Defect passivation engineering of wide-bandgap perovskites for high-performance solar cells
Xiao
Wu†
a,
Guoqing
Xiong†
a,
Ziyao
Yue
a,
Ziyao
Dong
b and
Yuanhang
Cheng
 *a
*a
aSchool of New Energy, Nanjing University of Science and Technology, Jiangyin, Jiangsu 214443, China. E-mail: yhcheng@njust.edu.cn
bSchool of Energy and Environment, Anhui University of Technology, Maanshan, Anhui 243002, China
First published on 10th November 2023
Abstract
Wide-bandgap (WBG) mixed-halide perovskite solar cells (PVSCs) exhibit a wide range of applicability, such as tandem photovoltaics (PVs), underwater PVs, space PVs, and building-integrated photovoltaics (BIPVs). However, the state-of-the-art WBG mixed-halide PVSCs still suffer from phase segregation and large open-circuit voltage (Voc) loss, which significantly limit the overall power conversion efficiency of devices. A dominant source of these limitations is the presence of defects within the mixed-halide perovskite lattice structure and at interfaces between the perovskite and carrier transport layers (CTLs). In response, various device engineering strategies have been implemented to passivate the defects and improve device performance. Therefore, in this comprehensive review, different types of defects inherent in WBG mixed-halide perovskites were firstly described, followed by their detrimental effects on perovskite materials and corresponding device performance. Furthermore, several device engineering strategies to passivate the defects at perovskite buried interface, perovskite bulk, and perovskite surface had been summarized, respectively. These defect passivation schemes provided a forward-oriented perspective on forthcoming strategies for WBG mixed-halide PVSCs. These strategies not only offered valuable guidance for realizing enhanced efficiency but also improved the phase stability of WBG mixed-halide PVSCs in the pursuit of high-performance PV technology.
1 Introduction
The advancement of human civilization heavily relies on the utilization of energy resources.1 However, the conventional use of fossil fuels is not only finite, but environmentally deleterious. Therefore, in pursuit of sustainable and ecologically harmonious living, resorting to renewable energy sources, especially solar energy, has gained paramount significance.2,3 This shift has led to the prominence of solar cells, pivotal technologies capable of converting photons into electrons through the photovoltaic (PV) effect, heralding an era of enduring and ecologically conscious progress.4–7 Within the PV community, beyond the Si-based solar cells and thin-film solar cells that have been successfully commercialized, perovskite solar cells (PVSCs) have been unequivocally regarded as the most promising devices throughout the past decade. This distinction is attributed to their outstanding optoelectronic characteristics,8–10 simple low-temperature solution processing,11–13 and low-cost fabrication methods.14–16Perovskites represent a class of materials characterized by the ABX3 crystal structure. In metal halide perovskites, the optoelectronic materials utilized in PVSCs, the A-site can be occupied by CH3NH3+ (MA+), HC(NH2)2+ (FA+), or Cs+, while the B-site is typically filled with Pb2+ or Sn2+, and X denotes halide anions such as I−, Br−, and Cl−.17 The perovskite materials, CH3NH3PbI3 and CH3NH3PbBr3, were firstly introduced as solar absorbers in dye-sensitized solar cells (DSSCs) in 2009.18 However, the liquid-based electrolytes within DSSC systems result in their rapid degradation. The turning point came in 2012 with the introduction of solid-state hole transport layer (HTL), facilitating the rapid and unprecedented development of PVSCs in the last decade.19 To date, the certified power conversion efficiency (PCE) of perovskite-based single junction solar cells has reached 26.1%,20 approaching that of Si-based solar cells. This could be attributed to outstanding optoelectronic characteristics of the organic–inorganic metal halide perovskites, including high absorption coefficients,21 long carrier diffusion lengths,22 long carrier lifetime,23 low recombination rates,24 and high defect tolerance.25 Moreover, the bandgaps of perovskites can be tuned by simply changing the composition,26 expanding their applications wider than any other PV technologies. Especially for organic–inorganic mixed-halide perovskites with a bandgap greater than 1.65 eV, which are referred to as wide-bandgap (WBG) perovskites, exhibit even more tremendous potential in various PV applications.27
As shown in Fig. 1a, the WBG perovskites can be employed as top cells in perovskite/silicon and perovskite/perovskite tandem PVs, which can surpass the efficiency limit of single-junction solar cell (33%), pushing the theoretical PCE towards a value of 46%.28,29 As materials with bandgaps over 1.8 eV hold the potential for absorbing blue-to-yellow light, the WBG PVSCs can also be applied to underwater PVs,30 as shown in Fig. 1b. Fig. 1c shows that WBG PVSCs can serve as important candidate technologies for space solar cells,31 which have apparent tolerance to high-energy proton radiation. Moreover, the colorful appearance and flexibility of WBG PVSCs make them promising candidates for building-integrated photovoltaics (BIPVs) (Fig. 1d).32 The WBG perovskites not only exhibit excellent response to outdoor and indoor lighting but offer additional aesthetic advantages due to their semi-transparency and various color choices.
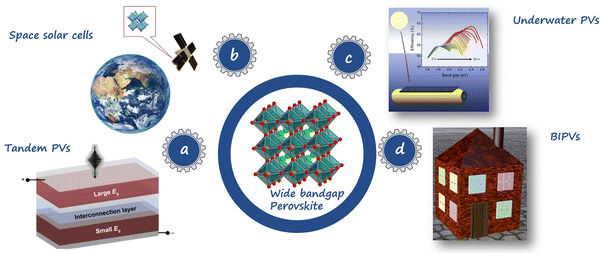 | ||
| Fig. 1 Various applications of WBG mixed-halide PVSCs, including (a) space PVs, (b) tandem PVs, (c) underwater PVs, and (d) BIPVs. Reproduced with permission from ref. 33. Copyright 2021, Springer Nature. Reproduced with permission from ref. 34. Copyright 2020, Elsevier. | ||
It has been demonstrated that the bandgap of metal halide perovskites primarily depends on the coupling between the B-site cation and the X-site anion.35 Reducing the lattice size of perovskites will increase the wavefunction overlap and thus result in a wider bandgap. Therefore, the WBG perovskites are generally obtained by replacing the iodine (I) ions with bromine (Br) ions.27,36 However, the state-of-the-art WBG mixed-halide PVSCs still suffer from issues of large open-circuit voltage (Voc) loss and phase segregation.27,37 Such issues limit the performance and applications of WBG perovskites, and the phase segregation caused by the migration of I and Br ions exacerbates the instability issue under illumination, making the degradation of WBG mixed-halide PVSCs even more rapid.38,39 A comprehensive understanding of the mechanisms behind these issues remains limited; however, the unavoidable generation of various defects during PVSCs fabrication processes is generally considered as one of the primary factors.27,40 On the one hand, defects act as non-radiative recombination centers, reducing the PCE of devices.41 On the other hand, defects act as transport channels for migration of ions, facilitating the phase segregation and causing the degradation of PVSCs.9,42
Both phase segregation and defects can contribute to instability in WBG perovskites.43 On the one hand, the migration of halide anions, facilitated by structural defects within the perovskite lattice, exerts a significant influence on phase segregation. Investigations have indicated that phase segregation predominantly manifests at grain boundaries, known for their heightened defect density compared to the grain interior.44 Therefore, it is suggested that defects are involved in initiating the phase segregation process.45 On the other hand, phase segregation will induce more defects in the perovskite lattice structure due to the migration of the ions. The emergence of these new defects accelerates the deterioration of device performance, thereby contributing to the instability of WBG mixed-halide perovskites.46 Nevertheless, the precise governing factor between phase segregation and defects in the context of WBG perovskite instability remains a topic of ongoing debate in the scientific community.
Despite that the whole picture of these issues is still uncovered, addressing the relevant part of defects can contribute to overall improvement of device performance. As a result, numerous defect passivation methods have been reported for achieving high-performance WBG mixed-halide PVSCs.47–58 Therefore, in this review, we firstly described different types of defects in WBG mixed-halide perovskites. Then, the impact of defects on the performances of both perovskite materials and devices has been discussed. Subsequently, passivation criteria along with strategies to passivate different types of defects are summarized, respectively. Finally, a forward-looking perspective on future passivation strategies for mixed-halide WBG PVSCs was provided, offering valuable guidance for the development of high-efficiency and phase-stable WBG mixed-halide PVSCs.
2 Defects in mixed-halide perovskites
The defects, namely the incomplete ion coordination within the perovskite materials, can form electron trap states within the bandgap, inducing the Voc loss and correspondingly lower PCEs.59 Defects primarily occur at perovskite interfaces and grain boundaries.27,60 Taking MAPbI3 as an example, the common defects include vacancy defects (VMA, VPb, and VI), interstitial defects (MAi, Pbi, and Ii), and antisite defects (MAPb, MAI, PbMA, PbI, IMA, and IPb).61In WBG perovskite materials, such as MAPbBr3 or MAPbI3−xBrx, both vacancies, interstitials, and antisite defects are also included. (1) For the vacancies, these defects occur when a lattice site within the crystal structure is unoccupied by an atom or ion. In WBG perovskites, vacancies can involve either the organic cation (e.g., VMA) or the halide anion (e.g., VI). These vacancies lead to the presence of uncoordinated lead or halogen ions within the crystal lattice.62 (2) For the interstitial defects, the interstitials refer to atoms or ions that occupy positions between the normal lattice sites. For WBG perovskites, interstitials can involve excess lead (e.g., Pbi), excess halogen (e.g., Ii or Bri), or excess organic cations (e.g., MAi) which disrupt the regular arrangement of atoms and introduce additional defect states.63 (3) For the antisite defects, they specifically arise when different types of atoms or ions exchange positions within the crystal lattice. In WBG perovskites, this can occur between the organic cations, lead cations and halide anions, such as MAI, MABr, IPb, BrPb and so on.64–66 Antisite defects can introduce energy levels within the bandgap, leading to trap states that degrade device performance.66
Owing to the ionic property of perovskite materials, as shown in Fig. 2a, defects are generally positively or negatively charged.67 The charged defects act as “traps” that can capture charge carriers of opposite polarity and thus induce non-radiative recombination, causing Voc loss and low PCEs in PV devices. Normally, the formation energies of vacancy defects are lower than those of interstitial defects, and the antisite defects have the highest formation energy.58,64,68–70 On the other hand, vacancy defects generally exhibit shallow energy levels, while most of interstitial and antisite defects are considered as deep-level defects that trigger the notorious non-radiative recombination in solar cells.71–73 For example, Z. Ni, et al. have elucidated that the negative (Ii−) or positive (Ii+) halide interstitials are the two main deep-level defects in the pure I-based perovskites.74
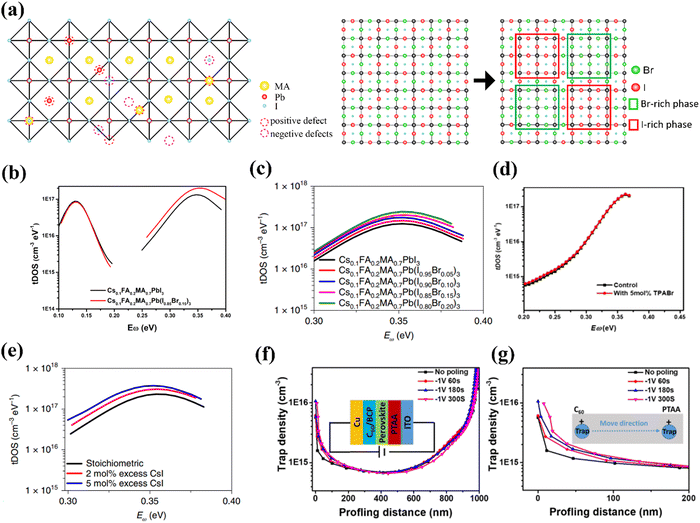 | ||
Fig. 2 (a) Various positively or negatively charged defects present in MAPbI3 and phase separation in WBG perovskites. (b) tDOS spectra of Cs0.1FA0.2MA0.7PbI3 and Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 determined from temperature dependent capacitance-frequency measurement. (c) tDOS spectra of Cs0.1FA0.2MA0.7Pb(I1−xBrx)3 (x = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5%, 10%, 15% and 20%) solar cells. (d) tDOS spectra of WBG devices with and without trimethylphenylammonium bromide (TPABr). (e) tDOS spectra of Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 solar cells with and without excess CsI. (f) Trap density of Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 perovskite solar cells before and after applying the reverse bias measured by drive-level capacitance profiling (DLCP) measurement (DLCP). (g) The magnified plots near the C60/perovskite interface in (f). Reproduced with permission from ref. 64. Copyright 2022, Springer Nature. 0, 5%, 10%, 15% and 20%) solar cells. (d) tDOS spectra of WBG devices with and without trimethylphenylammonium bromide (TPABr). (e) tDOS spectra of Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 solar cells with and without excess CsI. (f) Trap density of Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 perovskite solar cells before and after applying the reverse bias measured by drive-level capacitance profiling (DLCP) measurement (DLCP). (g) The magnified plots near the C60/perovskite interface in (f). Reproduced with permission from ref. 64. Copyright 2022, Springer Nature. | ||
In WBG mixed-halide perovskites, such as MAPb(I1−xBrx)3, it has been reported that phase segregation is more likely to occur when the bromide content falls in the range of 0.2 < x < 0.8 (Fig. 2a).27 Therefore, under such conditions, both phase segregation and defects play important roles in influencing the stability of perovskite materials and devices. However, under the condition with the bromide content lower than 20% or higher than 80%, the phase segregation is suppressed, and the defects may play a dominant role in determining the performance of perovskite devices. It is important to exclude the impact of phase segregation to identify the nature of the trap defects, as the phase segregation will make the defects types and densities more dynamic and more difficult to capture. Yang et al. have measured both the trap density of states (tDOS) and their energetic distribution in Cs0.1FA0.2MA0.7Pb(I1−xBrx)3 (x < 0.2) through thermal admittance spectroscopy (TAS).64 As shown in Fig. 2b, the two similar trap bands centered at ∼0.12 eV and ∼0.35 eV in Cs0.1FA0.2MA0.7PbI3 and Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 indicated that the Br incorporation did not introduce new types of charge traps. However, as shown in Fig. 2c, with the Br percentage increasing from 0 to 20%, tDOS considerably increased in the deep trap region (0.3–0.4 eV). Through measuring the tDOS of Cs0.1FA0.2MA0.7PbI3 and Cs0.1FA0.2MA0.7Pb(I0.85Br0.15)3 in an I-rich environment (Fig. 2d) and Br-rich environment (Fig. 2e), they found that the main deep charge traps are still I interstitials, similar in the pure I-based perovskites. To further identify whether the main defects are negative (Ii−) or positive (Ii+) halide interstitials, they also found that the deep traps will move towards the poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA) side, as depicted in Fig. 2f and g, indicating they are positively charges. However, this study did not furnish details concerning other defects present in the WBG perovskite, and the impact of phase segregation (when x > 0.2) on the defects formation was also not described. Although more works on the nature and properties of defects in WBG perovskites are required to elucidate their impacts on device performance, it is known that the defects are traps for charge carriers and they also serve as active sites for water and oxygen erosion, accelerating the degradation of perovskites.27
3 Passivators for defects
Passivation refers to the process of reducing defects and improving the surface properties of the perovskite materials, thereby enhancing its device performances and operational stability. Since WBG mixed-halide perovskites often suffer from severe Voc loss and instability issue due to their high defect densities, eliminating these defects is often of great significance to achieve better performance of the devices.General criteria for passivation is determined by the charged nature of these defects. For instance, in the case of positively charged defects caused by under-coordinated Pb2+ ions, such as vacancies, Lewis bases containing atoms capable of providing lone pairs of electrons (e.g., O, S, N, and P) can passivate them through coordination bonding.75,76 Alternatively, anions like iodide, bromide, and thiocyanate can passivate these defects through ionic bonding.77–79 The passivation of negatively charged defects resulting from excess electrons associated with under-coordinated I ions or I–Pb antisite defects can be achieved using Lewis acids capable of accepting pairs (e.g. fullerene) or structures containing abundant highly electronegative elements like F, which enable partial structures suitable for accepting electron pairs.80–82 Passivation can also occur through hydrogen bonding between iodide ions and hydrogen atoms attached to highly electronegative elements like O or N, or through ionic bonding with ammonium cations or metal cations.83,84
It is worth noting that from the aspect of passivating the defects, there is not much difference in defect passivation strategies between WBG and narrow-bandgap PVSCs. This is because WBG materials, such as MAPbBr3 or MAPbI3−xBrx, exhibit a similar composition of defect chemistry compared to their narrow-bandgap counterparts like MAPbI3, which has been described in the previous section. These compositions include vacancies (e.g., VMA or VI), interstitials (e.g., Pbi or Ii), and antisite defects (e.g., MAI or IPb). These defects result in uncoordinated lead or halogen ions (such as iodine or bromine ions) in the perovskite lattice structure and thus inducing the degradation of WBG PVSCs.
As shown in Fig. 3a, to passivate the uncoordinated lead ions in both wide bandgap or the narrow bandgap perovskites, some Lewis bases, alkali metal halides, crown ethers, small molecules, or polymers containing carboxylic acid or phosphoric acid groups can be used. For passivating the uncoordinated halide ions (bromide or iodide), Lewis acids, small molecules, or polymers containing amino or sulfonic acid groups can be employed. Additionally, during the fabrication process, different materials and strategies can be applied to eliminate defects at different positions in the PVSCs. As shown in Fig. 3b, the passivation strategies can also be divided into 3 categories, including the buried-interface defect passivation, the bulk defect passivation, and the surface defect passivation, which can be classified by the deposition of perovskites. The passivation method applied prior to perovskite deposition is denoted as “buried interface passivation”, which aims to mitigate the defects at the interface of perovskite and CTL. The term “bulk defects passivation” refers to the concurrent execution of the passivation method and perovskite deposition, with the objective of reducing the bulk defects within the provskite films. The post-implementation of the passivation method is specifically designed to minimize the perovskite surface defects, termed “surface defects passivation”.
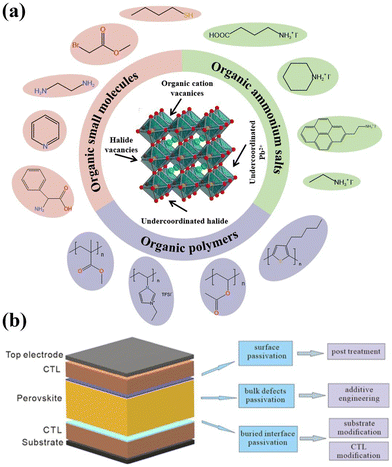 | ||
| Fig. 3 (a) Depiction of the passivator molecules for different defects in WBG perovskites (b) three categories of passivation methods for WBG PVSCs. | ||
To efficiently passivate the defects, it is important to select suitable strategies and corresponding passivators. For the WBG perovskites, another tough issue is the phase segregation of perovskites under illumination. Therefore, strategies to passivate defects and suppress the phase segregation at the same time for WBG perovskites were also introduced. In this section, we summarized the passivation methods have been proposed by researchers for WBG PVSCs so far and divided them into three categories, including buried interface passivation, bulk defects passivation and surface passivation. For each category we also introduced several different passivation strategies for different defect types.
4 Device passivation engineering for WBG PVSCs
4.1 Buried interface passivation
Self-assembled monolayers (SAMs) have been investigated as surface passivators in PVSCs to improve the device performance and stability, SAMs are organic molecules that can spontaneously arrange themselves into an ordered monolayer on the substrate, providing a tunable interface between the perovskite layer and the other functional layers within the device.86,87 The ordered array of organic molecules contains an anchoring group that can bond to the substrate and a functional headgroup.87 The headgroups often have hydrophilic functional groups to tune their surface properties. In addition, headgroups such as ammonium groups (–NH3) and carboxyl (–COOH) can passivate the defects at the upper perovskite, which will effectively enhance the PV performance of the device.88 As depicted in Fig. 4a, SAMs play the role of linkers, bonding the substrate and perovskite films tightly to eliminate interfacial voids. Moreover, SAMs are cheap, dopant-free, simple to process and intrinsically scalable compared to normal carrier transport materials (CTMs).89–91
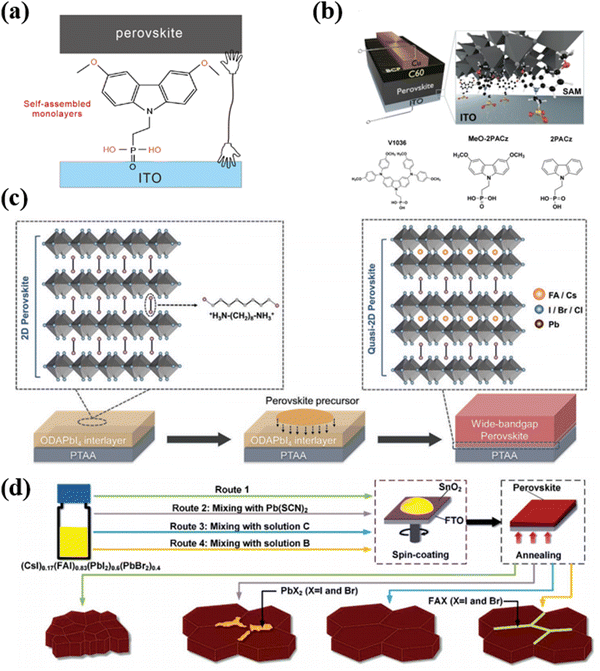 | ||
| Fig. 4 (a) The working mechanism of SAMs. (b) Schematic of the device structure, visualizing how the SAM molecules attach to the ITO surface and contact the upper perovskite. Reproduced with permission from ref. 89. Copyright 2019, Royal Society of Chemistry. (c) Schematic illustration of constructing the quasi-2D perovskite interface by the ODAPbI4 interlayer. Reproduced with permission from ref. 53. Copyright 2021, Wiley-VCH. (d) Schematic diagram of spin-coating stoichiometrically mixed precursor solution on the tin dioxide substrate. Reproduced with permission from ref. 49. Copyright 2018, Wiley-VCH. | ||
It is worth noting that while SAMs offer promising benefits, selecting appropriate molecules carefully, understanding their interactions with the perovskite materials, and optimizing their deposition techniques are crucial for achieving desired improvements in PVSCs. For example, Albrecht et al. have proposed new types of SAMs as the hole transport layers (HTL) for perovskite/CIGS tandem solar cells (TSCs).92 These new SAMs can offer a crucial advantage of conformal coverage on the rough CIGS film surface compared to spiro-OMeTAD and PTAA. In the work, they employed three different SAMs, namely (2-[3,6-bis[bis(4-methoxyphenyl)amino]-9H-carbazol-9-yl]ethyl)phosphonic acid (V1036), MeO-2PACz and [2-(9H-carbazol-9-yl)ethyl]phosphonic acid (2PACz). Fig. 4b illustrates three SAM molecules for PVSCs, among which the 2PACz exhibited the highest efficiency of 20.9% for the Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 solar cells and an efficiency of 23.26% for perovskite/CIGS TSCs.93 In addition, the methyl-substituted carbazole monolayer named Me-4PACz ([4-(3,6-dimethyl-9H-carbazol-9-yl)butyl]phosphonicacid) was also used as HTL, which can allow fast hole extraction and minimize non-radiative recombination. Meanwhile, the Me-4PACz substrate can especially improve the photostability of continuous illumination.93
In terms of application, different strategies and materials are adopted for corresponding substrates such as PTAA or SnO2. As shown in Fig. 4c, Wang et al. have developed an ingenious and effective way to achieve high-efficiency WBG PVSCs by adopting a 2D perovskite (ODAPbI4) interlayer on the top of PTAA.53 The long-chain ODA cation could diffuse into the upper WBG perovskite to modulate the crystal growth and reduce the charge trap density of the perovskite during film processing. Simultaneously, the formation of ODA-based quasi-2D perovskite at the hole-selective interface facilitates the effective hole extraction to HTL. These joint effects efficiently inhibited non-radiative recombination loss, improving the PCE of WBG PVSCs (Eg ≈ 1.66 eV) to 21.05% with an impressive Voc of 1.23 V.
For substrate SnO2, Zhou et al. proposed a way to reduce defects as shown in Fig. 4d.49 Firstly, the stoichiometrically mixed precursor solution was spin-coated on a hydrophilic SnO2 surface to get a perovskite thin film with low crystallinity and small grain size. Then the grain crystallization and surface chemistry were modified by selectively adding Pb(SCN)2, excess FAX, or a combination of both. The results greatly reflected the improved crystallinity, reduced impurity, and passivated grain boundary.
4.2 Additive engineering to passivate the perovskite bulk
Additive engineering includes incorporating passivating additives to the perovskite precursor solution, and has been widely used to passivate bulk defects within perovskites to improve the stability and efficiency of PVSCs. Additives can act as surfactants or stabilizers, improving the passivation quality and reducing bulk defects.50,96 The choice of additives depends on their compatibility with the perovskite material and their ability to reduce trap states.97 Various additives are constantly being discovered for corresponding defects.Potassium (K+) ion doping has been identified as an effective approach in additive engineering. Abdi-Jalebi et al. proposed that the K+ doping in mixed perovskites can reduce non-radiative recombination loss via passivating the grain boundary with potassium halide layers.54 Based on their viewpoint, the K+ doping would produce KI/KBr compounds which mainly distribute at the grain boundary, thus, immobilize the halide ions and vacancies, this can be vividly shown in Fig. 5a. In order to compensate for any halide vacancies, they introduced excess iodide to the perovskite precursor solutions by using potassium iodide. The excess halides are immobilized in the form of benign, potassium-rich, halide-sequestering species at the grain boundaries, filling these vacancies to passivate the non-radiative recombination, inhibiting halide migration and suppressing additional non-radiative recombination loss caused by interstitial halides.
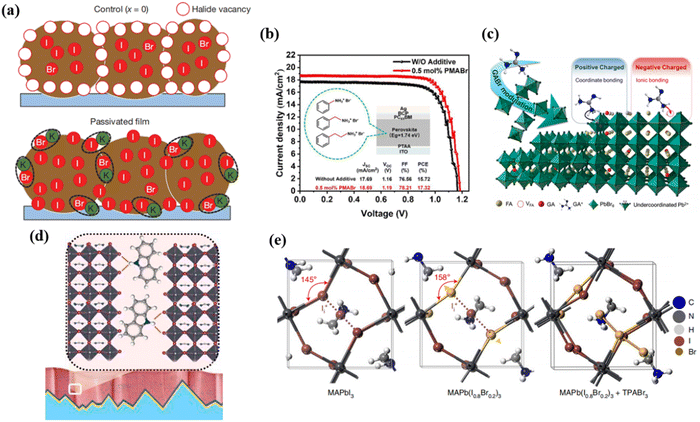 | ||
| Fig. 5 (a) Schematic of the passivation mechanism of K+, in which the excessive halide is immobilized through complexing with potassium into benign compounds at the grain boundaries. Reproduced with permission from ref. 54. Copyright 2018, Springer Nature. (b) J–V characteristics of the WB-PSCs with three additives. Reproduced with permission from ref. 56. Copyright 2022, ACS Appl. (c) Schematic mechanism of GABr modulation on FAPbBr3 based PVSCs. Reproduced with permission from ref. 51. Copyright 2022, Elsevier. (d) Schematic illustration of interaction of carbazole with perovskite films. Reproduced with permission from ref. 47. Copyright 2021, Elsevier. (e) Geometrical structures of MAPbI3 and MAPb(I0.8Br0.2)3 with iodide interstitials, and the calculated geometrical structure of MAPb(I0.8Br0.2)3 with Br3−. Reproduced with permission from ref. 58. Copyright 2021, Springer Nature. | ||
In addition, long chain ammonium cations were commonly used to passivate grain boundaries of perovskite films. Huo et al. reported a way of adding organic bromide salts with different chain lengths involving phenylammonium bromide (PhABr), phenmethylammonium bromide (PMABr) and phenethylammonium bromide (PEABr) to the perovskite precursor to passivate the defects in WBG PVSC (1.74 eV), as shown in Fig. 5b.56 They found that PMABr with moderate chain lengths achieved a PCE of 17.32% with enhanced Voc, performing the best compared to those of the standard device (15.72%) and the devices with PhABr (15.98%) or PEABr additives (16.83%).
It is widely known to use organic small molecules and polymers with Lewis acid or basic groups to passivate defects at grain boundaries of perovskite. Xu et al. added guanidinium bromide (GABr) to the FAPbBr3 precursor to passivate the charged defects of the perovskite.51 It was proposed that negatively charged (A-site vacancy) and positively charged (uncoordinated Pb2+) defects can be simultaneously passivated by the ionized –NH3+ group and unsaturated N atoms. As can be seen from Fig. 5c, the amine (and/or imine) group, namely that of a Lewis base, anchored with the under-coordinated Pb2+ by sharing lone-pair electrons. Conversely, the ammonium cation could passivate negatively charged defects by ionic bonding like A-site vacancy. Thereby, incorporation of GA+ simultaneously passivates both positively and negatively charged defects of the perovskite by multi-reactive site anchoring, transforming the perovskite from charge-rich to charge-neutral region. Liu et al. introduced carbazole to inhibit phase segregation and heal deep-level charged defects of WBG perovskites.47 Generally, the ion migration is activated by strain and starts from grain boundaries. As shown in Fig. 5d, through hydrogen bonding interactions with halides, the carbazole molecules can effectively stabilize the film surface, inhibiting ion migration from the surface to the bulk, thus preventing phase segregation. As a result, the carbazole-based WBG PVSC showed a PCE of 20.2%, giving rise to 28.2% efficiency for 2-T perovskite/silicon TSCs.
Yang et al. considered positive iodide interstitials as the deep-level defects in the WBG perovskite that greatly limit the PCE of PVSCs.64 Therefore, they subtly used trimethylphenylammonium tribromide (TPABr3) as a defect passivator and incorporated TPABr3 into the WBG perovskite precursor, inhibiting the formation of iodide interstitials. As proposed in Fig. 5e, Br− occupies the halide sites or fills halide vacancies, leaving two terminal Br atoms. Due to the space limit, the formation of iodide interstitials can be significantly suppressed.
4.3 Perovskite surface passivation
Surface passivation is another important engineering strategy aiming at improving the stability of perovskite materials and the device performance.98 It is a post treatment and adopted to modify the surface of perovskite after its generation.The perovskite surface is treated with certain organic molecules that can effectively passivate defects.99,100 These molecules improve the electronic properties of the perovskite surface by reducing trap states and minimizing non-radiative recombination processes. As the deep-level acceptor defects are the major causes of Voc, using ammonium salts as anti-solvent to passivate via surface gradient passivation (SGP) was proven to be an effective way to passivate defects on the perovskite layer. Yan et al. investigated that using a set of molecules sharing the phenylethylamine functional groups but with strategically varying positions of the F atom including PEAI (phenylethyl ammonium iodide), and ortho-, meta-, and para-FPEAI (o/m/p-FPEAI) through SGP can greatly passivate defects on the Cs0.05(FA0.77MA0.23)0.95Pb(I0.77Br0.23)3 perovskite surface as shown in Fig. 6a.58 Wherein, p-FPEAI shows the best passivation performance due to its longer distance between the electron-drawing group (F) and the positively charged terminal (–NH3+) which can increase the activity of ammonium ions. The PEA+ can effectively passivate IPb antisite defects and the F atom gives PEA+ an excellent ability to passivate IA antisite defects.
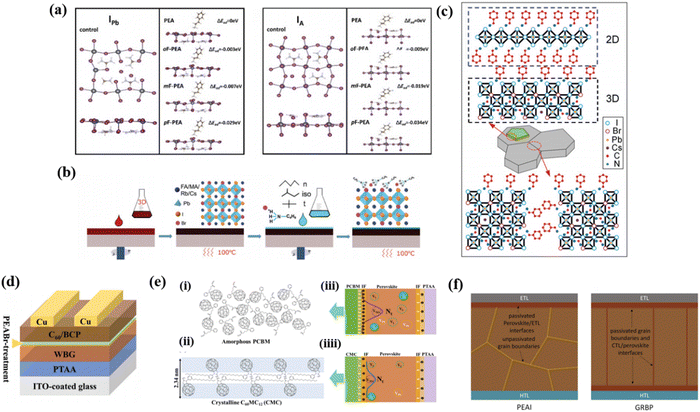 | ||
| Fig. 6 (a) Schematic diagram of the interaction between ammonium cations and the acceptor-like defects IPb and IA. Reproduced with permission from ref. 58. Copyright 2023, Wiley-VCH. (b) Schematic detailing the 2D treatment routes for the perovskite solar cell absorber layer by surface coating. Reproduced with permission from ref. 52. Copyright 2020, Wiley-VCH. (c) Schematic of BA modifcation on the Cs0.15FA0.85Pb(I0.73Br0.27)3 thin film. Reproduced with permission from ref. 48. Copyright 2017, Wiley-VCH. (d) Schematic illustration of the device structure with PEABr-treatment. Reproduced with permission from ref. 55. Copyright 2022, Wiley-VCH. (e) Schematic illustration of the mechanism for performance enhancement in device with crystalline fullerene derivatives. Reproduced with permission from ref. 57. Copyright 2018, ACS Appl. (f) Schematic of PEAI and GRBP post treated perovskite films. Reproduced with permission from ref. 50. Copyright 2022, Wiley-VCH. | ||
It is widely used to construct interfacial 2D/3D heterostructures by alkylamines to passivate defects, block ion migration, and suppress phase segregation of PVSCs. After coating different aliphatic alkylammonium bulky cations on the surface of the 3D perovskite, Duong et al. identified that a Ruddlesden–Popper quasi-2D perovskite phase can be formed on the surface of the 3D perovskite and effectively passivate surface defects.52 As shown in Fig. 6b, they took the quadruple-cation mixed-halide perovskite Rb0.05Cs0.095MA0.1425FA0.7125PbI2Br with a bandgap of 1.72 eV as the 3D perovskite and firstly spin the 3D perovskite film on a substrate based on a standard procedure, the substrate was subsequently annealed on a hot plate at 100 °C for 30 min. Then the 2D perovskite precursor (n-butylammonium bromide (n-BABr), iso-BABr, or t-BABr dissolved in 2-propanol with different concentrations) was spun on the 3D perovskite film, followed by annealing at 100 °C for 5 min. It is confirmed that the device performance has been greatly enhanced after that as the surface defects have been well modified. Zhou et al. introduced benzylamine (BA) post treatment for the 1.72 eV bandgap Cs0.15FA0.85Pb(I0.73Br0.27)3 perovskite via forming a 2D BA2PbI4 phase as illustrated in Fig. 6c.48 They have proven that constructing a 2D phase after perovskite formation can effectively passivate defective regions and prevent decomposition or phase segregation. In addition, the formation of 2D BA2PbI4 produced an energy cascade with the underneath 3D perovskite, which facilitated hole extraction and simultaneously blocked electron transportation. Consequently, the stability and efficiency of the PVSCs were significantly enhanced after BA modification.
Certain chemical treatments can be applied to the perovskite surface to passivate defects and reduce surface energy levels. For example, treatment with Lewis bases or halide salts can lead to the formation of a self-passivating layer on the perovskite surface, thereby improving the device performance and optimizing the surface electric field. As mentioned previously, surface post treatment using ammonium halides can effectively inhibit Voc deficit in WBG PVSCs. He et al. indicated a phenethylammonium bromide (PEABr) post-treatment strategy to precisely tailor the phase purity of 2D perovskites on the WBG perovskite at different annealing temperatures.55 As represented in Fig. 6d, after PEABr post-treatment, a pure 2D perovskite phase formed at 60 °C on the top of a 1.77 eV WBG perovskite. Consequently, this self-passivating layer significantly suppressed the defects on the surface, giving the 1.77 eV WBG PVSC a Voc of 1.284 V, which is the lowest Voc deficit (0.486 V) among WBG PVSCs whose bandgaps were higher than 1.75 eV.
Another method focuses on modifying the interface between the perovskite layer and the CTL. This can be achieved by using suitable interfacial materials that enhance charge extraction and reduce surface recombination. Khadka et al. synthesized long alkyl chain-substituted fullerene derivatives as the electron transport layer (ETL).57 Compared to amorphous PC61BM, the C60-fused N-methylpyrrolidine-meta-dodecyl phenyl (C60MC12) showed high crystallinity, which contribute to lower perovskite/ETL interfacial defects.57 The schematic diagrams explained the optimization mechanism. It is supposed that the surface molecules are randomly oriented in the amorphous PCBM film shown in Fig. 6e(i), while the crystalline CMC film forms well-ordered CMC molecules with a periodic bilayer structure with interlaced dodecyl chains as shown in Fig. 6e(ii). The CMC thin film with a bilayer crystalline structure generates an advantageous perovskite/ETL interface layer, effectively suppressing the traps at the interface. As represented in Fig. 6e(iii), the device with amorphous PCBM builds up more detrimental charges at the PCBM/perovskite interface, causing higher defect densities in the perovskite film. Moreover, the defect distribution may also diffuse into the perovskite layer. In contrast, the defects in the perovskite film of the device with the high crystallinity CMC film were effectively passivated (Fig. 6e(iiii)).
In addition, Liu et al. proposed a grain regrowth and bifacial passivation (GRBP) strategy to inhibit defects at both grain boundaries and perovskite/CTL interfaces simultaneously as shown in Fig. 6f.50 A mixture of methylammonium thiocyanate (MASCN) and phenethylammonium iodide (PEAI) was used to conduct a post treatment of perovskite films. The MASCN induces the regrowth of perovskite grains and can simultaneously facilitate the penetration of PEAI into the buried interface. Therefore, the bulk and interface non-radiative recombination losses are decreased, and the open circuit voltage of the device is substantially improved. Consequently, PCEs of 21.9% and 19.9% for the 1.65 eV bandgap opaque and semitransparent PVSCs are obtained.
Efficient defect passivation in WBG PVSCs is a vibrant research area. Researchers are continually exploring new materials, surface modification techniques, and interfacial engineering strategies to reduce trap densities, enhance charge carrier lifetimes, and improve the overall stability and efficiency of these devices.
5 Conclusion and outlook
Over the past decade, PVSCs have experienced rapid and unparalleled progress. As the bandgap can be easily tuned by composition engineering, the WBG mixed-halide PVSCs have been developed in various PV applications. Among them, the perovskite/Si or perovskite/perovskite tandem solar cells which use WBG PVSCs as the top cells to enhance total efficiency are regarded as one of the most promising PV technologies in the future. However, the WBG PVSCs suffer from large Voc deficits and instability issues and the underlying mechanisms are still unknown. Nevertheless, it is undisputed that one of the primary contributors to these issues is the inevitable occurrence of various defects during the perovskite preparation processes. These defects not only act as centers for non-radiative charge carrier recombination and vacancies for ion migration, but also serve as active sites for degradation caused by water and oxygen exposure. Generally, the defects can be classified as positively charged and negatively charged, as the two can be passivated by Lewis bases and Lewis acids, respectively. Furthermore, the defects can be classified by the position of the device, namely the buried-interface defects, the bulk defects, and the surface defects. To date, massive passivation strategies for these three defects have been introduced and relative devices also exhibited high performances.For future defect passivation strategies, we provide our perspectives as shown in Fig. 7. For the WBG perovskite buried interface passivation, we believe that using SAMs is one of the most promising methods in the future. Compared to the passivation interlayer engineering between the CTL and the perovskite layer, the SAM molecules can be designed for highly efficient hole or electron extraction, which is beneficial for device performance. On the other hand, the SAM molecules can be designed with certain headgroups, such as ammonium groups (–NH2) and carboxyl (–COOH) groups which can interact with undercoordinated Pb2+ and thus passivate the defects at the buried interface of the perovskite film. Therefore, material scientists should move forward to design and synthesize novel SAM molecules for perovskites to improve the efficiency and stability of WBG PVSCs in the future.
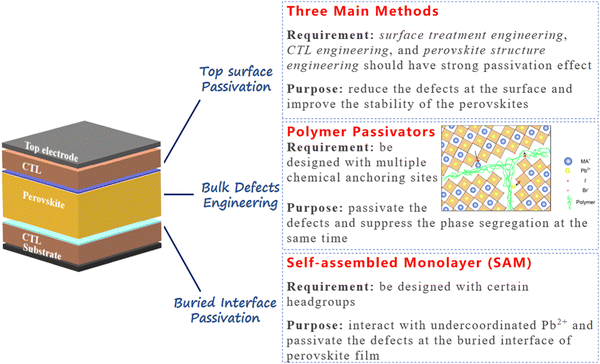 | ||
| Fig. 7 Some proposed strategies for passivating the defects at the buried interface of perovskite, in perovskite bulk, and at the top of perovskite surface. | ||
For the WBG perovskite bulk defects engineering, we recommend using polymer passivators with certain functional groups. As the WBG perovskites tend to exhibit phase segregation under light illumination due to the ion migration and remixing of the halide ions, it is helpful to find materials that not only passivate defects, but also suppress ion migration in the perovskite bulk. Using polymer additives in perovskite precursors shows potential to solve the above-mentioned issue of WBG perovskites. The polymer can be designed with multiple chemical anchoring sites for perovskite defect passivation. On the other hand, polymer additives are generally distributed at the grain boundaries of perovskites. Such network distribution will be a robust shield for ion migration from one grain to another. Therefore, polymer additives have great potential to passivate the defects and suppress phase segregation at the same time. Investigations of the perovskite precursor chemistry and interaction between perovskite and polymer additives are required to achieve high-efficiency and stable WBG PVSCs.
For defects passivation at the WBG perovskite top surfaces, there are three main methods including surface treatment engineering, CTL engineering, and perovskite structure engineering. For the perovskite surface treatment, novel materials are needed to have a strong passivation effect and at the same time it should be ultrathin to have a negligible effect on the charge transport. For CTL engineering, we believe new polymer-based CTLs with certain functional groups are needed to passivate the surface defects. For perovskite structure engineering, forming a thin low-dimensional perovskite layer on the top is a promising strategy to reduce the defects at the surface and improve the stability of the perovskites.
Finally, we believe that upscaling defect passivation engineering is a key step to push the commercialization of PVSCs. The current defect passivators and the passivation methods are not suitable for large-sale device fabrication. New low-cost functional materials as defect passivators are urgently required. Then how to prepare the passivation layer at the buried interface and perovskite top surface on a large-scale is a tricky problem, which requires more contributions from the PV community.
In conclusion, efficient defect passivation in WBG mixed-halide PVSCs remains an active area of research. Many novel materials, surface modification techniques, and interface engineering strategies have been introduced to reduce trap density, improve carrier lifetime, and enhance overall stability and efficiency of the corresponding PV devices. However, there is still a necessity to uncover the nature of the defects in WBG PVSCs. Disclosing the photophysical and chemical properties of the complicated defects is also important to develop novel materials and methods that can passivate the defects. Moreover, strategies to passivate the defects in perovskite bulk and the interface between the perovskite and the CTLs are still needed to enhance the performance of devices and inhibit phase segregation of the mixed halide perovskites. In addition, developing low-cost passivator materials and exploring defect passivation engineering for upscaling processing is of great importance for future commercialization of WBG mixed-halide PVSCs.
Conflicts of interest
There are no conflicts to declare.References
- S. R. Kellert, Building for life: Designing and understanding the human-nature connection, Renewable Resour. J., 2006, 24, 8 Search PubMed.
- Z. Yue, H. Guo and Y. Cheng, Toxicity of Perovskite Solar Cells, Energies, 2023, 16, 4007 CrossRef CAS.
- A. Alzoubi, Renewable Green hydrogen energy impact on sustainability performance, Int. J. Comput. Inf. Manuf., 2021, 1 DOI:10.54489/ijcim.v1i1.46.
- S. Kouro, J. I. Leon, D. Vinnikov and L. G. Franquelo, Grid-connected photovoltaic systems: An overview of recent research and emerging PV converter technology, IEEE Ind. Electron. Mag., 2015, 9, 47–61 Search PubMed.
- A. S. Bati, Y. L. Zhong, P. L. Burn, M. K. Nazeeruddin, P. E. Shaw and M. Batmunkh, Next-generation applications for integrated perovskite solar cells, Commun. Mater., 2023, 4, 2 CrossRef CAS.
- S.-P. Feng, Y. Cheng, H.-L. Yip, Y. Zhong, P. W. Fong, G. Li, A. Ng, C. Chen, L. A. Castriotta and F. Matteocci, Roadmap on commercialization of metal halide perovskite photovoltaics, J. Phys. Mater, 2023, 6, 320501 CrossRef.
- Z. Guo, A. K. Jena, G. M. Kim and T. Miyasaka, The high open-circuit voltage of perovskite solar cells: a review, Energy Environ. Sci., 2022, 15, 3171–3222 RSC.
- J. Islam and A. A. Hossain, Semiconducting to metallic transition with outstanding optoelectronic properties of CsSnCl3 perovskite under pressure, Sci. Rep., 2020, 10, 14391 CrossRef CAS PubMed.
- H. Kim, J. Lim, M. Sohail and M. K. Nazeeruddin, Superhalogen passivation for efficient and stable perovskite solar cells, Sol. RRL, 2022, 6, 2200013 CrossRef CAS.
- Y. Zhou, L. M. Herz, A. K. Jen and M. Saliba, Advances and challenges in understanding the microscopic structure–property–performance relationship in perovskite solar cells, Nat. Energy, 2022, 7, 794–807 CrossRef CAS.
- H. Zhang, L. Xu, B. Han, H. Wang, Y. Liu, P. Wang, P. Lin, X. Wu, X. Yu and C. Cui, PEIE-complexed SnO2 enabled low-temperature solution fabrication of high-performance CsPbI3 all-inorganic perovskite solar cells, J. Alloys Compd., 2023, 957, 170394 CrossRef CAS.
- S. Y. Hong, H. J. Lee, J. K. Park, J. H. Heo and S. H. Im, Acetylacetone modulated TiO2 nanoparticles for low-temperature solution processable perovskite solar cell, Int. J. Energy Res., 2022, 46, 22819–22831 CrossRef CAS.
- S. H. Reddy, F. Di Giacomo and A. Di Carlo, Low-temperature-processed stable perovskite solar cells and modules: a comprehensive review, Adv. Energy Mater., 2022, 12, 2103534 CrossRef CAS.
- L. Lin, T. W. Jones, T. C. J. Yang, N. W. Duffy, J. Li, L. Zhao, B. Chi, X. Wang and G. J. Wilson, Inorganic electron transport materials in perovskite solar cells, Adv. Funct. Mater., 2021, 31, 2008300 CrossRef CAS.
- Q. Liu, W. Cai, W. Wang, H. Wang, Y. Zhong and K. Zhao, Controlling Phase Transition toward Future Low-Cost and Eco-friendly Printing of Perovskite Solar Cells, J. Phys. Chem. Lett., 2022, 13, 6503–6513 CrossRef CAS PubMed.
- M. R. Samantaray, N. K. Rana, A. Kumar, D. S. Ghosh and N. Chander, Stability study of large-area perovskite solar cells fabricated with copper as low-cost metal contact, Int. J. Energy Res., 2022, 46, 1250–1262 CrossRef CAS.
- S. Kajal, Stability enhancement in solution-processed perovskite solar cells, PhD thesis, Ulsan National Institute of Science and Technology, 2020 Search PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- P. K. Kung, M. H. Li, P. Y. Lin, Y. H. Chiang, C. R. Chan, T. F. Guo and P. Chen, A review of inorganic hole transport materials for perovskite solar cells, Adv. Mater. Interfaces, 2018, 5, 1800882 CrossRef.
- National Renewable Energy Laboratory (NREL), Best Research-Cell Efficiencies, https://www.nrel.gov/pv/assets/pdfs/cell-pv-eff-emergingpv.pdf.
- B. Shi, L. Duan, Y. Zhao, J. Luo and X. Zhang, Semitransparent perovskite solar cells: from materials and devices to applications, Adv. Mater., 2020, 32, 1806474 CrossRef CAS PubMed.
- L. Yang, J. Shi, Y. Wu, X. Jin, T. Bie, C. Hu, W. Liang, Y. Gao, M. Xu and M. Shao, Long Carrier Diffusion Length and Efficient Charge Transport in Thick Quasi-Two-Dimensional Perovskite Solar Cells Enabled by Modulating Crystal Orientation and Phase Distribution, ACS Appl. Energy Mater., 2022, 5, 8930–8939 CrossRef CAS.
- X. Chu, Q. Ye, Z. Wang, C. Zhang, F. Ma, Z. Qu, Y. Zhao, Z. Yin, H.-X. Deng and X. Zhang, Surface in situ reconstruction of inorganic perovskite films enabling long carrier lifetimes and solar cells with 21% efficiency, Nat. Energy, 2023, 8, 372–380 CrossRef CAS.
- G. O. Odunmbaku, S. Chen, B. Guo, Y. Zhou, N. A. N. Ouedraogo, Y. Zheng, J. Li, M. Li and K. Sun, Recombination pathways in perovskite solar cells, Adv. Mater. Interfaces, 2022, 9, 2102137 CrossRef CAS.
- Y. He, I. Hadar and M. G. Kanatzidis, Detecting ionizing radiation using halide perovskite semiconductors processed through solution and alternative methods, Nat. Photonics, 2022, 16, 14–26 CrossRef CAS.
- H. Hegazy, G. M. Mustafa, A. Nawaz, N. Noor, A. Dahshan and I. Boukhris, Tuning of direct bandgap of Rb2ScTlX6 (X = Cl, Br, I) double perovskites through halide ion substitution for solar cell devices, J. Mater. Res. Technol., 2022, 19, 1271–1281 CrossRef CAS.
- F. Xu, M. Zhang, Z. Li, X. Yang and R. Zhu, Challenges and Perspectives toward Future Wide-Bandgap Mixed-Halide Perovskite Photovoltaics, Adv. Energy Mater., 2023, 13, 2203911 CrossRef CAS.
- J. Zheng, G. Wang, W. Duan, M. A. Mahmud, H. Yi, C. Xu, A. Lambertz, S. Bremner, K. Ding and S. Huang, Monolithic perovskite–perovskite–silicon triple-junction tandem solar cell with an efficiency of over 20%, ACS Energy Lett., 2022, 7, 3003–3005 CrossRef CAS.
- J. J. Yoo, S. S. Shin and J. Seo, Toward efficient perovskite solar cells: progress, strategies, and perspectives, ACS Energy Lett., 2022, 7, 2084–2091 CrossRef CAS.
- C. Liu, H. Dong, Z. Zhang, W. Chai, L. Li, D. Chen, W. Zhu, H. Xi, J. Zhang and C. Zhang, Promising applications of wide bandgap inorganic perovskites in underwater photovoltaic cells, Sol. Energy, 2022, 233, 489–493 CrossRef CAS.
- A. W. Ho-Baillie, H. G. Sullivan, T. A. Bannerman, H. P. Talathi, J. Bing, S. Tang, A. Xu, D. Bhattacharyya, I. H. Cairns and D. R. McKenzie, Deployment opportunities for space photovoltaics and the prospects for perovskite solar cells, Adv. Mater. Technol., 2022, 7, 2101059 CrossRef CAS.
- T. M. Koh, H. Wang, Y. F. Ng, A. Bruno, S. Mhaisalkar and N. Mathews, Halide perovskite solar cells for building integrated photovoltaics: Transforming building façades into power generators, Adv. Mater., 2022, 34, 2104661 CrossRef CAS PubMed.
- R. Wang, T. Huang, J. Xue, J. Tong, K. Zhu and Y. Yang, Prospects for metal halide perovskite-based tandem solar cells, Nat. Photonics, 2021, 15, 411–425 CrossRef CAS.
- J. A. Röhr, J. Lipton, J. Kong, S. A. Maclean and A. D. Taylor, Efficiency limits of underwater solar cells, Joule, 2020, 4, 840–849 CrossRef.
- A. Mahata, D. Meggiolaro and F. De Angelis, From large to small polarons in lead, tin, and mixed lead–tin halide perovskites, J. Phys. Chem. Lett., 2019, 10, 1790–1798 CrossRef CAS PubMed.
- M. Pratheek, P. Sujith, T. S. Hameed and P. Predeep, Effect of bromide doping on the phase stability and shelf life of the triple cation mixed halide perovskite single-crystals, Mater. Lett., 2022, 326, 132903 CrossRef CAS.
- P. Caprioglio, J. A. Smith, R. D. Oliver, A. Dasgupta, S. Choudhary, M. D. Farrar, A. J. Ramadan, Y.-H. Lin, M. G. Christoforo and J. M. Ball, Open-circuit and short-circuit loss management in wide-gap perovskite pin solar cells, Nat. Commun., 2023, 14, 932 CrossRef CAS PubMed.
- J. Bisquert, Interpretation of the Recombination Lifetime in Halide Perovskite Devices by Correlated Techniques, J. Phys. Chem. Lett., 2022, 13, 7320–7335 CrossRef CAS PubMed.
- Z. Cui, Q. Zhang, Y. Bai and Q. Chen, Issues of phase segregation in wide-bandgap perovskite, Mater. Chem. Front., 2023, 7, 1896–1911 RSC.
- A. Hassan, Z. Wang, Y. H. Ahn, M. Azam, A. A. Khan, U. Farooq, M. Zubair and Y. Cao, Recent defect passivation drifts and role of additive engineering in perovskite photovoltaics, Nano Energy, 2022, 107579 CrossRef CAS.
- D. A. Kara, D. Cirak and B. Gultekin, Decreased surface defects and non-radiative recombination via the passivation of the halide perovskite film by 2-thiophenecarboxylic acid in triple-cation perovskite solar cells, Phys. Chem. Chem. Phys., 2022, 24, 10384–10393 RSC.
- Y. Cheng and L. Ding, Pushing commercialization of perovskite solar cells by improving their intrinsic stability, Energy Environ. Sci., 2021, 14, 3233–3255 RSC.
- J. Zhao, A. S. Chesman, J. Yan, L. J. Sutherland, J. Jasieniak, J. Lu, W. Mao and U. Bach, Precursor engineering of lead acetate-based precursors for high-open-circuit voltage wide-bandgap perovskite solar cells, ACS Appl. Mater. Interfaces, 2023, 15, 18800–18807 CrossRef CAS PubMed.
- K. Ridzoňová, R. Grill, A. P. Amalathas, B. Dzurňák, N. Neykova, L. Horák, P. Fiala, X. Y. Chin, C. M. Wolff and Q. Jeangros, Correlating light-induced deep defects and phase segregation in mixed-halide perovskites, J. Mater. Chem. A, 2022, 10, 18928–18938 RSC.
- T. Huang, S. Tan, S. Nuryyeva, I. Yavuz, F. Babbe, Y. Zhao, M. Abdelsamie, M. H. Weber, R. Wang and K. N. Houk, Performance-limiting formation dynamics in mixed-halide perovskites, Sci. Adv., 2021, 7, eabj1799 CrossRef CAS PubMed.
- Y. Bai, Z. Huang, X. Zhang, J. Lu, X. Niu, Z. He, C. Zhu, M. Xiao, Q. Song and X. Wei, Initializing film homogeneity to retard phase segregation for stable perovskite solar cells, Science, 2022, 378, 747–754 CrossRef CAS PubMed.
- J. Liu, E. Aydin, J. Yin, M. De Bastiani, F. H. Isikgor, A. U. Rehman, E. Yengel, E. Ugur, G. T. Harrison and M. Wang, 28.2%-efficient, outdoor-stable perovskite/silicon tandem solar cell, Joule, 2021, 5, 3169–3186 CrossRef CAS.
- Y. Zhou, F. Wang, Y. Cao, J. P. Wang, H. H. Fang, M. A. Loi, N. Zhao and C. P. Wong, Benzylamine-treated wide-bandgap perovskite with high thermal-photostability and photovoltaic performance, Adv. Energy Mater., 2017, 7, 1701048 CrossRef.
- Y. Zhou, Y. H. Jia, H. H. Fang, M. A. Loi, F. Y. Xie, L. Gong, M. C. Qin, X. H. Lu, C. P. Wong and N. Zhao, Composition-tuned wide bandgap perovskites: From grain engineering to stability and performance improvement, Adv. Funct. Mater., 2018, 28, 1803130 CrossRef.
- Z. Liu, C. Zhu, H. Luo, W. Kong, X. Luo, J. Wu, C. Ding, Y. Chen, Y. Wang and J. Wen, Grain Regrowth and Bifacial Passivation for High-Efficiency Wide-Bandgap Perovskite Solar Cells, Adv. Energy Mater., 2023, 13, 2203230 CrossRef CAS.
- H. Xu, Z. Liang, J. Ye, S. Xu, Z. Wang, L. Zhu, X. Chen, Z. Xiao, X. Pan and G. Liu, Guanidinium-assisted crystallization modulation and reduction of open-circuit voltage deficit for efficient planar FAPbBr3 perovskite solar cells, Chem. Eng. J., 2022, 437, 135181 CrossRef CAS.
- T. Duong, H. Pham, T. C. Kho, P. Phang, K. C. Fong, D. Yan, Y. Yin, J. Peng, M. A. Mahmud and S. Gharibzadeh, High efficiency perovskite-silicon tandem solar cells: effect of surface coating versus bulk incorporation of 2D perovskite, Adv. Energy Mater., 2020, 10, 1903553 CrossRef CAS.
- D. Wang, H. Guo, X. Wu, X. Deng, F. Li, Z. Li, F. Lin, Z. Zhu, Y. Zhang and B. Xu, Interfacial engineering of wide-bandgap perovskites for efficient perovskite/CZTSSe tandem solar cells, Adv. Funct. Mater., 2022, 32, 2107359 CrossRef CAS.
- M. Abdi-Jalebi, Z. Andaji-Garmaroudi, S. Cacovich, C. Stavrakas, B. Philippe, J. M. Richter, M. Alsari, E. P. Booker, E. M. Hutter and A. J. Pearson, Maximizing and stabilizing luminescence from halide perovskites with potassium passivation, Nature, 2018, 555, 497–501 CrossRef CAS PubMed.
- R. He, Z. Yi, Y. Luo, J. Luo, Q. Wei, H. Lai, H. Huang, B. Zou, G. Cui and W. Wang, Pure 2D Perovskite Formation by Interfacial Engineering Yields a High Open-Circuit Voltage beyond 1.28 V for 1.77 eV Wide-Bandgap Perovskite Solar Cells, Adv. Sci., 2022, 9, 2203210 CrossRef CAS PubMed.
- X. Huo, Y. Li, Y. Lu, J. Dong, Y. Zhang, S. Zhao, B. Qiao, D. Wei, D. Song and Z. Xu, Suppressed halide segregation and defects in wide bandgap perovskite solar cells enabled by doping organic bromide salt with moderate chain length, J. Phys. Chem. C, 2022, 126, 1711–1720 CrossRef CAS.
- D. B. Khadka, Y. Shirai, M. Yanagida, T. Noda and K. Miyano, Tailoring the open-circuit voltage deficit of wide-band-gap perovskite solar cells using alkyl chain-substituted fullerene derivatives, ACS Appl. Mater. Interfaces, 2018, 10, 22074–22082 CrossRef CAS PubMed.
- N. Yan, Y. Gao, J. Yang, Z. Fang, J. Feng, X. Wu, T. Chen and S. Liu, Wide-Bandgap Perovskite Solar Cell Using a Fluoride-Assisted Surface Gradient Passivation Strategy, Angew. Chem., Int. Ed., 2023, 62, e202216668 CrossRef CAS PubMed.
- L. Wang, W. Zheng, F. Vitale, X. Zhang, X. Li, Y. Ji, Z. Liu, O. Ghaebi, C. T. Plass and R. Domes, Wide-Bandgap Double Perovskites with Multiple Longitudinal-Optical Phonon Scattering, Adv. Funct. Mater., 2022, 32, 2111338 CrossRef CAS.
- H. Jiao, Z. Ni, Z. Shi, C. Fei, Y. Liu, X. Dai and J. Huang, Perovskite grain wrapping by converting interfaces and grain boundaries into robust and water-insoluble low-dimensional perovskites, Sci. Adv., 2022, 8, eabq4524 CrossRef CAS PubMed.
- W. Wang, M. Cai, Y. Wu, K. Ji, B. Cheng, X. Liu, H. Lv and S. Dai, Defect healing of MAPbI3 perovskite single crystal surface by benzylamine, Symmetry, 2022, 14, 1099 CrossRef CAS.
- Z. Li, X. Zheng, X. Xiao, Y. An, Y. Wang, Q. Huang, X. Li, R. Cheacharoen, Q. An and Y. Rong, Beyond the Phase Segregation: Probing the Irreversible Phase Reconstruction of Mixed-Halide Perovskites, Adv. Sci., 2022, 9, 2103948 CrossRef CAS PubMed.
- L. A. Muscarella and B. Ehrler, The influence of strain on phase stability in mixed-halide perovskites, Joule, 2022, 6, 2016–2031 CrossRef CAS.
- G. Yang, Z. Ni, Z. J. Yu, B. W. Larson, Z. Yu, B. Chen, A. Alasfour, X. Xiao, J. M. Luther and Z. C. Holman, Defect engineering in wide-bandgap perovskites for efficient perovskite–silicon tandem solar cells, Nat. Photonics, 2022, 16, 588–594 CrossRef CAS.
- G. K. Grandhi, D. Hardy, M. Krishnaiah, B. Vargas, B. Al-Anesi, M. P. Suryawanshi, D. Solis-Ibarra, F. Gao, R. L. Hoye and P. Vivo, Wide-Bandgap Perovskite-Inspired Materials: Defect-Driven Challenges for High-Performance Optoelectronics, Adv. Funct. Mater., 2023, 2307441 CrossRef.
- Y. Yu, R. Liu, F. Zhang, C. Liu, Q. Wu, M. Zhang and H. Yu, Potassium tetrafluoroborate-induced defect tolerance enables efficient wide-bandgap perovskite solar cells, J. Colloid Interface Sci., 2022, 605, 710–717 CrossRef CAS PubMed.
- H. Xue, G. Brocks and S. Tao, Intrinsic defects in primary halide perovskites: A first-principles study of the thermodynamic trends, Phys. Rev. Mater., 2022, 6, 055402 CrossRef CAS.
- T. Nie, J. Yang, Z. Fang, Z. Xu, X. Ren, X. Guo, T. Chen and S. F. Liu, Amino-acid-type alkylamine additive for high-performance wide-bandgap perovskite solar cells, Chem. Eng. J., 2023, 468, 143341 CrossRef CAS.
- Y. Cheng, X. Liu, Z. Guan, M. Li, Z. Zeng, H. W. Li, S. W. Tsang, A. G. Aberle and F. Lin, Revealing the degradation and self-healing mechanisms in perovskite solar cells by sub-bandgap external quantum efficiency spectroscopy, Adv. Mater., 2021, 33, 2006170 CrossRef CAS PubMed.
- J. Kim, C.-H. Chung and K.-H. Hong, Understanding of the formation of shallow level defects from the intrinsic defects of lead tri-halide perovskites, Phys. Chem. Chem. Phys., 2016, 18, 27143–27147 RSC.
- J. Liu, M. Wang, J. Lin, G. Chen, B. Liu, J. Huang, M. Zhang, G. Liang, L. Lu and P. Xu, Mitigating deep-level defects through a self-healing process for highly efficient wide-bandgap inorganic CsPbI3−x Brx perovskite photovoltaics, J. Mater. Chem. A, 2022, 10, 17237–17245 RSC.
- T. Nie, Z. Fang, X. Ren, Y. Duan and S. Liu, Recent Advances in Wide-Bandgap Organic–Inorganic Halide Perovskite Solar Cells and Tandem Application, Nano-Micro Lett., 2023, 15, 70 CrossRef CAS PubMed.
- T. Wang, Y. Li, Q. Cao, J. Yang, B. Yang, X. Pu, Y. Zhang, J. Zhao, Y. Zhang and H. Chen, Deep defect passivation and shallow vacancy repair via an ionic silicone polymer toward highly stable inverted perovskite solar cells, Energy Environ. Sci., 2022, 15, 4414–4424 RSC.
- Z. Ni, H. Jiao, C. Fei, H. Gu, S. Xu, Z. Yu, G. Yang, Y. Deng, Q. Jiang and Y. Liu, Evolution of defects during the degradation of metal halide perovskite solar cells under reverse bias and illumination, Nat. Energy, 2022, 7, 65–73 CrossRef CAS.
- Y. Cai, J. Cui, M. Chen, M. Zhang, Y. Han, F. Qian, H. Zhao, S. Yang, Z. Yang and H. Bian, Multifunctional enhancement for highly stable and efficient perovskite solar cells, Adv. Funct. Mater., 2021, 31, 2005776 CrossRef CAS.
- C. Xu, Z. Zhang, S. Zhang, H. Si, S. Ma, W. Fan, Z. Xiong, Q. Liao, A. Sattar and Z. Kang, Manipulation of perovskite crystallization kinetics via Lewis base additives, Adv. Funct. Mater., 2021, 31, 2009425 CrossRef CAS.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer and M. Kim, Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- Y. Qi, K. A. Green, G. Ma, S. Jha, K. Gollinger, C. Wang, X. Gu, D. Patton, S. E. Morgan and Q. Dai, Passivation of iodine vacancies of perovskite films by reducing iodine to triiodide anions for high-performance photovoltaics, Chem. Eng. J., 2022, 438, 135647 CrossRef CAS.
- Y. Niu, D. He, X. Zhang, L. Hu and Y. Huang, Enhanced Perovskite Solar Cell Stability and Efficiency via Multi-Functional Quaternary Ammonium Bromide Passivation, Adv. Mater. Interfaces, 2023, 10, 2201497 CrossRef CAS.
- X. Gong, L. Guan, H. Pan, Q. Sun, X. Zhao, H. Li, H. Pan, Y. Shen, Y. Shao and L. Sun, Highly efficient perovskite solar cells via nickel passivation, Adv. Funct. Mater., 2018, 28, 1804286 CrossRef.
- L. Liu, Y. Yang, M. Du, Y. Cao, X. Ren, L. Zhang, H. Wang, S. Zhao, K. Wang and S. Liu, Self-Assembled Amphiphilic Monolayer for Efficient and Stable Wide-Bandgap Perovskite Solar Cells, Adv. Energy Mater., 2023, 13, 2202802 CrossRef CAS.
- Z. Xing, S.-H. Li and S. Yang, Targeted Molecular Design of Functionalized Fullerenes for High-Performance and Stable Perovskite Solar Cells, Small Struct., 2022, 3, 2200012 CrossRef CAS.
- Z. Fang, L. Jia, N. Yan, X. Jiang, X. Ren, S. Yang and S. Liu, Proton-transfer-induced in situ defect passivation for highly efficient wide-bandgap inverted perovskite solar cells, InfoMat, 2022, 4, e12307 CrossRef CAS.
- L. Yang, Y. Jin, Z. Fang, J. Zhang, Z. Nan, L. Zheng, H. Zhuang, Q. Zeng, K. Liu and B. Deng, Efficient Semi-Transparent Wide-Bandgap Perovskite Solar Cells Enabled by Pure-Chloride 2D-Perovskite Passivation, Nano-Micro Lett., 2023, 15, 111 CrossRef CAS PubMed.
- M. Jaysankar, B. A. Raul, J. Bastos, C. Burgess, C. Weijtens, M. Creatore, T. Aernouts, Y. Kuang, R. Gehlhaar and A. Hadipour, Minimizing voltage loss in wide-bandgap perovskites for tandem solar cells, ACS Energy Lett., 2018, 4, 259–264 CrossRef.
- F. Ali, C. Roldán-Carmona, M. Sohail and M. K. Nazeeruddin, Applications of self-assembled monolayers for perovskite solar cells interface engineering to address efficiency and stability, Adv. Energy Mater., 2020, 10, 2002989 CrossRef CAS.
- J. Zeng, L. Bi, Y. Cheng, B. Xu and A. K.-Y. Jen, Self-assembled monolayer enabling improved buried interfaces in blade-coated perovskite solar cells for high efficiency and stability, Nano Res. Energy, 2022, 1, e9120004 CrossRef.
- K. Choi, H. Choi, J. Min, T. Kim, D. Kim, S. Y. Son, G.-W. Kim, J. Choi and T. Park, A short review on interface engineering of perovskite solar cells: a self-assembled monolayer and its roles, Sol. RRL, 2020, 4, 1900251 CrossRef.
- A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis, G. Chistiakova, T. Bertram, J. A. Márquez, E. Köhnen and E. Kasparavičius, Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells, Energy Environ. Sci., 2019, 12, 3356–3369 RSC.
- Y. Hou, X. Du, S. Scheiner, D. P. McMeekin, Z. Wang, N. Li, M. S. Killian, H. Chen, M. Richter and I. Levchuk, A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells, Science, 2017, 358, 1192–1197 CrossRef CAS PubMed.
- Y. Yao, C. Cheng, C. Zhang, H. Hu, K. Wang and S. De Wolf, Organic hole-transport layers for efficient, stable, and scalable inverted perovskite solar cells, Adv. Mater., 2022, 34, 2203794 CrossRef CAS PubMed.
- I. M. Hermes, Y. Hou, V. W. Bergmann, C. J. Brabec and S. A. Weber, The interplay of contact layers: How the electron transport layer influences interfacial recombination and hole extraction in perovskite solar cells, J. Phys. Chem. Lett., 2018, 9, 6249–6256 CrossRef CAS PubMed.
- L. Zhou, M. Yan, G. Luo, L. Xu, Y. Fang and D. Yang, Self-Assembled Molecule Doping Enables High-Efficiency Hole-Transport-Layer-Free Perovskite Light-Emitting Diodes, Adv. Funct. Mater., 2023, 2303370 CrossRef CAS.
- Z. Chu, X. Chu, Y. Zhao, Q. Ye, J. Jiang, X. Zhang and J. You, Emerging low-dimensional crystal structure of metal halide perovskite optoelectronic materials and devices, Small Struct., 2021, 2, 2000133 CrossRef CAS.
- F. Meng, X. Shang, D. Gao, W. Zhang and C. Chen, Functionalizing phenethylammonium by methoxy to achieve low-dimensional interface defects passivation for efficient and stable perovskite solar cells, Nanotechnology, 2021, 33, 065201 CrossRef PubMed.
- J. Zhu, Y. Qian, Z. Li, O. Y. Gong, Z. An, Q. Liu, J. H. Choi, H. Guo, P. J. Yoo and D. H. Kim, Defect Healing in FAPb (I1-xBrx) 3 Perovskites: Multifunctional Fluorinated Sulfonate Surfactant Anchoring Enables> 21% Modules with Improved Operation Stability, Adv. Energy Mater., 2022, 12, 2200632 CrossRef CAS.
- H. Tan, F. Che, M. Wei, Y. Zhao, M. I. Saidaminov, P. Todorović, D. Broberg, G. Walters, F. Tan and T. Zhuang, Dipolar cations confer defect tolerance in wide-bandgap metal halide perovskites, Nat. Commun., 2018, 9, 3100 CrossRef PubMed.
- M. A. R. Laskar, W. Luo, N. Ghimire, A. H. Chowdhury, B. Bahrami, A. Gurung, K. M. Reza, R. Pathak, R. S. Bobba and B. S. Lamsal, Phenylhydrazinium iodide for surface passivation and defects suppression in perovskite solar cells, Adv. Funct. Mater., 2020, 30, 2000778 CrossRef CAS.
- J. Li, F. Gao, J. Wen, Z. Xu, C. Zhang, X. Hua, X. Cai, Y. Li, B. Shi and Y. Han, Effective surface passivation with 4-bromo-benzonitrile to enhance the performance of perovskite solar cells, J. Mater. Chem. C, 2021, 9, 17089–17098 RSC.
- J. Li, T. Bu, Z. Lin, Y. Mo, N. Chai, X. Gao, M. Ji, X.-L. Zhang, Y.-B. Cheng and F. Huang, Efficient and stable perovskite solar cells via surface passivation of an ultrathin hydrophobic organic molecular layer, Chem. Eng. J., 2021, 405, 126712 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © the Partner Organisations 2024 |