Open Access Article
Open Access ArticleEutectic solvents and low molecular weight gelators for next-generation supramolecular eutectogels: a sustainable chemistry perspective†
Giselle
de Araujo Lima e Souza
,
Maria Enrica
Di Pietro
 * and
Andrea
Mele
* and
Andrea
Mele
 *
*
Department of Chemistry, Materials and Chemical Engineering “G. Natta”, Politecnico di Milano, Piazza L. da Vinci 32, 20133 Milano, Italy. E-mail: mariaenrica.dipietro@polimi.it; andrea.mele@polimi.it
First published on 1st December 2023
Abstract
Eutectic solvents (ESs) have received a great deal of attention due to their advantageous physicochemical properties, low cost and high sustainability. Immobilization in gels greatly expands the combinatorial playground offered by these materials. Among the different strategies, the obtainment of supramolecular eutectogels by ES self-assembling in the presence of low molecular weight organic gelators is one of the most flourishing. This critical review highlights the advances in the last five years, introducing the different types of emerging supramolecular eutectogels and their main applications. A judicious analysis of opportunities and challenges is also discussed, identifying future optimisation directions. The recent literature is critically reviewed in light of the connection between the theory/practice of Green chemistry and the achievements of the UN Sustainable Development Goals.
Sustainability spotlightEutectogels are frontier materials easily obtainable from deep eutectic solvents (DESs) in the presence of a low amount of a suitable gelator. Low molecular weight gelators (LMWGs) are particularly convenient to form physical eutectogels whose preparation and main applications are discussed in this review from the viewpoint of sustainability and achievements of UN SDGs. Eutectogels are reviewed according to the class of compounds the LMWG belongs to. The final part of this review proposes two specific case studies of potential impact of eutectogels for the achievement of the civilization target “that no one is left behind” common to the UN SDGs: the recovery of metallic critical raw materials from end-of-life batteries and energy storage devices (in line with SDG12 and SDG14) and water remediation (towards SDGs 3, 6, 12, and 14). |
Introduction
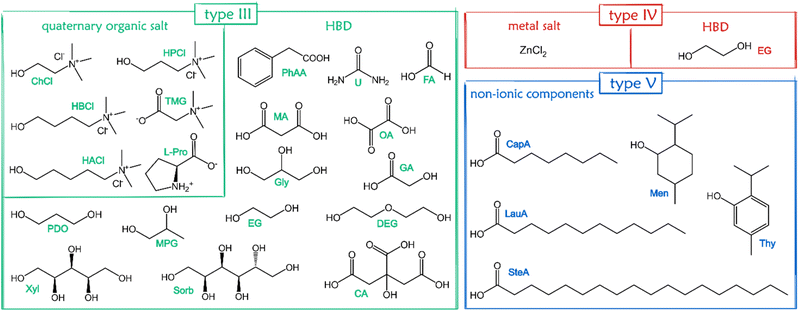
Eutectic solvents (ESs) have been known for a long time,1–3 but they were de facto rediscovered in 2003,4 when Abbott and coworkers coined the term “deep eutectic solvents” (DESs) to identify a binary mixture of choline chloride (ChCl) and urea (U). From then on, DESs have developed into a burgeoning field worthy of attention. Following the first definition – mixture of a Lewis base X− and a Brønsted base Y, with the general formula Cat+X−zY5 – an extremely high number of mixtures were just introduced as DESs without any strict characterization of their phase behaviour. According to mixed components, DESs are classified into 5 categories (Fig. 1).6,7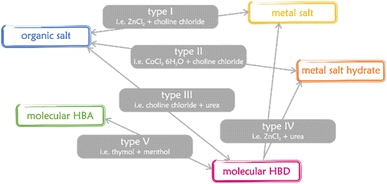 | ||
| Fig. 1 The main types of DESs.6,7 HBA and HBD stand for hydrogen bond acceptor and hydrogen bond donor, respectively. | ||
The fast growing popularity resulted in an overgeneralization of the definition of DES, with several misconceptions appearing in the literature.8 An ongoing debate exists on a strict definition of DES and on the depth of the melting point. The most widely recognised definition of a DES is that for a eutectic mixture to be labelled “deep”, a eutectic temperature much lower than that predicted by assuming a thermodynamic ideal behaviour of the liquid phase has to be observed as a result of stronger interactions between DES precursors than those present in pure compounds.9 Still, how deep the temperature deviation should be is unclear. Also, the resurgence of DESs made it clear that there exist eutectic solvents (ESs) which are promising regardless of whether they are ideal or not. A debate about the strict definition of a DES or a proper classification is outside the scope of this review. Therefore, we adopt here the abbreviation (D)ESs, to include all eutectic solvents, regardless of their deviation from ideality.
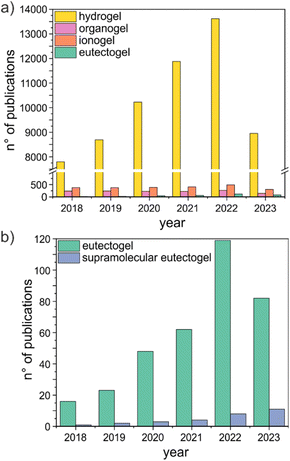
The potential as tunable solvents is one of the highest merits of (D)ESs.5 Gels can further expand the design space of (D)ESs, providing new perspectives towards the creation of sustainable soft materials. In this work, we will use “eutectogels” to refer to all gel-like systems obtained from (D)ESs. Soon after they appeared on the scientific scene, eutectogels have become a burgeoning field of study. They were suggested as promising materials in energy, (bio)electronics, drug delivery, analytical chemistry, and wastewater treatment, and their application in many sectors is now progressing at a rapid pace.10–12 The significant increase in the level of interest in eutectogels is illustrated by the rapid growth in the number of publications on the topic over the period 2018 to 2022 (Fig. 2). Even though the number of publications is more than 3 orders of magnitude lower than the current literature on hydrogels (gels having water as the liquid phase) and still much lower than the number of papers on organogels (gels having an organic solvent as the fluid component) and ionogels (gels having an ionic liquid as the fluid component) (Fig. 2a), the trend is increasing at a faster pace than other gel families.
Different strategies have been proposed to prepare eutectogels, mostly composed of a (D)ES immobilized within different matrices, such as linear polymers, polymer networks, biopolymers, or sol–gel derived silica networks.10–14 Here, we address specifically the promising family of supramolecular eutectogels obtained by self-assembling a low molecular weight gelator (LMWG), defined as an organic compound having molecular weight <2000 Da.15 Held together only by reversible intermolecular noncovalent interactions, these soft materials have been attracting particular attention, thanks to their fast and straightforward preparation and their thermal reversibility. Moreover, biocompatible or even natural gelators can be used, ensuring sustainability and low cost. The number of publications on supramolecular eutectogels still represents a minor percentage of all studies on eutectogels (Fig. 2b), but the continuous rapid advancements achieved ensure that the field will be at the forefront of the development and economic production of functional soft materials. In this review we illustrate the present state of research on preparation and applications of (D)ES-based supramolecular gels formed by LMWGs. In detail, the review covers a survey of the literature on the last quinquennial round, using the keywords “supramolecular” and “eutectogel*” or “eutectic solvent*” and “supramolecular” and “gel*”, in the Scopus database (last accessed on July 23, 2023). More papers were selected and added through forward and backward citation searching. Eutectogels formed by other strategies will not be reviewed, and the reader is referred to excellent reviews published in the past few years.10–14 Next to the preparation protocol and physicochemical characterization, target applications will be discussed. Promising research thrusts are given in the final section, aiming at sparking new thought and discussion on the current achievements and promoting further developments.
The sustainability space
Since the beginning of the research explosion on DESs, the claims of greenness have consistently appeared as the theme of many papers. Clear placement of (D)ESs and the novel class of eutectogels derived from them in the sustainability space is briefly introduced here before the discussion of the main features of eutectogels from LMWGs and future perspectives.Considering the (D)ESs before gelation, indeed several misconceptions stem from the arbitrary assumption of eutectic solvents as a green panacea. Suffice it to mention here some of them as paradigmatic examples. (1) Eutectic mixtures of natural products are intrinsically green solvents. A caveat on the inadequacy of testing protocols for toxicity has been reported,16 along with the need for an eco-toxicologic evaluation of DESs scored as “non-toxic” after in vitro tests.17 The overall message emerging from the literature is to evaluate the impact of the solvent within the frame of the process the solvent is involved in – also including the energy balance in manufacturing, purification, removal and disposal of the solvent – rather than naively attempt an absolute ranking.18,19 (2) (D)ESs are greener than… As remarked above, a solvent's impact should be assessed within the frame of a synthesis or a process adopting suitable green metrics.20 A nuts-and-bolts example of the use of green metrics for solvent selection was reported by Kisanthia et al.21 Additionally, ESs are green extractants for natural products, pollutants, added value products from biomasses, waste etc.,22 thus calling for the definition of sustainable protocols for analytical chemistry and principles of green analytical chemistry (GAC)23 flanking the well-assessed green chemistry principles (GCPs).24 The 12 GCPs and the 12 GAC principles are summarized in Tables S1 and S2,† respectively. In line with the modern vision of a classification of the environmental friendliness of a process using quantitative parameters, several green metrics targeted to analytical chemistry have been proposed,25–29 in a complementary way to the already mentioned green metrics for chemicals, synthetic routes and industrial processes, thus defining the new boundary of a chemical space as environmentally and socially acceptable.
While the factors contributing to the low environmental impact of chemicals and processes, commonly reported along with the “green” adjective, are necessary, they alone are not sufficient conditions for sustainability. Sustainability is a broader concept that encompasses “the priorities of economic competitiveness and societal concerns”.30
Environmental and societal issues are among the inspiring factors of the United Nations Sustainable Development Goals (SDGs), formulated and approved on 25 September 2015. SDGs defined the objectives to be achieved within 2030 “with a view towards ending all forms of poverty, fighting inequalities and tackling climate change while ensuring that no one is left behind”.31 The iconic poster summarizing the SDGs is reported in Fig. S1.†32
It is immediately clear that some of the goals are directly related to the GCPs and that the chemistry research and technology inspired by the GCPs is an important route to the achievement of many of the SDGs.33Fig. 3 shows a scheme of the correlation matrix between GCPs and SDGs. Although many of the rows of the matrix contain GCP correlation boxes, it is noteworthy that some missing connections are present. SDGs 1, 4, 5, 16 and 17 are indeed the most politically oriented objectives, and as such are not direct consequences of the good practices of GCPs. However, a growing consciousness of the transmission of the values of environmental respect, energy economy and waste reduction/valorization is a benign synergy contributing to reducing and eliminating inequalities. Education plays a crucial role (SDG 4) as it “defines the responsibility of educators in the chemical enterprise to provide sustainable education (target 4.7) to the world's learners with the knowledge and skills needed for a sustainable planet”.34
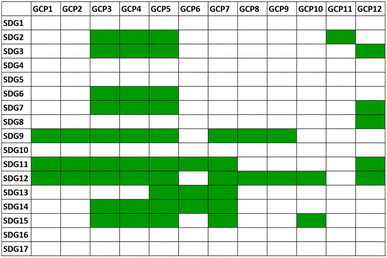 | ||
| Fig. 3 Correlation matrix between the 17 UN Sustainable Development Goals (SDGs) and the 12 principles of green chemistry (GCPs). The numbering and the definitions of the SDGs and GCPs are in Fig. S1 and in Table S1,† respectively. The green cells indicate a direct correlation between the connected elements in the matrix. | ||
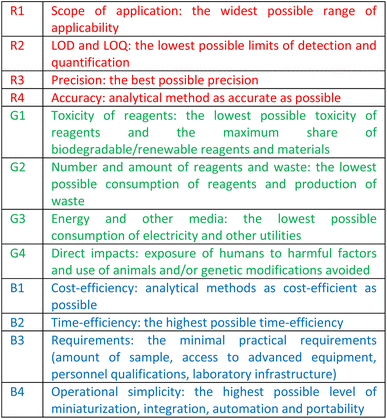
In an attempt to reconcile the greenness of the method with the priorities of economic competitiveness and societal concerns included in the concept of sustainable development,30 the red-green-blue (RGB) model has been introduced, where green represents compliance with GAC principles, red the analytical efficiency and blue the productivity and practical/economic efficiency.35 The saturation of each primary colour gives a “white” method, i.e. an ideal, fully complete and consistent method, which inspired the formulation of the 12 principles of white analytical chemistry (WAC), summarized in Table 1.36
Squeezing the 12 known GAC principles into 4 overarching “green” rules G1–G4 and flanking them with 4 “red” principles (R1–R4) and 4 “blue” principles (B1–B4), the RGB model and WAC idea seek a compromise between the environment, society, and economy, in the belief that sustainable developed methods should be at the same time green and “equitable”, in terms of price and applicability by everyday users.37
Having outlined the essential boundary conditions for sustainability, we anticipate here that the eligibility of (D)ESs and supramolecular eutectogels to solvents, reactants or matrices for sustainable chemistry can be mainly related to the atom economy close to 100%, limited energy consumption of their preparation, waste prevention, limited need for purification, and potential recyclability. Additional premises for the high sustainability of (D)ESs are the renewable natural sources of the components in the mixture and the often encouraging toxicologic and eco-toxicologic profile – if correctly assessed in the context of the application.38,39 The possibility to use relatively safe and even naturally occurring small gelators to form supramolecular eutectogels, together with their thermal reversibility and/or self-healing ability, represent other green claims. Yet, a reliable assessment of sustainability of a given method and application of a given (D)ES and eutectogel is difficult to predict and requires many factors to be considered on a case-by-case basis, ranging from safety and environmental friendliness to quality and economic impact of the results and compliance with social demands.
The recipe for supramolecular gels based on eutectic mixtures
The first ingredient: the (D)ES
The popularity of (D)ESs is related to their easy preparation protocol with no purification step and a 100% atom economy, mild energy consumption largely related to the heating required to turn the mixture of the components into a homogeneous liquid, together with the low cost and large availability of many precursors as bulk chemicals.40 The prototypical DES ChCl–U is for instance prepared from a nutritional supplement for livestock (choline) currently produced on a megaton per annum basis and a commercial fertilizer (urea) with a global production rate of ∼190 million tons per annum.5,41 Among type V DES constituents, cyclic terpene menthol is for instance the most used aroma compound, commonly present in sweets, oral care products, cosmetics and medicinal products, with a worldwide production around 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons per year, about 40% of which is extracted from mint plants.42
000 metric tons per year, about 40% of which is extracted from mint plants.42
Although DESs have been described as “green solvents”, mainly due to their low vapour pressure, not all DESs are inherently benign, i.e. DESs containing metal salts will have an innate toxicity.43 It is generally true that the most used constituents of (D)ESs and natural deep eutectic solvents (NADESs) are biodegradable and their toxicological properties are well characterized.5,44 However, the toxicity of the DES is different – eventually higher or much higher – than that of its individual components, and the need for a complete cytotoxicity profile is an important focus of the current research on ionic liquids and DESs.45–47 The key point is the capability of (D)ESs to permeate the lipid bilayer of cell membranes to a variable extent depending on the polarity and H-bond profile of the DES components. This factor should be kept in mind when evaluating the impact of a given (D)ES in a chemical process, thus in ranking the greenness/sustainability of (D)ES according to a chosen metric. Nevertheless, a critical viewpoint is mandatory, as the cytotoxicity data can be either positive or negative descriptors according to the application of (D)ESs, e.g. cancer therapy vs. cosmetic/pharmaceutic formulations.
When dealing with conventional hydrophilic DESs, it should be considered that they are very hygroscopic, and traces of water are often unavoidable when applied commercially.48 The presence of a controlled amount of water mitigates some drawbacks, e.g. viscosity, and does not disrupt the network of the intermolecular interactions characteristic of the DESs' local structuration.49,50 Alternative options are hydrophobic (D)ESs, which may overcome the viscosity issue associated with eutectics based on large quaternary ammonium salts and have better water-stability.51
A huge number of components have been mixed in a given molar ratio to form (D)ESs. For a general overview of DES components, the reader is referred to other excellent reviews.5,52 Here, we target only (D)ESs used to form supramolecular eutectogels, and a graphic summary of the chemical structures of such compounds is shown in Fig. 4. Type III are the materials used in the overwhelming majority of the supramolecular gels of interest here, with a few examples of eutectogels from types IV and V also reported. Most of the soft materials have been designed using type III DESs prepared with readily available, nontoxic precursors, mainly ChCl as HBA and various HBDs such as urea, glycerol, polyols, sugars, or organic acids.
The second ingredient: the low molecular weight gelator (LMWG)
Gels are ubiquitous soft solids that are widely used in everyday life and have received widespread attention. They are typically composed of a liquid entrapped in a three-dimensional network. The terms hydrogel or organogel are used when the liquid phase is water or an organic solvent, respectively.10,12,53 Main drawbacks consist in the poor temperature resistance of hydrogels and low conductivity and toxicity of organogels.10 Ionic liquids (ILs) have been introduced as an alternative liquid phase in gels, leading to formation of so-called ionogels or iongels.10,12 These soft materials combine the conductivity of hydrogels with the temperature resistance of organogels and have been successfully applied in a number of fields.10,12 Ionogels suffer from the typical limitations of ILs, mainly high cost, complex synthesis and questionable biocompatibility, hindering their practical applications. Sharing many advantageous properties with ILs, DESs have been identified as a logical, sustainable alternative to ILs in the preparation of gels.There are different strategies to form DES-based gels.10 A widely explored approach to prepare structured, gel-like systems consists in immobilizing DESs in silica matrices,54 biopolymers,55–62 or other cross-linked/entangled polymers.63–77 The immobilization of a (D)ES in a solid matrix opens up the possibility of synergistically combining the properties of both, i.e. physico-chemical properties of DESs and mechanical properties of polymers, limiting their drawbacks.78 Indeed, a semisolid DES-based soft material would practically keep the specific properties of (D)ESs except flowability.78 Polymerization can be initiated on monomers dissolved in the DESs, which then act as solvents, or the preformed polymer can be blended with the DESs. Another approach involves the direct polymerization of deep eutectic monomers (DEMs).79–83 Examples of DEMs include acrylic, methacrylic and itaconic acid.13,79,82,83 Additionally, colloidal gels have also been reported after dispersion of nanoparticles in DES, such as nanotubes or inorganic nanoparticles.84–86 All these different strategies provide versatility in the formation of DES gels, allowing for the development of tailored gel-like materials with specific properties and applications.
Another elegant way to structure DESs into gels relies on the addition of a small gelator (LMWG) that self-assembles into a nanoscale network as a result of intermolecular noncovalent interactions, generating eutectogels.87 A few examples exist where the DES itself is used as a supramolecular gelator. Florindo et al. reported for the first time a supramolecular DES gel obtained by adding a small amount of water to the hydrophobic DES composed of dodecanoate sodium salt and carboxylic acid.88 Since the DES was composed of hydrophobic long-chain fatty acids, the small amount of water acted as an anti-solvent precipitating the DES into a supramolecular gel. A transparent gel has also recently been obtained by mixing the DES lidocaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid 1
lauric acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 with water in a certain content range (DES content ca. 12–20 wt%).89
1.5 with water in a certain content range (DES content ca. 12–20 wt%).89
This critical review focuses more specifically on supramolecular eutectogels formed by self-assembling of a LMWG in (D)ES. From the viewpoint of sustainability, the points of strength of such an approach are the rapid and easy preparation protocol, the thermal reversibility and/or self-healing ability, and the possibility to use relatively safe and even naturally occurring small gelators. The self-assembly mechanism fulfills the requirements of several of the GCPs, such as atom economy, preventing waste and less energy consumption. Concerning the coherence with the remaining GCPs, a careful evaluation of the impact of the gelator is required in ranking the sustainability of the gel.
Broadly speaking, supramolecular gels, or low molecular weight gels, are bicomponent systems composed of a liquid entrapped in a three-dimensional network formed by a small gelator through nanostructures underpinned solely by reversible noncovalent interactions (hydrogen bonds, π–π stacking, van der Waals interactions, dipole–dipole, charge-transfer, ion coordination and solvophobic forces).15,53,90,91 Due to the reversible nature of their formation, they are also termed physical gels.92 As depicted in Fig. 5, above a critical concentration (called the minimum gelation concentration or critical gelation concentration CGC), the dissolved LMWGs form bulk gels through hierarchical self-assembly in a liquid-like continuous phase.15,93–95 On applying a proper trigger, individual gelator molecules assemble through non-covalent interactions to form primary fibrils, which then bundle together to form wider nanofibres. The resulting nanofibers tangle with one another to form an extended sample-spanning solid-like network able to immobilize the solvent through surface tension and capillary forces. This translates then into the bulk gel.
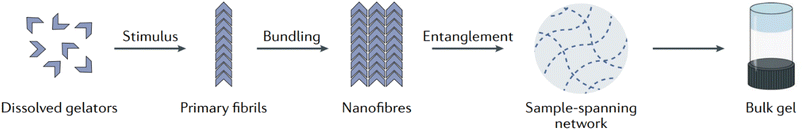 | ||
| Fig. 5 Schematic of supramolecular gel assembly. Reproduced from P. R. A. Chivers and D. K. Smith, Shaping and structuring supramolecular gels, Nat. Rev. Mater., 2019, 4, 463–478, https://doi.org/10.1038/s41578-019-0111-6. Copyright 2019, Springer Nature Limited. | ||
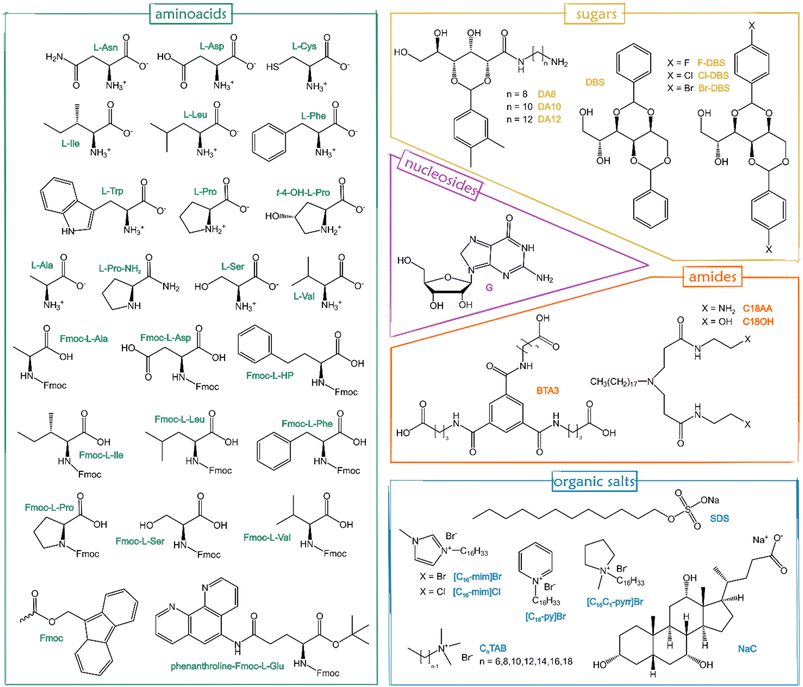
Although several stimuli (e.g. light, pH, solvent changes, and sonication) are able to switch gelation on or off, temperature is usually the simplest stimulus for gel formation: a hot solution containing the LMWG is cooled to a certain temperature, known as the sol–gel transition temperature (Tgel), to form the gel.15 Unlike permanently covalently cross-linked polymer gels, the process is thermodynamically reversible,91,96 and then heating the gel again leads to its disassembly into a sol. There exist many LMWGs nowadays,15,53,96,97 and many more are synthesized,91 or discovered.98Fig. 6 displays the gelators already applied to form supramolecular eutectogels in the last 5 years, including amino acids, sugar derivatives, nucleosides, amide derivatives, and surfactants or similar organic salts. It is worth underlining that the reversibility of physical gel formation and the temperature trigger often used to accomplish the assembly are in compliance with GCP1, GCP2 and GCP6.
Classes of supramolecular eutectogels
Table 2 gives an overview of the supramolecular eutectogels which will be reviewed in the following sections.| (D)ES | LMWG | Reference |
|---|---|---|
| Aminoacid-based eutectogels | ||
ChCl–PhAA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
L-Ile | Marullo et al.99 |
| L-Trp | ||
ChCl–PhAA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
L-Ile | Marullo et al.103 |
| L-Leu | ||
| L-Trp | ||
| L-Pro | ||
| L-Phe | ||
| Fmoc-L-Phe | ||
| Fmoc-L-Ile | ||
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
L-Pro | Saavedra et al.100 |
| L-Ser | ||
| L-Cys | ||
| L-Ile | ||
| L-Asn | ||
| L-Trp | ||
| L-Pro-NH2 | ||
| t-4-OH-L-Pro | ||
ChCl–MA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Fmoc-L-Ala | Cheng et al.101 |
ChCl–OA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Fmoc-L-Pro | |
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Fmoc-L-Val | |
| Fmoc-L-Ser | ||
| Fmoc-L-Phe | ||
| Fmoc-L-Ile | ||
| Fmoc-L-HP | ||
| Fmoc-L-Asp | ||
| Fmoc-L-Leu | ||
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Phenanthroline-Fmoc-L-Glu | Criado-Gonzalez et al.102 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Sugar-based eutectogels | ||
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1 2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
DBS | Ruiz-Olles et al.87 |
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–PDO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–MPG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–Xyl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–Sorb 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
F-DBS | Fan et al.104 |
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cl-DBS | |
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Br-DBS | |
ChCl–PDO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–MPG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
Men–Thy 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBS | de Araujo Lima e Souza et al.105 |
Men–SteA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
Men–LauA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
Men–CapA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
LauA–Cap 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
||
Thy–LauA 1.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
Thy–CapA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DA8 | Zhang et al.106 |
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DA10 | |
ChCl-Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DA12 | |
ChCl–PDO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–MPG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Nucleoside-based eutectogels | ||
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Guanosine (H3BO3/KOH) | Gu et al.107 |
ChCl–MPG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Guanosine | Qi et al.108 |
HPCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
HBCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
HACl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–MA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Amide derivative-based eutectogels | ||
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
C18AA | Delbecq et al.109 |
| C18OH | ||
ChCl–PhAA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
BTA3 | Zhang et al.110 |
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
BGA-10 | Wang et al.69 |
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–PDO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
BGA-12 | |
ChCl–MPG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Organic salt-based eutectogels | ||
| L-Pro-OA | [C16-mim]Br | Prieto Kullmer et al.111 |
| Men-LauA | [C16-py]Br | |
| EG-ZnCl2 | [C16C1-pyrr]Br | |
ChCl–FA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
[C16-py]Br | Parsana et al.112 |
ChCl–OA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
[C16-py]Cl | |
ChCl–CA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
CnTAB (n = 6, 8, 10, 12, 14, 16, 18) | Liang et al.113 |
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
SDS | Matthews et al.114 |
ChCl–DEG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Cetyldiethanolamine N-oxide | Marullo et al.115 |
TMG–DEG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
TMG–GA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
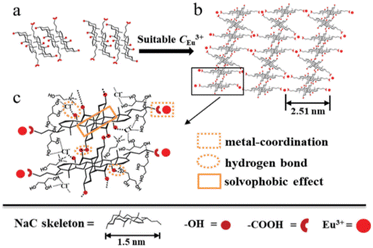
NaC | Sun et al.116 |
ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
NaC/Eu(NO3)3 | |
ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
Aminoacid-based eutectogels
In 2018 Marullo and coworkers introduced for the first time the concept of supramolecular eutectogels, using L-amino acids (isoleucine and tryptophan) in the type III DES ChCl–phenylacetic acid (PhAA) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99 The eutectogels exhibit high mechanical resistance and environmental friendliness. Gelation was achieved at 3 wt% loadings in a range of sol–gel transition Tgel between 34 and 38 °C via establishing a hydrogen bond between the L-amino acid and the DES, resulting in fully natural ionic soft materials which, nevertheless, retained an unexpectedly high degree of crystallinity. The same authors extended the investigation to a broader set of L-amino acids – phenylalanine, leucine, isoleucine, proline, and tryptophan, including some Fmoc-protected amino acids – to obtain low-impact materials for environmental remediation.99 Tests on model dyes such as methylene blue, Rhodamine B and methyl violet showed the good capability of L-Phe eutectogels in absorbing dye mixtures and a good level of recyclability. The dual role of organocatalysts and gelators exhibited by L-amino acids was exploited by the same group to obtain catalytic gel phases based on archetypical ChCl–U DES.100 The eutectogels were tested on the benchmark aldol condensation of acetone and p-nitrobenzaldehyde and the best performing L-Pro–ChCl–U eutectogel was applied both in aldol and Michael reactions. The good yields and the several elements of greenness of the eutectogel largely compensated for the modest enantiomeric excess (ee%) reported and the use of a volatile organic solvent to isolate the product.100
2.99 The eutectogels exhibit high mechanical resistance and environmental friendliness. Gelation was achieved at 3 wt% loadings in a range of sol–gel transition Tgel between 34 and 38 °C via establishing a hydrogen bond between the L-amino acid and the DES, resulting in fully natural ionic soft materials which, nevertheless, retained an unexpectedly high degree of crystallinity. The same authors extended the investigation to a broader set of L-amino acids – phenylalanine, leucine, isoleucine, proline, and tryptophan, including some Fmoc-protected amino acids – to obtain low-impact materials for environmental remediation.99 Tests on model dyes such as methylene blue, Rhodamine B and methyl violet showed the good capability of L-Phe eutectogels in absorbing dye mixtures and a good level of recyclability. The dual role of organocatalysts and gelators exhibited by L-amino acids was exploited by the same group to obtain catalytic gel phases based on archetypical ChCl–U DES.100 The eutectogels were tested on the benchmark aldol condensation of acetone and p-nitrobenzaldehyde and the best performing L-Pro–ChCl–U eutectogel was applied both in aldol and Michael reactions. The good yields and the several elements of greenness of the eutectogel largely compensated for the modest enantiomeric excess (ee%) reported and the use of a volatile organic solvent to isolate the product.100
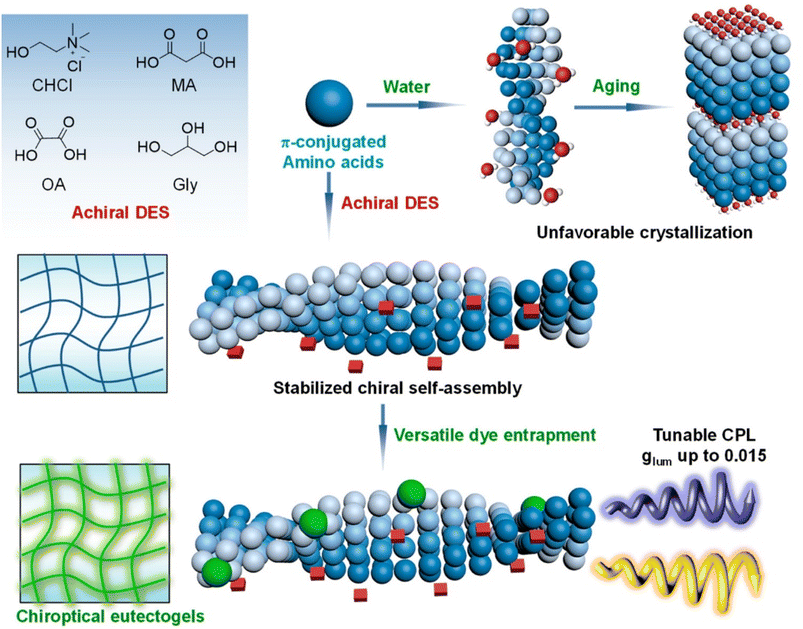
The chiral pool of amino acids was conveniently exploited to introduce supramolecular chirality in the eutectogel.101 The authors devised a successful strategy using achiral DES made of ChCl–malonic acid (MA) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Fmoc-protected L-amino acids (alanine (Ala), phenylalanine (Phe), valine (Val), homophenylalanine (HP), proline (Pro), serine (Ser), leucine (Leu), isoleucine (Ile), and aspartic acid (Asp)) as a chiral gelator to achieve supramolecular chiral media. The systems were then added to different fluorescent dyes, thus obtaining tunable circularly polarized luminescent materials. The key mechanistic points of the formation of the supramolecular gel are visually sketched in Fig. 7. Using water as solvent, the initial self-assembly of DES and amino acids evolved over time towards fibrous or crystalline architectures. Conversely, the DES self-assembly in the presence of homochiral amino acids resulted in stability over time due to the higher viscosity and reduced solute diffusivity. The gel thus obtained showed stable and well-defined chiroptical properties. Successive injection of fluorescent dyes, dissolved in volatile solvent that is easily removable, resulted in chirality transfer from the chiral gel to the dyes. Circularly polarized luminescence measurements highlighted good dissymmetry factors glum, a descriptor of the unbalance between left and right circularly polarized emission. The wavelength of the emitted radiation could be modulated according to the chemical structure of the dye able to interact with the chiral gelator.
1 and Fmoc-protected L-amino acids (alanine (Ala), phenylalanine (Phe), valine (Val), homophenylalanine (HP), proline (Pro), serine (Ser), leucine (Leu), isoleucine (Ile), and aspartic acid (Asp)) as a chiral gelator to achieve supramolecular chiral media. The systems were then added to different fluorescent dyes, thus obtaining tunable circularly polarized luminescent materials. The key mechanistic points of the formation of the supramolecular gel are visually sketched in Fig. 7. Using water as solvent, the initial self-assembly of DES and amino acids evolved over time towards fibrous or crystalline architectures. Conversely, the DES self-assembly in the presence of homochiral amino acids resulted in stability over time due to the higher viscosity and reduced solute diffusivity. The gel thus obtained showed stable and well-defined chiroptical properties. Successive injection of fluorescent dyes, dissolved in volatile solvent that is easily removable, resulted in chirality transfer from the chiral gel to the dyes. Circularly polarized luminescence measurements highlighted good dissymmetry factors glum, a descriptor of the unbalance between left and right circularly polarized emission. The wavelength of the emitted radiation could be modulated according to the chemical structure of the dye able to interact with the chiral gelator.
A phenanthroline derivative of Fmoc-protected L-glutamic acid (phenanthroline-Fmoc-Glu) was very recently identified as a gelator in ChCl–Gly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
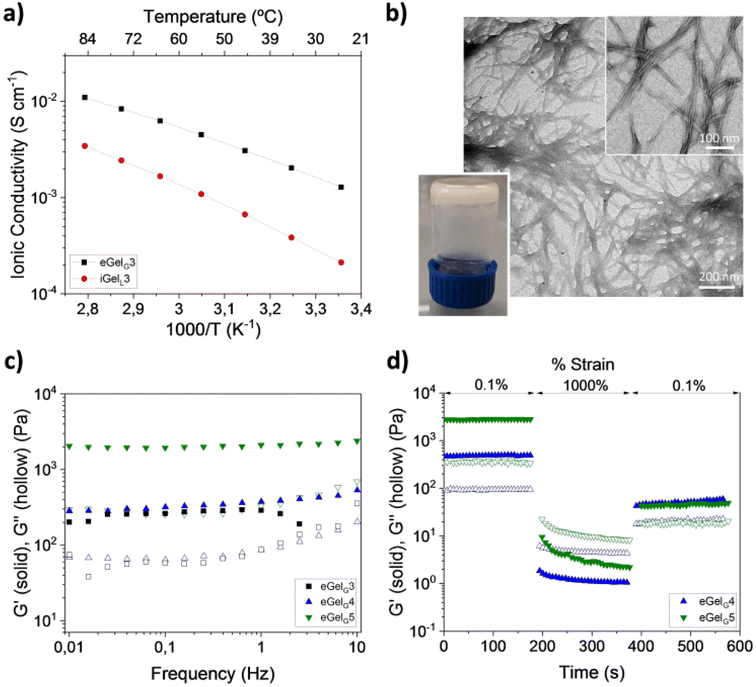
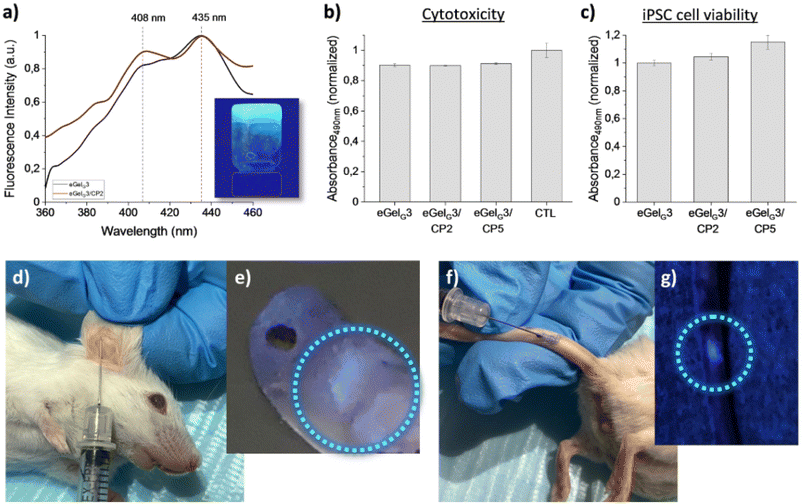
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.102 Out of the formed eutectogels (eGelGx, with x = % w/v of LMWG in the DES), only eGelG3 possessed self-healing and injectability properties and outperformed ionogels obtained using the same LMWG in the ILs cholinium lactate (iGelL) and cholinium glycolate (iGelGA), in terms of mechanical properties and ionic conductivity (Fig. 8). Testing the eutectogels' stability under physiological mimicking conditions (phosphate buffered saline, pH 7.4 and 37 °C), the authors observed a partial degradation of eGelG3 after 48 h. Stabilization was obtained by adding to the supramolecular formulation a conducting polymer, namely poly(3,4-ethylenedioxythiophene):chondroitin sulfate (PEDOT:ChS). Both the eutectogels and their composites showed no cytotoxicity when in contact with human induced pluripotent stem cells (iPSCs) compared to the control (Fig. 9b and c). The targeted application for the developed systems is as agents for local and minimally invasive bioimaging, thanks to the intrinsic fluorescence of the Fmoc and phenanthroline groups of the LMGW (Fig. 9a). Fig. 9d–g show a proof-of-concept assessed with mice, which illustrates clear localized fluorescence in the area of subcutaneous injection (mouse's ear or tail).
2.102 Out of the formed eutectogels (eGelGx, with x = % w/v of LMWG in the DES), only eGelG3 possessed self-healing and injectability properties and outperformed ionogels obtained using the same LMWG in the ILs cholinium lactate (iGelL) and cholinium glycolate (iGelGA), in terms of mechanical properties and ionic conductivity (Fig. 8). Testing the eutectogels' stability under physiological mimicking conditions (phosphate buffered saline, pH 7.4 and 37 °C), the authors observed a partial degradation of eGelG3 after 48 h. Stabilization was obtained by adding to the supramolecular formulation a conducting polymer, namely poly(3,4-ethylenedioxythiophene):chondroitin sulfate (PEDOT:ChS). Both the eutectogels and their composites showed no cytotoxicity when in contact with human induced pluripotent stem cells (iPSCs) compared to the control (Fig. 9b and c). The targeted application for the developed systems is as agents for local and minimally invasive bioimaging, thanks to the intrinsic fluorescence of the Fmoc and phenanthroline groups of the LMGW (Fig. 9a). Fig. 9d–g show a proof-of-concept assessed with mice, which illustrates clear localized fluorescence in the area of subcutaneous injection (mouse's ear or tail).
Sugar-based eutectogels
Ruiz-Olles et al. introduced novel supramoleculal gels obtained by the self-assembling of a cheap and environmentally friendly sugar-based gelator, 1,3:2,4-dibenzylidene-D-sorbitol (DBS), into different type III DES composed of ChCl and alcohols (ethylene glycol, propylene glycol, 1,3-propanediol, glycerol, sorbitol, and xylitol) or urea as the HBD.87 A scheme of the starting materials and a cartoon of the possible structuration of the supramolecular eutectogel are shown in Fig. 10.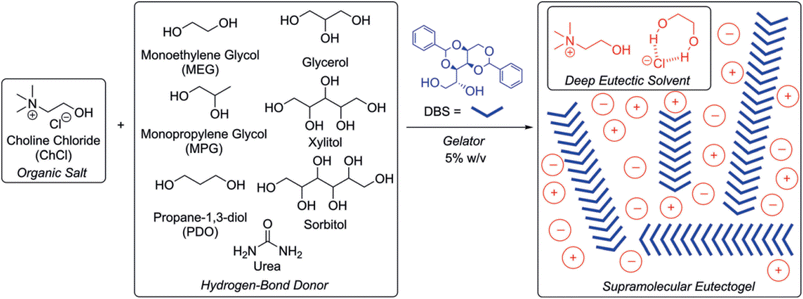 | ||
| Fig. 10 HBD and HBA used by Ruiz-Olles et al. to prepare DBS-based supramolecular eutectogels.87 In the right panel, the DES components are indicated with circles and the related charge, while the cross-linker DBS is sketched with blue wedges. Reprinted with permission from J. Ruiz-Olles, P. Slavik, N. K. Whitelaw and D. K. Smith, Self-Assembled Gels Formed in Deep Eutectic Solvents: Supramolecular Eutectogels with High Ionic Conductivity, Angew. Chem., Int. Ed., 2019, 58, 4173–4178. Copyright 2019 Wiley-VCH GmbH. | ||
DBS is a low-cost, commercially available building block, which self-assembles into nanofibrils through self-complementary H-bonds and π stacking and is considered the closest to a universal gelator that has ever been achieved.117,118 In the Ruiz-Olles work, the reported eutectogels had noticeably high thermal stability, with sol–gel interconversion temperatures Tgel in the range 110–140 °C for a DBS loading of 5% w/v and comparable electrical conductivity to the starting (D)ES. More in detail, gels prepared from ethylene glycol, propylene glycol and 1,3-propanediol exhibited ionic conductivities at room temperature higher than 1 mS cm−1. Moreover, the prepared eutectogels were tolerant to addition of metal cations, i.e. Li+, Mg2+, and Ca2+, at 1 M concentration, resulting in similar electrical conductivity of the prepared gels and electrolytes directly dissolved in DES. Interestingly, it was shown that the DES nature of the liquid-phase was retained within the underpinning nanofibrillar DBS networks.
However, loadings of at least 3% w/v were required in all systems to promote the DBS self-assembly and heating time of ca. 1 h, reduced to 10 s if assisted by ultrasonication. From a mechanistic viewpoint, it is understood that ionic ChCl suppresses the gel formation due to interaction of the chloride anion with the hydroxy groups on DBS.87
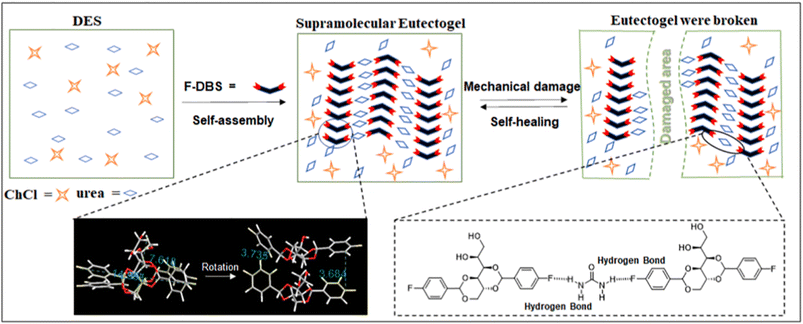
The introduction of a halogen atom covalently bound to the DBS molecular frame leading to X-DBS derivatives was examined by Fan et al.104 The aromatic rings of the DBS were halogenated in the para positions thus obtaining X-DBS, with X = F, Cl and Br. The authors successfully reported the preparation of X-DBS based on ChCl as the HBA and a variety of HBDs such as urea (U), ethylene glycol (EG), 1,2-propanediol (MPG), 1,3-propanediol (PDO), and glycerol (Gly). The best performing gelator was F-DBS at a 2% (w/v) concentration, able to generate eutectogels with remarkably high thermal stability (Tgel > 100 °C for ChCl–Gly and ChCl–U). The eutectogels showed interesting self-healing properties which could be rationalized by considering the specific F-mediated intermolecular interactions. Fig. 11 shows a scheme of gel formation, fracture through mechanical stress and self-healing due to the restoration of the intermolecular H-bonds after the end of the mechanical stress. It is worth mentioning the non-standard C–F⋯H–N intermolecular H-bond with the F atoms covalently bound to the sp2 hybridized C atom as an acceptor site.
The eutectogels mentioned above are characterized by the presence of one or more halogen atoms/ions in their formulation and thus raise some concerns from the viewpoint of the atom economy and, in a broader sense, the production of waste at the end-of-life. Additionally, LCA arguments may reinforce the suggestion of avoiding halogen containing components, in agreement with the CHON rule and the “design for degradation” principle (GCP10): in the case solvent recycling could not be achieved – or it would not be economically affordable – harmful secondary waste would be produced after incineration. If the halogen atom is a key structural feature for the assembly of the physical gel, as for the C–F⋯H–N interactions, careful evaluation of the trade-off between technological advancement and environmental costs should be done when performing the evaluation of the process sustainability.
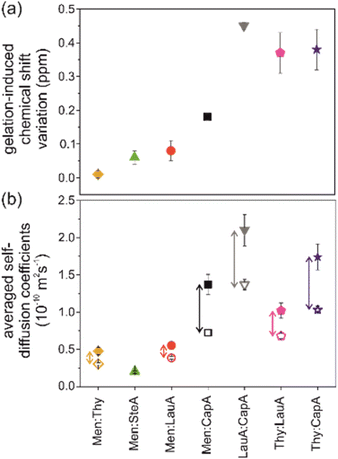
It is worth mentioning that the sugar-based LMWG DBS was recently used to extend for the first time the eutectogel chemical space to hydrophobic (D)ESs made of non-ionic components.105 The authors report the gelification of menthol (Men)- and thymol (Thy)-based (D)ESs with variable molecular weight saturated fatty acids by using DBS in the range 1–8% w/w. The Tgel spanned the range 110 °C for the Thy–caprylic acid (CapA) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ES with 8% w/w DBS to 153 °C for the Men–stearic acid (SteA) 1
1 ES with 8% w/w DBS to 153 °C for the Men–stearic acid (SteA) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ES with 8% w/w DBS. Rheological measurements pointed out the thermoreversible gel formation and self-healing properties. The authors measured and compared the self-diffusion coefficients of the DES components in the pure liquid and in the gel by using high-resolution magic angle spinning (HRMAS) NMR spectroscopy and pulsed field gradient spin-echo (PGSE) NMR methods. The results showed the counterintuitive acceleration effect of gelification on the diffusivity of the DES components, as illustrated in Fig. 12. In other words, the (D)ES components showed larger diffusion coefficients in the eutectogel compared to the pure liquid, despite the macroscopic difference in viscosity between the eutectogel and pristine DES. The authors invoked the interference of DBS in the network of interactions between the DES components as perturbation causing the acceleration effect. The intensity of the perturbation is inversely proportional to the strength of the intra-DES interactions. The authors quantify the latter with the gelation induced chemical shift variation Δδ = |δ(OH)DES − δ(OH)gel| and divide the eutectogels into the two categories of “responsive” (Δδ > 0.35) and “robust” (Δδ < 0.1). The correlation between gel robustness and acceleration effects is shown by the comparison of panels (a) and (b) in Fig. 12.
1 ES with 8% w/w DBS. Rheological measurements pointed out the thermoreversible gel formation and self-healing properties. The authors measured and compared the self-diffusion coefficients of the DES components in the pure liquid and in the gel by using high-resolution magic angle spinning (HRMAS) NMR spectroscopy and pulsed field gradient spin-echo (PGSE) NMR methods. The results showed the counterintuitive acceleration effect of gelification on the diffusivity of the DES components, as illustrated in Fig. 12. In other words, the (D)ES components showed larger diffusion coefficients in the eutectogel compared to the pure liquid, despite the macroscopic difference in viscosity between the eutectogel and pristine DES. The authors invoked the interference of DBS in the network of interactions between the DES components as perturbation causing the acceleration effect. The intensity of the perturbation is inversely proportional to the strength of the intra-DES interactions. The authors quantify the latter with the gelation induced chemical shift variation Δδ = |δ(OH)DES − δ(OH)gel| and divide the eutectogels into the two categories of “responsive” (Δδ > 0.35) and “robust” (Δδ < 0.1). The correlation between gel robustness and acceleration effects is shown by the comparison of panels (a) and (b) in Fig. 12.
A remark from the viewpoint of sustainability: the authors briefly examined the sustainability aspects of such a new class of materials by using van Aken's EcoScale metrics.119 Penalty points were only assigned to Thy-based systems due to their moderate toxicity and potential harm to aquatic environments. Energy consumption, with heating taking less than one hour for the entire eutectogel set, resulted in a deduction of 2 penalty points for each eutectogel. Overall, the EcoScale ranking ranged from 93 to 98 points, placing the preparation process of the eutectogels considered here in the “excellent” category.
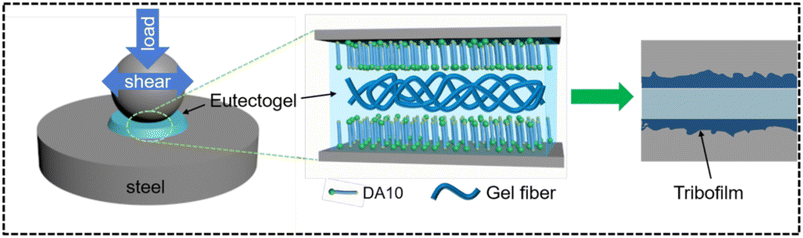
We end this section with one more example concerning the use of D-glucose acetal derivatives (DAn, with n the number of C atoms between the amido and amino functional groups) as gelators.106 Zhang and coworkers prepared and characterized many eutectogels for several ChCl–HBD pairs (HBD = U, EG, MPG, PDO, Gly). The best performing eutectogels, in terms of rheological properties and tribological behaviour, were made of 2–4% DA10/ChCl–Gly. The authors claim that such eutectogels, along with good properties of self-healing, resistance to corrosion and creep recovery, are the first examples of eutectogel lubricants. The general scheme of the lubrication mechanism of DA10/ChCl–Gly eutectogels is visualized in Fig. 13. The first step consists in the transformation of the gel into a quasi-liquid phase due to thixotropy. The DA10 gelator is adsorbed onto the steel surface via interaction of the hydroxyl, amido and amino groups with the steel surface. This process starts a cascade of complex chemical reactions at the interface mediated by the mechanic energy associated with the friction and electrochemical reactions. The result is the progressive growth of a strong tribofilm containing organic and inorganic oxides, as outlined by XPS studies. The presence of such a coating prevents the direct friction of the contacting surfaces, thus providing the desired lubricant effect.
Nucleoside-based eutectogels
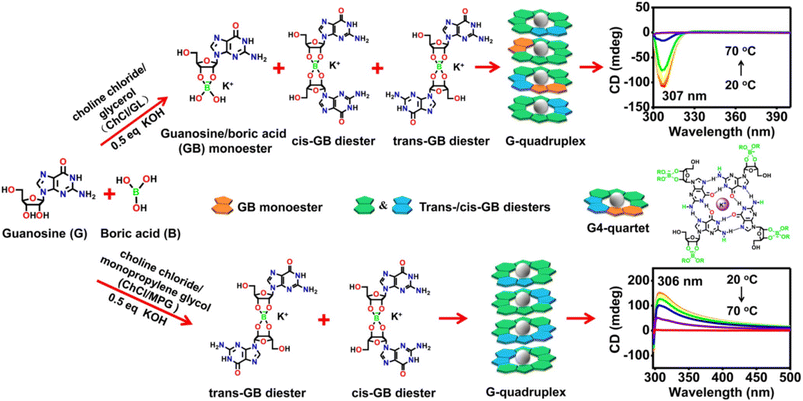
Natural nucleosides, and especially guanosine, are known to give rise to cyclic tetramers (G-quartet) via a strong network of inter-bases H-bonds with further stabilization by the inclusion of metal cations such as K+, Na+, Ba2+ or Sr2+. In turn, the G-quadruplet may generate supramolecular assemblies driven by π–π stacking of the nucleobases into the so-called G-quadruplexes.120 Gu et al. exploited this well-known capability of guanosine to fabricate suitable G-quartet derivatives suitable to carry out the challenging gelification in non-aqueous media.107 Guanosine-boronate esters were prepared in KOH-containing solutions of ChCl–Gly and ChCl–MPG. The replacement of KOH with LiOH only gave clear solutions which turned into stable eutectogels after further addition of KCl, suggesting the crucial role of guanosine and KOH for gelation. The reaction outcome, in terms of mono- and diester formation, turned out to be very much dependent on the DES type. Monoester and cis/trans diesters were formed in ChCl–Gly, while the cis/trans diesters were the only products in ChCl–MPG, as demonstrated by 11B-NMR spectroscopy. The different speciation was the driving force for the supramolecular chiral columnar assembly with opposite chiroptical properties, as sketched in Fig. 14.The G-quadruplex eutectogels with 3% w/w of guanosine showed good conductivity (ChCl–MPG eutectogel 7.78 mS cm−1 and ChCl–Gly eutectogel 1.36 mS cm−1), thixotropy (ChCl–MPG flows after vigorous stirring and reforms into a stable opaque gel after resting for 30 minutes and ChCl–Gly eutectogel, under similar conditions, recovers to a gel state within seconds), thermal stability (Tgel = 75.6 and 90.4 °C in ChCl–Gly and ChCl–MPG eutectogels, respectively) and injectability. These properties prompted the authors to claim these materials's good fitness for portable flexible electronic devices.
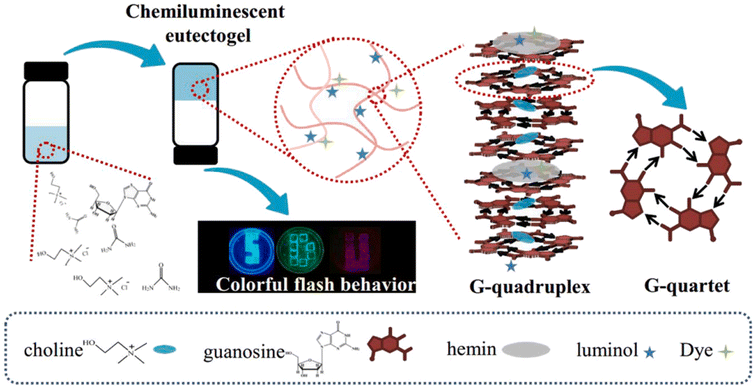
G-Quadruplex eutectogels were also formed by direct dissolution of guanosine powder in a series of chloride-based type III (D)ESs (ChCl–U, ChCl–Gly, ChCl–MA, and trimethylhydroxypropylammonium chloride–U; trimethylhydroxybutylammonium chloride–U, trimethylhydroxyamylammonium chloride–U).108 The authors hypothesized that the cation from the organic salt worked as the stabilizer of G-quadruplexes and the hydrogen bond networks derived from Ch–U synergistically reinforced the G-quadruplexes, with no need for additional ions. Hemin, luminol and fluorochrome were then added to the prepared eutectogels to create chemiluminescent luminophores, to be used for the chemiluminescence resonance energy transfer (CRET) platform, as depicted in Fig. 15.
Amide derivative-based eutectogels
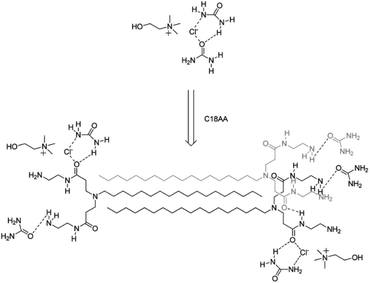
Delbecq et al. prepared supramolecular gels of ChCl–U (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) using two different long alkylaminoamide gelators, bearing two amide groups with two terminal hydroxyl (C18OH) or primary amine moieties (C18AA).109 The basic idea was to introduce, as gelators, species mimicking the structural motives of urea but, at the same time, introducing further sites of attractive interactions – dispersive forces in this case – able to generate non-covalent entanglements contributing to the formation of a compact gel. The possible mechanism for the gelation of standard ChCl–U (1
2) using two different long alkylaminoamide gelators, bearing two amide groups with two terminal hydroxyl (C18OH) or primary amine moieties (C18AA).109 The basic idea was to introduce, as gelators, species mimicking the structural motives of urea but, at the same time, introducing further sites of attractive interactions – dispersive forces in this case – able to generate non-covalent entanglements contributing to the formation of a compact gel. The possible mechanism for the gelation of standard ChCl–U (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), commonly labelled as reline, in the presence of C18AA is shown in Fig. 16.
2), commonly labelled as reline, in the presence of C18AA is shown in Fig. 16.
The C18AA – reline eutectogels were successfully employed as media for the synthesis of shape-controlled metal nanoparticles, such as Au nanospheres.109 This technology enables electrodeposition on diverse substrates, when loading DES gels with varied metal chloride concentrations. The benchmark of this technology is its ability to adjust gel conductivity by modifying intrinsic viscosity. This adaptability makes the technology versatile for tailored processes, paving the way for electrodeposition of metallic patterns on different substrates.
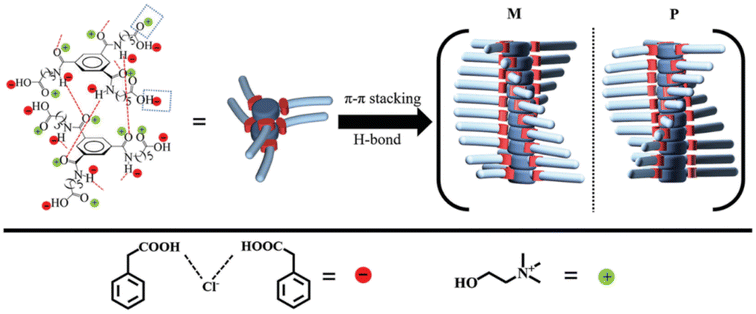
A very good example of versatile building blocks in supramolecular chemistry is the benzene-1,3,5-tricarboxamide structure (the BTA motif) due to the tendency of three amide groups to form intermolecular H-bonds.96 The BTA family may either derive from 1,3,5-benzenetricarboxylic acid or from 3,5-diaminoaniline. The corresponding derivatives are labelled as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O centered BTAs or N-centered BTAs, respectively. Thanks to the relatively simple synthesis and the possibility to append various different substituents to the side chain of the BTA scaffold, a wide range of BTA derivatives are available.96 A C
O centered BTAs or N-centered BTAs, respectively. Thanks to the relatively simple synthesis and the possibility to append various different substituents to the side chain of the BTA scaffold, a wide range of BTA derivatives are available.96 A C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O centered (i.e. with the amide groups attached to the benzene ring via the carbonyl group) and C3 symmetric (i.e. with three identical substituent groups) BTA homolog has been used by Zhang et al.110 The authors exploited the unique structural features of the BTA3 gelator to build supramolecular gels with the ChCl-PhAA 1
O centered (i.e. with the amide groups attached to the benzene ring via the carbonyl group) and C3 symmetric (i.e. with three identical substituent groups) BTA homolog has been used by Zhang et al.110 The authors exploited the unique structural features of the BTA3 gelator to build supramolecular gels with the ChCl-PhAA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 DES with well defined helicized columnar assemblies. The columnar architecture was the result of synergic action of three different factors: the threefold H-bond involving the amide functional groups, the intermolecular H-bond of the carboxylic acid groups at the end of the alkyl chain with the DES components, and the π–π stacking of the aromatic rings. The joint action of these factors is summarized in the scheme in Fig. 17.
2 DES with well defined helicized columnar assemblies. The columnar architecture was the result of synergic action of three different factors: the threefold H-bond involving the amide functional groups, the intermolecular H-bond of the carboxylic acid groups at the end of the alkyl chain with the DES components, and the π–π stacking of the aromatic rings. The joint action of these factors is summarized in the scheme in Fig. 17.
The authors report a detectable Cotton effect in the CD spectra of the eutectogel at 206 nm.110 A possible explanation relies upon the chiral stacking – with either a P or M twist – of BTA3 mediated by the directional threefold H-bonds. Although the P and M columnar assemblies were generated randomly, further assembly of columns with the same twist mode (P or M) into fibers resulted in symmetry breaking,121 thus leading to observable random CD signals.
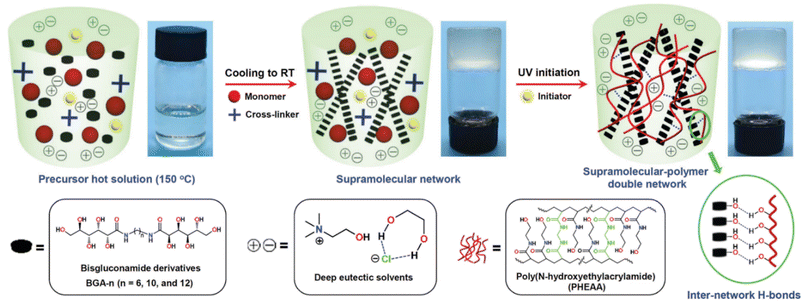
The class of bisgluconamide (BGA) gelators was used by Wang et al. within a complex strategy for the fabrication of small molecule-based supramolecular polymer double-network (SP-DN) eutectogels with outstanding thermal, mechanical, adhesive and self-healing properties.69 From the viewpoint of the molecular design, the strategy proposed by the authors can be summarized in some points (see Fig. 18 for a graphical guide).
(1) The BGA-n gelators are shown in the box on the left of Fig. 18. They can be classified as bolaamphiphilic molecules,122i.e. molecules with two polar heads and a variable spacer between them of opposite polarity. The authors first synthesized a collection of BGA-n compounds with n = number of C atoms in the alkyl spacer = 6, 10, 12. The combination of BGA-12 and the ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 DES showed good capability to form an eutectogel, thus highlighting the critical role of dispersive interactions in driving the sol–gel transition. Additionally, the authors proved the supramolecular assembly of the BGA-12 eutectogel in right-handed helical columns.
2 DES showed good capability to form an eutectogel, thus highlighting the critical role of dispersive interactions in driving the sol–gel transition. Additionally, the authors proved the supramolecular assembly of the BGA-12 eutectogel in right-handed helical columns.
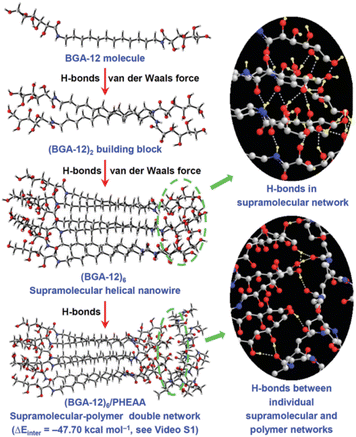
(2) The gelification was then carried out in the presence of N-hydroxyethylacrylamide (HEAA) monomers and N,N′-methylenebis-(acrylamide) (MBA) as cross-linkers and a suitable UV initiator. Once the eutectogel was formed, the photopolimetization of the monomers was carried out by UV irradiation affording the final BGA-12/PHEAA SP-DN eutectogel. The graphics in Fig. 18 clearly point out the interconnection of the non-covalent self-assembly of the eutectogel with the covalent, cross-linked network of the photopolymerized polyacrylamide. A detailed simulation study showed the role of van der Waals interactions among the BGA-12 units in driving the aggregation and the formation of helical superstructures and the presence of a complex equilibrium of H-bond interactions within BGA-12 aggregates and between the latter and the PHEAA backbone. An interaction energy of −47.70 kcal mol−1 was indeed calculated between the independent BGA-12 supramolecular network and PHEAA polymer network, confirming the improved stability after self-assembly. An explicative sketch is reported in Fig. 19.
Organic salt-based eutectogels
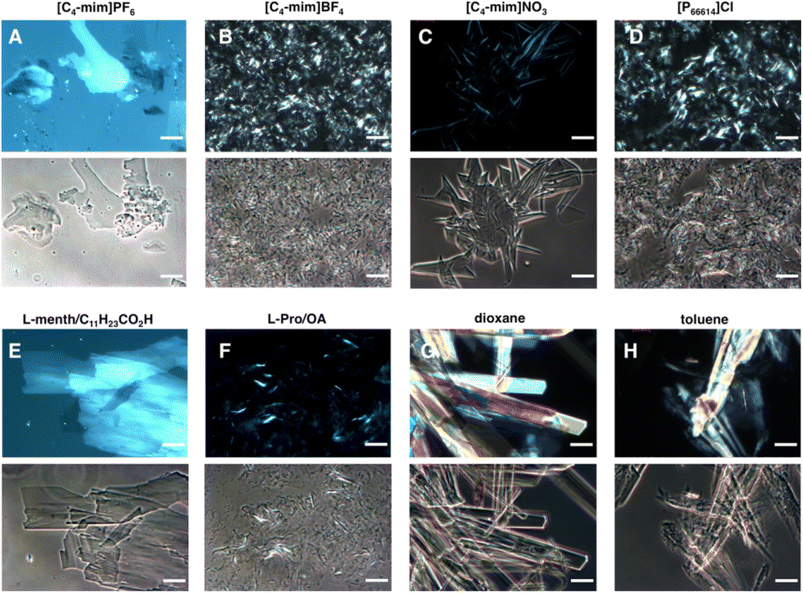
Another general approach to gelification in (D)ES relies upon the use of ionic or zwitterionic LMWGs such as surfactants, ionic liquids with long alkyl chains, and bile acid salts. The rationale is in the presence of a suite of intermolecular interactions of opposite signs, such as dispersive forces and coulombic/H-bond interactions. In many cases, the delicate interplay of these non-covalent interactions leads to complex and unexpected gelification mechanisms. A clear example is provided by Prieto Kullmer et al.111The authors introduce the use of long chain C16 organic salts, namely 1-cetyl-3-methylimidazolium [C16-mim], N-cetylpyridinium [C16-py], and N-cetyl-N-methylpyrrolidinium [C16C1-pyrr] bromide salts, to induce solvent gelation in a variety of representative ILs, molecular solvents and (D)ESs. The authors discovered the formation of ordered crystalline phases in the gelified materials by using non-destructive polarized optical microscopy (POM). The POM analysis showed the existence of optical anisotropy typical of the formation of crystalline phases. A visual summary of the gel morphologies is shown in Fig. 20.
The authors examined the Men-LauA DES in detail by NMR, XRD and DSC concluding that the gel contained crystalline gelator [C16-mim]Br in a crystal form different from the one observed for the pure gelator.111 The small dimensions of the crystals, the consequent large surface, and the uniform distribution of the crystals within the bulk DES generated a network capable of trapping the DES components. The overall effect was that of increasing stiffness and viscosity in the system up to the formation of the gel. Thus, the gelator crystallization in the cooling step induced the gelification of the DES solvent, in a quite elegant and uncommon gelation mechanism based on the formation of an ordered, crystalline phase. Concerning the sustainability of this approach, a caveat should necessarily be inserted at this stage. The use of long chain ionic liquids in the gel formulations introduces negative factors in the green metrics, due to the well-known toxicity brought about by long alkyl chain substituents.123 Nevertheless, once again it should be stressed that the evaluation of the malicious effects correlated to the toxicity should be performed relative to the target application, e.g. by considering that the same additive might have a positive or negative profile if used in cosmetic or in biocide formulations, respectively. This simple example calls for a critical use of toxicologic and ecotoxicologic data in claiming the greenness of materials and processes.
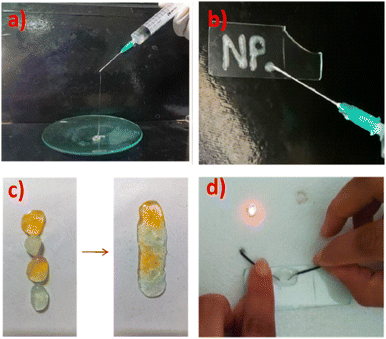
Very recently, [C16-py]Br and [C16-py]Cl were used for the gelification of type III DESs having ChCl as the HBA and mono-, di-, and trimeric acids (formic acid (FA), oxalic acid (OA), and citric acid (CA)) as HBDs, to be applied as delivery systems.112 Gels stable up to three months were formed at a 5% w/v surfactant concentration, thanks to the interplay of non-covalent (H-bond and hydrophobic) interactions, while gels were unstable already in a matter of few days (1 to 5) at a lower concentration of the surfactants (2.5% w/v), due to insufficient entanglement of the hydrophobic chains. Noteworthily, the injectability and self-healing properties were demonstrated, as displayed in Fig. 21, and the toxicity tested towards three human cell lines, showing no inhibitory effect on proliferation even at the maximum dose (2000 mg L−1). When used to encapsulate the anticancer drug curcumin, a 90-fold increase in the half-life and ca. 1000-fold increase in solubility were observed with respect to water.112 Moreover, the curcumin-loaded eutectogels showed increased antimicrobial properties against both Gram-positive and Gram-negative bacteria with respect to both pristine DESs and eutectogels. A sustained non-Fickian release of the drug was found, with complete release within 180 min at physiological pH.
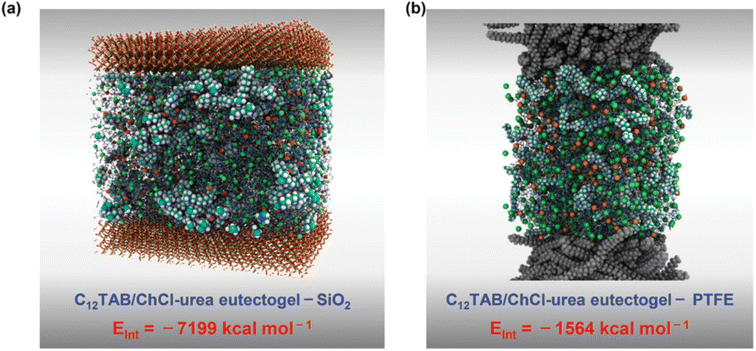
Liang et al. tested several alkyltrimethylammonium bromides CnTAB with n = 6, 8, 10, 12, 14, 16 and 18 and ChCl-based DESs to obtain high performance adhesive eutectogels.113 The best performing DES was the standard ChCl–U 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
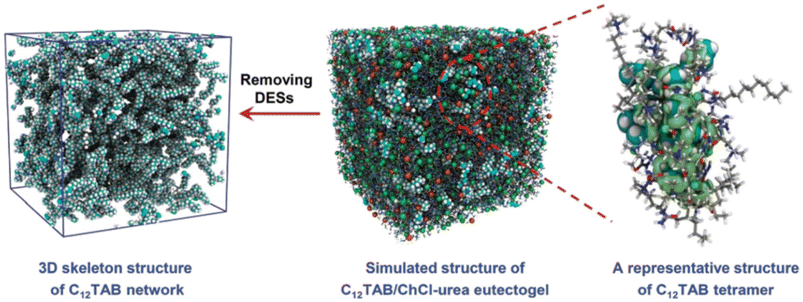
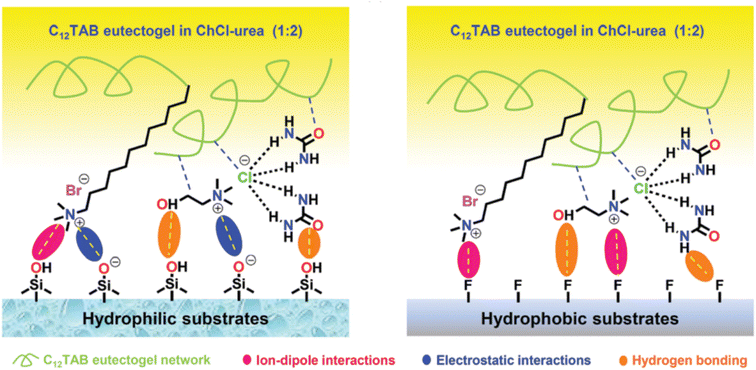
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, in which the self-assembly of C12TAB started at amounts as low as 0.2% w/w. Thorough characterization of the eutectogel with 25% w/w C12TAB highlighted the formation of micrometric dendritic fibrils and an overall dendritic fiber network. The role of each component in the gelification mechanism was elucidated by molecular dynamics simulations. A representative graphic is shown in Fig. 22. The central snapshot indicates a highly interconnected three-dimensional structure of ChCl, urea and C12TAB. This picture is the balance of three different factors: (i) solvophobic interactions between the C12 chains and the structured DES (characterized by a robust H-bond network); (ii) the competitive attitude of ChCl to give rise to weak but non-negligible dispersive interactions with the C12 chains. This point is important as the C12–ChCl interaction prevents the formation of C12TAB micelles, as it occurs in aqueous solvents; (iii) finally, the polar head of C12TAB is solvated by both the DES anion and urea. The specificity of such interactions is depicted in the right panel of Fig. 22 and it is the rationale for gel formation and stability. Additionally, the eutectogel C12TAB/ChCl–U showed outstanding adhesive properties, considering both the adhesive strength under extreme T and solvent conditions and the type of surface – hydrophilic or hydrophobic – the adhesive gel is capable of working on. The explanation of these tantalizing properties is depicted in Fig. 23. The MD simulation of the different surfaces is shown in Fig. 24. Although the interaction energy of the PTFE substrate (−1564 kcal mol−1) is lower than that of the SiO2 substrate (−7199 kcal mol−1), the C12TAB-based eutectogel is provided with such a broad toolkit of molecular interactions to cover the requirements for attractive interactions with the functional groups exposed on the surface of both receptive materials, irrespective of their polarity. Moreover, the DES acts not only as solvent for gelification but also plays an active role in the cohesion processes. For instance, the interfacial binding energies on the SiO2 substrate of the C12TAB network alone and of the ChCl–urea DES are −1886 and −5313 kcal mol−1, respectively, suggesting that stronger physical interactions exist with the C12TAB/ChCl–urea eutectogel than with the individual materials. This is an excellent example of design-for-versatility, based on a rational knowledge of the driving forces of self-assembly.
2, in which the self-assembly of C12TAB started at amounts as low as 0.2% w/w. Thorough characterization of the eutectogel with 25% w/w C12TAB highlighted the formation of micrometric dendritic fibrils and an overall dendritic fiber network. The role of each component in the gelification mechanism was elucidated by molecular dynamics simulations. A representative graphic is shown in Fig. 22. The central snapshot indicates a highly interconnected three-dimensional structure of ChCl, urea and C12TAB. This picture is the balance of three different factors: (i) solvophobic interactions between the C12 chains and the structured DES (characterized by a robust H-bond network); (ii) the competitive attitude of ChCl to give rise to weak but non-negligible dispersive interactions with the C12 chains. This point is important as the C12–ChCl interaction prevents the formation of C12TAB micelles, as it occurs in aqueous solvents; (iii) finally, the polar head of C12TAB is solvated by both the DES anion and urea. The specificity of such interactions is depicted in the right panel of Fig. 22 and it is the rationale for gel formation and stability. Additionally, the eutectogel C12TAB/ChCl–U showed outstanding adhesive properties, considering both the adhesive strength under extreme T and solvent conditions and the type of surface – hydrophilic or hydrophobic – the adhesive gel is capable of working on. The explanation of these tantalizing properties is depicted in Fig. 23. The MD simulation of the different surfaces is shown in Fig. 24. Although the interaction energy of the PTFE substrate (−1564 kcal mol−1) is lower than that of the SiO2 substrate (−7199 kcal mol−1), the C12TAB-based eutectogel is provided with such a broad toolkit of molecular interactions to cover the requirements for attractive interactions with the functional groups exposed on the surface of both receptive materials, irrespective of their polarity. Moreover, the DES acts not only as solvent for gelification but also plays an active role in the cohesion processes. For instance, the interfacial binding energies on the SiO2 substrate of the C12TAB network alone and of the ChCl–urea DES are −1886 and −5313 kcal mol−1, respectively, suggesting that stronger physical interactions exist with the C12TAB/ChCl–urea eutectogel than with the individual materials. This is an excellent example of design-for-versatility, based on a rational knowledge of the driving forces of self-assembly.
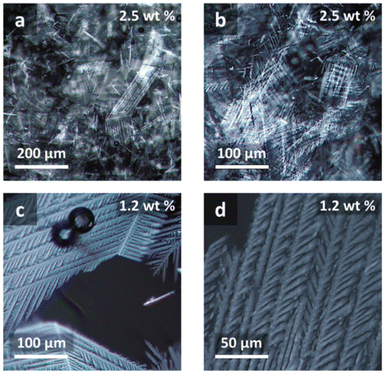
The formation of dendritic fractal aggregates has been highlighted by Matthews et al. in the gelification of ChCl–Gly (labelled by the authors as glyceline) in the presence of the surfactant sodium dodecyl sulfate (SDS).114 The authors proved that the assembly of SDS in DES glyceline afforded a dendritic, feather-like aggregation motif, with further growth to a complex dendritic fractal morphology, named fracto-eutectogel, comprising a central fiber supporting the growth of secondary fibers, in turn supporting a third generation and so forth. A polarized light microscopy (PLM) image of the feather-like aggregation motives is shown in Fig. 25. The authors highlighted that the dendrimer fractal morphology is detectable in ChCl–Gly but not in pure glycerol, thus adding evidence to the general observation of the templating effect of DES.
Another surfactant, cetyldiethanolamine N-oxide, was evaluated for the preparation of eutectogels suitable for iodine removal.115 Stable gels with Tgel in the range 32–48 °C were obtained only in ChCl–diethylene glycol (DEG) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, ChCl–EG 1
3, ChCl–EG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, trimethylglycine (TMG)–DEG 1
2, trimethylglycine (TMG)–DEG 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and TMG–glycolic acid (GA) 1
2 and TMG–glycolic acid (GA) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The surfactant was fully soluble in other type III DESs containing tetrabutylphosphonium or tetrabutylammonium salts, as well as in type V hydrophobic (D)ESs. The eutectogels with 5% loading of gelator were tested for I2 removal from hexane solutions, with ChCl–DEG displaying high removal efficiency in the model system over a wide range of initial I2 concentrations.
2. The surfactant was fully soluble in other type III DESs containing tetrabutylphosphonium or tetrabutylammonium salts, as well as in type V hydrophobic (D)ESs. The eutectogels with 5% loading of gelator were tested for I2 removal from hexane solutions, with ChCl–DEG displaying high removal efficiency in the model system over a wide range of initial I2 concentrations.
The last example of this section shows an interesting case of co-participation of DES and an inorganic additive in the formation of a functional eutectogel using a natural bile salt as a LMWG. Sun et al. reported the preparation of luminescent eutectogels incorporating Eu3+ ions.116 The main advantage of the entrapment of a lanthanide ion in a dense matrix such as that of the eutectogel is the reduction of the vibrational non-radiative transitions which depress the luminescence, thus enhancing the performance of the emitting material. The authors found that sodium cholate (NaC) natural bile salt formed eutectogels with ChCl–U, ChCl–Gly and ChCl–EG in the presence of Eu3+ ions. Interestingly, only ChCl–U was capable of gelification with NaC but with no Eu3+ added. The good photophysical response of the Eu3+ doped eutectogels is displayed in Fig. 26.
The spectroscopic and photophysical characterization prompted the authors to hypothesize the direct involvement of the lanthanide ions in the gel network.116 The mechanism is sketched in Fig. 27. In the presence of DES, NaC molecules show a limited aggregation propensity, forming mainly carboxylate H-bonded dimers with antiparallel orientation of the steroidal skeletons, interacting via solvophobic interactions, or small oligomers that behave as solutes in DES solvent without gelification. In the presence of a suitable concentration of Eu3+, the dimers coordinate the Eu3+ ions with the carboxylate. The fibrills grow and are stabilized in a three-dimensional network by the participation of the DES components, in an ensemble of coordination, intermolecular H-bonds, and dispersive interactions. At high Eu3+ concentrations, the coulombic repulsion dominates and the eutectogel structure collapses.
One step beyond: two research challenges
In this final part we present two case studies paradigmatic of the impact that research and innovation in the field object of this review may contribute to the civilization target “that no one is left behind”,31 specifically recalling SDG6, SDG8, SDG9 and SDG14: recovery of metallic critical raw materials from end-of-life batteries and energy storage devices, and water remediation.Critical raw materials recovery from reprocessing of end-of-life electrodes
The recycling of electrodes' components is acquiring tremendous importance for both sustainability issue – life to cradle consideration – and the geopolitical situation governing the critical raw materials supply chain. As highlighted by Graedel et al. since 2015, the issue of raw materials shortening cannot be faced by using a replacement strategy, as “the first thing to say is that for some materials and for some final products, we know of no suitable substitute. In other cases, product performance would suffer markedly under substitution”.124 With the increasing consciousness of environmental issues, the traditional categories considered for the criticality assessment – the supply risk and the vulnerability – were added to a third voice, the “environmental implications”, leading to a 3D criticality space for raw materials (Fig. 28). The latter aspect put hydrometallurgic (HM) processes as a convenient first step in the supply chain of metals125 and as an emerging approach to the recycling technology of spent lithium ion batteries126 in the spotlight. The simplest example of the HM process is the solvent leaching of the metal ore or spent electrodes to afford metal ions in aqueous solution followed by extraction/separation of metal salts for further processing. The natural evolution, in agreement with GCP3, GCP5, SDG6, SDG12 and SDG14 (see Section 2) is the replacement of the aqueous environment of HM with that of green and recyclable solvents, thus paving the way to the transition to solvometallurgy (SM). The reduction of waste, water consumption and a minor energy demand are the most intuitive pluses of SM compared to HM.127 A graphic comparison of HM vs. SM metal ore processing is sketched in Fig. 29. | ||
| Fig. 28 The 3D criticality space: the Yale analytical framework for the assessment of metal criticality based on the metrics discussed by Graedel et al.162 Reprinted with permission from T. E. Graedel, E. M. Harper, N. T. Nassar and B. K. Reck, On the materials basis of modern society, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6295–6300. | ||
In this general context, (D)ESs are gaining importance at a higher rate compared to other systems, typically ionic liquids. Fig. 30 shows the bibliometric relevance of different HM and SM methods for metal recovery, showing that DES related topics have seen the steepest increase in the number of publications in the last few years.128
Zhu et al. reviewed the current technologies of material recovery from LIBs.129 They stressed the point that several DESs are not only capable of leaching metal oxides, but they also act as reductant reagents, thus lowering the oxidation state of highly valent metal oxides in the cathode material and making metal oxide leaching easier. Remarkable leaching efficiency of ChCl–organic acid DESs, alternative to traditional strong inorganic acid leaching,130 and ChCl–U binary and ternary DESs,131 in the recovery of Li, Co, Ni and Mn, is also reported. From a sustainability perspective, the increased safety for the workers associated with the replacement of harmful strong mineral acid solutions with DES-based acidic media is a significant contribution to the achievement of SDGs 3, 6 and 8 (see Section 2). A critical unit operation in the process of recovery of precious elements from end-of-life electronic devices and electrodes is the liquid–liquid extraction. In the past few years, the application of hydrophobic DESs (or in general hydrophobic low melting mixtures, LMMs) has been proposed as a disruptive application of eutectic solvents. The possibility of metal ion extraction in water immiscible DES dates back to 2016. The mixtures lidocaine–decanoic acid at different molar ratios were shown to extract Co2+ from aqueous solutions, with excellent selectivity towards alkali metal ions.132 Indium, in the form of negatively charged chloride complexes, was successfully extracted in hydrophobic tetraheptylammonium chloride (THAC)–dodecanoic acid or THAC–oleic acid DES (hydrophobic type III).133 Interestingly, the authors report the unexpected extraction efficiency of the non-ionic hydrophobic type V eutectic mixture Men–dodecanoic acid at a low acidity level of the aqueous solution, a result which paves the way for the use of natural product derived hydrophobic type V ES in circular economy oriented processes. A recent and elegant application of type V hydrophobic DES was proposed by Hanada and Goto for the selective extraction of Li+ ions in a DES phase composed of suitable β-diketones and long chain alkylphosphine oxide or triphenylphosphate.134
In summary, the chemical space of solvometallurgy based on (D)ESs is new, wide and largely unexplored. More interestingly, at the moment there are no scientific publications on extraction/separation methods for the recovery of precious raw materials from spent electrodes using DES in the gel state. This is, in our opinion, the next step for innovation in materials science from the perspective of sustainable processes: a semi-solid active material for capturing critical species could, in principle, open the way to continuous extraction processes, increasing adsorption efficiency during column separations and enabling easier re-use of the ion-adsorbing phase with consequent improvement of green metrics factors related to the reduction of waste during the process, minor use of water, and a possibly lower energy consumption.
Water remediation
Water is a vital resource for human survival. According to the EU, only 40% of surface water bodies is in “good ecological status”.135 This calls for efficient and cheap technologies for water pollution monitoring and remediation, to drive serious sustainable and circular management and use of natural resources and guarantee healthy freshwater for all.Despite recent progress in improving water quality, water bodies are still at risk, and new contaminants of emerging concern (CEC) are in the spotlight.136 Among them, industrial CEC (ICEC) include priority (hazardous) substances listed in the EU Water Framework Directive.136 ICEC pollution is omnipresent, i.e. microplastics are detected practically in every water sample from EU lakes and rivers and also in 80% of our livestock feed, blood, milk and meat, and in the absence of action, the global annual plastic entering surface and groundwater is estimated to triple from 9–23 million metric tons in 2021 to around 53 by 2030.136
This has severe and irreversible implications. Many ICEC (e.g. nonylphenol, bisphenol A, and di-2-ethylhexyl phthalate) are endocrine disrupting chemicals, causing reproductive problems, cancer, cognitive deficits, obesity and diabetes, with health costs of €150 billion a year.136 Emblematic is the group of per- and polyfluoroalkyl substances (PFASs), detected at more than 70% of the groundwater measuring points in EU member states and classified as (very) persistent, (very) bioaccumulative and toxic, leading to negative effects for human health and biodiversity.136 No doubt, both social and economic triggers encourage a step change.
The removal of ICEC from water not only meets the goals of Cluster 6 of Horizon Europe (destination clean environment and zero pollution),137 but it is also in line with the objectives of the European Green Deal and the related initiatives targeting environmental challenges (zero pollution action plan and chemicals strategy for sustainability),138,139 as well as the United Nations SDG3, SDG6, SDG12, and SDG14 (see Fig. S1†).
Among water remediation technologies, the best-performing extraction procedures are currently based on dispersive liquid–liquid microextraction (DLLME). The fundamental principles of this technique were first reported by Rezaee et al.140 A scheme of DLLME is shown in Fig. 31.
Compared to conventional liquid–liquid extraction (LLE) and microextraction (LLME), merits of DLLME are low consumption of solvent and sample, high-speed and straightforward procedure, high recovery ratios and enrichment factors, and a low limit of detection.141–143 However, DLLME suffers from two main drawbacks that limit its application:
(i) A large set of chlorinated solvents (e.g. carbon tetrachloride, tetrachloroethane, dichloroethane, chlorobenzene, trichloromethane, and tetrachloroethylene) are the go-to choice. They are highly toxic and listed among the European Environment Agency list of priority pollutants144 and do not apply to all classes of contaminants.141
(ii) A disperser (1–2 mL of an environmentally unfriendly solvent, such as methanol, ethanol, acetone, acetonitrile or tetrahydrofuran) is usually added to decrease the partition coefficients for analyte extraction, at the expense of cost and environmental pollution.141,145
The logical way to address the solvent issue is to replace the toxic, volatile organic solvents (VOCs) used in DLLME with more sustainable solvents. Given the features outlined in the previous section, the choice of (D)ESs could be a logical option. Focusing on water remediation, some paradigmatic cases showed the potentiality of (D)ESs for water remediation,146,147 thus prompting a deeper and more systematic exploration – still missing in the literature – of their performance towards ICEC capture.
In comparison with LLE, solid-phase extraction (SPE) is easier and requires less solvent and sample.147 Despite being less amenable to automation, the miniaturized version, dispersive solid–liquid microextraction (DSLME), reduces and even eliminates the use of toxic and inflammable solvents.148 (D)ESs are an option to increase the selectivity, capacity and environmental friendliness of available sorbents.148 (D)ES-based DSLME for water treatment is, however, still a nascent technology, with a handful of new sorbents prepared only with type III ES and questionable materials.149,150
The further step of immobilizing (D)ESs in naturally derived LMWGs, resulting in a eutectogel for DSLME, remains a totally unexplored field strongly oriented towards the achievement of the UN SDGs 3, 6, 12, and 14.31 Supramolecular eutectogels hold great promise for applications in fields requiring good performance and environmental protection.99 An example of their benefits was demonstrated through the use of fully natural supramolecular eutectogels based on ChCl and PhAA with L-amino acids as gelators, which were tested as sorbents for dye removal in wastewater treatment.103 They showed an excellent water purification function and can be used at least 9 times without losing efficiency. The best results were obtained with the L-Phe-based gel, which turned out to be competitive with other gel-base sorbent systems, with a value of 1930 mg g−1 reached at a high concentration of rhodamine B (479 mg L−1). Moreover, when the gel was loaded on the column to decolorize flowing solutions, a removal efficiency of 85% could be achieved in only 10 min allowing its reuse for at least 4 cycles. This pioneering work demonstrated that sorbent materials based on supramolecular eutectogels are extremely promising. First, they have very low environmental impact, being entirely made of biobased components. Second, they can be recycled without intermediate washing, significantly decreasing the amount of waste generated from the whole process.
Outlook and future perspectives
The field of eutectogels has boomed in the past few years, greatly expanding the scope of soft-solid materials. We have overviewed the research status on supramolecular eutectogels, forming a self-assembling LMWG. Our analysis covers the recent literature from 2018 to 2023, and we have comprehensively discussed the current status in terms of preparation and applications of eutectogels.We illustrated the use of LMWGs as a simple, cost-effective, and environmentally friendly method for preparing supramolecular eutectogels with good temperature stability, conductivity, stretchability, and moderate mechanical strength. The main advantage of these soft materials lies in their biobased nature and straightforward preparation. Noteworthily, the possibility of using biobased chemicals for (D)ES formation and even as gelators provides exciting new perspectives in the design of green materials that fully embrace the principles of green chemistry and aligns with sustainability goals, including market and supply chain considerations.
We have addressed in detail two main research challenges where supramolecular eutectogels can make a difference in the near future. Other opportunities can be envisaged for the given eutectogel formulations:
(1) Sorbents in food safety and control: to monitor the contamination of food samples above the admitted maximum residue limits, it is crucial to develop effective methods to successfully separate and enrich the analytes before the instrumental analysis. (D)ESs have been variously combined with nanoparticles to form eutectogels used as sorptive materials in the environmentally safe extraction of antibiotics or pesticides from food samples.151,152 For LMWG-based eutectogels to be efficiently used as sorbents, it is first necessary to improve their mechanical properties. Indeed, eutectogels based on LMWGs are often difficult to manipulate and show poorer mechanical performance compared to other soft materials, as their non-covalent interactions make them relatively susceptible to breakdown.92 Hybrid sorptive materials can represent a viable option in this field. We foresee that LMWG-based supramolecular gelators may be combined with covalently cross-linked (bio)polymers to design more complex eutectogel matrices suitable for solid–liquid (micro)extraction.
(2) Components in (flexible) electronic devices: there is nowadays a growing demand for wearable sensors in the fields of bionic robots, health monitoring, and artificial intelligence, as well as quasi-solid electrolytes for energy storage devices to eliminate safety hazards. The challenge in such applications is to ensure both a high ionic conductivity and good mechanical properties. Amazing progress has been made with DES-based gel polymer electrolytes for lithium metal batteries or supercapacitors, showing high ionic conductivity without sacrificing the mechanical properties.153,154 Also, the biocompatibility and tunable properties of eutectogels make them extremely promising in sensing systems. Hydrophilic DESs have been incorporated in polymeric organohydrogels to develop soft human-motion sensors with balanced tensile strength, toughness and conductivity,155 while hydrophobic (D)ESs have been exploited to prepare eutectogels suitable for underwater sensing characterized by long-term underwater adhesion capability, high transparency and excellent biocompatibility.156,157 Compared to polymeric eutectogels, the overall profile of state-of-the-art supramolecular eutectogels is still inadequate for such applications. (D)ESs have usually lower conductivity than conventional electrolyte solutions, and the conductivity of supramolecular eutectogels is typically lower than that of liquid (D)ESs. Moreover, eutectogels based on LMWGs have poor mechanical properties.92 Future efforts should focus on developing high-conductivity supramolecular eutectogels with enhanced strength and stretch. If current compositions might still be unsuitable for real applications, the possibility to adjust the physical and chemical properties of eutectogels by selecting both the nature and the ratio of the (D)ES constituents, as well as the nature and content of the gelator, makes it possible to obtain plenty of novel soft electronic materials. Noteworthily, LMWG-based supramolecular gelators have already been combined with covalently cross-linked polymers in the construction of supramolecular polymer double-network eutectogels. The use of two distinct, complementary scaffolds can indeed improve stretchability, self-healing, adhesion, electrical conductivity, and long-term stability.14,158
(3) Drug delivery systems: many (D)ES components are pharmaceutical active ingredients; suffice to mention here that menthol and camphor are the basic ingredients in topical formulations in the market such as Tiger Balm and Vicks VapoRub. Their various combinations with (bio)polymers can then be used to prepare a plethora of materials for the sustained and/or controlled release of both incorporated drugs159 and (D)ES components themselves.160 The inherent reversibility of LWMG-based eutectogels, together with the biocompatibility of most components and the high dissolution ability of (D)ESs, make supramolecular eutectogels extremely promising in this area. The need for a complete cytotoxicity profile of the final delivery system – as highlighted in the previous section – represents here a compulsory step. Also, some DES compositions contain volatile compounds which may compromise the stability and/or volatility of the obtained soft materials and require careful examination.
It is clear that supramolecular eutectogels hold great potential but many challenges have yet to be faced to make state-of-the-art materials adequate for specific applications. Possible future research thrusts to facilitate the improvement include:
(1) Expanding the library, considering both the design freedom of (D)ES and LMWGs: (D)ESs offer a high plasticity in terms of composition due to the multiple possible combinations of starting materials and molar ratios. Most of the eutectogels reported so far use ChCl as the hydrogen bond acceptor, but many other HBAs can be explored. In addition, most of the reported supramolecular eutectogels are based on hydrophilic (D)ESs, which easily absorb water, resulting in poor weather resistance and a reduced voltage window of the resulting eutectogels. The use of hydrophobic eutectogels has been introduced only recently105 and may represent an important breakthrough. The increasing knowledge of the role of different intermolecular interactions – H-bonds, dispersive interactions, and steric hindrance – contributing to the local structuration of hydrophobic (D)ESs broadens the spectrum of systems potentially obtainable.161
The gelator ingredient provides additional variety. To preserve one of the most relevant (D)ES merits, i.e. preparation from inexpensive natural, renewable or bio-sourced starting materials, researchers should look for biobased gelators. This would represent an important step forward for truly green soft materials. Moreover, the multicomponent strategy already applied to supramolecular gels,94–96 could be extended to supramolecular eutectogels as well. In this case, multiple LMWGs are combined to form a more complex functional gel with desired behavior.
(2) Establishing an in-depth fundamental understanding of the molecular structure, formation mechanism, intermolecular interactions, and structure–property relationship of supramolecular eutectogels: if progress has been made in the prediction of gelation behavior in conventional solvents,117 it would be crucial to develop robust basic knowledge on whether a gelator will be sufficiently robust to form self-standing gels in a given (D)ES. Moreover, understanding the relationship between a gelator and (D)ES and how these affect the macroscopic performance of the gel would be of extremely high value to provide important guidelines for the design of efficient supramolecular eutectogels for target applications.
(3) Investigating further the gelation process and evaluating strategies for shaping and structuring supramolecular eutectogels: supramolecular (eutecto)gels based on LMWGs show in general poor rheological performance and a limited ability to be shaped with respect to other soft systems, such as polymer gels. It is known that the properties of the gels are highly process dependent, as a change in the parameters used during gelation may lead to significantly different outcomes.95 Hence, understanding and fine-tuning the gelation process would make it possible to access materials with very different properties from those of a single gelator. Moreover, different fabrication methods exist to impose different shapes onto supramolecular gels and for patterning structures within them,93 and these could be fruitfully applied to (D)ES based supramolecular gels.
To conclude, although more research is needed in the future to explore the novel properties and new applications of supramolecular eutectogels, they show in general great potential as a new generation of soft green materials and the investigation of their potentialities should be continued.
Author contributions
Giselle de Araujo Lima e Souza: data curation, writing – original draft, writing – review & editing. Maria Enrica Di Pietro: conceptualization, visualization, supervision, writing – original draft, writing – review & editing. Andrea Mele: conceptualization, funding acquisition, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was carried out within the MICS (Made in Italy – Circular and Sustainable) Extended Partnership and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.3 – D.D. 1551.11-10-2022, PE00000004). This manuscript reflects only the authors' views and opinions, and neither the European Union nor the European Commission can be considered responsible for them. AM would like to thank Politecnico di Milano for granting a one-year sabbatical at Elettra Sincrotrone.Notes and references
- W. G. McGavock, J. M. Bryant and W. W. Wendlandt, Urea Complexes of Lithium Chloride, Science, 1956, 123, 897 CrossRef CAS PubMed
.
- M. Gambino, P. Gaune, M. Nabavian, M. Gaune-Escard and J. P. Bros, Enthalpie de fusion de l’uree et de quelques melanges eutectiques a base d’uree, Thermochim. Acta, 1987, 111, 37–47 CrossRef CAS
.
- F. Guthrie, On eutexia, Lond. Edinb. Dublin Philos. Mag. J. Sci., 1884, 17, 462–482 CrossRef
.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chem. Commun., 2003, 70–71 RSC
.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Deep Eutectic Solvents: A Review of Fundamentals and Applications, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed
.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed
.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: the quest for type v des, Chem. Commun., 2019, 55, 10253–10256 RSC
.
- J. Afonso, A. Mezzetta, I. M. Marrucho and L. Guazzelli, History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: the unbalanced turn of deep eutectic solvents, Green Chem., 2022, 25, 59–105 RSC
.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, Insights into the Nature of Eutectic and Deep Eutectic Mixtures, J. Solution Chem., 2019, 48, 962–982 CrossRef CAS
.
- J. Wang, S. Zhang, Z. Ma and L. Yan, Deep eutectic solvents eutectogels: progress and challenges, Green Chem. Eng., 2021, 2, 359–367 CrossRef
.
- Y. Nahar and S. C. Thickett, Greener, faster, stronger: The benefits of deep eutectic solvents in polymer and materials science, Polymers, 2021, 13, 1–24 CrossRef PubMed
.
- L. C. Tomé and D. Mecerreyes, Emerging Ionic Soft Materials Based on Deep Eutectic Solvents, J. Phys. Chem. B, 2020, 124, 8465–8478 CrossRef
.
- J. D. Mota-Morales, R. J. Sánchez-Leija, A. Carranza, J. A. Pojman, F. del Monte and G. Luna-Bárcenas, Free-radical polymerizations of and in deep eutectic solvents: green synthesis of functional materials, Prog. Polym. Sci., 2018, 78, 139–153 CrossRef CAS
.
- M. J. Panzer, Holding it together: noncovalent cross-linking strategies for ionogels and eutectogels, Mater. Adv., 2022, 3, 7709–7725 RSC
.
- B. O. Okesola and D. K. Smith, Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal, Chem. Soc. Rev., 2016, 45, 4226–4251 RSC
.
- J. Torregrosa-Crespo, X. Marset, G. Guillena, D. J. Ramón and R. María Martínez-Espinosa, New guidelines for testing “Deep eutectic solvents” toxicity and their effects on the environment and living beings, Sci. Total Environ., 2020, 704, 135382 CrossRef CAS PubMed
.
- M. Vieira Sanches, R. Freitas, M. Oliva, A. Mero, L. De Marchi, A. Cuccaro, G. Fumagalli, A. Mezzetta, G. Colombo Dugoni, M. Ferro, A. Mele, L. Guazzelli and C. Pretti, Are natural deep eutectic solvents
always a sustainable option? A bioassay-based study, Environ. Sci. Pollut. Res., 2023, 30, 17268–17279 CrossRef CAS PubMed
.
- T. Welton, Solvents and sustainable chemistry, Proc. R. Soc. A, 2015, 471, 20150502 CrossRef PubMed
.
- K. L. Wilson, J. Murray, H. F. Sneddon, K. M. P. Wheelhouse and A. J. B. Watson, Connecting the Dots: Method Development Using Sustainable Solvents, Chem, 2017, 3, 365–368 CAS
.
- R. A. Sheldon, Metrics of Green Chemistry and Sustainability: Past, Present, and Future, ACS Sustain. Chem. Eng., 2018, 6, 32–48 CrossRef CAS
.
- R. Kisanthia, A. J. Hunt, J. Sherwood, L. O. Somsakeesit and C. Phaosiri, Impact of Conventional and Sustainable Solvents on the Yield, Selectivity, and Recovery of Curcuminoids from Turmeric, ACS Sustain. Chem. Eng., 2022, 10, 104–114 CrossRef CAS
.
- B. Kopilovic, A. I. Valente, A. M. Ferreira, M. R. Almeida, A. P. M. Tavares, M. G. Freire and J. A. P. Coutinho, Sustainability Towards the sustainable extraction and puri fi cation of non-animal proteins from biomass using alternative solvents, RSC Sustainability, 2023, 1, 1314–1331 RSC
.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef
.
-
P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press Inc., Oxford, 1998 Search PubMed
.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, Analytical Eco-Scale for assessing the greenness of analytical procedures, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef
.
- J. Płotka-Wasylka, A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index, Talanta, 2018, 181, 204–209 CrossRef PubMed
.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, AGREE - Analytical GREEnness Metric Approach and Software, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed
.
- M. Sajid and J. Płotka-Wasylka, Green analytical chemistry metrics: A review, Talanta, 2022, 238, 123046 CrossRef CAS PubMed
.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, AGREEprep – analytical greenness metric for sample preparation, TrAC, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS
.
- P. Marion, B. Bernela, A. Piccirilli, B. Estrine, N. Patouillard, J. Guilbot and F. Jérôme, Sustainable chemistry: how to produce better and more from less?, Green Chem., 2017, 19, 4973–4989 RSC
.
-
United Nations, Sustainable Development Goals, https://www.un.org/sustainabledevelopment/ Search PubMed
.
-
United Nations, Sustainable Development Goals: Communication Materials, https://www.un.org/sustainabledevelopment/news/communications-material Search PubMed
.
- T. L. Chen, H. Kim, S. Y. Pan, P. C. Tseng, Y. P. Lin and P. C. Chiang, Implementation of green chemistry principles in circular economy system towards sustainable development goals: challenges and perspectives, Sci. Total Environ., 2020, 716, 136998 CrossRef CAS PubMed
.
- J. E. Wissinger, A. Visa, B. B. Saha, S. A. Matlin, P. G. Mahaffy, K. Kümmerer and S. Cornell, Integrating Sustainability into Learning in Chemistry, J. Chem. Educ., 2021, 98, 1061–1063 CrossRef CAS
.
- P. M. Nowak and P. Kościelniak, What color is your method? Adaptation of the RGB additive color model to analytical method evaluation, Anal. Chem., 2019, 91, 10343–10352 CrossRef CAS PubMed
.
- P. M. Nowak, R. Wietecha-Posłuszny and J. Pawliszyn, White Analytical Chemistry: an approach to reconcile the principles of Green Analytical Chemistry and functionality, TrAC, Trends Anal. Chem., 2021, 138, 116223 CrossRef CAS
.
- R. Marcinkowska, J. Namieśnik and M. Tobiszewski, Green and equitable analytical chemistry, Curr. Opin. Green Sustainable Chem., 2019, 19, 19–23 CrossRef
.
- A. A. Quintana, A. M. Sztapka, V. d. C. Santos Ebinuma and C. Agatemor, Enabling Sustainable Chemistry with Ionic Liquids and Deep Eutectic Solvents: A Fad or the Future?, Angew. Chem., Int. Ed., 2022, 61, e202205609 CrossRef CAS PubMed
.
- S. Ražić, J. Arsenijević, S. Đogo Mračević, J. Mušović and T. Trtić-Petrović, Greener chemistry in analytical sciences: from green solvents to applications in complex matrices. Current challenges and future perspectives: a critical review, Analyst, 2023, 3130–3152 RSC
.
- M. H. Chakrabarti, F. S. Mjalli, I. M. Alnashef, M. A. Hashim, M. A. Hussain, L. Bahadori and C. T. J. Low, Prospects of applying ionic liquids and deep eutectic solvents for renewable energy storage by means of redox flow batteries, Renewable Sustainable Energy Rev., 2014, 30, 254–270 CrossRef CAS
.
- M. Malik, K. L. Ng and G. Azimi, Physicochemical characterization of AlCl3-urea ionic liquid analogs: speciation, conductivity, and electrochemical stability, Electrochim. Acta, 2020, 354, 136708 CrossRef CAS
.
- D. Dylong, P. J. C. Hausoul, R. Palkovits and M. Eisenacher, Synthesis of (−)-menthol: industrial synthesis routes and recent development, Flavour Fragrance J., 2022, 37, 195–209 CrossRef CAS
.
- E. L. Smith, Deep eutectic solvents (DESs) and the metal finishing industry: where are they now?, Trans. Inst. Met. Finish., 2013, 91, 241–248 CrossRef CAS
.
- C. A. Nkuku and R. J. LeSuer, Electrochemistry in deep eutectic solvents, J. Phys. Chem. B, 2007, 111, 13271–13277 CrossRef CAS PubMed
.
- S. J. Bryant, R. Atkin and G. G. Warr, Effect of Deep Eutectic Solvent Nanostructure on Phospholipid Bilayer Phases, Langmuir, 2017, 33, 6878–6884 CrossRef CAS PubMed
.
- Y. P. Mbous, M. Hayyan, W. F. Wong, A. Hayyan, C. Y. Looi and M. A. Hashim, Simulation of Deep Eutectic Solvents' Interaction with Membranes of Cancer Cells Using COSMO-RS, J. Phys. Chem. B, 2020, 124, 9086–9094 CrossRef CAS PubMed
.
- J. L. Trenzado, C. Benito, M. Atilhan and S. Aparicio, Hydrophobic Deep eutectic Solvents based on cineole and organic acids, J. Mol. Liq., 2023, 377, 121322 CrossRef CAS
.
- Y. Chen, D. Yu, W. Chen, L. Fu and T. Mu, Water absorption by deep eutectic solvents, Phys. Chem. Chem. Phys., 2019, 21, 2601–2610 RSC
.
- O. S. Hammond, D. T. Bowron and K. J. Edler, The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed
.
- M. E. Di Pietro, O. Hammond, A. van den Bruinhorst, A. Mannu, A. Padua, A. Mele and M. Costa Gomes, Connecting chloride solvation with hydration in deep eutectic systems, Phys. Chem. Chem. Phys., 2021, 23, 107–111 RSC
.
- C. L. Boldrini, N. Manfredi, F. M. Perna, V. Capriati and A. Abbotto, Designing Eco-Sustainable Dye-Sensitized Solar Cells by the Use of a Menthol-Based Hydrophobic Eutectic Solvent as an Effective Electrolyte Medium, Chem. –Eur. J., 2018, 24, 17656–17659 CrossRef CAS PubMed
.
- C. Florindo, L. C. Branco and I. M. Marrucho, Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents, ChemSusChem, 2019, 12, 1549–1559 CrossRef CAS PubMed
.
- J. W. Steed, Supramolecular gel chemistry: developments over the last decade, Chem. Commun., 2011, 47, 1379–1383 RSC
.
- B. Joos, T. Vranken, W. Marchal, M. Safari, M. K. Van Bael and A. T. Hardy, Eutectogels: A New Class of Solid Composite Electrolytes for Li/Li-Ion Batteries, Chem. Mater., 2018, 30, 655–662 CrossRef CAS
.
- V. Selvanathan, A. D. Azzahari, A. A. Adyani and R. Yahya, Ternary natural deep eutectic solvent (NADES) infused phthaloyl starch as cost efficient quasi-solid gel polymer electrolyte, Carbohydr. Polym., 2017, 167, 210–218 CrossRef CAS PubMed
.
- S. Ramesh, R. Shanti and E. Morris, Studies on the plasticization efficiency of deep eutectic solvent in suppressing the crystallinity of corn starch based polymer electrolytes, Carbohydr. Polym., 2012, 87, 701–706 CrossRef CAS PubMed
.
- V. K. Vorobiov, M. A. Smirnov, N. V. Bobrova and M. P. Sokolova, Chitosan-supported deep eutectic solvent as bio-based electrolyte for flexible supercapacitor, Mater. Lett., 2021, 283, 128889 CrossRef CAS
.
- J. Depoorter, A. Mourlevat, G. Sudre, I. Morfin, K. Prasad, A. Serghei, J. Bernard, E. Fleury and A. Charlot, Fully Biosourced Materials from Combination of Choline Chloride-Based Deep Eutectic Solvents and Guar Gum, ACS Sustain. Chem. Eng., 2019, 7, 16747–16756 CrossRef CAS
.
- M. A. Smirnov, A. L. Nikolaeva, V. K. Vorobiov, N. V. Bobrova, I. V. Abalov, A. V. Smirnov and M. P. Sokolova, Ionic conductivity and structure of chitosan films modified with lactic acid-choline chloride NADES, Polymers, 2020, 12, 350 CrossRef CAS PubMed
.
- M. L. Picchio, A. Gallastegui, N. Casado, N. Lopez-Larrea, B. Marchiori, I. del Agua, M. Criado-Gonzalez, D. Mantione, R. J. Minari and D. Mecerreyes, Mixed Ionic and Electronic Conducting Eutectogels for 3D-Printable Wearable Sensors and Bioelectrodes, Adv. Mater. Technol., 2022, 7, 2101680 CrossRef CAS
.
- H. Qin, R. E. Owyeung, S. R. Sonkusale and M. J. Panzer, Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing, J. Mater. Chem. C, 2019, 7, 601–608 RSC
.
- C. Zeng, H. Zhao, Z. Wan, Q. Xiao, H. Xia and S. Guo, Highly biodegradable, thermostable eutectogels prepared by gelation of natural deep eutectic solvents using xanthan gum: preparation and characterization, RSC Adv., 2020, 10, 28376–28382 RSC
.
- L. N. Sim, R. Yahya and A. K. Arof, Infrared studies of polyacrylonitrile-based polymer electrolytes incorporated with lithium bis(trifluoromethane)sulfonimide and urea as deep eutectic solvent, Opt. Mater., 2016, 56, 140–144 CrossRef CAS
.
- B. Joos, J. Volders, R. R. Da Cruz, E. Baeten, M. Safari, M. K. Van Bael and A. T. Hardy, Polymeric Backbone Eutectogels as a New Generation of Hybrid Solid-State Electrolytes, Chem. Mater., 2020, 32, 3783–3793 CrossRef CAS
.
- S. Hong, Y. Yuan, C. Liu, W. Chen, L. Chen, H. Lian and H. Liimatainen, A stretchable and compressible ion gel based on a deep eutectic solvent applied as a strain sensor and electrolyte for supercapacitors, J. Mater. Chem. C, 2020, 8, 550–560 RSC
.
- C. Mukesh, K. K. Upadhyay, R. V. Devkar, N. A. Chudasama, G. G. Raol and K. Prasad, Preparation of a Noncytotoxic Hemocompatible Ion Gel by Self-Polymerization of HEMA in a Green Deep Eutectic Solvent, Macromol. Chem. Phys., 2016, 217, 1899–1906 CrossRef CAS
.
- J. Wang, Z. Ma, Y. Wang, J. Shao and L. Yan, Ultra-Stretchable, Self-Healing, Conductive, and Transparent PAA/DES Ionic Gel, Macromol. Rapid Commun., 2021, 42, 1–5 Search PubMed
.
- Á. Miguel, N. García, V. Gregorio, A. López-Cudero and P. Tiemblo, Tough polymer gel electrolytes for aluminum secondary batteries based on urea: AlCl3, prepared by a new solvent-free and scalable procedure, Polymers, 2020, 12, 1336–1349 CrossRef PubMed
.
- K. Wang, H. Wang, J. Li, Y. Liang, X. Q. Xie, J. Liu, C. Gu, Y. Zhang, G. Zhang and C. Sen Liu, Super-stretchable and extreme temperature-tolerant supramolecular-polymer double-network eutectogels with ultrafast: In situ adhesion and flexible electrochromic behaviour, Mater. Horiz., 2021, 8, 2520–2532 RSC
.
- S. Wang, H. Cheng, B. Yao, H. He, L. Zhang, S. Yue, Z. Wang and J. Ouyang, Self-adhesive, stretchable, biocompatible, and conductive nonvolatile eutectogels as wearable conformal strain and pressure sensors and biopotential electrodes for precise health monitoring, ACS Appl. Mater. Interfaces, 2021, 13, 20735–20745 CrossRef CAS PubMed
.
- H. Qin and M. J. Panzer, Chemically Cross-Linked Poly(2-hydroxyethyl methacrylate)-Supported Deep Eutectic Solvent Gel Electrolytes for Eco-Friendly Supercapacitors, ChemElectroChem, 2017, 4, 2556–2562 CrossRef CAS
.
- E. J. Gachuz, M. Castillo-Santillán, K. Juarez-Moreno, J. Maya-Cornejo, A. Martinez-Richa, A. Andrio, V. Compañ and J. D. Mota-Morales, Electrical conductivity of an all-natural and biocompatible semi-interpenetrating polymer network containing a deep eutectic solvent, Green Chem., 2020, 22, 5785–5797 RSC
.
- J. Wang, Y. Deng, Z. Ma, Y. Wang, S. Zhang and L. Yan, Lignin promoted the fast formation of a robust and highly conductive deep eutectic solvent ionic gel at room temperature for a flexible quasi-solid-state supercapacitor and strain sensors, Green Chem., 2021, 23, 5120–5128 RSC
.
- C. Mukesh, R. Gupta, D. N. Srivastava, S. K. Nataraj and K. Prasad, Preparation of a natural deep eutectic solvent mediated self polymerized highly flexible transparent gel having super capacitive behaviour, RSC Adv., 2016, 6, 28586–28592 RSC
.
- R. Luo, H. Jiang, B. Du, S. Zhou and Y. Zhu, Preparation and application of solid polymer electrolyte based on deep eutectic solvent, AIP Adv., 2019, 9, 035341 CrossRef
.
- M. J. Deng, T. H. Chou, L. H. Yeh, J. M. Chen and K. T. Lu, 4.2 V wearable asymmetric supercapacitor devices based on a VOx//MnOx paper electrode and an eco-friendly deep eutectic solvent-based gel electrolyte, J. Mater. Chem. A, 2018, 6, 20686–20694 RSC
.
- M. W. Logan, S. Langevin, B. Tan, A. W. Freeman, C. Hoffman, D. B. Trigg and K. Gerasopoulos, UV-cured eutectic gel polymer electrolytes for safe and robust Li-ion batteries, J. Mater. Chem. A, 2020, 8, 8485–8495 RSC
.
- A. M. Navarro-Suárez and P. Johansson, Perspective—Semi-Solid Electrolytes Based on Deep Eutectic Solvents: Opportunities and Future Directions, J. Electrochem. Soc., 2020, 167, 070511 CrossRef
.
- S. Bednarz, M. Fluder, M. Galica, D. Bogdal and I. Maciejaszek, Synthesis of hydrogels by polymerization of itaconic acid-choline chloride deep eutectic solvent, J. Appl. Polym. Sci., 2014, 131, 1–8 CrossRef
.
- M. Isik, F. Ruiperez, H. Sardon, A. Gonzalez, S. Zulfi and D. Mecerreyes, Innovative Poly ( Ionic Liquid ) s by the Polymerization of Deep Eutectic Monomers, Macromol. Rapid Commun., 2016, 37, 1135–1142 CrossRef CAS PubMed
.
- L. Ren'Ai, K. Zhang, G. Chen, B. Su, J. Tian, M. He and F. Lu, Green polymerizable deep eutectic solvent (PDES) type conductive paper for origami 3D circuits, Chem. Commun., 2018, 54, 2304–2307 RSC
.
- J. D. Mota-Morales, M. C. Gutiérrez, I. C. Sanchez, G. Luna-Bárcenas and F. Del Monte, Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium, Chem. Commun., 2011, 47, 5328–5330 RSC
.
- S. Wierzbicki, K. Mielczarek, M. Topa-Skwarczyńska, K. Mokrzyński, J. Ortyl and S. Bednarz, Visible light-induced photopolymerization of Deep Eutectic Monomers, based on methacrylic acid and tetrabutylammonium salts with different anion structures, Eur. Polym. J., 2021, 161, 110836 CrossRef CAS
.
- A. R. Zarei, M. Nedaei and S. A. Ghorbanian, Application of deep eutectic solvent based magnetic colloidal gel for dispersive solid phase extraction of ultra-trace amounts of some nitroaromatic compounds in water samples, J. Mol. Liq., 2017, 246, 58–65 CrossRef CAS
.
- R. Wang, S. Zhang, Y. Su, J. Liu, Y. Ying, F. Wang and X. Cao, Semi-solid state electrochromic device based on deep eutectic solvent gel electrolyte and transparent Au modified FTO electrode, Electrochim. Acta, 2017, 258, 328–335 CrossRef CAS
.
- S. M. Yousefi, F. Shemirani and S. A. Ghorbanian, Deep eutectic solvent magnetic bucky gels in developing dispersive solid phase extraction: application for ultra trace analysis of organochlorine pesticides by GC-micro ECD using a large-volume injection technique, Talanta, 2017, 168, 73–81 CrossRef CAS PubMed
.
- J. Ruiz-Olles, P. Slavik, N. K. Whitelaw and D. K. Smith, Self-Assembled Gels Formed in Deep Eutectic Solvents: Supramolecular Eutectogels with High Ionic Conductivity, Angew. Chem., Int. Ed., 2019, 58, 4173–4178 CrossRef CAS PubMed
.
- C. Florindo, L. G. Celia-Silva, L. F. G. Martins, L. C. Branco and I. M. Marrucho, Supramolecular hydrogel based on a sodium deep eutectic solvent, Chem. Commun., 2018, 54, 7527–7530 RSC
.
- J. Wu and T. Yin, Amphiphilic Deep Eutectic Solvent Based on Lidocaine and Lauric Acid: Formation of Microemulsion and Gel, Langmuir, 2022, 38, 1170–1177 CrossRef CAS PubMed
.
- R. G. Weiss, The past, present, and future of molecular gels. What is the status of the field, and where is it going?, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed
.
- A. Dawn, T. Shiraki, S. Haraguchi, S. I. Tamaru and S. Shinkai, What kind of ‘soft materials’ can we design from molecular gels?, Chem. – Asian J., 2011, 6, 266–282 CrossRef CAS PubMed
.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Supramolecular materials, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC
.
- P. R. A. Chivers and D. K. Smith, Shaping and structuring supramolecular gels, Nat. Rev. Mater., 2019, 4, 463–478 CrossRef CAS
.
- E. R. Draper and D. J. Adams, How should multicomponent supramolecular gels be characterised?, Chem. Soc. Rev., 2018, 47, 3395–3405 RSC
.
- E. R. Draper and D. J. Adams, Low-Molecular-Weight Gels: The State of the Art, Chem, 2017, 3, 390–410 CAS
.
- A. J. Savyasachi, O. Kotova, S. Shanmugaraju, S. J. Bradberry, G. M. Ó'Máille and T. Gunnlaugsson, Supramolecular Chemistry: A Toolkit for Soft Functional Materials and Organic Particles, Chem, 2017, 3, 764–811 CAS
.
- S. Panja and D. J. Adams, Stimuli responsive dynamic transformations in supramolecular gels, Chem. Soc. Rev., 2021, 50, 5165–5200 RSC
.
- K. Zhi, H. Zhao, X. Yang, H. Zhang, J. Wang, J. Wang and J. M. Regenstein, Natural product gelators and a general method for obtaining them from organisms, Nanoscale, 2018, 10, 3639–3643 RSC
.
- S. Marullo, A. Meli, F. Giannici and F. D'Anna, Supramolecular Eutecto Gels: Fully Natural Soft Materials, ACS Sustain. Chem. Eng., 2018, 6, 12598–12602 CrossRef CAS
.
- B. Saavedra, A. Meli, C. Rizzo, D. J. Ramón and F. D'Anna, Natural eutectogels: Sustainable catalytic systems for C–C bond formation reactions, Green Chem., 2021, 23, 6555–6565 RSC
.
- Q. Cheng, A. Hao and P. Xing, Eutectogels as Matrices to Manipulate Supramolecular Chirality and Circularly Polarized Luminescence, ACS Nano, 2022, 16, 6825–6834 CrossRef CAS PubMed
.
- M. Criado-Gonzalez, N. Alegret, A. M. Fracaroli, D. Mantione, G. Guzmán-gonzález, R. Del Olmo, K. Tashiro, L. C. Tomé, M. L. Picchio and D. Mecerreyes, Mixed Conductive, Injectable, and Fluorescent Supramolecular Eutectogel Composites, Angew. Chem., Int. Ed., 2023, 62, e202301489 CrossRef CAS PubMed
.
- S. Marullo, A. Meli, N. T. Dintcheva, G. Infurna, C. Rizzo and F. D'anna, Environmentally friendly eutectogels comprising l-amino
acids and deep eutectic solvents: efficient materials for wastewater treatment, Chempluschem, 2020, 85, 301–311 CrossRef CAS PubMed
.
- K. Fan, L. Wang, W. Wei, F. Wen, Y. Xu, X. Zhang and X. Guan, Multifunctional self-healing eutectogels induced by supramolecular assembly for smart conductive materials, interface lubrication and dye adsorption, Chem. Eng. J., 2022, 441, 136026–136034 CrossRef CAS
.
- G. de Araujo Lima e Souza, M. E. Di Pietro, V. Vanoli, W. Panzeri, F. Briatico-Vangosa, F. Castiglione and A. Mele, Hydrophobic eutectogels: a new outfit for non-ionic eutectic solvents, Mater. Today Chem., 2023, 29, 101402–101408 CrossRef CAS
.
- B. Zhang, H. Sun, Y. Huang, B. Zhang, F. Wang and J. Song, Multifunctional supramolecular eutectogels for self-healable conductive materials and interface lubrication, Chem. Eng. J., 2021, 425, 131518–131528 CrossRef CAS
.
- C. Gu, Y. Peng, J. Li, H. Wang, X. Q. Xie, X. Cao and C. Sen Liu, Supramolecular G4 Eutectogels of Guanosine with Solvent-Induced Chiral Inversion and Excellent Electrochromic Activity, Angew. Chem., Int. Ed., 2020, 59, 18768–18773 CrossRef CAS PubMed
.
- P. Qi, L. Jia, M. Yi, E. Zhao, Y. Liu, A. Song and J. Hao, Chemiluminescent gels of G-quadruplexes in deep eutectic solvents, Colloids Surf., A, 2022, 655, 130319–130327 CrossRef CAS
.
- F. Delbecq, P. Delfosse, G. Laboureix, C. Paré and T. Kawai, Study of a gelated Deep Eutectic solvent metal salt solution as template for the production of size-controlled small noble metal nanoparticles, Colloids Surf., A, 2019, 567, 55–62 CrossRef CAS
.
- Y. Zhang, H. Wang, Q. Li and X. Chen, Gelation behavior and supramolecular chirality of a BTA derivative in a deep eutectic solvent, Soft Matter, 2022, 18, 3241–3248 RSC
.
- C. N. Prieto Kullmer, D. Ta, C. Y. Chen, C. J. Cieker, O. Annunziata and S. V. Dzyuba, Hexadecyl-Containing Organic Salts as Novel Organogelators for Ionic, Eutectic, and Molecular Liquids, ACS Omega, 2019, 4, 9400–9406 CrossRef CAS PubMed
.
- N. Parsana, S. Kumar, V. K. Aswal, O. El Seoud and N. I. Malek, Self-Healable , Injectable , and Conductive Supramolecular Eutectogel for the Encapsulation and Sustained Release of the Anticancer Drug Curcumin, ACS Appl. Eng. Mater., 2023, 1, 380–393 CrossRef CAS
.
- Y. Liang, K. Wang, J. Li, Y. Zhang, J. Liu, K. Zhang, Y. Cui, M. Wang and C. Sen Liu, Low-molecular-weight supramolecular adhesives based on non-covalent self-assembly of a small molecular gelator, Mater. Horiz., 2022, 9, 1700–1707 RSC
.
- L. Matthews, S. Ruscigno, S. E. Rogers, P. Bartlett, A. J. Johnson, R. Sochon and W. H. Briscoe, Fracto-eutectogels: SDS fractal dendritesviacounterion condensation in a deep eutectic solvent, Phys. Chem. Chem. Phys., 2021, 23, 11672–11683 RSC
.
- S. Marullo, M. Tiecco, R. Germani and F. D'Anna, Highly recyclable surfactant-based supramolecular eutectogels for iodine removal, J. Mol. Liq., 2022, 362, 119712 CrossRef CAS
.
- M. Sun, Q. Li and X. Chen, Self-assembled luminescent cholate gels induced by a europium ion in deep eutectic solvents, Soft Matter, 2021, 17, 2815–2822 RSC
.
- Y. Lan, M. G. Corradini, X. Liu, T. E. May, F. Borondics, R. G. Weiss and M. A. Rogers, Comparing and correlating solubility parameters governing the self-assembly of molecular gels using 1,3:2,4-dibenzylidene sorbitol as the gelator, Langmuir, 2014, 30, 14128–14142 CrossRef CAS PubMed
.
- B. O. Okesola, V. M. P. Vieira, D. J. Cornwell, N. K. Whitelaw and D. K. Smith, 1,3:2,4-Dibenzylidene-d-sorbitol (DBS) and its derivatives-efficient, versatile and industrially-relevant low-molecular-weight gelators with over 100 years of history and a bright future, Soft Matter, 2015, 11, 4768–4787 RSC
.
- K. Van Aken, L. Strekowski and L. Patiny, EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters, Beilstein J. Org. Chem., 2006, 2, 1–7 RSC
.
- G. M. Peters and J. T. Davis, Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs, Chem. Soc. Rev., 2016, 45, 3188–3206 RSC
.
- P. J. M. Stals, P. A. Korevaar, M. A. J. Gillissen, T. F. A. De Greef, C. F. C. Fitié, R. P. Sijbesma, A. R. A. Palmans and E. W. Meijer, Symmetry breaking in the self-assembly of partially fluorinated benzene-1,3,5-tricarboxamides, Angew. Chem., Int. Ed., 2012, 51, 11297–11301 CrossRef CAS PubMed
.
- N. Nuraje, H. Bai and K. Su, Bolaamphiphilic molecules: Assembly and applications, Prog. Polym. Sci., 2013, 38, 302–343 CrossRef CAS
.
- M. McLaughlin, M. A. Gilea, M. J. Earle, K. R. Seddon, B. F. Gilmore and S. A. Kelly, Characterization of ionic liquid cytotoxicity mechanisms in human keratinocytes compared with conventional biocides, Chemosphere, 2021, 270, 129432 CrossRef CAS PubMed
.
- T. E. Graedel, E. M. Harper, N. T. Nassar and B. K. Reck, On the materials basis of modern society, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6295–6300 CrossRef CAS PubMed
.
- R. Dominko, J. Bitenc, R. Berthelot, M. Gauthier, G. Pagot and V. Di Noto, Magnesium batteries: current picture and missing pieces of the puzzle, J. Power Sources, 2020, 478, 229027 CrossRef CAS
.
- J. Neumann, M. Petranikova, M. Meeus, J. D. Gamarra, R. Younesi, M. Winter and S. Nowak, Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling, Adv. Energy Mater., 2022, 12, 2102917 CrossRef CAS
.
- K. Binnemans and P. T. Jones, Solvometallurgy: An Emerging Branch of Extractive Metallurgy, J. Sustain. Metall., 2017, 3, 570–600 CrossRef
.
- G. J. P. Deblonde, A. Chagnes and G. Cote, Recent Advances in the Chemistry of Hydrometallurgical Methods, Sep. Purif. Rev., 2023, 52, 221–241 CrossRef
.
- A. Zhu, X. Bian, W. Han, D. Cao, Y. Wen, K. Zhu and S. Wang, The application of deep eutectic solvents in lithium-ion battery recycling: a comprehensive review, Resour., Conserv. Recycl., 2023, 188, 106690 CrossRef CAS
.
- B. Li, Q. Li, Q. Wang, X. Yan, M. Shi and C. Wu, Deep eutectic solvent for spent lithium-ion battery recycling: comparison with inorganic acid leaching, Phys. Chem. Chem. Phys., 2022, 24, 19029–19051 RSC
.
- M. Jafari, S. Z. Shafaie, H. Abdollahi and A. Entezari-Zarandi, Green recycling of spent Li-ion batteries by deep eutectic solvents (DESs): Leaching mechanism and effect of ternary DES, J. Environ. Chem. Eng., 2022, 10, 109014 CrossRef CAS
.
- D. J. G. P. Van Osch, D. Parmentier, C. H. J. T. Dietz, A. Van Den Bruinhorst, R. Tuinier and M. C. Kroon, Removal of alkali and transition metal ions from water with hydrophobic deep eutectic solvents, Chem. Commun., 2016, 52, 11987–11990 RSC
.
- E. E. Tereshatov, M. Y. Boltoeva and C. M. Folden, First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: indium extraction from hydrochloric and oxalic acids, Green Chem., 2016, 18, 4616–4622 RSC
.
- T. Hanada and M. Goto, Synergistic Deep Eutectic Solvents for Lithium Extraction, ACS Sustain. Chem. Eng., 2021, 9, 2152–2160 CrossRef CAS
.
-
European Commission, Water, https://environment.ec.europa.eu/topics/water_en Search PubMed
.
-
European Commission, Impact Assessment Report, https://environment.ec.europa.eu/system/files/2022-10/StaffWorkingDocumentExecutiveSummaryoftheImpactAssessmentReportaccompanyingtheProposal.pdf Search PubMed
.
-
European Commission, Cluster 6: Food, Bioeconomy, Natural Resources, Agriculture and Environment, https://research-and-innovation.ec.europa.eu/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe/cluster-6-food-bioeconomy-natural-resources-agriculture-and-environment_en Search PubMed
.
-
European Commission, Zero Pollution Action Plan, https://environment.ec.europa.eu/strategy/zero-pollution-action-plan_en Search PubMed
.
-
European Commission, Chemical Strategy for Sustainability, https://environment.ec.europa.eu/strategy/chemicals-strategy_en Search PubMed
.
- M. Rezaee, Y. Assadi, M. R. Milani Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, Determination of organic compounds in water using dispersive liquid-liquid microextraction, J. Chromatogr. A, 2006, 1116, 1–9 CrossRef CAS PubMed
.
- M. Sajid, Dispersive liquid-liquid microextraction: Evolution in design, application areas, and green aspects, TrAC, Trends Anal. Chem., 2022, 152, 116636 CrossRef CAS
.
- G. Li and K. H. Row, Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction, TrAC, Trends Anal. Chem., 2019, 120, 115651–115666 CrossRef CAS
.
- M. Rutkowska, J. Płotka-Wasylka, M. Sajid and V. Andruch, Liquid–phase microextraction: a review of reviews, Microchem. J., 2019, 149, 103989 CrossRef CAS
.
- European Environment Agency, WFD Reporting Guidance 2022 Search PubMed.
- P. Makoś, E. Słupek and J. Gębicki, Hydrophobic deep eutectic solvents in microextraction techniques–a review, Microchem. J., 2020, 152, 104384 CrossRef
.
- Y. Wang, N. Li, S. Jiang and X. Chen, Application of extraction and determination based on deep eutectic solvents in different types of environmental samples, Water, 2022, 14, 46–60 CrossRef CAS
.
- C. M. Chabib, J. K. Ali, M. A. Jaoude, E. Alhseinat, I. A. Adeyemi and I. M. Al Nashef, Application of deep eutectic solvents in water treatment processes: a review, J. Water Process. Eng., 2022, 47, 102663 CrossRef
.
- L. Nakhle, M. Kfoury, I. Mallard, D. Landy and H. Greige-Gerges, Microextraction of bioactive compounds using deep eutectic solvents: a review, Environ. Chem. Lett., 2021, 19, 3747–3759 CrossRef CAS
.
- M. S. Jagirani and M. Soylak, Deep eutectic solvents-based adsorbents in environmental analysis, TrAC, Trends Anal. Chem., 2022, 157, 116762 CrossRef CAS
.
- J. Werner, T. Grześkowiak and A. Zgoła-Grześkowiak, A polydimethylsiloxane/deep eutectic solvent sol-gel thin film sorbent and its application to solid-phase microextraction of parabens, Anal. Chim. Acta, 2022, 1202, 339666 CrossRef CAS PubMed
.
- M. Shirani, M. Faraji, H. Rashidi Nodeh, B. Akbari-adergani and S. Sepahi, An efficient deep eutectic magnetic nano gel for rapid ultrasound-assisted dispersive μ-solid phase extraction of residue of tetracyclines in food samples, J. Food Sci. Technol., 2023, 60, 2802–2812 CrossRef CAS PubMed
.
- Z. Asghari, H. Sereshti, S. Soltani, M. Taghizadeh, S. Karami, M. Esmaeili Bidhendi and S. Rezania, An alginate-based eutectogel impregnated with polyvinylpyrrolidone/benzoic acid deep eutectic solvent and magnetic carboxylated multiwalled carbon nanotubes: Evaluated as sorbent in green microextraction of pesticides, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2023, 1229, 123865 CrossRef CAS PubMed
.
- S. Yang, X. He, T. Hu, Y. He, S. Lv, Z. Ji, Z. Zhu, X. Fu, W. Yang, Y. Wang and A. Supertough, Nonflammable, Biomimetic Gel with Neuron-Like Nanoskeleton for Puncture-Tolerant Safe Lithium Metal Batteries, Adv. Funct. Mater., 2023, 2304727, 1–12 Search PubMed
.
- S. He, H. Ren, Y. Chen, D. Rong and Q. Rong, Full-device stretchable supercapacitors with superior thermal and self-healing stability based on recyclable polymeric eutectogels, J. Energy Storage, 2023, 72, 108619 CrossRef
.
- Y. Liang, D. Zou, Y. Zhang and Z. Zhong, Indirect method for preparing dual crosslinked eutectogels with high strength, stretchability, conductivity and rapid self-recovery capability as flexible and freeze-resistant strain sensors, Chem. Eng. J., 2023, 475, 145928 CrossRef CAS
.
- D. Du, J. Zhou, T. Kaneko, W. Dong, M. Chen and D. Shi, Stretchable and hydrophobic eutectogel for underwater human health monitoring based on hierarchical dynamic interactions, Chem. Eng. J., 2023, 474, 145704 CrossRef CAS
.
- X. Zhang, S. Liu, X. Wang, J. Peng, W. Yang, Y. Ma and K. Fan, Hydrophobic deep eutectic solvent-based eutectogels for underwater sensing, J. Colloid Interface Sci., 2024, 654, 1348–1355 CrossRef CAS PubMed
.
- H. Sun, B. Zhang, L. Lu, Z. Chen, Y. Huo, W. Li, B. Zhang and J. Song, D-gluconic acetal gelator-based supramolecular – Polymer dual network eutectogels for high performance temperature, strain, and pressure sensors, Chem. Eng. J., 2023, 451, 139051 CrossRef CAS
.
- S. N. Pedro, B. F. A. Valente, C. Vilela, H. Oliveira, A. Almeida, M. G. Freire, A. J. D. Silvestre and C. S. R. Freire, Switchable adhesive films of pullulan loaded with a deep eutectic solvent-curcumin formulation for the photodynamic treatment of drug-resistant skin infections, Mater. Today Bio, 2023, 22, 100733 CrossRef CAS PubMed
.
- A. Daou, S. M. Arakkal and A. M. Alkilany, Eutectics in Pharmacy Curriculum: A Simple Demonstration with Pharmaceutical Relevance, J. Chem. Educ., 2023, 100, 3533–3539 CrossRef CAS
.
- M. Busato, G. Mannucci, L. A. Rocchi, M. E. Di Pietro, A. Capocefalo, E. Zorzi, P. Casu, D. Veclani, F. Castiglione, A. Mele, A. Martinelli, P. Postorino and P. D'Angelo, The Complex Story Behind a Deep Eutectic Solvent Formation as Revealed by l-Menthol Mixtures with Butylated Hydroxytoluene Derivatives, ACS Sustain. Chem. Eng., 2023, 11, 8988–8999 CrossRef CAS
.
- L. Erdmann and T. E. Graedel, Criticality of non-fuel minerals: A review of major approaches and analyses, Environ. Sci. Technol., 2011, 45, 7620–7630 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00264k |
| This journal is © The Royal Society of Chemistry 2024 |