention as one of the top five priority atmospheric pollutants. NMVOCs encompass aromatic and aliphatic hydrocarbons ranging from C2 to C12 with varied chemical compositions and structures. It is well established that NMVOCs have a substantial impact on both human health and the environment, particularly upon exposure to them over extended periods of time. Additionally, NMVOCs contribute significantly to the photochemical generation of ozone and secondary organic aerosols.1,2Therefore, the continuous sensing of NMVOCs' concentration in the atmosphere represents an essential step towards enhancing air quality and safeguarding public health. However, it also presents challenges from the perspectives of active-sensing materials design and sensor-device assembly. Firstly, the atmosphere may contain a wide variety of different VOCs (ranging from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 100
000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) within the ppb to ppt range. Secondly, these NMVOCs are diluted within a matrix of other hydrophilic and hydrophobic gases, with water vapor being one of the prevalent and variable components of the atmosphere. And thirdly, current gas/VOC sensing technologies are either based on unselective semiconductor sensors or rely on complex and expensive gas chromatographic protocols.3 Despite their chemical variety and dilution degree in the atmosphere, NMVOCs share two common characteristics: high vapor pressure and hydrophobicity. These two characteristics can serve as a basis for designing technologies for their capture, degradation, and, most importantly, sensing.4
000) within the ppb to ppt range. Secondly, these NMVOCs are diluted within a matrix of other hydrophilic and hydrophobic gases, with water vapor being one of the prevalent and variable components of the atmosphere. And thirdly, current gas/VOC sensing technologies are either based on unselective semiconductor sensors or rely on complex and expensive gas chromatographic protocols.3 Despite their chemical variety and dilution degree in the atmosphere, NMVOCs share two common characteristics: high vapor pressure and hydrophobicity. These two characteristics can serve as a basis for designing technologies for their capture, degradation, and, most importantly, sensing.4
Capacitive sensors based on Metal–Organic Frameworks (MOFs) have emerged as a promising alternative to conventional technologies to tackle this challenge.5 Capacitive gas sensors operate by monitoring variations in the dielectric constant or thickness of the dielectric active material caused by the adsorption of specific molecules (e.g., VOCs).6 The chemical tunability of MOFs offers a unique advantage in this context. By precisely manipulating the components of MOFs, such as inorganic nodes, organic linkers, and pore space, their selectivity towards VOC capture from the atmosphere can be improved.7 This selective adsorption is critical for the subsequent sensing of VOCs via the variation of the dielectric properties of the MOF itself.8 Unfortunately, the low dielectric response of conventional MOFs still exhibits limitations in terms of the sensitivity response of a sensor device. Despite efforts to miniaturize the electronic circuit to enhance the signal-to-noise ratio, the subtle variation of the electric signal recorded during gas adsorption remains a challenge. As capacitive sensors rely on charge rearrangements and concentrations within the active material, interphases, and electrodes, the incorporation of positive–negative charge pairs or dipole-based decorations within the MOF structure is a way to enhance capacitance variation during gas adsorption.9 Thus, this strategy has been successfully applied by (i) modifying the MOF structure itself via post-synthetic grafting and (ii) incorporating ionic liquids (ILs) into its pore space.5
Ionic liquids, defined as liquid molten salts at room temperature, can be easily integrated into the pore space of MOFs due to their liquid nature. In addition, the combination of MOF and IL chemistries to design composite porous ionic conductors, along with their rich chemistry and versatility, makes MOF/IL composites highly appealing for assembling capacitive sensors with tuned cross-selectivity and sensitivity responses. Indeed, the simplicity of the MOF/IL assembly enables the employment of market-ready spray and screen-printing technologies for the bottom-up integration of MOF/IL composites into smart, flexible, affordable, lightweight and low-energy-demand capacitive sensors compatible with the Industry 4.0 and the Internet of Things concepts.10
However, since the first publication of MOF/IL composites used for gas sensing, there is room for development.5,11–13 Previous literature has explored the use of a UiO-66–NH2 MOF with ILs for humidity sensing11,12 and an HKUST-1 MOF with ILs for CO2 sensing.13 However, while these studies focus on MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL materials, our research delves into different MOF and IL compositions, encompassing both non-metal-based and metal-based ILs. Additionally, beyond humidity sensing, we assess sensor responses to NMVOCs. Notably, our approach utilizes printing technologies for sensor development, distinguishing it from the drop-casting, dip coating, and vapor-assisted conversion techniques employed in prior studies.
IL materials, our research delves into different MOF and IL compositions, encompassing both non-metal-based and metal-based ILs. Additionally, beyond humidity sensing, we assess sensor responses to NMVOCs. Notably, our approach utilizes printing technologies for sensor development, distinguishing it from the drop-casting, dip coating, and vapor-assisted conversion techniques employed in prior studies.
This pioneering study has prompted further investigation into whether gas sensing sensitivity and selectivity in MOF/IL composites are primarily governed by the pore chemistry of the MOF or by the interaction between the VOC and the ILs when confined in an ordered porosity. To systematically explore these parameters, the dielectric response of the parent MOF and IL components as well as the MOF/IL intermediates has been studied in this research for: (i) MOF/IL porous conductors (low IL loading) and (iii) IL-supersaturated porous MOF/IL inks (scheme in Fig. 1). All these MOF/IL composites have been integrated into parallel capacitors to engineer a MOF/IL capacitive sensor array with varied responses. Overall, the research has been dedicated to address an environmentally relevant challenge, which is the cross-selectivity of VOCs. To achieve this goal, the ZIF-8 material has been selected as the host due to its hydrophobic nature and its affinity for imidazolium-based IL and VOC adsorption. Three different 1-methyl-3-methylimidazolium-based ILs were employed as guest ionic molecules in capacitive sensors, each with various anions conferring differing hydrophilic characteristics. These combinations allow a systematic investigation of how different ILs interact with the MOF and impact the sensitivity and selectivity of the resulting capacitive sensors for VOCs in the presence of water vapor.
Experimental
Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) (99%, Thermo Scientific), 2-methylimidazole (97%, Thermo Scientific), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][TFSI]2) (IL TFSI, 99%, Iolitec), bis(1-butyl-3-methylimidazolium)tetrachloronickelate ([Bmim]2[NiCl4]) (IL Ni, 99%, in-house according to the recipe described in ref. 14), bis(1-ethyl-3-methylimidazolium)tetrathiocyanatocobaltate ([Emim]2[Co(SCN)4]) (IL Co, 99%, Iolitec), methanol (99.8%, Labkem), and ethanol (96%, Labkem) were used as provided.Processing of MOF/IL samples
The production of MOF/IL dispersions using the zeolitic imidazolate framework (ZIF-8) was carried out using the following two-step procedure.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 10 min to recover the ZIF-8 nanoparticles. Finally, the solid was dried at 80 °C, and the resulting powder was ground with an agate mortar to homogenize the sample for its characterization.
500 rpm for 10 min to recover the ZIF-8 nanoparticles. Finally, the solid was dried at 80 °C, and the resulting powder was ground with an agate mortar to homogenize the sample for its characterization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL samples with weight ratios of 1
IL samples with weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1
0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 and 1
0.4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The ZIF-8 and IL mixture was stirred overnight in a hermetically closed vial under mild heating conditions (60 °C). Afterwards, the vials were opened to trigger the full evaporation of ethanol to obtain the final ZIF-8
1, respectively. The ZIF-8 and IL mixture was stirred overnight in a hermetically closed vial under mild heating conditions (60 °C). Afterwards, the vials were opened to trigger the full evaporation of ethanol to obtain the final ZIF-8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL composites. Powdered and slurry-like samples were obtained depending on the degree of IL addition to the MOF. For 1
IL composites. Powdered and slurry-like samples were obtained depending on the degree of IL addition to the MOF. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 ratios, the forced impregnation gives rise to powdered ZIF
0.4 ratios, the forced impregnation gives rise to powdered ZIF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL samples. When the MOF
IL samples. When the MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio was adjusted to 1
IL ratio was adjusted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a slurry-like sample was formed, with the ZIF-8 component completely saturated in IL. All the ZIF-8/IL samples were activated overnight at 80 °C and sealed in Eppendorf tubes before their integration into the transduction system.
1, a slurry-like sample was formed, with the ZIF-8 component completely saturated in IL. All the ZIF-8/IL samples were activated overnight at 80 °C and sealed in Eppendorf tubes before their integration into the transduction system.
Sensor fabrication
Experiments for the detection of volatile organic compounds
The MOF/IL sensors' response to increasing gas concentrations of acetone, ethanol, water and isopropanol (IPA) was evaluated in real time. A custom-made experimental setup was employed to control the composition of the vapor-mixture, as described in ref. 5. Then, the electrical signal (capacitance) obtained from the experiment was measured with an Agilent E4980A Precision LCR Meter. The whole experiment was controlled using LabView software responsible for electrical data gathering, as well as the gas flow control on two mass flow controllers, one for a base N2 flux (vN2) and the other for the solvent (vVOC). The concentration of the gas in the final flux in contact with the capacitive-sensor was estimated using eqn (1):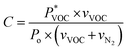 | (1) |
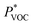 is the theoretical vapor pressure of a determinate VOC at RT (24.55, 5.85, 2.32 and 4.44 kPa for acetone, ethanol, water and IPA, respectively), vVOC is the nitrogen flux going through the solvent vessel (sccm), Po is the atmospheric pressure (101 kPa), and vN2 is the nitrogen flux (sccm).16 All experiments were carried out at RT.
is the theoretical vapor pressure of a determinate VOC at RT (24.55, 5.85, 2.32 and 4.44 kPa for acetone, ethanol, water and IPA, respectively), vVOC is the nitrogen flux going through the solvent vessel (sccm), Po is the atmospheric pressure (101 kPa), and vN2 is the nitrogen flux (sccm).16 All experiments were carried out at RT.
Characterization of the active MOF, IL and MOF/IL materials
All the diffraction patterns were analysed by a two-fold protocol. The incorporation of the IL within the ZIF-8 structure induces a significant change of the intensity ratio of different diffraction maxima of the patterns at 2θ > 7°. In order to quantify this intensity variation, the intensity of the diffraction maxima was obtained by (i) the fitting of the individual maximum, and (ii) the pattern-matching analysis of the whole diffraction pattern. The intensity ratio between the (110)/(211) maxima was monitored with the IL loading into the ZIF-8. In addition, the envelope density was computed from the intensity of the diffraction maxima obtained from the simulated XRD pattern of the ZIF-8, and from the intensity of the diffraction maxima of the ZIF-8/IL samples obtained by pattern matching analyses. The comparison between the electron density maps of the ZIF-8 and ZIF-8/IL gives qualitative information about the location of the IL molecules within the crystal structure of the ZIF-8 compound.
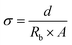 | (2) |
Results and discussion
Structural properties of ZIF-8/IL samples
ZIF-8, known for its intrinsic hydrophobicity, was selected as the porous matrix based on its ultra-large surface area (1821 m2 g−1), high thermal stability, and excellent chemical resistance, to incorporate three different ionic liquids.20 The selection of these three ILs was based on their varying hydrophobic/hydrophilic nature as well as the presence or absence of metal-based anions in their chemical structure. Throughout the study, the very similar cationic components, Emim and Bmim, were kept constant, while the anions were varied. The hydrophobic bis(trifluoromethylsulfonyl)imide anion was selected for the IL-TFSI, and the more hydrophilic Bmim−[NiCl4] and Emim-[Co(SCN)4] metal-based anions were chosen for the IL-Ni and IL-Co ionic liquids, respectively. For simplicity, the selected ILs will be denoted henceforth as IL-TFSI, IL-Ni, and IL-Co. Additionally, for each MOF-IL combination, three MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL molar-loading degrees for each IL were investigated (for TFSI: 1
IL molar-loading degrees for each IL were investigated (for TFSI: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, 1
0.7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7; for Ni: 1
7; for Ni: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, 1
0.6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; and for Co: 1
6; and for Co: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, MOF
5, MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL calculated based on a Zn12(C4N2H5)24 ZIF-8 cage).
IL calculated based on a Zn12(C4N2H5)24 ZIF-8 cage).
Considering the pore volume of the ZIF-8 cage (∼1006 Å3 per mol-cage (Zn12(C4N2H5)24)) and the volume of each IL molecule (IL-TFSI: 615 Å3, IL-Co: 638 Å3, and IL-Ni: 523 Å3), it is feasible to confine a maximum of approximately 2 IL molecules within each ZIF-8 cage21,22 (Fig. S1, ESI).† Beyond this threshold, the ZIF-8 porosity becomes saturated, and any additional IL is integrated into the ZIF-8 sample's surface and intraparticle space. Nevertheless, as demonstrated by A. Padua et al.,23 the misfit between the volume of the ZIF-8 cages and that of the IL components could give rise to remnant porosity, even if IL molecules continue to accumulate at the surface particle level.
To confirm the ability of the IL cations and anions to enter the ZIF-8 pore cage, a comparison was made between the diameter of the ILs and the window pore size of ZIF-8.24 ZIF-8 forms micropores with a diameter of 11.6 Å that are interconnected in a three-dimensional manner, linked by eight six-membered ring apertures with diameters of 3.4 Å. During gas adsorption, a gate opening effect occurs, increasing the size from 3.4 to 4 Å.25 Consequently, all IL cations/anions possess dimensions that allow them to fit and enter the ZIF-8 pore (Fig. S1†).
As illustrated in the scheme of Fig. 1, by modifying the IL loading in the ZIF-8/IL system, it is possible to transit from porous non-ionic conductors (ZIF-8) to porous ionic conductors with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 weight ratio of ZIF-8/IL (1
0.1 weight ratio of ZIF-8/IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65 molar ratio) and 1
0.65 molar ratio) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 weight ratio (1
0.4 weight ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 molar ratio), and finally into porous ionic conducting inks when a 1
2.6 molar ratio), and finally into porous ionic conducting inks when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-MOF/IL weight ratio (or 1
1-MOF/IL weight ratio (or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5 molar ratio) is applied. In this last case, the ZIF-8 intraparticle space would be partially or completely covered by IL molecules in the liquid state.
6.5 molar ratio) is applied. In this last case, the ZIF-8 intraparticle space would be partially or completely covered by IL molecules in the liquid state.
Once the MOF and IL components were integrated through forced-impregnation into a single composite material, their structure, as well as their spectroscopic and thermal properties, were thoroughly studied before evaluating their potential as the active component of capacitive sensors.
ZIF-8 nanoparticles with an average particle diameter of <50 nm were successfully synthesized, according to SEM and TEM pictures (Fig. S2 and S3†). The XRD pattern of the parent ZIF-8 is consistent with the simulated diffraction pattern calculated from the structural model obtained from ref. 26. The pattern matching analysis of the XRD data further confirms that there are no additional diffraction maxima aside from those of the ZIF-8 compound. The integration of the ILs into the pore space of ZIF-8 does not cause a shift in the position of the main diffraction maxima; however, it does significantly alter their relative intensities (Fig. 2a, S4 and S5†). Furthermore, above a certain threshold of IL loading, the XRD patterns start to show a significant rise in the background, thus suggesting that the extra amount of IL contributes to the amorphous (incoherent) fraction of the scattering.
As a result, after the pore space of the ZIF-8 is saturated, the IL begins to be integrated as a liquid phase coating the MOF/IL particles of the sample. That is, an ink with a rheological behaviour midway between IL and MOF materials is obtained. Notably, the cell parameters derived from the pattern matching fittings are strikingly similar (e.g. 17.0395 Å, 17.0128 Å, 17.0190 Å and 17.0152 Å for ZIF-8, ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, ZIF-8/TFSI 1
0.1, ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, and ZIF-8/TFSI 1
0.4, and ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 samples). Accordingly, the pattern matching analysis shows that MOF/IL Co and MOF/IL Ni gave similar results in terms of the crystallographic cell (Table S1†) (Fig. 2b, c, S6 and S7† for the 1
1 samples). Accordingly, the pattern matching analysis shows that MOF/IL Co and MOF/IL Ni gave similar results in terms of the crystallographic cell (Table S1†) (Fig. 2b, c, S6 and S7† for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 ratio samples). The similarity in the cell parameters of the ZIF-8/IL and ZIF-8 indicates that the incorporation of IL into the pore space of ZIF-8 does not strongly affect its framework. Looking closely at the results, almost no expansion was noticed for the ZIF-8/TFSI system, while a minor expansion for the ZIF-8/Ni and ZIF-8/Co systems was detected. Similarly, the variation of the relative intensities of the most intense diffraction maxima (Fig. 2d) indicates that the IL is immobilized following the long-range crystallographic order of the ZIF-8 pore system, rather than adopting a fully disordered arrangement into the structure.
0.4 ratio samples). The similarity in the cell parameters of the ZIF-8/IL and ZIF-8 indicates that the incorporation of IL into the pore space of ZIF-8 does not strongly affect its framework. Looking closely at the results, almost no expansion was noticed for the ZIF-8/TFSI system, while a minor expansion for the ZIF-8/Ni and ZIF-8/Co systems was detected. Similarly, the variation of the relative intensities of the most intense diffraction maxima (Fig. 2d) indicates that the IL is immobilized following the long-range crystallographic order of the ZIF-8 pore system, rather than adopting a fully disordered arrangement into the structure.
The difference envelope density (DED) maps of the ZIF-8 with different IL loadings were calculated from the (hkl)-Fobs2 data obtained from the pattern matching.27 When ZIF-8/IL-TFSI samples are compared to neat ZIF-8, additional electronic density zones arise, as evidenced by a comparison of the DED maps. In both, most of the electron density regions fit with the zinc ions and imidazole organic linkers of the ZIF-8 framework. Furthermore, the electronic density resulting from IL-TFSI is positioned around the Zn ions of the ZIF-8 structure (Fig. 3a). The ZIF-8/Ni-IL and ZIF-8/Co-IL systems show as well very similar electronic density maps, which are detailed in the ESI (Fig. S8 and S9†).
Carbon dioxide high-pressure isotherms at 0°C were measured to follow up the evolution of the porosity of the MOF/IL materials. All materials exhibit an important adsorption contribution at low pressures attributed to the MOF/IL micro-porosity and a second adsorption step at higher pressures associated with pore condensation effects (Fig. 3b). As expected, the CO2 adsorption in the lower pressure regime decreases when the ZIF-8 is loaded with ILs, but still a remnant porosity is observed for these IL-over-saturated ZIF-8/IL inks. The decrease of the surface areas from 1756 and 1097 m2 g−1 in ZIF-8 and ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 835 and 779 m2 g−1 for ZIF-8/TFSI 1
0.1 to 835 and 779 m2 g−1 for ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 and ZIF-8/TFSI 1
0.4 and ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 further confirms the hypothesis and is in good agreement with the literature.28 Thus, as reported in previous studies, the misfit between the caged pores of ZIF-8 and the size of IL molecules gives rise to a remnant porosity even when the sample is fully saturated. That is, once saturated, the remnant porosity in the ZIF-8 cages is smaller than that of the components of the IL, but still large enough for small molecules like CO2 to access it. Hence, this remnant porosity still leaves room for molecules of water and volatile organic compounds to be adsorbed onto the system and ultimately to trigger a sensing reaction.29–31
1 further confirms the hypothesis and is in good agreement with the literature.28 Thus, as reported in previous studies, the misfit between the caged pores of ZIF-8 and the size of IL molecules gives rise to a remnant porosity even when the sample is fully saturated. That is, once saturated, the remnant porosity in the ZIF-8 cages is smaller than that of the components of the IL, but still large enough for small molecules like CO2 to access it. Hence, this remnant porosity still leaves room for molecules of water and volatile organic compounds to be adsorbed onto the system and ultimately to trigger a sensing reaction.29–31
The FTIR-ATR data (Fig. 4a) confirm the successful incorporation of the ILs into the MOF. The incorporation of IL-TFSI into ZIF-8 causes the formation of additional absorption bands associated with the –CH stretching vibrations of the imidazolium cations and the –S–N–S and –C–N stretching vibrations of the TFSI, and Co-based anions of the selected ILs, respectively (Fig. S10 and S11†).32,33 Importantly, the inclusion of an IL in the MOF causes no appreciable displacement of the absorption bands, indicating weak interactions between ZIF-8 and IL.
 | ||
Fig. 4 Characterization of ZIF-8, IL TFSI, and ZIF-8/TFSI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1 0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 and 1 0.4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites: FTIR-ATR spectra (a), TGA curves (b), and DSC curves in different temperature ranges (c and d). 1) composites: FTIR-ATR spectra (a), TGA curves (b), and DSC curves in different temperature ranges (c and d). | ||
The weight loss mechanism related to the IL component in the ZIF-8/IL composites can be determined by TGA measurements. Specifically, as the IL loading increases, so does the overall weight loss percentage shown in the TGA curve, and vice versa (Fig. 4b, S12 and S13†). Additionally, the incorporation of IL-TFSI into the framework leads to a decrease in the temperature of ZIF-8 degradation. On the other hand, the degradation profiles of IL-Co and IL-Ni are hidden in the degradation profile of ZIF-8. IL-Ni has three degradation steps at (i) ∼35 °C, (ii) ∼325 °C and (iii) ∼410 °C;34 therefore, the degradation step at ∼550 °C is relative to the degradation of the MOF, similarly with what happens with IL-Co. These observations suggest that different types of ILs affect the thermal stability of the ZIF-8 framework differently. This effect is explained by the chemical interactions established between the IL anion/cation and the MOF, which can be affected by the length of the alkyl chain in the case of the IL cations and changes in the size and electronic structure in the case of the IL anions.35
No phase transitions were observed for pure ZIF-8 from the calorimetry data between 200 and 400 °C (Fig. 4c and d), prior to the beginning of its decomposition, which occurs at about 550 °C.36 Nevertheless, endothermic peaks between 370 and 390 °C (represented by the arrows), associated with the samples' decomposition, emerged when IL was added to the MOF. Interestingly, the MOF@IL system's initial decomposition temperature decreases as IL loading in the MOF increases. Furthermore, the DSC curves from ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 samples show small but broad endothermic peaks throughout the −40 to 150 °C range, highlighted by an orange square in Fig. 4d. This feature is tentatively attributed to the internal reorganization of the IL within the free volume left in the MOF, as suggested by the absence of peaks in both pristine TFSI and ZIF-8/TFSI 1
0.4 samples show small but broad endothermic peaks throughout the −40 to 150 °C range, highlighted by an orange square in Fig. 4d. This feature is tentatively attributed to the internal reorganization of the IL within the free volume left in the MOF, as suggested by the absence of peaks in both pristine TFSI and ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
For a deeper understanding of the internal morphology of the samples at the nanometric scale, SANS measurements were conducted. From the data depicted in Fig. 5a it can be observed that the incorporation of IL TFSI into ZIF-8 produces a change in the nanostructure of the system, as the SANS signal changes with the variation of the MOF/IL composition and concentration. Moreover, the shape of the curves indicates a middle way behaviour between a monodisperse and a polydisperse system, since purely monodisperse inhomogeneities exhibit distinctive oscillations in the SANS profile.37 Interestingly, the incorporation of IL-TFSI into ZIF-8 smoothens the SANS curve, showing almost no signal of inhomogeneities for the highest loading of IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). All SANS profiles from the ZIF-8/TFSI system have been fitted using a combined power law (in the smallest q region) and the form factor of polydisperse ellipsoids (in a q-range of 0.154–4.5 nm−1) and Gaussian peak model (in a q-range of 4.5–6.5 nm−1) using SasView software (Fig. 5a and b).38 It is important to note that the SANS features are only visible when there is enough scattering length density (SLD) contrast between the components (SLD difference of at least ∼1 cm−2). In our case, the SLD for air, ZIF-8, IL-TFSI, IL-Ni and IL-Co is 0 × 1010, 1.52 × 1010, 2.41 × 1010, 1.45 × 1010 and 1.68 × 1010 cm−2, respectively.
1). All SANS profiles from the ZIF-8/TFSI system have been fitted using a combined power law (in the smallest q region) and the form factor of polydisperse ellipsoids (in a q-range of 0.154–4.5 nm−1) and Gaussian peak model (in a q-range of 4.5–6.5 nm−1) using SasView software (Fig. 5a and b).38 It is important to note that the SANS features are only visible when there is enough scattering length density (SLD) contrast between the components (SLD difference of at least ∼1 cm−2). In our case, the SLD for air, ZIF-8, IL-TFSI, IL-Ni and IL-Co is 0 × 1010, 1.52 × 1010, 2.41 × 1010, 1.45 × 1010 and 1.68 × 1010 cm−2, respectively.
On one hand, for low q-values, a power-law decay is observed, reflecting the presence of larger structures generated from the agglomeration of individual inhomogeneities. The observed power law decay indicates a dense mass-fractal organization for almost all samples with P values between 2.8 and 2.9 (which correspond to mass-fractal dimension Dm = 2.8–2.9). For the highest loading of IL-TFSI, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, there is a transition to a surface-fractal organization with a P value of 3.1 (corresponding to surface-fractal dimension Ds = 2.9). The fitting of the data at higher q-values to an ellipsoid model with a polydisperse polar radius reveals inhomogeneities with an ellipsoid shape with the polar radius and equatorial radius varying between 3 and 11 nm and 21 and 129 nm, respectively, depending on the loading of IL-TFSI. Finally, at very high q-values (0.55 Å−1), there is a Gaussian peak which corresponds to a real space distance of 11.4 Å. This d-spacing is consistent with the crystal structure of the MOF (for ZIF-8 and ZIF-8/IL 1
1, there is a transition to a surface-fractal organization with a P value of 3.1 (corresponding to surface-fractal dimension Ds = 2.9). The fitting of the data at higher q-values to an ellipsoid model with a polydisperse polar radius reveals inhomogeneities with an ellipsoid shape with the polar radius and equatorial radius varying between 3 and 11 nm and 21 and 129 nm, respectively, depending on the loading of IL-TFSI. Finally, at very high q-values (0.55 Å−1), there is a Gaussian peak which corresponds to a real space distance of 11.4 Å. This d-spacing is consistent with the crystal structure of the MOF (for ZIF-8 and ZIF-8/IL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 and 1
01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 samples), of around 12 Å, extracted from the fittings to the XRD data measured on these samples. Furthermore, a decrease in the intensity of this peak is evident with increasing IL loading in the sample. This is attributed to an increase in the incoherent scattering contribution from the sample, which is ascribed to its amorphous IL component, while the coherent scattering contribution coming from the crystal structure of the MOF decreases.
0.4 samples), of around 12 Å, extracted from the fittings to the XRD data measured on these samples. Furthermore, a decrease in the intensity of this peak is evident with increasing IL loading in the sample. This is attributed to an increase in the incoherent scattering contribution from the sample, which is ascribed to its amorphous IL component, while the coherent scattering contribution coming from the crystal structure of the MOF decreases.
Overall, for the ZIF-8 parent sample, the inhomogeneities are related to the MOF interparticle space (air pores) between agglomerates of ZIF-8. Agglomeration of ZIF-8 with a size of 170 nm was detected by DLS. The loading of IL-TFSI into the ZIF-8 sample gives rise to the smothering and decrease of the scattering signal for the ZIF-8/IL-TFSI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sample, arising from the MOF pores and interparticle space being fully filled with the excess of IL. Accordingly, for the samples with moderate loading of IL (1
1) sample, arising from the MOF pores and interparticle space being fully filled with the excess of IL. Accordingly, for the samples with moderate loading of IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4), these inhomogeneities also arise from MOF pores and interparticle space partially filled with low and intermediate amounts of IL (Fig. 5c). We assume that the anisotropic ellipsoid-like shape of the inhomogeneities arises from the pellet processing, where a uniaxial force is being perpendicularly applied to compact the material, using 2 bar of pressure for a few seconds.
0.4), these inhomogeneities also arise from MOF pores and interparticle space partially filled with low and intermediate amounts of IL (Fig. 5c). We assume that the anisotropic ellipsoid-like shape of the inhomogeneities arises from the pellet processing, where a uniaxial force is being perpendicularly applied to compact the material, using 2 bar of pressure for a few seconds.
Similar results and behaviour were observed for the samples with the ILs of Co and Ni (Fig. S14 and S15†), except for the ZIF-8/IL-Ni 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample, where a different structure is detected although with similar power law decay.
1 sample, where a different structure is detected although with similar power law decay.
Electrical properties of ZIF-8/IL samples
The dielectric properties of all ZIF-8/IL composites were investigated using Electrochemical Impedance Spectroscopy (EIS) at room temperature (Fig. 6a, S16 and S17†). The electrical response of each MOF/IL material is presented as a Nyquist plot, which displays the real (Z′) and imaginary (Z′′) parts of the impedance measurements. Ionic/protonic conductors typically exhibit an arc placed at high frequencies in the Nyquist plots, followed by a barb placed at low frequencies. The resistance observed in the high-frequency range, corresponding to the initial part of the arc in the Nyquist plot, is attributed to the electrical response of the bulk sample and the grain interphase. Conversely, the resistance observed in the low-frequency range, represented by the barb in the Nyquist plot, is associated with ionic mobility.39The intersection of the arc and the barb is employed to calculate the ionic conductivity of ionic conductor materials. In the specific case of the MOF/IL system, the ionic mobility can arise from the MOF or the IL components themselves. Vapor adsorption makes the interpretation of the electrical response even more complex, since the gas molecules can disrupt the charge mobility of the MOF and/or IL, or even contribute to the electrical response by generating new ionic species. These positive or negative deviations on the base-ground electrical signature enable a gas sensing procedure based on a cross-selectivity between the varied responses of MOF/IL systems to gases of varying compositions.
Neat ZIF-8 presents an electrical insulating behaviour, and therefore, the electrical output is below the measurable threshold of the equipment. Nevertheless, when confining 0.65 molecules of IL-TFSI into the cage of ZIF-8, its ionic conductivity increases up to 2.24 × 10−8 S cm−1. Consequently, increasing the amount of IL-TFSI integrated into the ZIF-8 pore space leads to increased conductivities of 2.72 × 10−7 S cm−1 for porous solid ZIF-8/TFSI-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, and 1.66 × 10−5 S cm−1 when the combination of the MOF and the IL gives rise to ZIF-8/TFSI-1
0.4, and 1.66 × 10−5 S cm−1 when the combination of the MOF and the IL gives rise to ZIF-8/TFSI-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 porous ion-conductive ink. As expected, the conductivity strongly increases when the systems transition from solid-type MOF-IL conductors to ink-liquid-like hybrid systems. ZIF-8/IL systems based on Co and Ni follow the same tendency, indicating that an increase in the concentration of IL leads to an increase in the ionic conductivity of the sample, with the conductivity being significantly higher for these composites containing the IL in the liquid phase. Overall, it is reasonable to assume that the majority of the ionic conductivity observed in ZIF-8/IL materials can be attributed to the mobility of the IL trapped within the pore space (confirmed by the XRD and difference envelope density maps) or in the interparticle space between agglomerated MOF nanoparticles (confirmed by SANS data).
1 porous ion-conductive ink. As expected, the conductivity strongly increases when the systems transition from solid-type MOF-IL conductors to ink-liquid-like hybrid systems. ZIF-8/IL systems based on Co and Ni follow the same tendency, indicating that an increase in the concentration of IL leads to an increase in the ionic conductivity of the sample, with the conductivity being significantly higher for these composites containing the IL in the liquid phase. Overall, it is reasonable to assume that the majority of the ionic conductivity observed in ZIF-8/IL materials can be attributed to the mobility of the IL trapped within the pore space (confirmed by the XRD and difference envelope density maps) or in the interparticle space between agglomerated MOF nanoparticles (confirmed by SANS data).
The electrical behaviour of ZIF-8/TFSI was further studied by EIS at a frequency range between 1 and 106 Hz, after exposure to saturated VOC vapors for 1 h, namely ethanol (Fig. 6b), water vapor (Fig. 6c), IPA (Fig. 6d), and acetone (Fig. 6e). The ionic conductivity results were extracted from the Nyquist plots and are summarized in Table 1. The experimental data are fitted to the appropriate reference circuit model as shown in Fig. 6f. In the model, R1 represents the ohmic resistance of the electrolyte in the MOF material, in series with a constant phase element of the electrical double layer forming at the electrodes, Q1, in parallel combination with a constant phase element of the geometric capacitance, Q2.40,41
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1
0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 and 1
0.4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) after drying the samples and after exposing them to saturated vapors of ethanol, water, acetone and IPA
1) after drying the samples and after exposing them to saturated vapors of ethanol, water, acetone and IPA
ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
|---|---|---|---|
| Dried sample | 8.67 × 10−9 | 3.46 × 10−7 | 8.24 × 10−6 |
| Ethanol | 1.38 × 10−8 | 1.82 × 10−6 | 7.42 × 10−5 |
| Water | 1.21 × 10−8 | 1.76 × 10−8 | 9.37 × 10−6 |
| IPA | 3.11 × 10−7 | 1.14 × 10−5 | 1.80 × 10−5 |
| Acetone | 1.16 × 10−6 | 8.94 × 10−4 | 2.73 × 10−3 |
From the Nyquist plots we can conclude that the exposure of ZIF-8/TFSI composites to the four tested VOCs generically improves the ionic conductivity of the materials. This increase is clearly observed for acetone and isopropanol, where the typical semi-circle for conductive samples is observed, leading to higher conductivity values as shown in Table 1. Moreover, it is observed that for samples with higher loading of IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), higher conductivities are found, as the presence of more ionic charges contributes to higher ionic conductivity.
1), higher conductivities are found, as the presence of more ionic charges contributes to higher ionic conductivity.
MOF/IL-capacitive sensors' response to water vapor and C2–C3 alcohols
The MOF/IL composites were integrated by spraying them onto a transducer designed as four parallel capacitors, as detailed in the Experimental section. The response of the MOF/IL capacitive sensors was evaluated by exposing them to a flow with a given concentration of the target vapor while simultaneously measuring their capacitance variation. As described in detail in the Experimental section, the flow controllers allow the VOC concentration to be varied within certain ranges that depend on the VOC's vapor pressure and the flux of the N2 and N2/VOC streams. Before each measurement, the sensors were preheated at 80 °C for 30 minutes to remove any traces of humidity/VOC adsorbed on the MOF/IL sample.The measurements for the three VOCs explored in this work (ethanol, acetone, and isopropanol) were taken with a constant flow of 100 sccm of N2 and changing the N2/VOC flux from 0 to 200 sccm. The concentration of the VOC was increased in steps of 20 sccm of N2/VOC stream, with steady periods of 5 min with the VOC/N2 stream activated, and another 5 min with only the N2 flux passing through the sensor. Additionally, for acetone and IPA, additional measurements were performed from 0 to 20 sccm with a step of 2 sccm to investigate a lower VOC concentration range comparable to the one obtained for water vapor and ethanol. The capacitance response, sensitivity, limit of detection (LoD), and reversibility curves were measured for all the ZIF-8/IL samples. For the sake of simplicity, the data for ZIF-8, ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, and IL-TFSI are shown in Fig. 7. Also, the data measured for ZIF-8/TFSI 1
0.4, and IL-TFSI are shown in Fig. 7. Also, the data measured for ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and those obtained for the ZIF-8/Ni-IL (1
1, and those obtained for the ZIF-8/Ni-IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, 1
0.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 0
0.1 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ZIF-8/Co-IL (1
1) and ZIF-8/Co-IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, 1
0.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 0
0.1 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are summarized in the ESI (Fig. S18–S23†).
1) are summarized in the ESI (Fig. S18–S23†).
The response of the MOF/IL sensors has been analyzed using a three-fold strategy. Firstly, the magnitude and speed of response of the ZIF-8, ZIF-8/IL, and IL-based sensors over time are presented when increasing the water vapor and VOC concentrations (Fig. 4a–c). Even though the N2 and N2/VOC fluxes applied for all the experiments are equal, it is important to consider that the concentration of the vapors depends on their volatility. Therefore, under the same experimental conditions, different VOC/water concentrations are obtained when employing the same flux configurations. Thus, in a second step, the LoD, sensitivity, and the ΔCp with respect to the Cp base ground value of MOF/ILs when exposed to a given concentration of VOC or water vapors of the MOF/IL have been analysed (Fig. 7d–f). Last but not least, the selectivity of the sensors has been compared when exposed to similar VOC concentrations (29k, 27k and 27k ppm of IPA, acetone and ethanol). In parallel, the response of the devices to exposure to 40% and 60% relative humidity was explored, in accordance with the values employed as a reference in the literature.42 In addition, it is important to note that the response recorded for IPA by some of the MOF/IL sensors is within the concentration range that is relevant for its monitoring in the areas of indoor air quality monitoring and leakage monitoring.3
From the response of the sensors when exposed to different VOC/N2 fluxes (Fig. 7a–c) it is confirmed that ZIF-8/IL samples have a linear, fast, and recoverable response. In detail, the MOF/IL sensors take less than 10 seconds to reach 90% of the maximum response at each measurement step. The recovery of the background signal after stopping the VOC flow takes a little bit longer but is still below 1 minute. In the case of water vapor detection, the base ground signal recovery is longer due to its low volatility when compared to the studied VOCs. The ZIF-8 shows as well a swift response, although the capacitance variation is in most cases below that of the ZIF-8/IL sensors. Conversely, IL sensors exhibit a very large capacitance fluctuation but longer responses, in particular during VOC desorption, which could require more than 5 minutes to restore the starting capacitance value.
When studying the variation of capacitance (ΔCp) versus VOC concentration (ppm) (Fig. 7d–f), an exponential variation of ΔCpversus VOC concentration is observed, compared to the linear correlation derived from the capacitance variation (ΔCp) versus VOC flux. Overall, the experimental data indicate that the integration of the IL into ZIF-8 improves the sensor's response to some VOCs. Moreover, it is evident that the IL exhibits a significant response to all of the VOCs. Based on these results and the previous electrical characterization, it is assumed that the ΔCp mostly arises from the charge rearrangement/mobility of the IL during the VOC adsorption. Even though the data for the ZIF-8/TFSI system is shown as a reference for the MOF/IL system, other ZIF-8 combinations with Co and Ni ILs show similar behaviour, but with different selectivity responses to some VOCs and between the VOCs and the water vapor (Fig. S18, S19, S21 and S22†). The results from ΔCp change with the variation of VOC concentration match well with the EIS data, in the sense that the MOF/IL materials producing the highest ΔCp are the ones with the highest ionic conductivity (IL-TFSI), and the ones with lower ΔCp are the ones with lower ionic conductivity (IL-Ni). Additionally, the exponential response of the MOF/IL sensors to VOCs and water vapor can be parametrized by fitting the linear tendency observed in the ΔCpversus VOC concentration plots, so the sensitivity (i.e. slope) and the LoD (smallest measurement that can be detected with reasonable certainty) for each MOF/IL–VOC pair have been calculated and are summarized in Tables 2 and S2.†
| Sample | Vapor | LoD (ppm) | Sensitivity (pF ppm−1) | Sensitivitya (pF ppm−1) |
|---|---|---|---|---|
| a In the case of samples with two different linear regressions (one at low [VOC] and one at high [VOC]). | ||||
| ZIF-8 | Water | 3690 | 9.4 × 10−6 | — |
| Ethanol | 9703 | 8.0 × 10−6 | ||
| IPA | 2851 | 6.2 × 10−6 | ||
| Acetone | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215 215 |
2.5 × 10−6 | ||
ZIF-8/TFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
Water | 1928 | 2.7 × 10−5 | 5.0 × 10−5 |
| Ethanol | 7490 | 1.8 × 10−5 | 8.3 × 10−5 | |
| IPA | 4328 | 2.4 × 10−5 | — | |
| Acetone | 2982 | 1.3 × 10−4 | 2.3 × 10−3 | |
| IL TFSI | Water | 1835 | 4.2 × 10−2 | — |
| Ethanol | 4711 | 3.3 × 10−2 | 7.1 × 10−2 | |
| IPA | 4499 | 3.1 × 10−2 | — | |
| Acetone | 6655 | 7.2 × 10−2 | — | |
From a fundamental perspective, both capacitance and ionic conductivity depend on the generation, accumulation, and transport of new charges upon gas adsorption or gas molecule dissolution processes in the MOF, MOF/IL or IL matrices. So, considering the sensitivity parameters depicted in Tables 2 and S2,† the IL is the component that has the best response in terms of capacitance variation, but its sensitivity towards the detection of different VOCs (slope of ΔCpvs. VOC concentration plots) is comparable. In contrast, the ZIF-8 porous framework shows a small response but noticeable differences in the sensitivities towards the detection of different VOCs and water vapor. That is, the presence of the IL enhances the response, and the ZIF-8 imparts the selectivity. ZIF-8/IL sensors (Fig. 7g) still maintain a significant signal response when exposed to gas vapors, but also retain the selectivity that comes from the pore structure of the MOF. This explanation agrees with the experimental data since, for the samples with the higher amount of IL (MOF/IL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the ΔCp decreases, which is attributed to the saturation of the MOF pores by the IL, the loss of space for adsorption of gases, and hence, the attenuation of the capacitance variation coming from the IL. In summary, from all ILs, TFSI is the one presenting the lower LoD for all the tested gases, except for IPA which is lower for IL-Ni. When comparing the MOF/IL systems, the MOF/IL-TFSI samples present a lower LoD for all gases except for water, which is lower for the MOF itself. In the case of sensitivity, the three different ILs and MOF/IL systems show different sensitivities towards each gas.
1) the ΔCp decreases, which is attributed to the saturation of the MOF pores by the IL, the loss of space for adsorption of gases, and hence, the attenuation of the capacitance variation coming from the IL. In summary, from all ILs, TFSI is the one presenting the lower LoD for all the tested gases, except for IPA which is lower for IL-Ni. When comparing the MOF/IL systems, the MOF/IL-TFSI samples present a lower LoD for all gases except for water, which is lower for the MOF itself. In the case of sensitivity, the three different ILs and MOF/IL systems show different sensitivities towards each gas.
The determination of sensitivity and LoD of the developed sensors needs to be further explored, as the used experimental setup does not allow the sensitivity of the system to be evaluated at low VOC concentrations due to experimental limitations of the setup to reduce the VOCs' flows even further. The minimum concentration that we can detect is 4.1k, 10.2k, 5.0k, and 0.9k ppm for water, ethanol, acetone, and IPA, respectively. This is a limitation considering that ethanol begins to be an eye irritant above 1k ppm, and acetone concentration above 500 ppm is toxic upon long-time exposure. Nevertheless, our experimental setup is able to detect IPA at relevant concentrations, since it begins to be harmful above 3k ppm and we can detect it from 900 ppm.43 Thus, there are some MOF/IL combinations that could perform IPA detection in this concentration range, even in the presence of water vapor. Another approach to enhance sensitivity and reduce the LoD involves enhancing the electronic transduction system. This can be achieved by employing more intricate transduction designs, such as interdigitated configurations, downsizing the electrode transducers, and implementing hardware signal processing techniques.
Last but not least, Fig. 7h summarizes the response of the different sensors for the same VOC concentration, as well as their selectivity for VOC and water pairs for a set of sensors. In addition, we have compared this response to the one obtained in an environment of 40 and 60% relative humidity. It is interesting to note that we have not obtained a pure selectivity of a MOF/IL sensor for an individual VOC, but that a cross-selective system can be assembled when combining different ZIF-8/IL sensors. The response to the presence of water-moisture of some of the sensors (ZIF-8/Co-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) is very low in comparison to the one obtained for VOCs. Additionally, once the water variable is removed from the equation, some MOF and IL combinations are partially selective to certain VOCs compared to others. Considering the selectivity demonstrated by these MOF/IL sensors towards the NMVOCs herein studied, it would be interesting to broaden the scope by testing these sensors under more demanding analyte atmospheres (e.g. CO2, and formaldehyde and toluene VOCs).
0.4) is very low in comparison to the one obtained for VOCs. Additionally, once the water variable is removed from the equation, some MOF and IL combinations are partially selective to certain VOCs compared to others. Considering the selectivity demonstrated by these MOF/IL sensors towards the NMVOCs herein studied, it would be interesting to broaden the scope by testing these sensors under more demanding analyte atmospheres (e.g. CO2, and formaldehyde and toluene VOCs).
Conclusions
Harnessing the hybridization of metal–organic frameworks and ionic liquids opens the door to balancing the porosity, magnitude, and selectivity of the electrical response of MOF/IL hybrids upon vapor gas adsorption from air. Depending on the MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL ratio, materials ranging from pure porous non-ionic conductive MOFs to MOF
IL ratio, materials ranging from pure porous non-ionic conductive MOFs to MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL porous ionic conductors, MOF
IL porous ionic conductors, MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL porous ion-conductive inks and slurry-like ionic liquids can be achieved. XRD data and BET measurements, among other complementary measurements including SANS, have confirmed the internal and external hybridization of IL molecules inside and outside the internal structure of porous ZIF-8 nanoparticles. Even when transitioning from IL to MOFs the electric response upon VOC and water vapor exposure of the system is attenuated; the MOF
IL porous ion-conductive inks and slurry-like ionic liquids can be achieved. XRD data and BET measurements, among other complementary measurements including SANS, have confirmed the internal and external hybridization of IL molecules inside and outside the internal structure of porous ZIF-8 nanoparticles. Even when transitioning from IL to MOFs the electric response upon VOC and water vapor exposure of the system is attenuated; the MOF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL composite exhibits a fast and recoverable reaction compared to pure IL-based sensors. In contrast, when transitioning from a MOF to IL, the porosity is diminished, but the composites still maintain some selectivity in gas adsorption and, consequently, in detection. The dispersion and homogeneous integration of an IL into the MOF or the MOF-nanoparticle matrix offers advantages in terms of a fast, selective, and recoverable response of the sensors, but also for their processing. The liquid nature of ILs is a barrier when it comes to producing and applying reliable and long-lifetime sensors. Therefore, their support in porous materials like MOFs not only allows the modification of their intrinsic properties as active materials for sensing but also their processability by printing technologies.
IL composite exhibits a fast and recoverable reaction compared to pure IL-based sensors. In contrast, when transitioning from a MOF to IL, the porosity is diminished, but the composites still maintain some selectivity in gas adsorption and, consequently, in detection. The dispersion and homogeneous integration of an IL into the MOF or the MOF-nanoparticle matrix offers advantages in terms of a fast, selective, and recoverable response of the sensors, but also for their processing. The liquid nature of ILs is a barrier when it comes to producing and applying reliable and long-lifetime sensors. Therefore, their support in porous materials like MOFs not only allows the modification of their intrinsic properties as active materials for sensing but also their processability by printing technologies.
It is important to note that ZIF-8 not only serves as a supporting material, as the incorporation of different concentrations of the same IL produces different capacitance outputs towards the same VOC. Hence, the contribution to the selectivity of the MOF/IL composites can be further improved if the pore characteristics and inner surface chemistry of the MOF host are tuned towards the adsorption of specific molecules. Furthermore, there is scope for further fine-tuning the MOF/IL capacitive sensors through varying the MOF/IL active layer thickness. Advanced printing technologies, more complex interdigitated designs, downsized electrode transducers and hardware signal processing can push forward sensor sensitivity.
Author contributions
Conceptualization: Bruna F. Gonçalves and Roberto Fernandez de Luis. Funding acquisition: Senentxu Lanceros-Mendez and Roberto Fernandez de Luis. Investigation: Bruna F. Gonçalves, Viktor Petrenko, Jose Maria Porro, Leide P. Cavalcanti, Karmele Urtiaga, José M. S. S. Esperança, Daniela M. Correia, Felix Fernandez-Alonso, and Roberto Fernandez de Luis. Methodology: Bruna F. Gonçalves, Eduardo Fernandez, Ainara Valverde, Mattia Gaboardi, Hugo Salazar, and Karmele Urtiaga. Supervision: Senentxu Lanceros-Mendez and Roberto Fernandez de Luis. Validation: Bruna F. Gonçalves. Writing – original draft: Bruna F. Gonçalves, Senentxu Lanceros-Mendez, and Roberto Fernandez de Luis. Writing – review & editing: Bruna F. Gonçalves, Eduardo Fernandez, Ainara Valverde, Mattia Gaboardi, Hugo Salazar, Viktor Petrenko, Jose Maria Porro, Leide P. Cavalcanti, José M. S. S. Esperança, Daniela M. Correia, Felix Fernandez-Alonso, Senentxu Lanceros-Mendez, and Roberto Fernandez de Luis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support for this work has been secured through Grants PID2020-114506GB-I00 funded by MCIN/AEI/10.13039/501100011033; TED2021-129457B-I00 funded by MCIN/AEI/10.13039/501100011033, EVOLMOF PID2021-122940OB-C31 (AEI/FEDER, UE) (including FEDER financial support), ENZYMOF (TED2021-130621B-C42). This study forms part of the Advanced Materials Programme and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1). We acknowledge the continued support received from the IKUR Strategy under the collaboration agreement between Ikerbasque Foundation and the Materials Physics Center and BCMaterials on behalf of the Department of Education of the Basque Government. Basque Government Industry and Education Departments under the ELKARTEK and PIBA (PIBA-2022-1-0032) programs are also acknowledged. The MSCA-RISE-2017 (No. 778412) INDESMOF, which received funding from the European Union's Horizon 2020 Research and Innovation Programme, is also acknowledged. Finally we acknowledge the Portuguese Foundation for Science and Technology (FCT) through projects UID/QUI/00686/2020, UIDB/50006/2020, UIDP/50006/2020, LA/P/0008/2020, and 2022.05932.PTDC and an investigator FCT Contract 2020.02915.CEECIND (D. M. C.).References
- K. Oliveira, M. Guevara, O. Jorba, X. Querol and C. P. García-Pando, Sci. Total Environ., 2023, 867, 161449 CrossRef CAS PubMed.
- P. S. Monks, C. Granier, S. Fuzzi, A. Stohl, M. L. Williams, H. Akimoto, M. Amann, A. Baklanov, U. Baltensperger, I. Bey, N. Blake, R. S. Blake, K. Carslaw, O. R. Cooper, F. Dentener, D. Fowler, E. Fragkou, G. J. Frost, S. Generoso, P. Ginoux, V. Grewe, A. Guenther, H. C. Hansson, S. Henne, J. Hjorth, A. Hofzumahaus, H. Huntrieser, I. S. A. Isaksen, M. E. Jenkin, J. Kaiser, M. Kanakidou, Z. Klimont, M. Kulmala, P. Laj, M. G. Lawrence, J. D. Lee, C. Liousse, M. Maione, G. McFiggans, A. Metzger, A. Mieville, N. Moussiopoulos, J. J. Orlando, C. D. O'Dowd, P. I. Palmer, D. D. Parrish, A. Petzold, U. Platt, U. Pöschl, A. S. H. Prévôt, C. E. Reeves, S. Reimann, Y. Rudich, K. Sellegri, R. Steinbrecher, D. Simpson, H. ten Brink, J. Theloke, G. R. van der Werf, R. Vautard, V. Vestreng, C. Vlachokostas and R. von Glasow, Atmos. Environ., 2009, 43, 5268–5350 CrossRef CAS.
- Z. Zhai, X. Zhang, X. Hao, B. Niu and C. Li, Adv. Mater. Technol., 2021, 6, 2100127 CrossRef CAS.
- M. Khatib and H. Haick, ACS Nano, 2022, 16, 7080–7115 CrossRef CAS PubMed.
- E. Fernandez, P. G. Saiz, N. Peřinka, S. Wuttke and R. de Luis, Adv. Funct. Mater., 2021, 31, 2010703 CrossRef CAS.
- N. Garg, A. Deep and A. L. Sharma, Coord. Chem. Rev., 2021, 445, 214073 CrossRef CAS.
- B. Siu, A. R. Chowdhury, Z. Yan, S. M. Humphrey and T. Hutter, Coord. Chem. Rev., 2023, 485, 215119 CrossRef CAS.
- H.-Y. Li, S.-N. Zhao, S.-Q. Zang and J. Li, Chem. Soc. Rev., 2020, 49, 6364–6401 RSC.
- D. Matatagui, A. Sainz-Vidal, I. Gràcia, E. Figueras, C. Cané and J. M. Saniger, Sens. Actuators, B, 2018, 274, 601–608 CrossRef CAS.
- M. Moshiri, A. Charles, A. Elkaseer, S. Scholz, S. Mohanty and G. Tosello, in Procedia CIRP, Elsevier B.V., 2020, vol. 93, pp. 32–37 Search PubMed.
- K. Wu, X. Miao, H. Zhao, S. Liu, T. Fei and T. Zhang, ACS Appl. Nano Mater., 2023, 6, 9050–9058 CrossRef CAS.
- K. Wu, Y. Yu, Z. Hou, X. Guan, H. Zhao, S. Liu, X. Yang, T. Fei and T. Zhang, Sens. Actuators, B, 2022, 355, 131136 CrossRef CAS.
- P. Qin, S. Okur, Y. Jiang and L. Heinke, J. Mater. Chem. A, 2022, 10, 25347–25355 RSC.
- C. Zhong, T. Sasaki, A. Jimbo-Kobayashi, E. Fujiwara, A. Kobayashi, M. Tada and Y. Iwasawa, Bull. Chem. Soc. Jpn., 2007, 80, 2365–2374 CrossRef CAS.
- H. H.-M. Yeung, A. F. Sapnik, F. Massingberd-Mundy, M. W. Gaultois, Y. Wu, D. A. X. Fraser, S. Henke, R. Pallach, N. Heidenreich, O. V. Magdysyuk, N. T. Vo and A. L. Goodwin, Angew. Chem., Int. Ed., 2019, 58, 566–571 CrossRef CAS PubMed.
- D. W. Green and R. H. Perry, in Perry's Chemical Engineers' Handbook, McGraw-Hill Education, New York, 8th edn, 2008 Search PubMed.
- B. F. Gonçalves, et al., STFC ISIS Neutron and Muon Source, DOI:10.5286/ISIS.E.RB2220418.
- G. D. Wignall and F. S. Bates, J. Appl. Crystallogr., 1987, 20, 28–40 CrossRef CAS.
- K. C. Kim, T.-U. Yoon and Y.-S. Bae, Microporous Mesoporous Mater., 2016, 224, 294–301 CrossRef CAS.
- T. Shi, S. Hussain, C. Ge, G. Liu, M. Wang and G. Qiao, Rev. Chem. Eng., 2023, 39, 911–939 CrossRef CAS.
- I. A. Baburin and V. A. Blatov, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 447–452 CrossRef PubMed.
- V. A. Blatov, Crystallogr. Rev., 2004, 10, 249–318 CrossRef CAS.
- M. C. Gomes, L. Pison, C. Červinka and A. Padua, Angew. Chem., Int. Ed., 2018, 57, 11909–11912 CrossRef PubMed.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC.
- T. Ueda, T. Yamatani and M. Okumura, J. Phys. Chem. C, 2019, 123, 27542–27553 CrossRef CAS.
- O. Karagiaridi, M. B. Lalonde, W. Bury, A. A. Sarjeant, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 18790–18796 CrossRef CAS PubMed.
- A. A. Yakovenko, J. H. Reibenspies, N. Bhuvanesh and H.-C. Zhou, J. Appl. Crystallogr., 2013, 46, 346–353 CrossRef CAS.
- T. J. Ferreira, R. P. P. L. Ribeiro, J. P. B. Mota, L. P. N. Rebelo, J. M. S. S. Esperança and I. A. A. C. Esteves, ACS Appl. Nano Mater., 2019, 2, 7933–7950 CrossRef CAS.
- J. Avila, C. Corsini, C. M. Correa, M. Rosenthal, A. Padua and M. Costa Gomes, ACS Nano, 2023, 17, 19508–19513 CrossRef CAS PubMed.
- Z. Wang, A. Ozcan, G. A. Craig, F. Haase, T. Aoyama, D. Poloneeva, K. Horio, M. Higuchi, M.-S. Yao, C. M. Doherty, G. Maurin, K. Urayama, A. Bavykina, S. Horike, J. Gascon, R. Semino and S. Furukawa, J. Am. Chem. Soc., 2023, 145, 14456–14465 CrossRef CAS PubMed.
- M. Z. Ahmad and A. Fuoco, Curr. Res. Green Sustainable Chem., 2021, 4, 100070 CrossRef CAS.
- J. Kiefer, J. Fries and A. Leipertz, Appl. Spectrosc., 2007, 61, 1306–1311 CrossRef CAS PubMed.
- L. C. Fernandes, R. M. Meira, D. M. Correia, C. Ribeiro, E. Fernandez, C. R. Tubio and S. Lanceros-Méndez, Nanomaterials, 2022, 12(17), 3072 CrossRef CAS PubMed.
- F. A. Yassin, F. Y. El Kady, H. S. Ahmed, L. K. Mohamed, S. A. Shaban and A. K. Elfadaly, Egypt. J. Pet., 2015, 24, 103–111 CrossRef.
- M. Zeeshan, V. Nozari, S. Keskin and A. Uzun, Ind. Eng. Chem. Res., 2019, 58, 14124–14138 CrossRef CAS.
- V. Nozari, C. Calahoo, J. M. Tuffnell, D. A. Keen, T. D. Bennett and L. Wondraczek, Nat. Commun., 2021, 12, 5703 CrossRef CAS PubMed.
- O. V. Tomchuk, D. S. Volkov, L. A. Bulavin, A. V. Rogachev, M. A. Proskurnin, M. V. Korobov and M. V. Avdeev, J. Phys. Chem. C, 2015, 119, 794–802 CrossRef CAS.
- M. Doucet, et al., Zenodo Search PubMed.
- L. A. Middlemiss, A. J. R. Rennie, R. Sayers and A. R. West, Energy Rep., 2020, 6, 232–241 CrossRef.
- Z. Zhang, C. Li, A. Chandresh and L. Heinke, Ionics, 2022, 28, 487–494 CrossRef CAS.
- A. Chandresh, Z. Zhang and L. Heinke, Materials, 2021, 14, 4352 CrossRef CAS PubMed.
- A. V. Arundel, E. M. Sterling, J. H. Biggin and T. D. Sterling, Environ. Health Perspect., 1986, 65, 351–361 CAS.
- European Chemicals Agency, http://echa.europa.eu/, accessed 5 October 2023.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta00959b |
| This journal is © The Royal Society of Chemistry 2024 |






