Oxygen defect engineering on low-crystalline iron(III) oxyhydroxide as a highly efficient electrocatalyst for water oxidation†
Yaning
Fan
a,
Junjun
Zhang
 *a,
Kongliang
Luo
a,
Xuanyu
Zhou
a,
Jiahua
Zhao
b,
Weiwei
Bao
c,
Hui
Su
d,
Nailiang
Wang
*a,
Pengfei
Zhang
*a,
Kongliang
Luo
a,
Xuanyu
Zhou
a,
Jiahua
Zhao
b,
Weiwei
Bao
c,
Hui
Su
d,
Nailiang
Wang
*a,
Pengfei
Zhang
 ab and
Zhenghong
Luo
ab and
Zhenghong
Luo
 ab
ab
aState Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan, Ningxia 750021, China. E-mail: zhangjj089@nxu.edu.cn; wangnl@nxu.edu.cn
bSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, P. R. China
cNational & Local Joint Engineering Laboratory for Slag Comprehensive Utilization and Environmental Technology, School of Material Science and Engineering, Shaanxi University of Technology, Hanzhong, Shaanxi, 723000 P. R. China
dDepartment of Chemistry, FRQNT Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke Street W., Montreal, QC H3A 0B8, Canada
First published on 9th November 2023
Abstract
Improving the water oxidation performance of non-precious nanoelectrocatalysts is the key to developing green hydrogen energy. Herein, we developed a simple method to synthesize FeOOH nanocatalysts with low crystallinity and oxygen vacancies (VO). These catalysts demonstrate excellent electrocatalytic performance for water oxidation. The VO-FeOOH catalyst exhibits an overpotential of 255 mV at 10 mA cm−2 and maintains stability for more than 120 hours at a high current output (50 mA cm−2). DFT calculations show that the rate-determining step (RDS) of VO-FeOOH and FeOOH is O* to OOH* (the Gibbs free energy (ΔG) of the RDS is 1.65 eV and 1.91 eV, respectively). This result indicates that VO can effectively reduce the energy barrier from *O to *OOH of the OER process, thus improving the activity of the VO-FeOOH nanocatalysts. Our focus was on utilizing one of the abundant metallic elements to fabricate defect-rich OER electrocatalysts with improved performance through a convenient one-step synthesis approach. This methodology shows great promise for the development of high-performance catalysts.
1. Introduction
With the continuous economic development and increase in population, the continuously depleting fossil fuels and related environmental issues have attracted increasing attention from the public. As a result, there is an escalating demand among humanity for clean, renewable energy and environmental friendly energy conversion technologies.1,2 In light of this, people began to develop energy conversion technologies, such as electrolyzers, zinc-air batteries, and fuel cells.3 The advancement of renewable hydrogen energy plays a crucial role in addressing future energy crises and achieving objectives, such as carbon neutrality.4The electrolysis of water, a hydrogen production process characterized by mild reaction conditions and minimum equipment requirements, has been continuously evolving.5 Water electrolysis can be mainly divided into the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), where the OER plays a crucial role throughout the reaction, but its inherent slow kinetic process hinders its development.6 The R&D of high-performance catalysts can effectively reduce the overpotential required for the OER process, and noble metal-based catalysts are currently the most efficient OER electrocatalysts.7 However, the scarcity of raw materials severely limits the large-scale application and commercial production.8,9 Therefore, the preparation of low-cost, efficient, and stable non-precious metal-based nanocatalysts is a key strategy to improve the OER and promote water electrolysis.10,11
In recent years, transition metal-based phosphides,12 borides,13 nitrides,14 hydroxides,15 and sulfides16 have attracted much interest as OER catalysts. Hydroxides exhibit superior electrocatalytic activity compared to noble metals IrO2 and RuO2.17 However, due to the low conductivity and the cumbersome preparation process, the utilization of FeOOH catalyst is in the initial stage.18 Three main factors influence the intrinsic property of iron(III) oxyhydroxide. First, FeOOH can be classified into four crystal structures: α (orthorhombic), β (tetragonal), δ (hexagonal), and γ (orthorhombic),19 of which β-FeOOH exhibits the highest OER activity.20,21 Furthermore, recent studies have reported that the performance of mixed-phase FeOOH is superior to that of single-phase crystals, mainly due to its higher VO.22 The second factor affecting its performance is crystallinity. Amorphous FeOOH provides abundant random orientations and unsaturated bonds for water molecules and intermediate reactions.23 The results indicate that the performance of mixed amorphous-crystalline structures surpasses that of single-phase amorphous or highly crystalline species.24 The last factor is the formation of cation or anion vacancies. Their formation leads to electron modulation and the formation of unsaturated bonds,25 thus improving the conductivity of species and OER electrocatalytic performance. At present, the main methods of generating oxygen vacancies are thermal treatment, anion/cation doping, plasma treatment, and other technical treatments (laser, flame, stripping, and template strategy).26 The reduction of NaBH4 can effectively introduce VO on the surface of the catalyst material. So far, NaBH4 reduction has been widely used to produce VO in Co3O4, CoFe2O4, NiMn2O4, Fe–Co oxides, etc.27–29
Herein, we proposed a one-step reaction method to prepare FeOOH nanosheets with low crystallinity and abundant defects. This nanocatalyst was attached to a nickel foam (NF) surface using an organic binder, and its electrochemical results demonstrated excellent catalytic properties (activity and stability). As an OER catalyst, VO-FeOOH exhibits good catalytic activity, characterized by low overpotential, reaction dynamics, and stability. At a current output of 10 milliamperes per cm2, the required overpotential was 255 mV. It exhibited stability exceeding 120 h at a current output of 50 milliamperes per cm2, surpassing most similar catalysts in terms of efficiency and stability. The low crystalline structure of the FeOOH nanosheet and the presence of VO resulted in a high specific surface area with open spaces, abundant transfer channels,18 and exposure to more active sites, as well as the formation of unsaturated coordination bonds, which accelerated charge transfer. Through detailed theoretical calculation and analysis, it is shown that the energy barrier of key rate-limiting steps is reduced by the introduction of oxygen defects. This is of certain guiding significance for the development of OER nanocatalysts.
2. Experimental section
Preparation of the VO-FeOOH sample: the VO-FeOOH sample materials were prepared by the oxidation conversion method at room temperature.30 First, 4.5 mmol Fe(NO3)3·9H2O and 15 mmol NaBH4 were dissolved in 75 ml deionized water, respectively. After fully dissolved, the NaBH4 solution was slowly added to Fe(NO3)3·9H2O solution under stirring, and a dark green liquid was obtained by stirring for 1.5 h under a N2 atmosphere. The liquid was centrifuged and washed with deionized water and anhydrous ethanol until the solid became an orange-red solid. Finally, the solid obtained by centrifugation was dried overnight at 60 °C, obtaining the final red-brown solid powder (Fig. S2†), which was recorded as the VO-FeOOH sample.3. Results and discussion
3.1 Materials synthesis and characterization
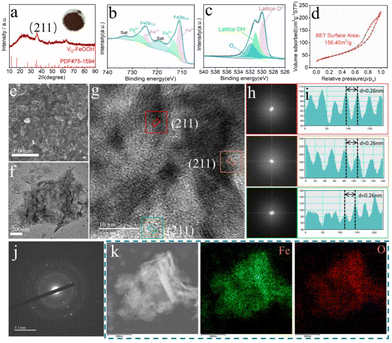
The production of low-cost catalysts for electrocatalytic hydrogen production is of great significance. The iron element is abundant in the earth's crust, and the iron-based catalyst has high catalytic activity, which is very suitable for large-scale production of electrolytic water catalysts. Low-crystalline VO-FeOOH reddish-brown powders containing VO were successfully synthesized using a straightforward oxidation conversion method at ambient temperature.30 Detailed synthesis and characterization are presented in the ESI (Fig. S1–S3†). The phase structure of this catalyst was analyzed by X-ray diffraction (XRD), as depicted in Fig. 1a. The diffraction peaks at 35.30°, 46.64°, and 64.72° correspond to the crystal planes (211), (411) and (541) of the VO-FeOOH sample (PDF#75-1594), respectively. The remaining portion exhibited an amorphous nature, indicating the successful synthesis of low-crystallinity FeOOH in this study. Simultaneously, the surface of the VO-FeOOH material was subjected to analysis using X-ray photoelectron spectroscopy (XPS). The full spectrum of XPS, as shown in Fig. S4,† reveals the coexistence of Fe and O in the compound VO-FeOOH.31Furthermore, the high-resolution Fe 2p spectrum of VO-FeOOH, as depicted in Fig. 1b, exhibits two prominent peaks centered at 711.1 eV and 724.4 eV, which correspond to Fe 2p1/2 and Fe 2p3/2.32 It shows that Fe (II) is the main component of the VO-FeOOH sample, and it also shows that unsaturated coordination bonds are formed in the sample, respectively. Additionally, two satellite peaks centered at 718.1 eV and 732.8 eV are attributed to these respective Fe 2p peaks.33 According to the data presented in Fig. 1c, the spectrum of the nuclear energy level of the O 1s exhibits three distinct peaks. These peaks, located at 529.8 eV, 531 eV, and 531.8 eV, can be attributed to O2− species, OH− groups, and adsorbed oxygen species, respectively.34
In addition, scanning electron microscopy (SEM) analysis revealed that the VO-FeOOH sample exhibited a nanosheet morphology (Fig. 1e). This nanosheet structure provided an increased number of exposed active sites on the surface, thereby facilitating adsorption and dissociation reactions within the reaction medium. Furthermore, the corresponding Brunauer Emmett Teller (BET) analysis also indicated that the VO-FeOOH sample had a significant specific surface area and a mesoporous structure concentrated in the range of 2–50 nm (Fig. 1d and S5†). According to the image presented in Fig. 1f, the nanosheet structure was further characterized using transmission electron microscopy (TEM), revealing distinct morphological features with a thin lamellar structure. To determine the thickness of VO-FeOOH nanosheets, atomic force microscopy (AFM) measurements were performed, yielding a measured thickness of approximately 150 nm, which is consistent with the results observed by the SEM image (Fig. S6†). As the OER catalytic layer, the thickness of the nanosheets not only facilitates the exposure of more active sites but also resists the impact of bubble release, thus stabilizing the catalytic process.35 Additionally, HRTEM investigations indicated that the lattice structure of VO-FeOOH exhibited a low-crystalline configuration. In particular, lattice fringes corresponding to the crystal plane of VO-FeOOH (211) were discernible in selected regions (Fig. 1g), providing evidence of the inherent low crystallinity of the VO-FeOOH sample. To directly study the phase structure of VO-FeOOH, the fast Fourier transform (FFT) of HRTEM images was used for analysis. Fig. 2g (I), (II), and (III) show three different FFT modes extracted from the red, orange, and green squares of Fig. 2g, which exhibit two wide and blurred halo diffraction rings (Fig. 2h) without any identifiable diffraction points, further revealing the characteristics of long-range disorder but short-term order.36 The lattice fringe width of VO-FeOOH was measured to be 0.26 nm from Fig. 1i. Compared with the (211) crystal plane of standard FeOOH, the lattice of the (211) crystal plane of low crystalline VO-FeOOH was increased by about 2.36%. The formation of low crystalline species likely causes the lattice of FeOOH to produce tensile strain.37
The tensile strain of the VO-FeOOH lattice was further studied by lattice images obtained from HRTEM images by inverse fast Fourier transform (IFFT) mode (Fig. S7†). The corresponding IFFT image shows that the lattice fringes on the (211) crystal plane have obvious tensile strain. The selected area electron diffraction (SAED) mode of VO-FeOOH shows that the diffraction ring is widened, which belongs to the typical low crystalline state (Fig. 1j).38 The energy dispersive X-ray (EDS) element mapping image of VO-FeOOH demonstrated an even distribution of Fe and O within the VO-FeOOH sample (Fig. 1k and S8†). Therefore, the coordination of unsaturated bonds and micro-pores and nanosheet structures of low-crystalline species have significant electrolyte permeability advantages, which are conducive to the rapid migration of electrons and electrolyte ions, thereby improving the catalytic performance.
3.2 Electrocatalytic OER performance
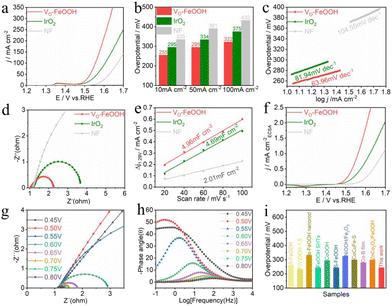
A comprehensive performance analysis of the prepared catalyst was carried out. To determine the ideal quantity of the VO-FeOOH coating per unit area of NF, the following volumes were applied: 25, 50, 75, 100, and 125 μL, respectively. Fig. S9† illustrates that the OER catalytic activity was optimized using a coating amount of 100 μL. The electrocatalytic activity was tested by LSV in 1.0 M potassium hydroxide after iR compensation. As illustrated in Fig. 2a, VO-FeOOH exhibited catalytic activity with an overpotential of 255, 295, and 335 mV at 10, 50, and 100 mA cm−2, respectively, better than IrO2 (295, 334, and 391 mV) and NF (335, 391, and 432 mV) (Fig. 2b). Furthermore, the overpotential of VO-FeOOH prepared in this work at 10 mA cm−2 surpasses that of some FeOOH catalysts and other types of nanocatalysts, as depicted in Fig. 2i and Table S1.†The kinetics of the VO-FeOOH samples were further evaluated by electrochemical impedance spectroscopy (EIS) and Tafel slopes. The results revealed that VO-FeOOH (63.96 mV dec−1) has a lower Tafel slope than the commercial catalysts IrO2 (81.94 mV dec−1) and NF (104.55 mV dec−1), elucidating that the catalyst has faster OER dynamics (Fig. 2c). The measured EIS data were fitted using an equivalent circuit diagram (Fig. S10†), and the results revealed that VO-FeOOH has a smaller charge transfer resistance and faster electrochemical OER catalytic characteristics (Fig. 2d). To further reveal the activity of VO-FeOOH, IrO2, and NF, the electrochemical active surface area (ECSA) (Fig. S11†) was determined by electric double layer capacitance (Cdl). The Cdl value of VO-FeOOH is 4.96 mF cm−2, which is higher than that of other contrast samples (Fig. 2e), indicating that there are more active sites exposed on the surface of VO-FeOOH and the OER activity is higher. The ECSA of VO-FeOOH was calculated to be 124 cm2, and its large active area should be due to its nanosheet morphology and porous structure (Fig. 2a and S5†). When the LSV curve is normalized to ECSA, VO-FeOOH exhibits higher catalytic activity than that of IrO2 and NF (Fig. 2f), demonstrating that the large specific surface area and the structure of the oxygen vacancy improve its intrinsic activity.
The intrinsic reaction kinetics of the electrode/electrolyte interface was studied by in situ electrochemical impedance spectroscopy (EIS).39Fig. 2g and S12† depict Nyquist plots for various catalysts, revealing that the resistance decreases with increasing external voltage. When compared to IrO2 and NF catalysts, the VO-FeOOH catalyst exhibits the lowest resistance across all potentials, indicating that the presence of oxygen vacancies significantly enhances the adsorption kinetics of oxygen-containing reactive species during the alkaline water oxidation process. The Bode diagram illustrates the phase relaxation process of the catalyst sample, as demonstrated in Fig. 2h and S13† for the VO-FeOOH, IrO2, and NF samples. Peaks in the low and high-frequency regions correspond to the charge transfer reaction occurring at the electrolyte and catalyst interface, as well as electron conduction within the catalyst's inner layer at high frequencies.40 As the potential increases, the electron transfer resistance in the reaction process decreases notably. In the case of VO-FeOOH, IrO2, and NF electrodes, as the potential increases, the phase angle shifts towards the high-frequency region. Compared with that of the carbon cloth, the first phase angle of NF appears at 0.55 V, while that of the carbon cloth appears at 0.6 V (Fig. S14†),41 indicating that the substrate itself has high intrinsic activity. The catalyst supported on the NF surface effectively promotes the electron transfer between the electrolyte and the catalyst, thereby promoting electron conduction. These results indicate that VO-FeOOH, IrO2, and NF share similar interfacial reaction characteristics.35,42,43
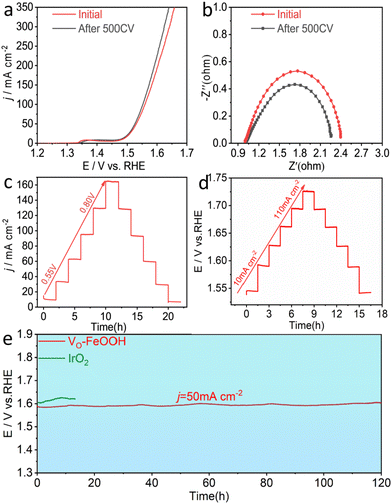
For electrocatalysis, good stability is an important application index.44 The LSV curve and EIS of the VO-FeOOH sample after 500 cycles in Fig. 3a and b show that the activity of VO-FeOOH after CV cycles is slightly improved, and the charge transfer resistance is slightly reduced. This may be due to the incomplete activation of the catalyst in the early stage, demonstrating that the VO-FeOOH catalyst has good stability under dynamic current. Fig. 3c and d show that the VO-FeOOH catalyst can remain stable at different current potentials and densities. The chronopotentiometry curve in Fig. 3e and Fig. S15† shows that the VO-FeOOH catalyst has good durability after the 120 h and 45 h OER test at 50 mA cm−2 and 100 mA cm−2, respectively, and the potential change can be ignored. However, the commercial catalyst IrO2 has a significant performance decline in less than 20 hours of operation. The VO-FeOOH sample has a higher catalytic stability than the IrO2 catalyst. It can also be explained that the VO-FeOOH coated on the surface of NF does not fall off, which proves that it is a feasible method to coat the nanocatalyst on the surface of NF.
To further evaluate the overall water splitting performance of the Vo-FeOOH catalyst, we used Pt/C and VO-FeOOH to assemble an electrolytic cell (Fig. S16a and b†). The results show that, under the same conditions, the VO-FeOOH||Pt/C electrolytic cell has better performance than the RuO2||Pt/C commercial catalyst electrolytic cell (Fig. S16c†). It is worth noting that the performance of the catalyst did not show a downward trend in the long-term stability at 10 mA cm−2, which is consistent with the results of the three-electrode system, indicating that it has excellent stability (Fig. S16d†). To explore the effect of catalyst morphology on stability, we characterized the catalyst supported on NF by SEM and TEM. The corresponding results showed that the morphology of the catalyst did not change significantly during the long-term stability test (Fig. S17a–c†). At the same time, HRTEM also proved that the phase of the catalyst did not change before and after the OER operation (Fig. S17d and e†).
To compare the electrocatalytic performance and mechanism of highly crystalline FeOOH and low crystalline VO-FeOOH, we prepared highly crystalline FeOOH samples using the previously reported wet chemical method (Fig. S18†).21 The HRTEM image of FeOOH clearly shows that it has neatly arranged lattice fringes with a width of 0.151 nm, which belongs to the (002) crystal plane of FeOOH, indicating that it has high crystallinity (Fig. S19†). We have experimentally studied the defects of catalysts and demonstrated that the defects enhance the electron transfer and the activity of individual sites in the catalytic process. Raman spectroscopy is known to be sensitive to defects and lattice disorder.45 As depicted in Fig. 4a, the conspicuous Raman peaks at 215, 279, and 391 cm−1 correspond to the asymmetric stretching vibrations between the metal and hydroxide ions in the highly crystalline FeOOH catalyst.46 These observations align with the characteristic crystalline features of FeOOH, providing further confirmation of its high crystallinity. The corresponding peak positions indicate that FeOOH exhibits Raman activity.47 Meanwhile, the Raman spectrum of VO-FeOOH showed a reduction in peak intensities corresponding to the characteristic peaks and the formation of a broad band, which aligns with the spectroscopic features of low crystallinity.48,49 The coexistence of Fe and O in the FeOOH sample was observed through the full spectrum of XPS (Fig. S20†). By fitting the high-resolution O 1s spectra of VO-FeOOH and FeOOH in Fig. S21,† it was observed that the VO peak area of the VO-FeOOH sample was distinctly larger than that of FeOOH, pointing that VO-FeOOH has more VO than FeOOH.
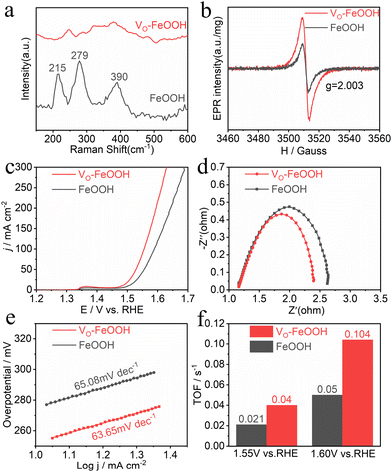 | ||
| Fig. 4 (a) The Raman spectra, (b) EPR spectra, (c) LSV curve, (d) EIS spectrum, (e) Tafel slope of VO-FeOOH and FeOOH, and (f) calculated TOF values of VO-FeOOH and FeOOH at 1.55 V and 1.60 V. | ||
Electron paramagnetic resonance (EPR) spectroscopy analysis also confirmed this conclusion (Fig. 4b). At g = 2.003, VO-FeOOH exhibited a stronger symmetric EPR signal compared to FeOOH, confirming the formation of VO in VO-FeOOH. At the same time, by comparing the peak area of Fe2+ in VO-FeOOH and FeOOH (Fig. S22†), it can be found that the area of Fe2+ in VO-FeOOH is larger than that in FeOOH, indicating that the formation of low valence Fe2+ is caused by VO species.50 Subsequent electrochemical results revealed that low-crystallinity VO-FeOOH exhibited higher electrochemical catalytic activity (Fig. 4c) and low-overpotential (Fig. S23†), faster electron transfer rate (Fig. 4d), and smaller Tafel slope (Fig. 4e) compared to high-crystallinity FeOOH. Further Cdl results also revealed that VO-FeOOH had a larger effective electrochemical specific surface area (ECSA) compared to FeOOH (Fig. S24†).
The intrinsic property of nanocatalysts is closely related to the turnover frequency (TOF),44 as shown in Fig. 4f. The TOF values of VO-FeOOH and FeOOH at potentials of 1.55 V and 1.60 V were 0.04 and 0.021 s−1, and 0.104 and 0.04 s−1, respectively, indicating that the presence of low crystallinity and VO species enhanced the property of the VO-FeOOH electrode. The corresponding BET results also indicated that the low-crystallinity VO-FeOOH had a larger specific surface area (156.40 m2 g−1) compared to the high-crystallinity FeOOH (123.58 m2 g−1) (Fig. 1d and S25a†), together with a more concentrated mesoporous distribution (Fig. S5 and S25b†). In conclusion, the research results suggest that low-crystallinity VO-FeOOH facilitates the formation of VO, thereby enhancing the formation of unsaturated sites and conductivity and thus improving the electrocatalytic activity of the OER process.
3.3 DFT simulation and catalytic mechanism
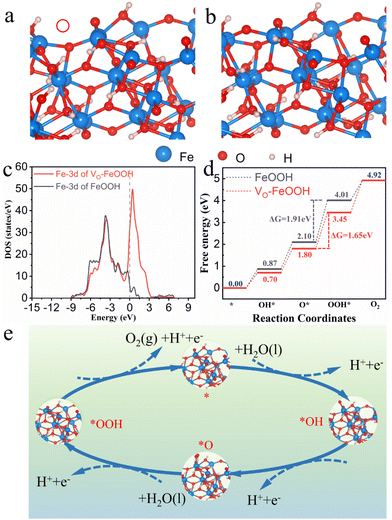
To further study the specific role of defects in the catalytic process, we carried out theoretical calculations on the catalytic process. Here, based on the experimental results depicted in Fig. 4c, the theoretical models of VO-FeOOH and FeOOH structures were constructed for different active intermediates using the AEM mechanism under alkaline conditions (Fig. 5a and b). The density of states (DOS) for VO-FeOOH and FeOOH is shown in Fig. 5c. VO has a significant impact on the DOS of FeOOH. The d-band of the VO-containing structure is closer to the Fermi level compared to FeOOH, indicating lower adsorption energy for OER active intermediates as compared to the FeOOH counterpart. VO-containing FeOOH exhibits significantly higher DOS at the Fermi level compared to FeOOH, suggesting that higher DOS enables more charge carriers to directly participate in catalytic reactions, which can significantly enhance the electrode's OER property. Furthermore, the intensity of the Fermi level aligns well with electron transfer resistance (Fig. 4d), and an analysis of different elements reveals that the presence of VO can effectively enhance the DOS of different atoms (Fig. S26†), introducing more active catalytic sites, thereby facilitating the progress of the OER process.51,52Then we continue to study the OER catalytic activity of VO-FeOOH, and further calculate the different models (Fig. S27 and S28†) based on the ΔG change of the 4e− pathway to elucidate the water oxidation energy barrier of VO-FeOOH and FeOOH counterpart.53 The OER process of the widely accepted AEM mechanism can be divided into four stages, including three intermediate states (OH*, O*, and OOH*) (Fig. 5d). All possible reaction paths on VO-FeOOH are shown in Fig. 5d and e, and the reaction path with the highest energy required is O* → OOH*, indicating that this step is the RDS in VO-FeOOH and FeOOH counterpart. The ΔGmax in VO-FeOOH is 1.65 eV, which is less than the 1.91 eV in FeOOH, indicating that VO contributes to the formation of more strong active sites in VO-FeOOH, thereby enhancing the OER performance of VO-FeOOH samples. The DFT calculation showed that the defects optimized the adsorption capacity of the intermediates during the corresponding catalytic process, effectively reduced the energy barrier, and finally improved the catalytic efficiency and stability. On the whole, we improved the oxygen defect level of the FeOOH nanocatalyst through a simple strategy, and used the defects to improve the activation ability of the rate-limiting step of the water oxidation process, and finally improved the activity and stability of cheap iron(III) oxyhydroxide.
4. Conclusions
In summary, the VO-FeOOH nanosheets with low crystallinity and abundant VO were synthesized by a simple oxidation conversion method under an inert N2 atmosphere. The results showed that VO-FeOOH nanosheets provided a larger reaction area for the OER process and provided a fast channel for charge transfer, exposing more potential active sites. Defect-enriched VO-FeOOH exhibits excellent catalytic performance in the water oxidation process. The overpotential at 10 mA cm−2 is 255 mV, and it can operate stably for 120 h (j@50 mA cm−2), and the reaction kinetics are better than those of the FeOOH counterpart. DFT calculations show that VO plays an important role in the OER process. VO can effectively promote the d-band to approach the Fermi level and improve the conductivity of the VO-FeOOH sample. At the same time, the ΔG of the RDS in the OER process was reduced from 1.65 eV to 1.91 eV, which improved the OER catalytic activity of VO-FeOOH. The study provides an effective path for the synthesis of low-cost, high-performance energy conversion electrodes with a defective structure and lays a valuable theoretical foundation for the structure and performance of OER catalysts.Author contributions
Yaning Fan: Data acquisition, writing-review & editing. Junjun Zhang: Funding acquisition, writing-review & editing. Kongliang Luo: Data acquisition, writing-review & editing. Xuanyu Zhou: Visualization. Weiwei Bao: Data acquisition, writing-review & editing. Hui Su: Writing & editing. Jiahua Zhao and Nailiang Wang: Writing & editing. Pengfei Zhang: Visualization and acquisition of funds. Zhenghong Luo: Supervision.Conflicts of interest
The authors declare that they have no conflict of interests.Acknowledgements
This work was supported by Ningxia Key Research and Development Program (No. 2021BEE03007), Inner Mongolia R & D Program Plan (2021ZD0042, 2021EEDSCXSFQZD006) and National Natural Science Foundation of China (Grant No. 21902123). Junjun Zhang thanks the Chinese Academy of Sciences Western Young Scholar Program for the scholarships and the Scientific Research Start-Up Project Program of the Ningxia University.References
- H. Xie, Z. Zhao, T. Liu, Y. Wu, C. Lan, W. Jiang, L. Zhu, Y. Wang, D. Yang and Z. Shao, A membrane-based seawater electrolyser for hydrogen generation, Nature, 2022, 612, 673–678 CrossRef CAS.
- Z. Shi, X. Zhang, X. Lin, G. Liu, C. Ling, S. Xi, B. Chen, Y. Ge, C. Tan, Z. Lai, Z. Huang, X. Ruan, L. Zhai, L. Li, Z. Li, X. Wang, G.-H. Nam, J. Liu, Q. He, Z. Guan, J. Wang, C.-S. Lee, A. R. J. Kucernak and H. Zhang, Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution, Nature, 2023, 621, 300–305 CrossRef CAS.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- L. Chong, G. Gao, J. Wen, H. Li, H. Xu, Z. Green, J. D. Sugar, A. J. Kropf, W. Xu, X. M. Lin, H. Xu, L.-W. Wang and D.-J. Liu, La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis, Science, 2023, 380, 609–616 CrossRef CAS.
- X. Liang, K. X. Zhang, Y. C. Shen, K. Sun, L. Shi, H. Chen, K. Y. Zheng and X. X. Zou, Perovskite-type water oxidation electrocatalysts, J. Electrochem., 2022, 28, 2214004 Search PubMed.
- L. Zhou, C. Yang, W. Zhu, R. Li, X. Pang, Y. Zhen, C. Wang, L. Gao, F. Fu, Z. Gao and Y. Liang, Boosting alkaline hydrogen evolution reaction via an unexpected dynamic evolution of molybdenum and selenium on MoSe2 Electrode, Adv. Energy Mater, 2022, 12, 2202367 CrossRef CAS.
- Z. Nie, T. Liu, Y. Chen, P. Liu, Y. Zhang, Z. Fan, H. He, S. Chen and F. Zhang, In-situ growing low-crystalline Co9S8Ni3S2 nanohybrid on carbon cloth as a highly active and ultrastable electrode for the oxygen evolution reaction, Electrochim. Acta, 2022, 402, 139558 CrossRef CAS.
- Y. Sun, W. Cao, X. Ge, X. Yang, Y. Wang, Y. Xu, B. Ouyang, Q. Shen and C. Li, Built-in electric field induced interfacial charge distributions of Ni2P/NiSe2 heterojunction for urea-assisted hydrogen evolution reaction, Inorg. Chem. Front., 2023, 10, 6674–6682 RSC.
- J. Zhao, Q. Niu, J. Zhang and P. Zhang, Core–shell construction of metal@carbon by mechanochemically recycling plastic wastes: towards an efficient oxygen evolution reaction, Green Chem., 2023, 25, 8047–8056 RSC.
- A. K. Tareen, G. S. Priyanga, K. Khan, E. Pervaiz, T. Thomas and M. Yang, Nickel-based transition metal nitride electrocatalysts for the oxygen evolution reaction, ChemSusChem, 2019, 12, 3941–3954 CrossRef CAS.
- F. Yue, C. Wang, W. Duan, H. Pang, T. Wei, K. Xue, D. Wang, F. Fu and C. Yang, Selenium vacancies regulate d-band centers in Ni3Se4 toward paired electrolysis in anion-exchange membrane electrolyzers for upgrading N-containing compounds, Sci. China: Chem., 2023, 66, 2109–2120 CrossRef CAS.
- Y. Ren, Z. Li, B. Deng, C. Ye, L. Zhang, Y. Wang, T. Li, Q. Liu, G. Cui, A. M. Asiri, Y. Luo and X. Sun, Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt, Int. J. Hydrogen Energy, 2022, 47, 3580–3586 CrossRef CAS.
- Y. Bai, Y. Wu, X. Zhou, Y. Ye, K. Nie, J. Wang, M. Xie, Z. Zhang, Z. Liu, T. Cheng and C. Gao, Promoting nickel oxidation state transitions in single-layer NiFeB hydroxide nanosheets for efficient oxygen evolution, Nat. Commun., 2022, 13, 6094 CrossRef CAS.
- S. Anantharaj, S. Kundu and S. Noda, “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts, Nano Energy, 2021, 80, 105514 CrossRef CAS.
- Y. Hu, Y. Zheng, J. Jin, Y. Wang, Y. Peng, J. Yin, W. Shen, Y. Hou, L. Zhu, L. An, M. Lu, P. Xi and C.-H. Yan, Understanding the sulphur-oxygen exchange process of metal sulphides prior to oxygen evolution reaction, Nat. Commun., 2023, 14, 1949 CrossRef CAS.
- R. Yu, C. Wang, D. Liu, Z. Wu, J. Li and Y. Du, Bimetallic sulfide particles incorporated in Fe/Co-based metal–organic framework ultrathin nanosheets toward boosted electrocatalysis of the oxygen evolution reaction, Inorg. Chem. Front., 2022, 9, 3130–3137 RSC.
- X. Liang, S. Wang, J. Feng, Z. Xu, Z. Guo, H. Luo, F. Zhang, C. Wen, L. Feng, C. Wan and M.-M. Titirici, Structural transformation of metal–organic frameworks and identification of electrocatalytically active species during the oxygen evolution reaction under neutral conditions, Inorg. Chem. Front., 2023, 10, 2961–2977 RSC.
- Y. Zhang, X.-A. Teng, Z.-Q. Ma, R.-M. Wang, W.-M. Lau and A.-X. Shan, Synthesis of FeOOH scaly hollow tubes based on Cu2O wire templates toward high-efficiency oxygen evolution reaction, Rare Met., 2023, 42(6), 1836–1846 CrossRef CAS.
- X. Xu, F. Song and X. Hu, A nickel iron diselenide-derived efficient oxygen-evolution catalyst, Nat. Commun., 2016, 7, 12324 CrossRef CAS.
- Y. Sakamoto, Y. Noda, K. Ohno, K. Koike, K. Fujii, T. M. Suzuki, T. Morikawa and S. Nakamura, First principles calculations of surface dependent electronic structures: a study on β-FeOOH and γ-FeOOH, Phys. Chem. Chem. Phys., 2019, 21, 18486–18494 RSC.
- J. J. Zhang, W. W. Bao, M.-Y. Li, C. M. Yang and N.-N. Zhang, Ultrafast formation of an FeOOH electrocatalyst on Ni for efficient alkaline water and urea oxidation, Chem. Commun., 2020, 56, 14713–14716 RSC.
- J. Hu, S. Li, J. Chu, S. Niu, J. Wang, Y. Du, Z. Li, X. Han and P. Xu, Understanding the phase-induced electrocatalytic oxygen evolution reaction activity on FeOOH nanostructures, ACS Catal., 2019, 9, 10705–10711 CrossRef CAS.
- L. Wu, M. Ning, X. Xing, Y. Wang, F. Zhang, G. Gao, S. Song, D. Wang, C. Yuan, L. Yu, J. Bao, S. Chen and Z. Ren, Boosting oxygen evolution reaction of (Fe,Ni)OOH via defect engineering for anion exchange membrane water electrolysis under industrial conditions, Adv. Mater., 2023, 2306097 CrossRef CAS.
- Y. Qiu, Q. Jia, S. Yan, B. Liu, J. Liu and X. Ji, Favorable amorphous−crystalline Iron oxyhydroxide phase boundaries for boosted alkaline water oxidation, ChemSusChem, 2020, 13, 4911–4915 CrossRef CAS.
- D. Yan, Y. Li, J. Huo, R. Chen, L. Dai and S. Wang, Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions, Adv. Mater., 2017, 29, 1606459 CrossRef.
- K. Zhu, F. Shi, X. Zhu and W. Yang, The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction, Nano Energy, 2020, 73, 104761 CrossRef CAS.
- S. Peng, F. Gong, L. Li, D. Yu, D. Ji, T. Zhang, Z. Hu, Z. Zhang, S. Chou, Y. Du and S. Ramakrishna, Necklace-like multishelled hollow spinel oxides with oxygen vacancies for efficient water electrolysis, J. Am. Chem. Soc., 2018, 140, 13644–13653 CrossRef CAS.
- H. Sun, Y. Zhao, K. Mølhave, M. Zhang and J. Zhang, Simultaneous modulation of surface composition, oxygen vacancies and assembly in hierarchical Co3O4 mesoporous nanostructures for lithium storage and electrocatalytic oxygen evolution, Nanoscale, 2017, 9, 14431–14441 RSC.
- K. L. Yan, X. Shang, Z. Z. Liu, B. Dong, S. S. Lu, J. Q. Chi, W. K. Gao, Y. M. Chai and C.-G. Liu, A facile method for reduced CoFe2O4 nanosheets with rich oxygen vacancies for efficient oxygen evolution reaction, Int. J. Hydrogen Energy, 2017, 42, 24150–24158 CrossRef CAS.
- J. Lu, Z. Wang, Y. Guo, Z. Jin, G. Cao, J. Qiu, F. Lian, A. Wang and W. Wang, Ultrathin nanosheets of FeOOH with oxygen vacancies as efficient polysulfide electrocatalyst for advanced lithium–sulfur batteries, Energy Storage Mater., 2022, 47, 561–568 CrossRef.
- X. Bai, Y. Fan, C. Hou, T. Tang and J. Guan, Partial crystallization of Co–Fe oxyhydroxides towards enhanced oxygen evolution activity, Int. J. Hydrogen Energy, 2022, 47, 16711–16718 CrossRef CAS.
- H. l. Wei, A. D. Tan, S. Z. Hu, J. H. Piao and Z. Y. Fu, Efficient spinel iron-cobalt oxide/nitrogen-doped ordered mesoporous carbon catalyst for rechargeable zinc-air batteries, Chin. J. Catal., 2021, 42, 1451–1458 CrossRef CAS.
- P. Ma, S. Luo, Y. Luo, X. Huang, M. Yang, Z. Zhao, F. Yuan, M. Chen and J. Ma, Vertically aligned FeOOH nanosheet arrays on alkali-treated nickel foam as highly efficient electrocatalyst for oxygen evolution reaction, J. Colloid Interface Sci., 2020, 574, 241–250 CrossRef CAS.
- G. Ge, M. Liu, C. Liu, W. Zhou, D. Wang, L. Liu and J. Ye, Ultrathin FeOOH nanosheets as an efficient cocatalyst for photocatalytic water oxidation, J. Mater. Chem. A, 2019, 7, 9222–9229 RSC.
- J. J. Zhang, W. W. Bao, X. H. Feng, C. M. Yang, N. L. Wang, Y. J. Qiu, J. T. Li, P. F. Zhang and Z. H. Luo, Layered double hydroxides as a robust catalyst for water oxidation through strong substrate-catalytic layer interaction, Int. J. Hydrogen Energy, 2023, 48, 35038–35049 CrossRef CAS.
- J. Feng, L. Wu, S. Liu, L. Xu, X. Song, L. Zhang, Q. Zhu, X. Kang, X. Sun and B. Han, Improving CO2−to-C2+ Product electroreduction efficiency via atomic lanthanide dopant-induced tensile-strained CuOx catalysts, J. Am. Chem. Soc., 2023, 145, 9857–9866 CrossRef CAS.
- Y. Zhou, Y. Wu, D. Guo, J. Li, Y. Li, X. Yang, S. Fu, G. Sui and D. F. Chai, Novel strain engineering combined with a microscopic pore synergistic modulated strategy for designing lattice tensile-strained porous V2C-MXene for high-performance overall water splitting, ACS Appl. Mater. Interfaces, 2023, 15, 15797–15809 CrossRef CAS.
- Y. Tian, M. Li, Z. Wu, Q. Sun, D. Yuan, B. Johannessen, L. Xu, Y. Wang, Y. Dou, H. Zhao and S. Zhang, Edge-hosted atomic Co−N4 sites on hierarchical porous carbon for highly selective two-electron oxygen reduction reaction, Angew. Chem., Int. Ed., 2022, 61, e202213296 CrossRef CAS.
- D. Wang, S. Ruan, P. Ma, R. Wang, X. Ding, M. Zuo, L. Zhang, Z. Zhang, J. Zeng and J. Bao, Confinement synergy at the heterointerface for enhanced oxygen evolution, Nano Res., 2023, 16, 8793–8799 CrossRef CAS.
- L. Deng, S.-F. Hung, Z.-Y. Lin, Y. Zhang, C. Zhang, Y. Hao, S. Liu, C.-H. Kuo, H.-Y. Chen, J. Peng, J. Wang and S. Peng, Valence oscillation of Ru active sites for efficient and pobust acidic water oxidation, Adv. Mater., 2023 DOI:10.1002/adma.202305939.
- T. Wu, S. Xu, Z. Zhang, M. Luo, R. Wang, Y. Tang, J. Wang and F. Huang, Bimetal modulation stabilizing a metallic heterostructure for efficient overall water splitting at large current density, Adv. Sci., 2022, 9, 2202750 CrossRef CAS.
- Y. Li, W. Bao, J. Zhang, T. Ai, D. Wu, H. Wang, C. Yang and L. Feng, Ultrathin MoS2 nanosheets decorated on NiSe nanowire arrays as advanced trifunctional electrocatalyst for overall water splitting and urea electrolysis, J. Ind. Eng. Chem., 2023, 121, 510–518 CrossRef CAS.
- C. Yang, C. Wang, L. Zhou, W. Duan, Y. Song, F. Zhang, Y. Zhen, J. Zhang, W. Bao, Y. Lu, D. Wang and F. Fu, Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation via coupling electrocatalytic oxidation 5-hydroxymethylfurfural, Chem. Eng. J., 2021, 422, 130125 CrossRef CAS.
- C. Wang, B. Yan, Z. Chen, B. You, T. Liao, Q. Zhang, Y. Lu, S. Jiang and S. He, Recent advances in carbon substrate supported nonprecious nanoarrays for electrocatalytic oxygen evolution, J. Mater. Chem. A, 2021, 9, 25773–25795 RSC.
- Y. Cao, L. Shen, X. Hu, Z. Du and L. Jiang, Low temperature desulfurization on Co-doped α-FeOOH: Tailoring the phase composition and creating the defects, Chem. Eng. J., 2016, 306, 124–130 CrossRef CAS.
- J. Zhao, B. Wu, X. Huang, Y. Sun, Z. Zhao, M. Ye and X. Wen, Efficient and Durable Sodium, Chloride-doped Iron oxide-hydroxide nanohybrid-promoted capacitive deionization of saline water via synergetic pseudocapacitive process, Adv. Sci., 2022, 9, 2201678 CrossRef CAS.
- C. F. Li, L. J. Xie, J. W. Zhao, L. F. Gu, H. B. Tang, L. Zheng and G. R. Li, Interfacial Fe−O−Ni−O−Fe bonding regulates the active Ni sites of Ni-MOFs via iron doping and decorating with FeOOH for super-efficient oxygen evolution, Angew. Chem., Int. Ed., 2022, 61, e202116934 CrossRef CAS.
- Y. Zhang, P. Yan, Q. Wan, K. Wu and N. Yang, Morphology-dependent electrochemistry of FeOOH nanostructures, Electrochem. Commun., 2016, 68, 10–14 CrossRef CAS.
- S. Ye, J. Wang, J. Hu, Z. Chen, L. Zheng, Y. Fu, Y. Lei, X. Ren, C. He, Q. Zhang and J. Liu, Electrochemical construction of low-crystalline CoOOH nanosheets with short-range ordered grains to improve oxygen evolution activity, ACS Catal., 2021, 11, 6104–6112 CrossRef CAS.
- W. G. Lim, C. Jo, A. Cho, J. Hwang, S. Kim, J. W. Han and J. Lee, Approaching ultrastable high-rate Li–S Batteries through hierarchically porous titanium nitride synthesized by multiscale phase separation, Adv. Mater., 2019, 31, 1806547 CrossRef.
- H. Wu, Q. Lu, Y. Li, J. Wang, Y. Li, R. Jiang, J. Zhang, X. Zheng, X. Han, N. Zhao, J. Li, Y. Deng and W. Hu, Rapid joule-heating synthesis for manufacturing high-entropy oxides as efficient electrocatalysts, Nano Lett., 2022, 22, 6492–6500 CrossRef CAS.
- B. Qiu, L. Cai, Y. Wang, Z. Lin, Y. Zuo, M. Wang and Y. Chai, Fabrication of nickel–cobalt bimetal phosphide nanocages for enhanced oxygen evolution catalysis, Adv. Funct. Mater., 2018, 28, 1706008 CrossRef.
- E. Wang, M. Guo, J. Zhou and Z. Sun, Reasonable Design of MXene-supported dual-atom catalysts with high catalytic activity for hydrogen evolution and oxygen evolution reaction: A first-principles investigation, Materials, 2023, 16, 1457 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi02043f |
| This journal is © the Partner Organisations 2024 |