Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reaction of methionine with chlorine: kinetics, product formation, and potential use as a scavenger in chlorine dioxide-based systems†
Mischa
Jütte
 a,
Josephine
Heyns
a,
Mohammad Sajjad
Abdighahroudi
a,
Josephine
Heyns
a,
Mohammad Sajjad
Abdighahroudi
 a,
Christoph
Schüth
bc and
Holger V.
Lutze
a,
Christoph
Schüth
bc and
Holger V.
Lutze
 *acd
*acd
aTechnical University of Darmstadt, Institute IWAR, Chair of Environmental Analytics and Pollutants, Franziska-Braun-Straße 7, 64287 Darmstadt, Germany. E-mail: h.lutze@iwar.tu-darmstadt.de
bTechnical University of Darmstadt, Institute of Applied Geosciences, Schnittspahnstr. 9, 64287 Darmstadt, Germany
cIWW Water Centre, Moritzstraße 26, D-45476 Mülheim an der Ruhr, Germany
dCentre for Water and Environmental Research (ZWU), Universitätsstraße 5, D-45141 Essen, Germany
First published on 4th November 2023
Abstract
The present study investigates the reaction of methionine with free available chlorine (FAC) and estimates the usability of methionine as a selective scavenger for intrinsically formed FAC in chlorine dioxide (ClO2)-based reactions. It has been reported that ClO2 forms chlorite and FAC in the reaction with phenolic compounds. However, for some reactive moieties such as reduced sulfur-containing compounds, no quantification of FAC could be carried out yet, due to the limitation of the current methods which arise from the high reactivity of these compounds with both ClO2 and FAC. Methionine reacts fast with FAC (kapp = 6.8 × 108 M−1 s−1 at pH 7), and very slow with ClO2 (kapp = 10−2 M−1 s−1 at pH 7). Methionine sulfoxide and chloride are formed in equal parts in the reaction of methionine with FAC, as stable products. Hence the yield of methionine sulfoxide and chloride can principally be used to quantify intrinsically formed FAC. While the results for phenol were in accordance with the literature (50% chlorite and 50% FAC), hydroquinone and 1,4-dimethylpiperazine also resulted in methionine sulfoxide yields, even though no FAC formation has been reported for these compounds. It is possible that reactive organic intermediates are formed, which additionally causes methionine sulfoxide formation (e.g., semiquinone or phenoxy radicals). Based on this study, the FAC formation in the reaction of glutathione with ClO2 can be confirmed and is expected to be 50%. However, since methionine sulfoxide can be formed by reactive organic intermediate species, quantitative statements should be handled with care.
Water impactThis study showed that methionine can be used to scavenge intrinsically formed FAC in reactions of organic compounds with ClO2. This finding will increase the knowledge about ClO2 reactions and thus help to improve ClO2-based water treatment. |
Introduction
Chlorine dioxide (ClO2) based oxidation is increasingly used in water treatment since it is less prone to form halogenated disinfection by-products (DBPs) compared to chlorine which forms DBPs such as trihalomethane (THM).1,2 Indeed, ClO2 shows less THM formation compared to chlorine.3 However, it is proven that in its reactions with specific reactive moieties (e.g., phenols) free available chlorine (FAC) is formed as a reaction product.4–8 Although the most abundant reaction product is chlorite (ClO2−), in the reaction with most phenolic moieties, FAC and ClO2− are formed in 50% ratios each.4–8 In the reaction with saturated nitrogen-containing heterocycles, ClO2 is reported to react mainly via electron transfer, and nearly 100% ClO2− is formed.9 Other studies showed that ClO2 could react with specific amino acids and form 50% FAC and 50% ClO2−.7,10,11 The fate of this intrinsically formed FAC has not been fully investigated yet. Intrinsically formed FAC may cause the formation of harmful DBPs in ClO2 treatment. However, FAC as secondary oxidant might positively affect pollutant degradation and disinfection.12,13 For instance, the reaction with phenols and amines, which are reactive moieties of a broad range of micropollutants, differs strongly between ClO2 and FAC. While phenolic compounds react fast with ClO2,12 they react slow with FAC.14 On the other hand, FAC reacts very fast with primary amines, which react slow with ClO2. This synergy might allow to degrade a broader spectrum of pollutants during ClO2 based (waste)water treatment.4,12The challenge of determining intrinsic FAC formation is to outcompete all other reactions that FAC might undergo in the corresponding reaction system (cf. reactivities of FAC).14 This can only be done with a selective scavenger (for FAC reactions) which provides full scavenging of FAC without reacting with ClO2. Different approaches have been used so far. One reported approach is the addition of ammonia as a selective scavenger.5 Thereby, ammonia reacts several orders of magnitude faster with FAC than ClO2 (kapp (FAC + NH3) = 1.3 × 104 M−1 s−1 at pH 7, kapp (ClO2 + NH3) = <10−6 M−1 s−1 at pH 7)14,15 and forms chloramine (NH2Cl), which can be quantitatively detected.5 Terhalle et al., used bromide (Br−) as FAC scavenger. Br− reacts fast with FAC (kapp = 5.3 × 103 M−1 s−1 at pH 7)14 and forms free available bromine (FAB), which further reacts with phenol to form bromophenols and the quantification of intrinsic FAC was carried out by quantification of formed bromophenols.4 Glycine has also been used as a selective scavenger.6–9 Glycine reacts fast with FAC (kapp = 1.5 × 105 M−1 s−1 at pH 7)14 and slow with ClO2 (kapp = <10−5 M−1 s−1 at pH 7)15 and is, therefore, a suitable scavenger for intrinsically formed FAC. The reaction of FAC with glycine forms chloro–glycine (Cl–Gly), which can be detected photometrically in the presence of iodide in excess16 or with IC simultaneous to other chlorine species formed in the reaction (glycine method).17 The sensitivity of the glycine method can be further increased by installing post column reaction using potassium iodide as reactant and ammonium molybdate as a catalyzer.17 However, it has been shown that Cl–Gly can undergo follow-up reactions with intrinsically formed reactive species (e.g., ortho-benzoquinone) which would result in a wrong interpretation of the FAC yields.8
All methods mentioned above have the drawback that the second-order reaction rate constant of FAC with the scavenging compound is the limiting factor. Thus, most sulfur-containing compounds can hardly be investigated in terms of FAC formation because the reaction rate of FAC with these sulfur-containing compounds is several orders of magnitude faster compared to the reaction rate of FAC with the aforementioned scavengers.14 For full scavenging of FAC high scavenger-surpluses over the compound under study are required, which may result in undesired ClO2 scavenging. Ison et al. gave evidence that FAC might be formed in the reactions of cysteine and glutathione (GSH) with ClO2.18 However, the available FAC determination methods are not capable of quantifying the FAC yields in these reactions. This study investigates methionine as a novel scavenger for the determination of intrinsically formed FAC in the reaction of ClO2 with sulfur-containing compounds. The reaction rate for methionine with FAC is kapp = 6.8 × 108 M−1 s−1 at pH 7 (ref. 14) and methionine has been reported to be unreactive toward ClO2 at pH 6.19 A proposed pathway for the reaction of methionine shows that the reaction stoichiometry of FAC with methionine is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and yields 1 molecule of methionine sulfoxide (MSO) and one molecule of chloride (Cl−).14
1 and yields 1 molecule of methionine sulfoxide (MSO) and one molecule of chloride (Cl−).14
Material and methods
Chemicals, instruments, & methods
The chemicals used in this study and their purpose of use are listed in Table S1.† Table S2† provides an overview of the instruments used in this study. Finally, Tables S3 and S4† show the IC and LC method used for detection, respectively.Production of ClO2 and FAC solutions
ClO2 solutions were produced on-site by using the persulfate–chlorite method. Detailed information on the procedure is provided in literature.4 Since ClO2 is an unstable and volatile oxidant, the concentration of the ClO2 stock solution needs to be measured before every experiment. This was carried out by direct absorption measurement at λ = 359 nm (ε359 = 1250 M−1 s−1).20 ClO2 stock solutions were stored in the dark at 4 °C and were used until the concentration of ClO2 dropped below 80% of the initial concentration after production. Detailed information about the impurities of the stock solution can be found in previous work.8FAC solutions were prepared daily before every experiment. A 15% FAC solution was 400-fold diluted in pure water and the FAC concentration was measured by direct absorption measurement at λ = 292 nm (ε292 = 350 M−1 cm−1) (note that the concentration is determined by measuring absorption of OCl− thus, pH should be above 10 (pKa = 7.54)).17
Determination of reaction kinetics
The second-order reaction constant (kapp) for the reaction of methionine with ClO2 has been determined by pseudo-first-order kinetics by monitoring the UV absorbance of ClO2 over time. Therefore, a reaction solution containing different concentrations of methionine and ClO2 was mixed in a 3 mL quartz cuvette (optical path length 1 cm), and the adsorption of ClO2 at λ = 359 nm was monitored over time (data points were measured every 2 seconds for 30 minutes). Furthermore, the reaction solution contained 5 mM phosphate buffer to ensure a constant pH of 7. ClO2 was added with two different molar ratios towards methionine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). All experiments were carried out in triplicates.
100). All experiments were carried out in triplicates.
By plotting the ClO2 degradation as ln([ClO2]/[ClO2]0) over time, the pseudo-first-order rate constant (kobs) of the reaction can be calculated. The slope of this plot (kobs) needs to be divided by the initial concentration of the compounds in excess ([A]), which gives the second-order reaction rate constant according to eqn (1).
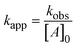 | (1) |
Formation of chloride and MSO in methionine oxidation by FAC
The inorganic reaction products of the reaction of methionine with FAC have been determined via IC. Therefore, 100 μM methionine solution reacted with different concentrations of FAC (20–100 μM) at pH 7. After a reaction time of 10 minutes, to ensure full turnover of FAC (kapp (FAC + methionine) = 6.8 × 108 M−1 s−1),14 the aliquots were transferred into IC vials (polypropylene (PP)) and measured with the IC method described in Table S3.† One has to take into account that the stock solution of FAC contains high impurities of Cl−. Therefore, the determination of Cl− impurities is necessary. For this purpose, identical FAC concentrations were dosed into a 10 mM glycine solution (further containing 5 mM phosphate buffer at pH 7) to scavenge all FAC species. The remaining Cl− can be quantified and later subtracted from the measured Cl− concentration in the reaction of methionine with FAC.The formation of MSO as a reaction product was measured via LC–MS/MS, described in Table S4.† The formation of MSO was measured as the reaction product of methionine with FAC and calibrated with the commercially available standard. To determine the reaction products of methionine and FAC, different doses of FAC were added to 100 μM methionine solution, buffered at pH 7. Formed MSO was quantified using a calibration based on external standards which were commercially available. All experiments were carried out in triplicates.
Model compounds
To test the suitability of the developed method, four model compounds were chosen with different chlorine balances (sum of different chlorine species formed per consumed ClO2). While phenol is reported to show 50% FAC and 50% ClO2− formation,4,5 1,4-dimethylpiperazine (DMP) and hydroquinone have been observed to form 100% ClO2− in ClO2-based reactions as inorganic by-products.6,9 GSH was investigated as a sulfur-containing model compound.Reaction solutions contained 100 μM of the model compound, 1 mM methionine, and 5 mM phosphate buffer adjusted to pH 7. ClO2 was added in different doses (50, 100, and 200 μM). After a reaction time of 30 min, aliquots were transferred to LC (glass) and IC (PP) vials and were measured by IC and LC–MS/MS described in Tables S3 and S4.† All chlorine balances were determined in triplicates. For determination of FAC recovery a defined dose of FAC (100 μM) was added to an aliquot of the exact same reaction solutions which was used for ClO2 treatment. The FAC recovery rate was determined by the ratio of measured vs. added FAC. By this any matrix effects of the reaction solution could be observed/ruled out. Note, that is approach does not cover the interference by intrinsically formed species.8
Follow-up reactions
To investigate the follow-up reactions of GSH with ClO2−, different GSH doses were dosed to 100 μM ClO2− in presence of 10 mM glycine at pH 7. The chlorine balance of these solutions were measured after 5 min, 24 hours, and 48 hours.To investigate if formed ClO2− is causing MSO formation by reacting with methionine, a long-term monitoring experiment was conducted. Thereby 100 μM ClO2− and 100 μM methionine were mixed in the presence of 5 mM phosphate buffer at pH 7. Then ClO2−, Cl−, and MSO were monitored over time. To achieve comparable results, it was ensured that IC and LC were always measured simultaneously (same time of sample injection). The total period was set to 24 hours. Cl− samples need to be blank corrected and the impurity of ClO2− solutions (purity = 80%) also contains Cl−. Thus, the Cl− concentration of the impurity needs to be determined as well and subtracted from the final result.
To investigate the follow-up reaction of methionine with benzoquinone, as reactive organic transformation product, 100 μM methionine and 100 μM benzoquinone were mixed at pH 7 and MSO formation was monitored for 18 hours.
Results and discussion
Reaction kinetics
To investigate if methionine is a suitable scavenger for the intrinsically formed FAC as the secondary oxidant in ClO2-based reactions, two features are necessary. First, the second-order reaction rate constant for the reaction with FAC should be high, which has been reported for methionine to be kapp = 6.8 × 108 M−1 s−1 at pH 7.14 Furthermore, the second-order reaction rate constant for the reaction with ClO2 should be several orders of magnitude lower. The second-order rate constant for the reaction of methionine and ClO2 has not been reported in the literature yet. Table 1 shows the obtained pseudo-first-order reaction rate constants and the second-order reaction rate constants for different ratios between methionine and ClO2.Molar ratios methionine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ClO2 ClO2 |
k obs [s−1] | k app [M−1 s−1] |
|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
5.05 (± 0.02) × 10−5 | 1.01 (± 0.01) × 10−2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5.82 (± 0.02) × 10−4 | 1.16 (± 0.01) × 10−2 |
Overall, the second-order rate constant of methionine with ClO2 is low (10−2 M−1 s−1) and ten orders of magnitude lower than the corresponding reaction with FAC. Thus, methionine can effectively and selectively scavenge FAC in the presence of ClO2.
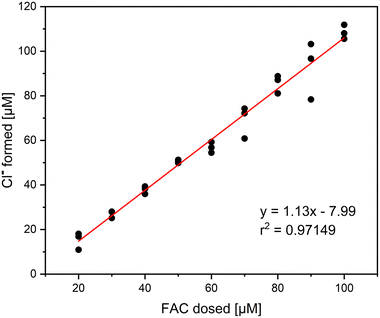
Formation of chloride and MSO
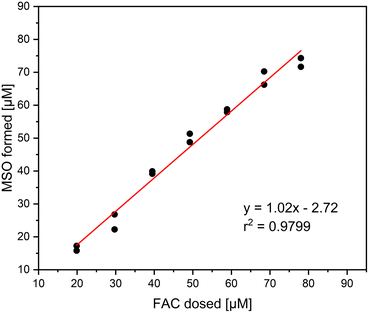
It was investigated if Cl− is the only inorganic reaction product of the reaction of methionine and FAC. In that, different concentrations of FAC were added to a 0.1 mM methionine solution and measured with IC. The relative yields based on the dosed FAC concentration are shown in Fig. 1. It is visible that Cl− is the main reaction product in this reaction, with yields ranging between 90 and 110%. The standard deviation of the triplicate measurement is relatively high in this experiment, which can be explained by the high Cl− background concentrations in the FAC stock solution. Despite the high Cl− background concentrations, precise determination of Cl− is possible. It has to be mentioned that no other chlorine species (i.e., ClO2−, chlorate (ClO3−)) were detected in this experiment, indicating that the reaction of FAC with methionine forms 100% Cl− which is according to the proposed reaction mechanism (Fig. S1†).14The formation of MSO as the reaction product of the reaction of methionine with FAC has been investigated as well. Thereby, the focus was to determine the yield of MSO per FAC consumed. The MSO yields are later required to correlate with intrinsically formed FAC yields when methionine is applied as a FAC scavenger. For this purpose, FAC was added in different concentrations to methionine and the formed MSO was measured (determined using commercially available MSO standards). The amount of FAC added was than correlated with MSO formed to determine the MSO yield (Fig. 2).
The correlation of MSO formation with added FAC shows a linear trend. Based on the slope of the plot, which is very close to 1, it can be stated that the stoichiometry of the proposed reaction mechanism is 1, i.e., one molecule of MSO is formed per molecule of FAC consumed.
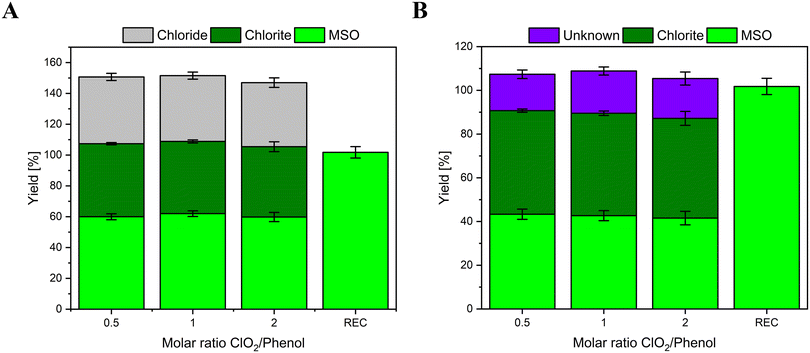
Chlorine mass balance of model compounds during reactions with chlorine dioxide
Phenol, hydroquinone, and DMP have been chosen as model compounds since their chlorine balance is known. While phenol is reported to form 50% FAC and 50% ClO2−,4,5 hydroquinone and DMP are reported to form 100% chlorite.6,8,9,21,22 These data will later be compared to the chlorine balances measured with the methionine method.The determined chlorine mass balance for phenol is shown in Fig. 3. Fig. 3A shows all species formed in this reaction (Cl−, ClO2−, and MSO). Additionally, direct addition of FAC to an aliquot of the reaction solution displays close to 100% turnover to MSO (recovery (REC)). This indicates that methionine is added in sufficient concentrations to effectively scavenge intrinsically formed FAC (if formed) and yield MSO.
As demonstrated before, FAC reacts with methionine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio and forms 1 mole of Cl− and MSO. Therefore, if MSO and Cl− are included in the mass balance, the total yield would be >100%. For instance, if a compound forms 50% FAC and 50% ClO2− in the reaction with ClO2, FAC would react with methionine and form 50% Cl− and 50% MSO (relative to the dosed molar ClO2 concentration). Hence, the Cl− and MSO yields need to be converted into FAC yields. Thereby it has to be considered that Cl− and MSO can form from reactions not involving FAC, while equal yields of Cl− and MSO are formed in the reaction of FAC with methionine. Accordingly, formed FAC equals the yield of Cl− if MSO is formed at higher concentrations than Cl− and vice versa. (i.e., [FAC] = [MSO], if [MSO] ≤ [Cl−]; [FAC] = [Cl−] if [MSO] ≥ [Cl−]). In this case, the formed Cl− yields (gray columns in Fig. 3A) represents the formed FAC in this reaction, which is in accordance with previously reported literature values for phenol (40–50%).4,5 After subtracting Cl− yields from MSO yields, a fraction of MSO remains, labeled as unknown in Fig. 3B, which is not formed from the reaction of FAC with methionine. Thus, MSO must be formed during a different reaction pathway involving different reaction partners. This could point to a reaction of ClO2− with methionine. However, in this experiment, ClO2− is formed with a 50% yield of the dosed ClO2 concentration, which is in accordance with the previously reported literature.4,5 Therefore, MSO might be formed by reactive organic species, which are formed during the reaction of phenol with ClO2. It is postulated that the reaction of ClO2 with phenol forms benzoquinone,21 a weak oxidant with a standard reduction potential of 0.6992 V,23 which might be reactive toward methionine. Another explanation could be the formation of reactive intermediates (i.e., phenoxy radicals),24 which could also react with methionine. The reaction of these reactive products or intermediates with methionine would also explain the lower Cl− yields compared to MSO.
1 stoichiometric ratio and forms 1 mole of Cl− and MSO. Therefore, if MSO and Cl− are included in the mass balance, the total yield would be >100%. For instance, if a compound forms 50% FAC and 50% ClO2− in the reaction with ClO2, FAC would react with methionine and form 50% Cl− and 50% MSO (relative to the dosed molar ClO2 concentration). Hence, the Cl− and MSO yields need to be converted into FAC yields. Thereby it has to be considered that Cl− and MSO can form from reactions not involving FAC, while equal yields of Cl− and MSO are formed in the reaction of FAC with methionine. Accordingly, formed FAC equals the yield of Cl− if MSO is formed at higher concentrations than Cl− and vice versa. (i.e., [FAC] = [MSO], if [MSO] ≤ [Cl−]; [FAC] = [Cl−] if [MSO] ≥ [Cl−]). In this case, the formed Cl− yields (gray columns in Fig. 3A) represents the formed FAC in this reaction, which is in accordance with previously reported literature values for phenol (40–50%).4,5 After subtracting Cl− yields from MSO yields, a fraction of MSO remains, labeled as unknown in Fig. 3B, which is not formed from the reaction of FAC with methionine. Thus, MSO must be formed during a different reaction pathway involving different reaction partners. This could point to a reaction of ClO2− with methionine. However, in this experiment, ClO2− is formed with a 50% yield of the dosed ClO2 concentration, which is in accordance with the previously reported literature.4,5 Therefore, MSO might be formed by reactive organic species, which are formed during the reaction of phenol with ClO2. It is postulated that the reaction of ClO2 with phenol forms benzoquinone,21 a weak oxidant with a standard reduction potential of 0.6992 V,23 which might be reactive toward methionine. Another explanation could be the formation of reactive intermediates (i.e., phenoxy radicals),24 which could also react with methionine. The reaction of these reactive products or intermediates with methionine would also explain the lower Cl− yields compared to MSO.
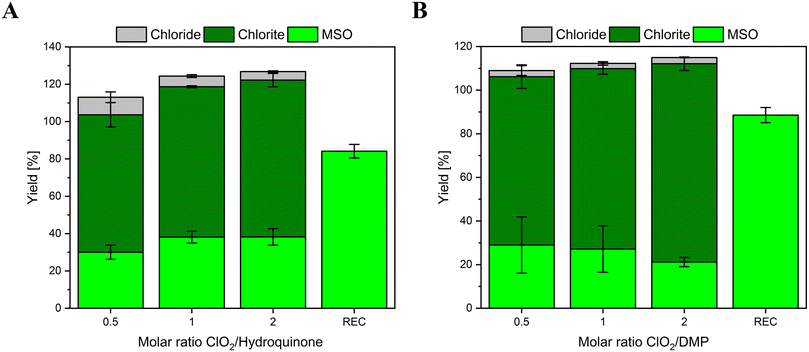
The chlorine balances of two other compounds have been investigated as well. Hydroquinone is also a phenolic moiety with a second hydroxyl group in the para-position. Hydroquinone is reported to form 100% ClO2−.6,8,21 DMP is also reported to form 100% ClO2−;9 however, the reactive moiety of DMP is a tertiary amine. This compound is investigated as well to discover if different functional groups have different outcomes in the chlorine balances. Fig. 4 shows the chlorine balances of hydroquinone (A) and DMP (B).
In both cases, the main inorganic product is ClO2−, which is in accordance with the literature. However, the yields of ClO2− are below 100%. Additionally, the recoveries of MSO by dosing 100 μM FAC directly into aliquots of either reaction solution without ClO2 ranges between 80–90%, which is lower compared to the results of phenol. An increase in the methionine concentration might support better results; however, due to the low solubility of methionine, the initial concentration could not be increased much further.
Based on literature data, no FAC should be formed in the reaction of hydroquinone and DMP with ClO2. However, in this experiment, 20–30% MSO is detected. The simultaneously formed Cl− concentrations are much lower, which indicates, that not FAC but another reactive organic species is formed during the reaction. The hypothesized reactive species may cause the oxidation of methionine to MSO similar to phenol (e.g., benzoquinone, semiquinone, or phenoxy radical in case of hydroquinone, cation radical in case of DMP).
Another critical aspect is the overall chlorine recovery. The sum of Cl− and ClO2− is around 80% which is lower than the dosed ClO2 concentration. A loss in the chlorine mass balance typically indicates the formation of halogenated products. However, from the reaction of DMP and hydroquinone with ClO2, no halogenated reaction products are reported.12 It might be possible that the presence of methionine interferes with the reaction pathway and causes the chlorination of organic compounds. For both compounds, the overall chlorine mass balance shows an increasing trend with increasing ClO2 dose, mainly due to the increasing yields of ClO2−. This might be caused by the fact that the effect of side reactions is reduced with a higher degree of reactant oxidation by ClO2. Further experiments are necessary to investigate if this effect is canceled out completely at one point, where the [ClO2]/[reactant] fold increased.
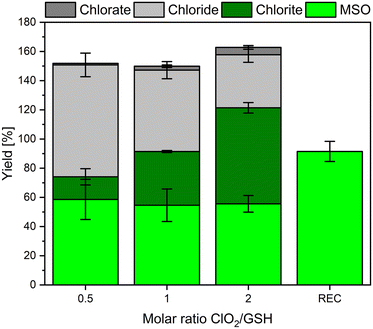
To investigate if the developed method can be applied to study sulfur-containing compounds, GSH has been chosen as a model compound. The determined chlorine mass balance and the FAC recovery are shown in Fig. 5. Please note that minor yields (<5%) of ClO3− were detected as well (dark gray columns Fig. 5). The low formation of highly oxidized form of chlorine might be caused by side reactions of e.g., ClO2 with FAC.12 The FAC recovery (REC), measured as formed MSO, is nearly complete (92%). Thus, if FAC is formed in this reaction, it will be scavenged by methionine. For GSH, the yields of inorganic chlorine species show a strong dependency on the ClO2 dose. If ClO2 is dosed in a molar ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
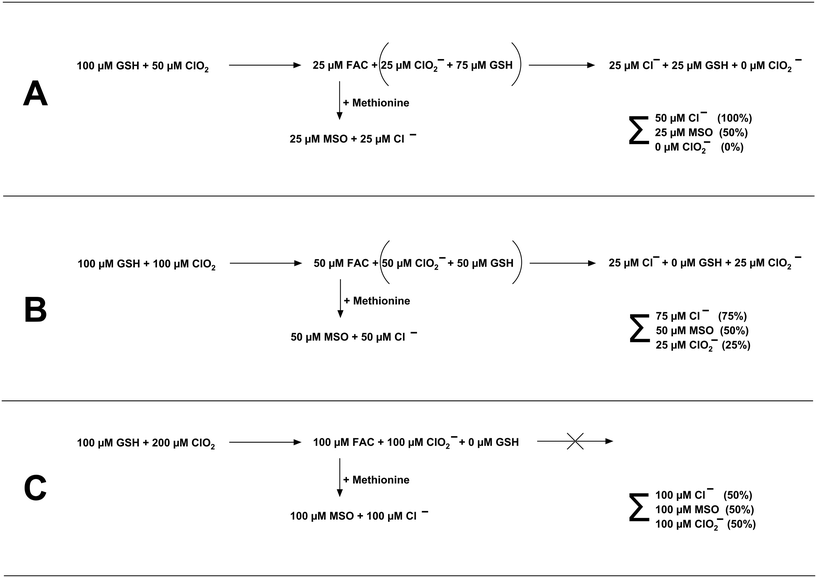
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to GSH, the most dominant reaction product is Cl−. The higher the stoichiometric ratio, the more dominant ClO2− becomes. This might be explainable by follow-up reactions of ClO2− with residual GSH concentration. The reaction of ClO2− with GSH is very slowly and forms Cl− (see Fig. S2†). The stoichiometry of this reaction has been determined to be two moles of GSH reacting with one mole of ClO2− (see Fig. S4†), which is in accordance with reported literature values.18 Please note that the Cl− yields were determined by experiments where ClO2− was in two to five-fold excess over GSH. However, further experiments are necessary to determine if the chlorine balance changes significantly if GSH is present in excess over ClO2− (i.e., shift in the molar yields of Cl−). The principle of the reaction of GSH and ClO2 for the dosed ClO2 concentrations is shown in Fig. 6.
1 to GSH, the most dominant reaction product is Cl−. The higher the stoichiometric ratio, the more dominant ClO2− becomes. This might be explainable by follow-up reactions of ClO2− with residual GSH concentration. The reaction of ClO2− with GSH is very slowly and forms Cl− (see Fig. S2†). The stoichiometry of this reaction has been determined to be two moles of GSH reacting with one mole of ClO2− (see Fig. S4†), which is in accordance with reported literature values.18 Please note that the Cl− yields were determined by experiments where ClO2− was in two to five-fold excess over GSH. However, further experiments are necessary to determine if the chlorine balance changes significantly if GSH is present in excess over ClO2− (i.e., shift in the molar yields of Cl−). The principle of the reaction of GSH and ClO2 for the dosed ClO2 concentrations is shown in Fig. 6.
In case 50 μM ClO2 is dosed to 100 μM GSH (Fig. 6A), ClO2 will be fully consumed, and 50% of ClO2 will be transformed to FAC (25 μM), and 50% will be transformed to ClO2− (25 μM), which leaves 75 μM GSH (reaction stoichiometry of ClO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GSH 2
GSH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 see Text S1 and Fig. S3†). This quantity of GSH will react with ClO2− in a 2
1 see Text S1 and Fig. S3†). This quantity of GSH will react with ClO2− in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S4†), eventually forming 25 μM Cl− and leaving a fraction of 25 μM GSH. In this case, in total, 50 μM Cl−, 25 μM MSO, and 0 μM ClO2− should be formed in the final sample, which are 100, 50, and 0% of the dosed ClO2 concentration, respectively. This changes if the dosed ClO2 concentration is increased (Fig. 6B and C), whereby the fraction of GSH available for follow-up reaction with ClO2− is getting reduced. The theoretical values in Fig. 6 are in good accordance with the measured values in Fig. 5. The differences in yields of ClO2− can be explained by the different reaction times used to generate the data in Fig. 5 and S2.† Since the reaction of ClO2− with GSH is very slow, the reaction in Fig. 5 did not fully proceed. Based on the observed results, it can be concluded that FAC is formed in the reaction of ClO2 with GSH during a two-step reaction mechanism, which is shown in Fig. 7.
1 stoichiometry (Fig. S4†), eventually forming 25 μM Cl− and leaving a fraction of 25 μM GSH. In this case, in total, 50 μM Cl−, 25 μM MSO, and 0 μM ClO2− should be formed in the final sample, which are 100, 50, and 0% of the dosed ClO2 concentration, respectively. This changes if the dosed ClO2 concentration is increased (Fig. 6B and C), whereby the fraction of GSH available for follow-up reaction with ClO2− is getting reduced. The theoretical values in Fig. 6 are in good accordance with the measured values in Fig. 5. The differences in yields of ClO2− can be explained by the different reaction times used to generate the data in Fig. 5 and S2.† Since the reaction of ClO2− with GSH is very slow, the reaction in Fig. 5 did not fully proceed. Based on the observed results, it can be concluded that FAC is formed in the reaction of ClO2 with GSH during a two-step reaction mechanism, which is shown in Fig. 7.
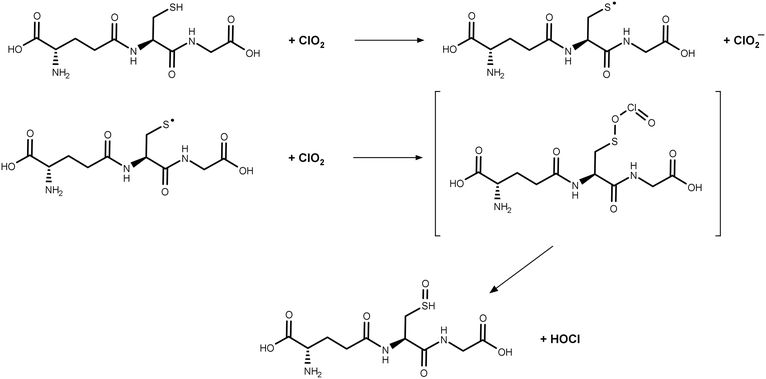 | ||
| Fig. 7 Proposed reaction mechanism for glutathione with chlorine dioxide. GSH reacts with two molecules of ClO2 and forms ClO2− and HOCl. | ||
MSO formation by follow-up reactions
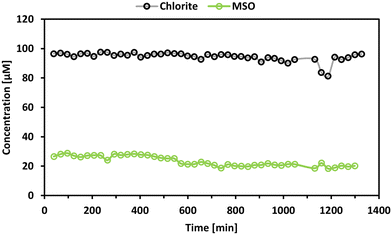
Previous results indicated a slow but possible reactivity between methionine and some transformation products. To investigate this hypothesis, ClO2− and methionine were mixed with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the evolution of ClO2− and formation of MSO was monitored for 24 h (sampling rate: 1 sample per 40 min). The results are shown in Fig. 8.
1, and the evolution of ClO2− and formation of MSO was monitored for 24 h (sampling rate: 1 sample per 40 min). The results are shown in Fig. 8.
It can be stated that ClO2− is not significantly degraded during the investigated time frame. In contrast, MSO is formed instantaneously by around 30% (of the initial molar methionine concentration) and shows a slightly decreasing trend during the investigated period. Cl− was also monitored in this experiment. However, after subtracting the Cl− concentration of the blank samples and the impurities of the ClO2− solution, the remaining value residual concentration for Cl− was below zero. It has to be noted that the sensitivity of the Cl− measurement is strongly affected due to the high Cl− background concentration in the ClO2− solution. Based on these results, it can be stated that ClO2− does not react with methionine. However, MSO is formed from an unknown source. So far, it cannot be explained why MSO is formed in the presence of ClO2− and is not formed in the absence of ClO2−, although no ClO2− degradation can be observed. One possibility might be the presence of 20% impurities in ClO2− stock solutions. Although the impurities mainly consist of Cl−, it might be possible that other impurities may be reactive towards methionine. Note that ClO3− and OCl− were not detected in ClO2− standards. The slow degradation of MSO over time might be explainable by the formation of methionine sulfone. It is reported that sulfoxides might be further oxidized to sulfones.25 However, the formation of methionine sulfone was not observed in this study.
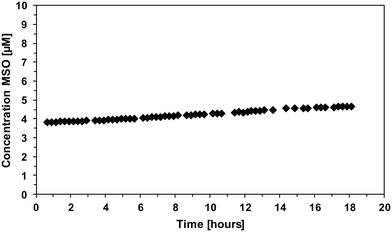
The follow-up reaction of methionine with benzoquinone has been investigated as well. Therefore, benzoquinone and methionine were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and MSO formation was monitored over time (Fig. 9).
1 ratio and MSO formation was monitored over time (Fig. 9).
The results show that MSO also forms in the reaction of methionine and benzoquinone; however, only small yields of MSO have been formed after 18 h reaction time (4–5% per added benzoquinone). This shows that methionine can react with benzoquinone to form MSO but it does not explain the 30% MSO yields in case of hydroquinone or 1,4-DMP. This indicates that MSO formation from other reactants than FAC is a complex interplay of different intrinsically formed reactive species such as semiquinone or phenoxy radicals.
Conclusion and outlook
Based on the results of this study, it can be concluded that methionine can scavenge intrinsically formed FAC in ClO2-based reaction mechanisms and form a product which is not reactive. Thus, the scavenger is less prone in causing interferences compared to e.g., glycine which forms Cl–Gly. However, further investigations are necessary to use this method for intrinsically formed FAC quantification. Even though the reaction of methionine and FAC leads to the formation of MSO and Cl− in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the additional formation of MSO from a yet unknown source (i.e., potential reaction of reactive intermediates with methionine) might bias the final results. It seems that this unknown reaction with methionine does not form Cl−. Therefore it is possible to quantify the MSO yields caused by the reaction with FAC. However, if the chlorine balance is not known many assumptions have to be made, which makes quantification of FAC yields difficult. Especially, in the case of GSH, where a stoichiometry-dependent chlorine balance was observed, it is hard to predict exactly which fraction of MSO is formed by FAC. One possible option to solve this problem is combining this method with an existing method. It would be for example possible to quantify the ClO2− yields of the reaction of GSH with ClO2 in presence of glycine as FAC scavenger to avoid any interferences by FAC.17 Then only the FAC yields are unknown and could be determined by using the methionine method, since MSO is more stable than Cl–Gly.8 Additionally, it still needs to be investigated which other reactive species or generally which factors may contribute in to MSO formation. Even though the methionine method does not seem suitable for quantification of FAC yields, it can be used to make qualitative statements about FAC formation. For instance, GSH seems to form FAC, however, precise FAC yields are yet unclear and their determination requires further investigations.
1 ratio, the additional formation of MSO from a yet unknown source (i.e., potential reaction of reactive intermediates with methionine) might bias the final results. It seems that this unknown reaction with methionine does not form Cl−. Therefore it is possible to quantify the MSO yields caused by the reaction with FAC. However, if the chlorine balance is not known many assumptions have to be made, which makes quantification of FAC yields difficult. Especially, in the case of GSH, where a stoichiometry-dependent chlorine balance was observed, it is hard to predict exactly which fraction of MSO is formed by FAC. One possible option to solve this problem is combining this method with an existing method. It would be for example possible to quantify the ClO2− yields of the reaction of GSH with ClO2 in presence of glycine as FAC scavenger to avoid any interferences by FAC.17 Then only the FAC yields are unknown and could be determined by using the methionine method, since MSO is more stable than Cl–Gly.8 Additionally, it still needs to be investigated which other reactive species or generally which factors may contribute in to MSO formation. Even though the methionine method does not seem suitable for quantification of FAC yields, it can be used to make qualitative statements about FAC formation. For instance, GSH seems to form FAC, however, precise FAC yields are yet unclear and their determination requires further investigations.
Safety precautions
Chemicals can be hazardous and have to be used with care. Especially ClO2 is toxic, volatile, and explosive. Hence, the production and application of ClO2 have to be done in a suitable fume hood. Measures have to be applied for disposing ClO2-containing solutions after work. All precautions of the manufacturers regarding instruments and chemicals have to be studied and applied with care. All general laboratory safety rules have to be applied carefully.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Technical University of Darmstadt for supporting the presented study.References
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res., Rev. Mutat. Res., 2007, 636(1–3), 178–242, DOI:10.1016/j.mrrev.2007.09.001.
- H. Gallard and U. von Gunten, Chlorination of natural organic matter: Kinetics of chlorination and of THM formation, Water Res., 2002, 36(1), 65–74, DOI:10.1016/S0043-1354(01)00187-7.
- X. Zhang, S. Echigo, R. A. Minear and M. J. Plewa, Characterization and comparison of disinfection by-products of four major disinfectants, ACS Symp. Ser., 2000, 761, 299–314, DOI:10.1021/bk-2000-0761.ch019.
- J. Terhalle, P. Kaiser, M. Jütte, J. Buss, S. Yasar, R. Marks, H. Uhlmann, T. C. Schmidt and H. V. Lutze, Chlorine Dioxide—Pollutant Transformation and Formation of Hypochlorous Acid as a Secondary Oxidant, Environ. Sci. Technol., 2018, 52(17), 9964–9971, DOI:10.1021/acs.est.8b01099.
- V. Rougé, S. Allard, J. P. Croué and U. von Gunten, In Situ Formation of Free Chlorine during ClO2 Treatment: Implications on the Formation of Disinfection Byproducts, Environ. Sci. Technol., 2018, 52(22), 13421–13429, DOI:10.1021/acs.est.8b04415.
- K. Hupperich, X. A. M. Mutke, M. S. Abdighahroudi, M. Jütte, T. C. Schmidt and H. V. Lutze, Reaction of chlorine dioxide with organic matter – formation of inorganic products, Environ. Sci.: Water Res. Technol., 2020, 6(9), 2597–2606, 10.1039/D0EW00408A.
- M. Jütte, J. V. Große, M. S. Abdighahroudi, C. Schüth and H. V. Lutze, Novel insights into chlorine dioxide based disinfection mechanisms – investigation of the reaction with amino acids, Environ. Sci.: Water Res. Technol., 2022, 8(3), 630–639, 10.1039/D1EW00812A.
- M. Jütte, J. A. Wilbert, M. Reusing, M. S. Abdighahroudi, C. Schüth and H. V. Lutze, Reaction Mechanisms of Chlorine Dioxide with Phenolic Compounds—Influence of Different Substituents on Stoichiometric Ratios and Intrinsic Formation of Free Available Chlorine, Environ. Sci. Technol., 2023, 571(47), 18846–18855, DOI:10.1021/acs.est.2c09496.
- M. S. Abdighahroudi, X. A. M. Mutke, M. Jütte, K. Klein, T. C. Schmidt and H. V. Lutze, Reaction of Chlorine Dioxide with Saturated Nitrogen-Containing Heterocycles and Comparison with the Micropollutant Behavior in a Real Water Matrix, Environ. Sci. Technol., 2022, 56(16), 11589–11601, DOI:10.1021/acs.est.1c08381.
- M. J. Napolitano, B. J. Green, J. S. Nicoson and D. W. Margerum, Chlorine dioxide oxidations of tyrosine, N-acetyltyrosine, and dopa, Chem. Res. Toxicol., 2005, 18(3), 501–508, DOI:10.1021/tx049697i.
- D. J. Stewart, M. J. Napolitano, E. V. Bakhmutova-Albert and D. W. Margerum, Kinetics and mechanisms of chlorine dioxide oxidation of tryptophan, Inorg. Chem., 2008, 47(5), 1639–1647, DOI:10.1021/ic701761p.
- M. S. Abdighahroudi, M. Jütte, K. Hupperich, X. A. M. Mutke, T. C. Schmidt and H. V. Lutze, Mechanisms and byproduct formation in the application of chlorine dioxide, in Comprehensive Analytical Chemistry, 2021, pp. 51–83, DOI:10.1016/bs.coac.2021.01.003.
- M. Jütte, M. S. Abdighahroudi, T. Waldminghaus, S. Lackner and H. V. Lutze, Bacterial inactivation processes in water disinfection – mechanistic aspects of primary and secondary oxidants – A critical review, Water Res., 2023, 231, 119626, DOI:10.1016/j.watres.2023.119626.
- M. Deborde and U. von Gunten, Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: A critical review, Water Res., 2008, 42(1–2), 13–51, DOI:10.1016/j.watres.2007.07.025.
- J. Hoigné and H. Bader, Kinetics of reactions of chlorine dioxide (OClO) in water—I. Rate constants for inorganic and organic compounds, Water Res., 1994, 28(1), 45–55, DOI:10.1016/0043-1354(94)90118-X.
- J. Houska, E. Salhi, N. Walpen and U. von Gunten, Oxidant-reactive carbonous moieties in dissolved organic matter: Selective quantification by oxidative titration using chlorine dioxide and ozone, Water Res., 2021, 207, 117790, DOI:10.1016/j.watres.2021.117790.
- M. S. Abdighahroudi, T. C. Schmidt and H. V. Lutze, Determination of free chlorine based on ion chromatography—application of glycine as a selective scavenger, Anal. Bioanal. Chem., 2020, 412(28), 7713–7722, DOI:10.1007/s00216-020-02885-1.
- A. Ison, I. N. Odeh and D. W. Margerum, Kinetics and mechanisms of chlorine dioxide and chlorite oxidations of cysteine and glutathione, Inorg. Chem., 2006, 45(21), 8768–8775, DOI:10.1021/ic0609554.
- H. Tan, A. C. Sen, W. B. Wheeler, J. A. Cornell and C. I. Wei, A Kinetic Study of the Reaction of Aqueous Chlorine and Chlorine Dioxide with Amino Acids, Peptides and Proteins, J. Food Sci., 1987, 52(6), 1706–1711, DOI:10.1111/j.1365-2621.1987.tb05910.x.
- D. J. Gates, G. Ziglio and K. Ozekin, State of the science of chlorine dioxide in drinking water, Water Research Foundation/Fondazione AMGA, Published online 2009 Search PubMed.
- J. E. Wajon, D. H. Rosenblatt and E. P. Burrows, Oxidation of Phenol and Hydroquinone by Chlorine Dioxide, Environ. Sci. Technol., 1982, 16(7), 396–402, DOI:10.1021/es00101a006.
- W. Gan, S. Huang, Y. Ge, T. Bond, P. Westerhoff, J. Zhai and X. Yang, et al. Chlorite formation during ClO2 oxidation of model compounds having various functional groups and humic substances, Water Res., 2019, 159, 348–357, DOI:10.1016/j.watres.2019.05.020.
- J. Weiss, Handbook of Ion Chromatography, Wiley-VCH Verlag GmbH & Co. KGaA, 2016, DOI:10.1002/9783527651610.
- L. K. Folkes, M. Trujillo, S. Bartesaghi, R. Radi and P. Wardman, Kinetics of reduction of tyrosine phenoxyl radicals by glutathione, Arch. Biochem. Biophys., 2011, 506(2), 242–249, DOI:10.1016/j.abb.2010.12.006.
- A. Lopez, G. Mascolo, G. Tiravanti, M. Santori and R. Passino, Oxidation of Sulfur-containing s-Triazines during groundwater hypochlorination, Water Sci. Technol., 1994, 30(7), 53–59, DOI:10.2166/wst.1994.0304.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00216k |
| This journal is © The Royal Society of Chemistry 2024 |