Advances in 3D tissue models for neural engineering: self-assembled versus engineered tissue models
Shuqian
Wan
a,
Ulises
Aregueta Robles
a,
Laura
Poole-Warren
ab and
Dorna
Esrafilzadeh
 *a
*a
aGraduate School of Biomedical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia. E-mail: d.esrafilzadeh@unsw.edu.au
bTyree Foundation Institute of Health Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
First published on 21st May 2024
Abstract
Neural tissue engineering has emerged as a promising field that aims to create functional neural tissue for therapeutic applications, drug screening, and disease modelling. It is becoming evident in the literature that this goal requires development of three-dimensional (3D) constructs that can mimic the complex microenvironment of native neural tissue, including its biochemical, mechanical, physical, and electrical properties. These 3D models can be broadly classified as self-assembled models, which include spheroids, organoids, and assembloids, and engineered models, such as those based on decellularized or polymeric scaffolds. Self-assembled models offer advantages such as the ability to recapitulate neural development and disease processes in vitro, and the capacity to study the behaviour and interactions of different cell types in a more realistic environment. However, self-assembled constructs have limitations such as lack of standardised protocols, inability to control the cellular microenvironment, difficulty in controlling structural characteristics, reproducibility, scalability, and lengthy developmental timeframes. Integrating biomimetic materials and advanced manufacturing approaches to present cells with relevant biochemical, mechanical, physical, and electrical cues in a controlled tissue architecture requires alternate engineering approaches. Engineered scaffolds, and specifically 3D hydrogel-based constructs, have desirable properties, lower cost, higher reproducibility, long-term stability, and they can be rapidly tailored to mimic the native microenvironment and structure. This review explores 3D models in neural tissue engineering, with a particular focus on analysing the benefits and limitations of self-assembled organoids compared with hydrogel-based engineered 3D models. Moreover, this paper will focus on hydrogel based engineered models and probe their biomaterial components, tuneable properties, and fabrication techniques that allow them to mimic native neural tissue structures and environment. Finally, the current challenges and future research prospects of 3D neural models for both self-assembled and engineered models in neural tissue engineering will be discussed.
1. Introduction
Neural function relies on systematic interactions between the external environment and the exquisite nervous system structures comprising more than 100 billion neuronal cells, extracellular matrix (ECM), signalling molecules and electrical signals.1,2 Damage to neural tissue disrupts connections, leading to permanent loss of sensory and motor functions, isolating affected individuals.3,4 Laboratory research often focuses on 2D cell culture systems, which have limitations such as lack of in vivo-like structures, limited cell–cell interaction, and cellular heterogeneity.5,6 To better understand neural damage mechanisms and develop repair methods, studying biology on the bench needs to be more representative of human neural physiology. The emergence of in vitro 3D human cell culture techniques has great potential for overcoming the limitations of 2D approaches.7 However, despite the advances in developing 3D culture approaches, the high complexity of neural tissues challenges the current capacity to accurately model neural tissue on the bench.Neural tissue intricacy includes networks formed by neurons through dendrites and axons, crucial for connecting tissues and transmitting signals.8 The network is highly spatially organized comprising complex and high fidelity features with axon diameters ranging from 1 to 100 micrometres with lengths that can extend to over 1 meter.9,10 Given this complexity, studying nerve tissue regeneration or repair strategies can be greatly supported by replicating the neural system microenvironment in vitro integrating biochemical, mechanical, physical, and electrical cues.11 This review explores 3D models in neural tissue engineering, emphasizing self-assembled organoids and hydrogel-based engineered 3D models. As summarised in Fig. 1, self-assembled models allow stem cells to organize into aggregates and tissue-like arrangements, while engineered models involve designing structures with relevant cell types and biomaterial scaffolds. However, both approaches have limitations in replicating tissue architectures and appropriate ECM and cellular signalling.
 | ||
| Fig. 1 Schematic overview of two main categories of 3D models for in vitro neural tissue engineering. Self-assembled 3D tissue models (left) and engineered 3D tissue models (right). | ||
Self-assembled models are generated by providing appropriate environments for stem cells to organise and develop into 3D structures such as spheroids, organoids, or assembloids (Fig. 1).12,13 These models are typically exploited to study the behaviour and interactions of differentiated cell types in environments that more accurately reflect the cellular and ECM composition of neural tissues than 2D cultures.14 In contrast, engineered models are generated by designing the desired structures and fabricating them through the integration of relevant cell types with biomaterial scaffolds, fulfilling the tissue design requirements (Fig. 1).15 Through manipulating the microenvironment surrounding the cells, engineered 3D tissues promote specific cellular behaviours, such as proliferation or differentiation.16 However, both approaches have limitations. Structures in most self-assembled 3D neural tissue models do not replicate tissue architectures that occur in the nervous system,17,18 whereas engineered models rely on fabricated structures that may not mimic the appropriate ECM and cellular signalling.12,13
Both self-assembled and engineered tissue structures have a role in generation of new knowledge of neural cell and tissue functions. Self-assembled tissues have been applied to studying cell, tissue, and disease development and are ideal for evaluating interactions of drugs with tissues.19,20 Engineered tissue models are typically centred on applications in regenerative medicine, including approaches to repair and replace tissues.7,21,22 One of the applications of 3D engineered tissue models which attracts significant interest is the development of tissue and organ systems which can be used to test medical devices on the bench.23,24 Such models require complex anatomical structures combined with living cells to study how devices impact on surrounding tissues and microenvironment as well as to support rapid throughput studies to accelerate product development.25,26 Given that self-assembled models are not able to develop these complex architectures, researchers must consider how engineered 3D structures could be optimised to provide better models of neural tissue. Therefore, the selection of biomaterial components, manipulation of properties, and choice of fabrication techniques emerge as pivotal considerations during the development of engineered models.
Many different biomaterials have been developed and studied for fabricating tissue engineered scaffolds. However, soft neural tissue structures ideally require similarly soft ECM-like materials to provide appropriate biochemical and mechanical environment.27,28 Neural ECM is a complex mixture of matrix molecules including large glycoproteins, laminin, collagen, and fibronectin that assembles into fibrils or other complex macromolecular structures.29,30 ECM plays a crucial role in neural development in vivo and is a major niche element serving as a scaffold for cell support to regulate cell behaviours.31,32 Hydrogels, comprising both biological and synthetic polymers, have potential to be engineered to mimic neural tissue ECM, thereby providing an ideal model for physiologically relevant ECM environments.33,34 However, considering the microenvironment in neural tissue, the 3D scaffold is expected to meet biochemical, mechanical, and electrical requirements at the same time in a system to comprehensively reproduce the native tissue.35
Such 3D tissue-mimicking substrates can be fabricated into complex shapes and tailored to provide specific mechanical properties. With the capacity to integrate ECM and soluble biological molecules, hydrogels provide an ideal platform for developing functional and organised structures.34,36 The combination of hydrogels with other fabrication techniques, such as mould casting and bioprinting, allows fabrication of highly complex and multi-functional neural tissue models that more accurately reflect the natural tissue microenvironment.23,33 However, to represent the natural cellular environment comparable to that observed in native tissue, and to a degree in self-assembled models, engineered models require more advanced hydrogel based biomaterials and fabrication approaches.
Self-assembled and engineered models both offer unique advantages and limitations, and the choice of which model to use depends on the specific final application. While engineering materials and fabrication approaches have great promise for development of biomimetic neural tissue models, there remain significant challenges with recapitulating native ECM structures, situating cells in the appropriate niches and integrating chemical and electrical signalling. This review will systematically discuss the advantages, challenges, and future perspectives associated with self-assembled and engineered models, especially in hydrogel-based biomaterials, their properties, and fabrication for engineered models, shedding light on their contributions to our understanding of neural development and potential therapeutic applications for neurological disorders.
2. Self-assembled 3D neural tissue models
Human self-assembled 3D tissue models are generated by human pluripotent stem cells (hPSCs), which include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).37 Self-assembled models are known for their remarkable self-organization when spontaneously aggregated into 3D structures. These can be classified based on their developmental complexity level as spheroids, organoids, or assembloids (Fig. 1).14 With the development of stem cell research and advancement of techniques, more intricate organ-like models have been created, which provide researchers with promising platforms to study complex physiological processes and offer novel experimental models that, in part, bridge the gap between animal models and human biology.38–40 This section will discuss each of these self-assembly models in more detail.2.1. Spheroids, organoids, and assembloids
Spheroids are simple 3D spherical cellular aggregates that form primarily through cell-to-cell adhesion (Fig. 1). They offer a platform to study intricate cell–cell and cell–matrix interactions, as well as differentiation processes and drug responses.38,41 While spheroids do not have complex tissue structures, they can overcome some of the limitations of traditional 2D cell culture approaches to replicate natural cellular responses in vitro, as shown in Fig. 2.42 Generating spheroids can be achieved through various techniques that spatially concentrate monodispersed cells to promote cell–cell adhesion and impede interaction with other substrates.43 In approximately two weeks cells aggregate into spherical-shaped, multilayered structures that range in size from several hundred micrometres to several millimetres,44,45 which can be maintained over long-term culture.20,46 With the implementation of diverse tissue culture techniques spheroids can be grown to display features of the neuronal tissue milieu that closely represent those in vivo.20,45 | ||
| Fig. 2 A representation of the advantages and disadvantages of the common models in neural tissue engineering. | ||
Spheroids have been used to mimic essential characteristics of neural structures like brain tissues, including diverse cell types, electrical activity, production of ECM, and mechanical properties. For instance, spheroids formed from neonatal rat cortex cells have been shown to develop electrically active neural networks, secrete relevant ECM molecules like laminin, and present an elastic modulus comparable to that of the native in vivo tissue.47 Spheroids also allow manipulation of specific cell populations, which can be useful for studying the role of different cell types in tissue development and function.48 Biopsy samples or surgical resections can isolate tumour cells to grow as spheroids, which can be used to model disease development.49 For example, Seidel et al. used human neuroblastoma cell line (SH-SY5Y) which came from bone marrow of a patient with neuroblastoma, to generate spheroids with overexpress EGFP-fused tau as a model to study the pathologies of tau protein in Alzheimer Disease.50
The development and differentiation of spheroids can be promoted and regulated by adjusting cell types and cell ratios. Song et al., investigated the effect of cell ratio on the expression of neural trophic factors, ECM molecules, and neural differentiation in vitro.51 This study showed that co-culturing iPSC with human mesenchymal stem cells (hMSC) at a ratio of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 supported a significantly higher expression of neuronal markers like β-III tubulin as compared to 50
75 supported a significantly higher expression of neuronal markers like β-III tubulin as compared to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 75
50 and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 ratios, and substantially higher than that of iPSC-only cultures. In addition, adjusting cell ratio and cell type can provide control of ECM deposition and thus influence cellular behaviour. For instance, the 25
25 ratios, and substantially higher than that of iPSC-only cultures. In addition, adjusting cell ratio and cell type can provide control of ECM deposition and thus influence cellular behaviour. For instance, the 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 iPSC-MSC group exhibited increased expression of collagen IV, while the expression of laminin appeared to rise with the proportion of hMSC. Also, the incorporation of hMSC can substantially upregulate the expression of proliferating and tissue forming cytokines like transforming growth factor-β1 and prostaglandin E2.51
75 iPSC-MSC group exhibited increased expression of collagen IV, while the expression of laminin appeared to rise with the proportion of hMSC. Also, the incorporation of hMSC can substantially upregulate the expression of proliferating and tissue forming cytokines like transforming growth factor-β1 and prostaglandin E2.51
While the cellular organization into 3D spheroids is largely defined by random cell interactions, the arrangement of cells into tissue structures can be influenced through incorporation of heterotypic cells prior and following formation of spheroid structure. For instance, Liqing et al.,51 showed that in co-cultures where both hMSC and iPSC are monodispersed and mixed, cell organization is driven based on the relative degree of adhesiveness of each cell type. With hMSC showing stronger cell–cell interactions than iPSC, this co-culture generated spheroids with a core of hMSC and an outer shell of iPSC. Whereas culturing monodispersed hMSC with pre-formed iPSC spheroids allows for the formation of an iPSC core with a hMSC outer shell. While spheroids can better represent native tissue compared to traditional 2D counterparts, they are not able to mimic the in vivo structure and functionality of native tissues and organs.38 More advanced tissue models are required to present the complex physiological structure and environment.
Under specific conditions, the hPSC self-organize into complex, multicellular structures resembling mini organs, called organoids (Fig. 1).6,52,53 Spheroids and organoid models have overlapping and distinct purposes (Fig. 2). Both can develop tissue models to study neuronal cell development under healthy and pathological conditions in 3D, thus enabling the study of previously inaccessible aspects in a controlled laboratory setting. The key difference is that organoids with more complex structures can often better represent the cellular heterogeneity and physiological functionality of organs as illustrated in Fig. 2. Organoids more closely resemble the native tissue both histologically and genetically.6 Brain organoids can model many features of the early development of the human brain and are a powerful in vitro system to model congenital brain disorders induced by neurotrophic viral infections, or inherited genetic mutations.54 For example, Latour et al., generated GLB1 knockout cerebral organoids from a isogenic iPSC lines with lysosomal β-gal deficiency by employing CRISPR/Cas9 genome editing to model human GM1 gangliosidosis in the central nervous system.55 These features of organoids support a wide range of applications in neural development research, including the study of neural diseases, neural injury repair, and the establishment of fundamental knowledge in the field.
Although organoids have an important role as a research tool in medicine and biology, they do not recreate the body's interconnectedness and are only adapted for studies on a single organ type.56 To address these limitations, studies have explored strategies to optimize the culture conditions for organoids, for example development of a vascularised system to provide a closer-to-ideal growth environment. Vascularisation can encourage the formation of structural units that partially resemble structure and function of organs, and promote maturity and support nutrient delivery.57 Worsdorfer et al., incorporated human mesodermal progenitor cells (MPCs) into neural organoids that resulted in a vascularised and well-structured neuroepithelium.58
Integration of organoids with other organoids as well as spheroids or engineered tissue models, known as assembloids, has allowed creation of more complex and physiologically relevant systems for neural tissue engineering.52 Assembloids have emerged as an advanced tool that facilitate the spatial organization of multiple types of cells, tissues, or organoids (Fig. 1).59 Their primary function is to replicate the complex microenvironment and intricate interactions between multiple organs and tissues, thereby enabling more accurate representation of tissue structure, function, and molecular mechanisms involved in development and disease modeling.60
By integrating different tissue types into a single 3D model, assembloids allow for the study of inter-organ and inter-tissue interactions, which are crucial for understanding physiological processes and disease pathogenesis.61,62 These interactions can involve cell–cell communication, cell–ECM interactions, and the formation of functional units that resemble natural tissue architecture.59 Assembloids have a potential to more closely mimic the complexity and heterogeneity of native neural tissue including cell–cell interactions and circuit formation, as compared with spheroids or organoids(Fig. 2).60 For instance, to study the human cortico-striatal pathway and how its dysfunction leads to neuropsychiatric disease, Miura and colleagues developed a method to model long-range neuronal connectivity in human brain assembloids by generating 3D spheroids resembling specific domains of the nervous system and then integrating them physically to allow axonal projections and synaptic assembly.63 Furthermore, assembloids can be constructed from multiple organoids to create “multi-region assembloids”, which further enhances their capability to mimic the complexity of native tissue structures and interactions across different regions of the body.64
Despite their potential, one significant drawback of assembloids is the technical complexity involved in their construction.65 Integrating multiple types of cells, tissues, or organoids into a single 3D model requires precise control over spatial organization and cell–cell interactions, which can be challenging to achieve consistently.66 Moreover, assembloids may struggle to accurately replicate the dynamic and intricate physiological structures found in vivo, leading to limitations in their ability to fully recapitulate complex tissue behaviours.59,67 Additionally, the scalability and reproducibility of assembloids across different experimental setups may be hindered by their intricate nature and the variability inherent in their construction process.56 These challenges highlight the need for further refinement and optimization of assembloid technologies to fully harness their potential in neural tissue engineering and disease modelling.68
In summary, spheroids, organoids, and assembloids as self-assembling tissue models represent remarkable advancements in neural tissue engineering, offering versatile platforms for studying complex neural processes in vitro. These tissue structures, mimicking the architecture and functionality of native tissues to varying extents, have revolutionized our approach to understanding neural development, and disease mechanisms. However, despite their potential, challenges and limitations persist in effectively recapitulating in vivo tissue complexity with self-assembled models.
2.2. The challenges of self-assembled 3D neural tissue model
Self-assembled 3D systems go part of the way to bridging the gap between conventional 2D in vitro models and animal models. However, the potential for assembloids as in vivo tissue surrogates is still challenged. While limited access to primary tissue for comparison purposes has been one factor,64 the challenges extend further to encompass various tissue design elements, summarised in Fig. 2, which have not been successfully addressed. As discussed in this section, such challenges include long developmental times, poor reproducibility, uncontrolled structure, size and shape, and insufficient biological maturation.69,70A significant challenge in self-assembled systems is the long developmental time to differentiate from stem cells to 3D organoids.71 Reported development times for organoids and assembloids in the neural tissue engineering field are up to 6 months, depending on the system complexity.72 For instance, assembloids modelling spinal cord multi-synaptic circuitry in vitro can take up to 50 days,73 whereas brain assembloids require 3 to 4 months to develop.63 Similarly, organoids targeting the formation of neuromuscular junctions74 as well as cerebral cortex75 and cortico-motor models73 can require up to 5 months for development. Generating and maintaining assembloids throughout this time frames can be difficult and costly, often requiring precise timing in culture conditions and several iterations to adjust cell-type ratios.64 Notably, the process requires complex multidisciplinary skills and know-how,56 overall challenging their reproducibility.
While organoids produced from the same cellular batches or in replicate batches under similar experimental conditions are expected to be morphologically and phenotypically similar, there is notable variation in the shape and size of individual organoids within the same batch. Typically, these organoids are smaller than the actual size of the organ in vivo.14 For instance, cerebral organoids modelling the human cerebral cortex have been reported to have a diameter of approximately 4 mm, substantially smaller than the target tissue with a diameter close to 5 cm.17 Moreover, the culture of self-assembled models requires the use of animal-based ECM, such as Matrigel™ or Basement Membrane Extract.6,76 These extracts have inherent batch-to-batch variability in their composition, which may affect the reproducibility of experiments.77,78 For instance organoids grown on Matrigel substrates have variable architectures as compared to organoids grown on synthetic scaffolds with chemically defined composition that yield more reproducible structures.70,79 Moreover, the inconsistent mechanical and biochemical properties observed both within individual batches and across different batches have resulted in ambiguity and reduced reproducibility in cell culture experiments.80,81
Incomplete biological maturation is another significant challenge faced by self-assembled systems in accurately modelling native tissue in vitro. This can manifest as a lack of specific cell types, an inability to replicate functional tissue structures, and undersized structures. For instance, current cortical spheroids have been shown to generate various cortical cell types, including neural progenitors, mature neuron subtypes, and astrocytes. These cells self-organize into distinct cortical layers and establish functional neural networks. However, they still lack oligodendrocytes, the myelinating glia of the central nervous system.82
Guiding the development of specific organ structures poses significant challenges, as these structures are vital for tissue functionality, encompassing ducts, canals, ventricles, and vascular networks crucial for facilitating mass transfer of physiological fluids.17,83,84 In particular, the limited diffusion of nutrients and oxygen represents a well-documented constraint in organoid culture,71 and self-assembling tissue models, impeding the formation of higher order tissue structures. As organoids grow in size, diffusion-dependent nutrient supply and waste removal become less efficient, often resulting in necrosis at the centre of organoids.52,85 These mass transfer limitations are a key factor that affects the terminal size and maturation of organoids and typically results in a necrotic core in larger tissue masses. To overcome these limitations, organoid culture protocols often use dynamic systems like spinning bioreactors to enhance diffusion.52 However, these dynamic culture processes can generate mechanical stress such as shear forces during rotation, which can affect the shape and structure of organoids.86 Recent advancements in engineering technology have aimed to address this issue, through the development of sophisticated devices that facilitate nutrient transportation into the core of the organoid and by co-culturing blood vessels alongside the organoid.87
In addition, stem cells, in their spontaneous organization, conform to specific structures influenced by the complex composition of ECM and soluble factors. Orchestrating such complex microenvironment in vitro is a key challenge to address for enabling control on the organization and structure of organoids during the culture process.88 It is expected that these challenges can be overcome when combining self-assembling systems with emerging advanced biomaterial-based cell supporting technologies. The next section discusses the potential to develop engineered tissues, crafted from a combination of cells, substrates, and bioactive factors. This holds significant promise in addressing several challenges of self-assembled models, such as structural control, reproducibility, and maturation, in a precise manner.36
3. Engineered 3D neural tissue model
Engineering neural tissue models involves integrating cells with biomaterials and culture media that provide supporting growth and developmental factors.89 These models have emerged as a promising approach to overcome some of the challenges of self-assembled models.90,91 Moreover, the engineered tissue models with isolated cells from patients can be applied on functional tissue regeneration and replacement, or disease modelling to optimize current disease treatments.92 While differentiation and maturation of neural cells still requires biological processes that are difficult to direct, engineered models aim to present cells with a defined supporting milieu, theoretically improving on reproducibility and development time.93 However, designing and developing functional nerve tissue models requires considering several factors and environmental conditions that support neuronal tissue grow and function.7,94 As described in this section, this involves biochemical, mechanical, physical, as well as electrical factors reported in literature that support grow, differentiation, survival, and function of neural cells.95Selection of biomaterials is crucial for engineering neural tissue models in vitro.33 Hydrogels, among various technologies, show great promise and are widely employed as scaffolds in nerve tissue engineering.25,34,96Table 1 summarises common uses of hydrogel biomaterials for neural tissue engineering. They exhibit high cyto-compatibility, permeability to nutrients, and mechanical properties closely resembling those of biological tissue.97 Hydrogels, which are polymer systems extensively investigated for tissue engineering,98 form networks through physical or chemical crosslinks.99 The design of these networks can allow for incorporation of bioactive molecules in substrates with various degradation profiles or may be non-degradable.100 Also, the structure and mechanical properties of hydrogel networks are controllable through the selection of fabrication techniques and chemical compositions.101
| Biomaterials | Cell types | Application for neural tissue engineering | Ref. | |
|---|---|---|---|---|
| Natural | Synthetic | |||
| Fibrinogen | N/A | Transgenic mouse ESCs | Neurons and astrocytes differentiation | 102 |
| Collagen-1, gelatine, laminin, poly-D-lysine, fibronectin, Matrigel, hyaluronic acid | N/A | Mouse ESCs | Neural and glial cells differentiation and maturation | 103 |
| Fibrin, hyaluronic acid, laminin | N/A | Human neural stem/progenitor cells (SC27 and SC23) | Neurons and astrocytes differentiation; vascularization | 111 |
| Chitosan | N/A | Motor neuron-like neural stem cells-34 cell line | Cell survival and neuronal differentiation | 105 |
| Collagen | N/A | Mouse endogenous neural stem cells | Neurogenesis and inhibited astrogliogenesis | 112 |
| Matrigel | N/A | iPSC line HCS1–2 | Neuronal survival, differentiation, and maturation | 104 |
| Geltrex, gelatin methacrylate (GelMA), arginine-glycine-aspartate acid (RGD) | N/A | hiPSCs, human neuroblastoma SH-SY5Y | Stemness maintain and neuronal differentiation support | 113 |
| Laminin 111 alginate | N/A | hiPSCs | Long-term survival, differentiation into neurons and astrocytes as well as synaptogenesis | 114 |
| Alginate functionalized with RGD | N/A | Human SH-SY5Y cells | Cell survival and neuronal differentiation | 115 |
| Alginate, gellan gum, laminin | N/A | hiPSC-IMR90 lines | Differentiate into neurons and astrocytes and display spontaneous intracellular calcium signals | 116 |
| GelMA | N/A | Bone mesenchymal stem cells (MSCs), neural stem cells | Survival, proliferation, neural stem cells differentiated more toward neurons and oligodendrocytes than toward astrocytes | 117 |
| PuraMatrix | N/A | Adipose derived stem cells | Proliferation, adhesion, and differentiation into motor neuron-like cells | 118 |
| N/A | PNIPAAm-PEG | Human ESCs lines H1 and H9 | hPSCs expansion and differentiation | 106 |
| Peptide | PEG | Hb9:GFP mouse ESCs | Motor neuron differentiation and axonal outgrowth | 107 |
| Sericin, gelatin | PVA | PC12s Schwann cells (SCs) | SCs and PC12s developed neuronal networks | 23 |
| Velvet antler polypeptides, sodium alginate (SA) | PVA | iPSCs | iPSCs differentiated into neurons, astrocytes, and oligodendrocytes, with the presence of VAPs promoting oligodendrogenesis in a dose-dependent manner | 108 |
| GelMA | PEG diacrylate (PEGDA) | SH-SY5Y cell line | Cell survival in GelMA hydrogel, but not in PEGDA hydrogel | 119 |
| Collagen hyaluronic acid | PEG | Primary mouse microglial cells, cortical neurons, and astrocytes | Cell metabolic activity up to 21 days and synapse formation | 120 |
| Collagen | Polypyrrole (PPy) | PC12 cells | Cell extension and neuronal functional expression | 121 |
| Silk fibroin | Graphene | Rat SCs, PC12 cells, mouse embryonic stem cells | Regulating nerve cell behaviours | 122 |
| Poly (ethylene glycol) dimethacrylate (PEGDMA) | Multi-walled carbon nanotubes (CNTs) | PC12 cells | Neural differentiation | 123 |
Hydrogels are commonly fabricated from natural materials such as decellularized tissue matrix, ECM proteins, animal or plant derivatives, polysaccharides and protein-engineered materials,102–105 or synthetic materials like poly(vinyl alcohol) (PVA), poly(ethylene glycol) (PEG), and hybrid matrices.106–108 While synthetic polymers enable the precise control of hydrogel mechanical properties, they often lack cellular activity due to the absence of bioactive elements.97 On the contrary, natural polymers support enhanced cellular interactions but tend to form constructs that are less mechanically and dimensionally stable compared to synthetic hydrogels.109 Co-hydrogel or biosynthetic systems aim to combine the advantages of both natural and synthetic polymers, allowing for greater control over polymer properties.110 The following sections will discuss the capability of hydrogels to incorporate and deliver cells with crucial growth cues which are essential for neural tissue modelling.
3.1. Tuneable properties of 3D hydrogel system
As illustrated in Fig. 3, cell-compatible hydrogels can be designed to integrate and control biochemical, mechanical, and electrical cues. This allows modulation of cell behaviours, such as proliferation, differentiation, adhesion, migration, and cell–cell and cell-microenvironment interaction.35,124 However, the key challenge consists in tailoring hydrogel technologies to fulfil all of the design requirements for neural tissue modelling in a single system. This section discusses recent findings about integrating biochemical, mechanical, physical, and electrical cues into hydrogels to promote neural cells development, separately. | ||
| Fig. 3 Schematic of hydrogel systems with various tunable properties to instruct cellular behaviors in modeling 3D neural tissue in vitro. | ||
| Hydrogel material | Biochemical cues | Cell/tissue type | Key findings | Ref. |
|---|---|---|---|---|
| Matrigel, hyaluronic acid | Retinoic acid (RA), sonic hedgehog (Shh), anti-a6, anti-b1 | mESC | Blocking a6 or b1 integrin subunits inhibited effects on neural differentiation and neurite outgrowth; RA and Shh promote differentiation into neural and astrocyte lineages | 103 |
| Fibrin nanofiber hydrogel | AFG and fSAP | SCs | AFG/fSAP hydrogel exhibit significantly higher density of myelinated nerve fibres and innervated muscles and motor function, compared with AFG group | 126 |
| Acrylated PLA-b-PEG-b-PLA | CNTF | Mouse retinas | CNTF released from a degradable hydrogel above an explanted retina could stimulate outgrowth of neurites | 127 |
| Silk fibroin nanofibers | NGF | Rat SCs; PC12 cells; rat neural stem cells, implantation to rat spinal cord | NGF was optimized to regulate the neural/astroglial differentiation and to obtain the differentiation ratios | 128 |
| Matrigel | Basic fibroblast growth factor (bFGF) | Mouse neural stem cells | When combined with bFGF, electric field stimulation establishes neural tissue with a proper neuronal cell number, highly branched neurites, and a well-developed neuronal network | 130 |
| Collagen type I | EpoB-loaded PCL microspheres | hEnSCs | The collagen hydrogel containing the EpoB improves MN-like cell differentiation and maturation of hEnSCs | 136 |
| Silk fibroin/collagenhydrogel | Continuous spatial biochemical gradient, NGF | DRG neurons | Scaffold with nerve growth factor gradient along oriented microstructure promote nerve regeneration and directional elongation | 137 |
| AFG/fSAP | KLT and RGI peptides | Implantation to rat spinal cord | AFG/fSAP can facilitate spinal cord regeneration via guiding regenerated tissues, accelerating axonal regrowth and remyelination, and promoting angiogenesis | 145 |
| Elastin-like polypeptide hydrogels | RGD ligand | Chicks DRG | Increasing the cell-adhesive RGD ligand density led to a significant increase in the rate, length, and density of neurite out-growth | 146 |
| PCL microfiber | Oxymatrine (OMT) Decellularized spinal cord ECM | Neural stem cells, implantation to rat spinal cord | Composite scaffold could guide the directional growth of axons, reduce scarring, and promote the recovery of motor function in rats | 147 |
| Pentapeptide IKVAV-functionalized poly (lactide ethylene oxide fumarate) (PLEOF) hydrogel | Acrylated IKVAV(Ac-IKVAV) peptide and YIGSR (Ac-YIGSR) peptide | Rat neural stem cells, L929 (fibroblasts), rabbit red blood cells | Neural stem cells could be readily formed as spheroids that can attached and proliferated within the 3D hydrogel constructs; new blood vessels formed in vivo, and few inflammatory responses were observed in 4-week implantation study | 148 |
| Methacrylated hyaluronic acid (MeHA)/collagen-I hydrogel | NGF, GDNF | SCs, dorsal root ganglia | MeHA/collagen-I bioink improved cell viability, and was conducive for cell adhesion, growth factor sequestration, and neural cell infiltration | 138 |
| Acellularized spinal cord matrix and gelatine-acrylated-β-cyclodextrin-polyethene glycol diacrylate hydrogel, aligned PCL microfibers | WAY-316606, an activator of Wnt/β-catenin signalling | Rat neural stem cells, implantation to rat spinal cord | Composite hydrogel could significantly recover the motor function of rats after spinal cord injury | 149 |
ECM-integrating hydrogels aim to mimic the composition the tissue matrix integrating single ECM components such as laminin,114 collagen,112 hyaluronic acid,120 and fibronectin70 or multiple components through decellularized matrix129 or commercially available tissue-derived substances like Matrigel,130 and Geltrex.113 Notably, naturally sourced hydrogels can readily present ECM components, whereas synthetic variants necessitate exogenous incorporation through physical or chemical methods. It is essential to allow for spatiotemporal control of ECM components independent of the hydrogel material as their presence and relative distribution can influence neuronal outgrowth and behaviours.131 For instance, in peripheral neurons a laminin-rich substrate supports elongation of neurites whereas collagen-IV rich substrates promote the growth of dendritic processes for innervation of target tissue.132,133
Hydrogels can also be crosslinked with designed peptides by physical or chemical bonding to optimise their biocompatibility. Li et al., generated synthetic peptide hydrogels composed of collagen-like peptide (CLP) and CLP with an integrin-binding motif arginine-glycine-aspartate.134 These hydrogels facilitated the spontaneous organization of primary cerebellar cells into tissue-like clusters which exhibited fast-rising calcium signals in the soma, indicative of action potential generation.135
Soluble molecule-encapsulating hydrogels can be engineered to release growth factors, which are important for the survival, proliferation, and differentiation of neural cells.128,136 Nerve growth factor (NGF) is commonly used to support differentiation and neurite outgrowth of nerve cells along with brain derived neurotrophic factor (BDNF) and ciliary-neurotrophic factor (CNTF).127,128,137 The incorporation of other factors like Glial cell line-derived neurotrophic factor (GDNF) supports the survival and growth of various types of neuronal and glial cells.138
The primary requirement for neural cells development hinges upon a biochemical environment that supplies adhesion sites and sustains crucial biological functions for cell survival, growth, migration, and differentiation.139 Neural cells, in turn, perceive their environment through adhesion molecules known as integrins. These integrins engage with the cell cytoskeleton and modulate gene expression, proliferation, and survival via bidirectional signalling with the biochemical milieu. For instance, collagen, a key ECM component, is frequently employed in vitro for cell culture studies.120 Epothilone B (EpoB), a microtubule-stabilizing agent known for its ability to penetrate the blood–brain barrier, exhibits promise in inducing axon regeneration and elongation in damaged nerve cells.136 Mahmoodi et al. demonstrated this potential by fabricating a composite of 3D collagen hydrogel containing EpoB-loaded polycaprolactone (PCL) microspheres with varying concentrations of EpoB, as depicted in Fig. 4A.136 Their findings indicated that EpoB-loaded PCL microspheres within the 3D collagen hydrogel could enhance the adhesion, proliferation, and motor neuron differentiation efficacy of human endometrial stem cells (hEnSCs).136 Neural cells can sense the environment through adhesion molecules, termed integrins.140 The integrins interact with the cell cytoskeleton and influence gene expression, proliferation, and survival through bidirectional signalling with the biochemical environment.141
 | ||
| Fig. 4 Incorporating biochemical cues into 3D hydrogel system for neural tissue engineering. (A) Adhesion, and proliferation of human endometrial stem cells (hEnSCs) regulated by different concentrations of Proanthocyanidin (PA) and epothilone B (EpoB) in collagen hydrogels.136 Reproduced from ref. 136 with permission from Springer Nature, copyright 2021. (B) Nerve growth factor gradients 3D microchannels directed axonal orientation of dorsal root ganglia on the longitudinal sections of silk fibroin/collagen hydrogel.137 Reproduced from ref. 137 with permission from American Chemical Society, copyright 2020. (C) Aligned fibrin hydrogel (AFG) and functionalized self-assembling peptides (fSAP) nanofiber composite hydrogel facilitate spinal cord regeneration via guiding regenerated tissues, accelerating axonal regrowth and remyelination, and promoting angiogenesis.145 Reproduced from ref. 145 with permission from Biomaterials, copyright 2021. | ||
Hydrogels can be modified to present spatial gradients of biochemical cues or developed to present specific structures designed to regulate cell behaviour aiming to recapitulate native microenvironment.142 For example, 3D printed silk fibroin/collagen hydrogel displaying continuous spatial NGF gradient can direct the axonal outgrowth of dorsal root ganglia (DRG) toward high-NGF concentration side as shown in Fig. 4B.137 Self-assembled 3D neural tissue models naturally generate a dynamic microenvironment through cell–cell interactions. In the process, signaling molecules or extracellular matrices are secreted spatiotemporally.143 However, it is still necessary to offer regulations over the microenvironment in self-assembled models to guide cells behaviors.144 By combining hierarchically aligned fibrin hydrogel (AFG) and functionalized self-assembling peptides (fSAP), nanofiber composite hydrogel AFG/fSAP can present both biochemical and topographical cues for SCs in the microenvironment, illustrated in Fig. 4C.145 The addition of fSAP improved the water content of the interpenetrating hydrogel and supplied more adhesion sites for cells.145In vivo results showed that AFG/fSAP facilitated spinal cord regeneration via guiding tissue regeneration, accelerating axonal regrowth and remyelination, and promoting angiogenesis.145
Overall, hydrogels can be modified to support various biochemical composition, concentration, and structure, which can change hydrogel microenvironment to guide cell behaviors through bioactive signaling and cell–ECM interaction.115 In addition to biochemical cues, mechanical and physical cues also exert significant influence on the regulation of neural cell behaviors.
 | ||
| Fig. 5 The effects of stiffness of hydrogel on cellular behaviours for neural tissue engineering. (A) Brain organoids development and differentiation were significantly affected by matrix stiffness in Matrigel hydrogel modified with an interpenetrating network of alginate.167 Reproduced from ref. 168 with permission from American Chemical Society, copyright 2022. (B) The higher modulus 16 kPa PVA-sericin/gelatine supports development of neuronal networks when Schwann cells were co-encapsulated with PC12s.23 Reproduced from ref. 23 with permission from Acta Biomaterialia, copyright 2019. (C) Mechanical stretching enhance neurite extension and axon elongation of N2a cells in 3D GelMA-PEGDA hydrogel.151 Reproduced from ref. 151 with permission from Springer Nature, copyright 2022. | ||
 | ||
| Fig. 6 The effects of topography of substrate on cellular behaviours for neural tissue engineering. (A) Schematic illustration of different featured topographies and their effects on cell behaviours.150 Reproduced from ref. 150 with permission from J Mater Chem B, copyright 2021. (B) Axons aligning along a horizontally imprinted pattern (200 nm width and 400 nm pitch) in polymethylmethacrylate (PMMA)-covered silicon chips.157 Reproduced from ref. 157 with permission from Biomaterials, copyright 2006. (C) Nanotopographic substrates promote human pluripotent stem cell neuroepithelial conversion on glass substrates functionalized with human vitronectin.158 Reproduced from ref. 158 with permission from Nanoscale, copyright 2018. | ||
| Hydrogel materials | Mechanical properties | Cell types | Key findings | Ref. |
|---|---|---|---|---|
| Poly (vinyl alcohol) copolymerised with sericin and gelatine (PVA-SG) | Compressive moduli of 16 kPa and 2 kPa | PC12 cells, SCs | The higher modulus PVA-SG supports development of neuronal networks when SCs were co-encapsulated with PC12s. | 23 |
| Glycosaminoglycan-binding polyacrylamide hydrogels | Gel stiffness (0.7 kPa, 3 kPa and 10 kPa) | hESC lines (H1, H7, H9, H9 Syn-GFP, and SA02) | Stiff substrates maintain hESC proliferation and pluripotency; compliant hydrogels facilitate hESC neuronal differentiation | 182, 183 |
| Laminin coated polyacrylamide (PA) gels | Shear modulus (200 Pa, 250 Pa, 2150 Pa, 9000 Pa) | Rat neuronal and glial cells | Laminin-coated soft gels encourage attachment and growth of neurons while suppressing astrocyte growth | 153 |
| Alginate hydrogels | Shear modulus (183 Pa, 1028 Pa, 1735 Pa, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 Pa) 700 Pa) |
Rat neural stem cells | The rate of proliferation and differentiation of neural stem cells decreased with the increase in modulus | 186 |
| Collagen I-Matrigel hydrogel | Matrix stiffness (5.1 kPa to 9.8 kPa) | SCs | Collagen I-50% Matrigel hydrogel with 9.8 kPa matrix stiffness could support Schwann cell spread and grow over 14 days | 187 |
| Methacrylamide chitosan hydrogel | Young's elastic modulus (E(Y)) (1 kPa to 30 kPa) | Rat neural stem/progenitor cell (NSPC) | NSPCs exhibited maximal proliferation on 3.5 kPa surfaces; neuronal differentiation was favoured on the softest surfaces with (E(Y)) < 1kPa; oligodendrocyte differentiation was favoured on stiffer scaffolds (>7 kPa) | 164 |
| Elastin-like polypeptide hydrogels | Elastic moduli of 0.5, 1.5, or 2.1 kPa | Chicks DRG | Most compliant materials (0.5 kPa) led to the greatest out-growth, with some neurites extending over 1.8 mm by day 7 | 146 |
| Poly-L-lysine and laminin coated polydimethylsiloxane (PDMS) and polyacrylamide | Young's modulus (5 kPa, 500 kPa) | Rat primary rat cortical neurons | Migration of cortical neurons is enhanced on soft substrates, leading to a faster formation of neuronal networks | 188 |
| Laminin coated polyacrylamide (PAA) hydrogel | Shear modulus (1 kPa, 0.1 kPa) | Xenopus eye primordia | Retinal ganglion cell axons grown on stiff substrates were significantly longer than those grown on soft substrates | 189 |
| Polyacrylamide gels | Young's modulus (170 Pa–3200 Pa) | Rat hippocampal cells | Soft substrates (170 Pa) promoted the formation of neurites than stiff substrates (3200 Pa) | 190 |
| Polyethylene glycol diacrylate-gelatine-methacryloyl (GelMA) hydrogel | Tensile modulus (60 kPa–10 kPa) and compressive modulus (6 kPa–0.8 kPa) | Mouse neuroblastoma cell line Neuro2a | Mechanical stretching could significantly enhance neurite extension and axon elongation and the neurites were more directionally oriented to the stretching direction | 151 |
| Vinyl sulfone and RGD functionalized dextran hydrogel | Young's moduli ranging from 0.1 kPa to 6 kPa | Human mesenchymal stromal cell | Matrix degradability regulates cell spreading kinetics, while matrix stiffness dictates the final spread area once cells achieve equilibrium spreading | 166 |
| Gelatin methacrylate and gelatin mixed with fibrin hydrogels | Single channel size: 150 × 300 × 5000 (w × h × l) μm; | iPSC – derived spinal neuronal progenitor cells (sNPCs) and oligodendrocyte progenitor cells (OPCs) | The bioprinted sNPCs differentiated and extended axons throughout microscale scaffold channels | 155 |
| Poly(lactide-co-glycolide) | A bridge mould (1.5 × 2.6 × 4 (w × h × l) mm) with channels of 250 or 150 μm diameter. | Bridge implantation in rat spinal cord lateral hemisection injury model | Bridges supported substantial cell infiltration, aligned cells within the channels, axon growth across the channels, and high levels of transgene expression at the implant site with decreasing levels on rostral and caudal portions | 156 |
Differentiation of stem cells towards a specific lineage can be influenced by external stimulus such as the mechanical stiffness of the supporting substrate.161,162 This stiffness level has been shown to support neuronal differentiation of hMSCs and primary neural stem cells.163–165 To investigate how cells sense matrix stiffness in 3D environments, Long et al. co-polymerised vinyl sulfone with RGD-functionalised dextran to develop a hydrogel in which matrix stiffness can be tuned independently.166 Hydrogel stiffness was modulated by tuning the ratio of components, resulting in Young's moduli ranging from 0.1 kPa to 6 kPa. They found that hMSCs spread more extensively on stiffer hydrogels. Furthermore, as shown in Fig. 5A, Cassel de Camps et al., found that the development of organoids is significantly affected by matrix stiffness.167 They encapsulated iPSCs derived brain organoids in 3D modified Matrigel hydrogel. The Matrigel matrix was modified with an interpenetrating network of alginate to tune the mechanical properties with matrix stiffness ranged from 1 Pa to 1000 Pa.167 Gross morphological differences developed with the stiffest hydrogel beyond 2 weeks, as organoids in the stiffest matrix grew to be rounder and significantly smaller than those in the softer variants.167 Additionally, the number of neural rosette structures, indicative of ESCs differentiating into neuroprogenitors, decreased over threefold in stiff gels. While there were no notable differences in the proportion of dopaminergic neurons (as indicated by TH expression), a significant increase in the proportion of mature neurons (as indicated by MAP2 expression) was observed in the stiffest matrix formulation tested.167 Overall, supporting that hydrogel mechanics can impact on self-organizing capacity and development of organoids.
Other studies have shown that careful tailoring of matrix stiffness can support neural cell development and survival in complex co-culture systems.168 For example, Aregueta-Robles et al. co-cultured SCs and neuron-like PC12 cells within a degradable PVA-based hydrogel (Fig. 5B).23 In this scenario, cells are expected to grow and extend a supporting network before the hydrogel completely degrades. To achieve this, the hydrogel stiffness was tailored to transiently support SCs growth, allowing cells to expand processes and form a supporting substrate for the PC12 cells. SCs and PC12 cells were able to grow neuronal networks when the initial hydrogel compressive modulus was 16 kPa.23 As the hydrogel degraded SCs were presented with a substrate stiffness around 1kPa–2 kPa, which is a mechanical modulus reported to support development of cytoplasmic processes.153 Hydrogels with a mechanical modulus lower than 1 kPa were not able to support effective growth and development of neural cells.23 Indeed, SCs have been reported to express less adhesion proteins as the substrate stiffness decreases,169 and to grow less processes when grown on soft substrates (<1 kPa).23
Mechanical stretching is another property reported to influence cell behaviour.170 Mechanical stretching has been shown to enhance neurite extension and axon elongation (Fig. 5C).151 Quanjing and colleagues studied the differentiation of mouse neuroblastoma cells Neuro2a (N2a) encapsulated in GelMA hydrogels co-polymerised with PEGDA. These cells were subjected to mechanical stretching at 0%, 25%, and 50% of the initial hydrogel length (Fig. 5C).151 Results showed that stretching induced significant neurite outgrowth with longer neurites than the unstretched control group. Moreover, cells subjected to stretching exhibited up to a threefold increase in Tuj-1 (neuron-specific class III β-tubulin) expression and a twofold increase in GFAP (Glial fibrillary acidic protein, GFAP) expression compared to non-stretched controls.151 Notably, cell cultures were not highly confluent, most likely due to the confinement of the non-degradable matrix.151 However, these studies support hydrogel stretching as a potential strategy to promote neural differentiation towards specific lineages and to direct axon outgrowth.151
Providing topographical cues is a common approach to directly impacting cell adhesion, migration, morphology, and neurite outgrowth.171Fig. 6 shows different approaches to guide neurite outgrowth and promote neuronal differentiation into specialised lineage. Typical topographical factors include grooves and surface features with variable arrangement patterns (Fig. 6A).150 Hydrogels in particular have been engineered to provide topographical cues, such as channels, grooves or ridges, and various degrees of surface roughness that can ultimately lead to alterations in the growth, differentiation, and proliferation of cells during neural development.172In vivo studies have shown that tubular-shaped hydrogels can support regeneration of severed nerves.173,174 In this scenario, the pathway for cell growth is constrained by the hydrogel substrate, thus forcing unidirectional growth. In vitro studies have also shown that directional growth of DRG neurons can be directed by spatially defining their 3D environment.175 Notably, while hydrogels serve to guide neurite outgrowth, the degree of regeneration is substantially greater, comparable to autografts, when the substrate is supplemented with ECM or growth factors.157 Autografts represent the gold standard approach for peripheral nerve regeneration but repairing gaps longer than 5 mm proves difficult,176 in part because regenerating axons taper as they grow.173 Hydrogel nerve guidance conduits have shown some success of nerve repair in greater gaps up to 40 mm, with larger defects of up to 5 cm requiring incorporation of living cells within the graft.177 However, matching the regenerative level of autografts has not been accomplished.
Micropatterning growth substrates has also been used as a strategy to drive directed cell growth. Berkovitch and Seliktar encapsulated DRG neurons in PEG hydrogels conjugated with fibrinogen, gelatin, and albumin.178 They showed that neurons grew and extended axonal processes through laser ablated microchannels of the order of 10 to 70 μm. Similarly, Li et al., micropatterned a polyacrylamide hydrogels grafted with YIGSR peptides.179 The patterning consisted of aligned ridge/groove structures, both of 30 μm, and tested its potential to align Schwann cells. Hydrogel micropatterns effectively controlled the aligned growth of Schwann cells and increased the expression of genes related to the cytoskeleton, indicating promising potential for their use in nerve regeneration applications.179 Of note, cell growth over microfabricated materials is complex. Goldner et al. found an unusual capability of a subpopulation of neonatal rat DRG neurons to extend neurites that spanned across the grooves, with no underlying solid support, which is of interest to designing biomaterials for 3D axon guidance.180 Johansson et al. fabricated nano-printed patterns in polymethylmethacrylate (PMMA)-covered silicon chips on which patterns consisted of parallel grooves with depths of 300 nm and varying widths of 100 to 400 nm to test the effect of nano-printed patterns on axonal outgrowth (Fig. 6B).157 After 1 week of incubation, axons displayed contact guidance on all patterns and the nerve cell processes preferred to grow on ridge edges and elevations in the patterns rather than in grooves.157
Changes to substrate roughness can also impact on cell growth and behaviour. As shown in Fig. 6C, Chen et al. showed that conversion of hiPSCs and hESCs into neuroepithelial cells can be promoted by increasing the surface roughness of cell culture substrates.158 Stem cells cultured on vitronectin-coated glasses with varied surface roughness differentiated into neuroepithelial cells as early as day 2 on substrates with local surface features of 200 nm, as evidenced through expression of PAX6, a marker of early neuroectodermal differentiation.158 This was half the time required for start observing PAX6+ cells in smoother surfaces (1 nm).158 In addition, the rougher substrates yielded over 88% PAX6+ neuroepithelial cells by day 8, compared to approximately 32% in the smooth variants.158 This suggests that nano-topographic cues can provide potent regulatory signals to mediate adult stem cell behaviours, including self-renewal and differentiation.158 However, there has been minimal research on application of such physical features to hydrogels. In a study by Hou et al., the impact of surface roughness and stiffness on the mechanical response and osteogenesis of hMSCs was investigated.181 GelMA hydrogels were patterned with nano- and micro-aggregates, resulting in variable surface roughness ranging from 200 nm to 1.2 μm and mechanical properties ranging from 3.8 kPa to 31.3 kPa.181 It was observed that the mechano-response and osteogenesis of hMSCs were significantly enhanced on the rougher regions of soft hydrogels and on regions with intermediate roughness on stiff hydrogels.181 This suggests a synergistic effect between roughness and stiffness in driving cellular mechano-response.181
Comprehensive studies are still required to fine-tune the mechanical microenvironment for neural tissue engineering.182,183 However, tailoring the mechanical and physical cues can only address in part the guidance of neuronal growth and cell behaviors.184 Combinations of biochemical, mechanical and physical factors are all critical contributors to nerve growth guidance.185 While their relative contribution is not clear, it is likely all of these factors are interdependent.185 In addition to these three factors, electrical signals also play a key role in neural tissue development and function.
 | ||
| Fig. 7 Incorporating electrical cues into 3D hydrogel system for neural tissue engineering. (A) Electrical stimulation promotes axon outgrowth, Schwann cell migration and myelination in nanofiber hydrogels incorporated with carbon nanotubes.201 Reproduced from ref. 201 with permission from American Chemical Society, copyright 2020. (B) Rat cortical neurite outgrowth at 24 h in a silk hydrogel after electrical stimulation of varying frequencies showed a preference of orientation towards the embedded electrodes, but not affected by AC field frequencies.203 Reproduced from ref. 203 with permission from Brain Research, copyright 2018. (C) Thermo-sensitive electroactive hydrogel that made by poly (ethylene glycol)-co-polyvaline (mPEG-PLV) polymer grafted with tetraniline and loaded with nerve growth factor combined with electrical stimulation promoted endogenous neurogenesis and improved motor function.204 Reproduced from ref. 204 with permission from Springer Nature, copyright 2021. | ||
| Hydrogel materials | Electrical components | Cell type | Key findings | Ref. |
|---|---|---|---|---|
| Silk/collagen hydrogel | Gold wires, alternating field of 80 mV mm−1, 0.5 Hz–2 kHz | Rat primary cortical neurons | Alternating electric field stimulation could direct axon 3D length growth and orientation | 203 |
| PEGDMA | CNTs | PC12 cells | PEGDMA with randomly distributed CNTs promote neural differentiation | 165 |
| PEG-PEGDA hydrogel | Polyimide stimulating electrodes, biphasic electrical stimulation of 100, 500 and 1000 μA, with a 100 Hz frequency at 100 μs | Neural stem cells | Scaffolds promoted neural stem cell proliferation and early neuronal differentiation; 500 μA current promoted neuronal maturity | 197 |
| Silk fibroin and graphene oxide hydrogel | Graphene oxide nanosheets | SCs | Soft substrates supported SC survival, proliferation, spreading, and gene expression of neurotrophic factors, while the increased conductivity may also be beneficial to SC functional behaviours | 206 |
| Polydopamine functionalized reduced graphene oxide (rGO-PDA) with PVA | Graphene oxide | PC12 cells | Highly efficient neuronal differentiation was also observed both with and without electrical stimulation | 198 |
| mPEG-PLV polymer with nerve growth factor | Tetraniline | PC-12 cells, neural stem cells isolated from newborn Sprague–Dawley rat | Electrical stimulation promoted the neuronal differentiation of neural stem cells and axonal growth | 204 |
| Amino-functionalized graphene crosslinked collagen | Electric (3.8 ± 0.2 m Siemens per cm); electric stimulation (100 mV mm−1) | Raw 264.7 cells, bone marrow derived MSCs | Suppressing the neuro-inflammation and promoting the neuronal cellular migration and proliferation | 199 |
| Silk-gelatine/polylactic acid | NGF-incorporated Fe3O4-graphene nanoparticles | PC12 | A biologically and electrically conductive cell passage with one-dimensional directionality was constructed to allow for a controllable constrained geometric effect on neuronal adhesion, differentiation, and neurite orientation | 207 |
| 3D Matrigel with bFGF | Direct current electric field via 5% FBS agar bridges (150 mV mm−1 DC). | Neural stem cell from the fatal brain tissue of C57BL/6 mice at E12–14 | EF-stimulated neural stem cells in 3D Matrigel mainly differentiated into neurons, bFGF promoted neural differentiation with highly branched neurites, and neuronal network development | 130 |
| PEG-based hydrogel | Poly(3,4-ethylenedioxythiophene) (PEDOT) | C2C12 myoblasts | Combination of topographical and electrical cues maximized the differentiation of C2C12 myoblasts into myotubes | 208 |
| Collagen–polypyrrole hybrid hydrogel | PPy nanoparticles, 100 mV cm−1 | PC12 cells | The oriented fibrous microstructures enhanced neuron-like cells aligning with fibres’ axon; the matrix conductivity improved cell extension and upregulated neural related gene expression; external electrical stimulation further promoted the neuronal functional expression | 121 |
| Silk fibroin | Graphene | Rat SCs, PC12 cells, mouse embryonic stem cells | Multiple cues including nanofibrous structure, aligned topography, stiffness, bioactive graphene sheets and hydrogel state were successfully introduced into SF-based materials, resulting in synergistic effects on nerve cell behaviours | 122 |
| Poly (terephthalate) | PEDOT:PSS, indium tin oxide on poly(terephthalate) conductive back electrode, zinc oxide electron transfer layer, poly(3-hexylthiophene) photoactive layer | Primary hippocampal neuron, primary cardiac myocyte | These devices enable stimulation of individual hippocampal neurons and photocontrol of beating frequency of cardiac myocytes via safe charge-balanced capacitive currents | 209 |
| Matrigel | Electrotactic chamber via 5% FBS agar bridges, 150 mV mm−1 DC | Human neural stem cells, mice neural stem cells | Electrical stimulation could induce neuronal differentiation, the orderly growth of neurites and the maturation of neuron subtypes to construct a well-developed neuronal network with synapses and myelin sheaths | 210 |
He et al. incorporated carbon nanotubes into self-assembling peptide hydrogels to study the effect of electrical stimulation on axon outgrowth and Schwann cell migration (Fig. 7A).201 DRG spheres were encapsulated in the conductive hydrogel substrate and subjected to 1 mA at 10 Hz for 30 minutes per day over 30 days.201 Electrical stimulation promoted axon outgrowth, Schwann cell migration away from DRG spheres, and promoted myelination of growing neurites.201 Additionally, the stimulation approximately doubled the expression of the myelin basic protein gene and the c-Jun gene,201 the latter known for initiating a repair process in Schwann cells and facilitating the development of specialized cells that support regeneration.202 Of note, the incorporation of carbon nanotubes had a negative impact on neurite outgrowth compared to a non-conductive hydrogel control.201 Thus, while electrical stimulation has potential to enhance nerve regeneration, careful consideration of material properties is crucial for optimizing outcomes.
Lee et al. further elaborated on this concept, demonstrating how electroconductive scaffolds augmented neural stem cell proliferation and differentiation.197 By integrating amine-functionalized multi-walled carbon nanotubes (MWCNTs) within PEGDA polymer, they observed that MWCNT-incorporated scaffolds facilitated neural stem cell proliferation and early neuronal differentiation compared to scaffolds lacking MWCNTs.197 Moreover, biphasic pulse stimulation with 500 μA current at 100 Hz for 4 days promoted neuronal maturation, as evidenced by increased RNA expression of Tuj1, Nestin, and GFAP protein.197
Furthermore, Wu et al. emphasized the synergistic benefits of combining matrix orientation with electroconductivity to enhance neural function.121 Electroconductive PPy nanoparticles were synthesized with modified hydrophilicity to enhance their uniform distribution within collagen hydrogels.121 Findings showed that collagen–PPy hydrogel microfibers with aligned microstructures could effectively guide neurite growth of PC12 cells along the matrix-defined direction.121 Additionally electrical stimulation further promoted neurogenesis, improved cell extension, and significantly upregulated the expression of tubulin-βIII, neurofilament protein, and voltage-gated calcium channels genes. Therefore, alongside electroconductivity, the oriented microstructure and desired bioactivity of the matrix should be considered in the design of biomimetic ECM for neurogenesis.121
While hydrogel materials inherently lack conductive elements, their hydrophilic nature enables them to act as passive conductors. Tang-Schomer et al., for instance, introduced electrodes into a hydrogel system to stimulate axon growth in rat cortical neurons (Fig. 7B).203 This system consisted of a 3D silk/collagen hydrogel with embedded gold wires to deliver an alternating field of 80 mV mm−1 at 0.5 Hz to 2 kHz for up to 4 days, in which rat cortical neurons were exposed.203 Results showed that 0.5 to 20 Hz can promote axon growth, with 2 Hz producing the biggest effect of about 30% increase in axon length compared to control cultures.203 Interestingly, neurite outgrowth showed a preference of orientation towards the embedded electrodes.203
Building upon these findings, Liu et al. took a comprehensive approach by combining biochemical, physical, and electrical cues within a thermosensitive polymer electroactive hydrogel (TPEH) to repair spinal cord injuries, shown in Fig. 7C.204 This hydrogel consisted of poly (ethylene glycol)-co-polyvaline (mPEG-PLV) polymer grafted with tetraniline and loaded with NGF.204 They found that electrical stimulation promoted the differentiation of neural stem cells and axonal growth in vitro.204 The average neurite length in the TPEH + NGF + ES group was 136.30 μm, which was significantly longer than that in the control (25.48 μm), TPEH + NGF (38.64 μm), and TPEH + ES (45.12 μm) groups.204 In a rat model of spinal cord injury, the rate of neurogenesis in the group of TPEH + NGF + ES was 1.2 and 3.1 times higher than that of the Gel + NGF, and Gel + ES groups, respectively.204 Their study revealed that electrical stimulation, alongside the delivery of nerve growth factor, not only promoted neuronal differentiation and axonal growth in vitro but also stimulated endogenous neurogenesis, ultimately leading to improved motor function.204
Overall, this integration highlights the importance of incorporating multifaceted growing cues in the design of biomimetic extracellular matrices for neurogenesis and tissue repair.205 The next section discusses common fabrication technologies for engineering 3D hydrogels, offering insights into how these technologies can be leveraged to design cell supporting substrates with tailored properties conducive to neural tissue regeneration and repair.
3.2 The fabrication technologies for engineering 3D hydrogel
Identifying fabrication techniques that bridge the gap between the natural, uncontrolled microenvironment characteristic of self-assembled models and the controlled, fabricated synthetic scaffold used in engineered structures is critical for developing functional and representative 3D neural tissue models. A significant challenge in engineering nerve tissue involves creating nerve guiding conduits with supporting substrates that enable control over cell growth and organization into functional neural architectures.211 Shaping hydrogels into nerve guiding conduits has been achieved through conventional methods like mould-casting and more recently, 3D printing techniques. However, each approach presents advantages and limitations, necessitating a balance between bioactivity and structural fidelity.The ability to precisely manipulate the arrangement and combination of transplanted cells holds great potential for tissue engineering, whether it is for growing nerve tissue in the lab or regenerating tissue at injury sites. However, incorporating cells into hydrogel bioinks presents additional challenges due to their impact on ink rheology and the potential effects of ink components on cellular viability.213,220Fig. 8 illustrates the representative technologies of 3D bioprinting to fabricate hydrogel systems for neural tissue engineering. For instance, in techniques like stereolithography (SLA) or digital light processing (DLP) printing, the presence of photoinitiators221 and photoabsorbers222 may pose toxicity concerns for cells depending on their concentration and exposure.222 Similarly, in extrusion-based printing, factors such as printing process parameters like speed, time and pressure, and bioink biocompatibility, can dramatically impact cell viability.223 Additionally, cell density can influence the mechanical and rheological properties of the ink.213
 | ||
| Fig. 8 3D bioprinting technologies for neural tissue engineering. (A) High cell viability, good cell attachment and improved proliferation of dermal fibroblasts in 3D printed platelet lysate and gelatine methacryloyl hydrogel.215 Reproduced from ref. 215 with permission from Acta Biomaterialia, copyright 2021. (B) Biomimetic 3D-printed polyethylene glycol-gelatine methacrylate (PEG-GelMA) bioink loaded with neural progenitor cells (NPCs) by digital light processing (DLP) printing for spinal cord injury repair.229 Reproduced from ref. 229 with permission from Springer Nature, copyright 2019. | ||
Despite these challenges, recent advancements in printing techniques and materials offer promising solutions. Specialized bioinks, such as the one developed by Daikuara et al., have been engineered to balance printing requirements with cell biocompatibility (Fig. 8A).215 This bioink, specifically designed for extrusion-based printing, consisted of platelet lysate and GelMA, aiming to mimic native skin tissue in vitro.215 This bioink exhibited favorable rheological properties and shape fidelity, along with tunable mechanical properties to the stiffness of match dermal skin.215 Within the printed hydrogel, dermal fibroblasts exhibited high viability, good attachment, and enhanced proliferation. Consequently, developing bioinks involves multiple iterations to identify an optimal component ratio that aligns printing requirements with cell biocompatibility.215 Of note, 3D printing large tissue structures can entail varying durations, ranging from minutes to hours. Prolonged printing and photopolymerization processes may potentially compromise cell health while they are suspended within the bioink.217
While 3D printing holding promise for creating nerve guiding-like structures, challenges persistent in simultaneously achieving biochemically supportive, mechanically robust tissue structures with finely resolved microscale features.224 In extrusion-based platforms, aligning hydrogel formulations with the rheology criteria of the printer often yields soft and poorly resolved structures.225 Recent advances in extrusion-based techniques, such as negative sacrificial templates226 or supporting baths,213 have facilitated the creation of highly intricate 3D hydrogels structures, yet achieving fine resolution remains a persistent challenge.227 Fine resolution is crucial for seamless cell perfusion and the precise placement of cells within the tissue-supporting structure.
Alternatively, SLA or DLP printing methods can generate structures with higher resolution, but further characterisation is essential to match the dynamic ratios of crosslinker density, photo-absorber, and photo-initiator for precise control over features during polymerization.228 These adjustments necessitate additional characterization steps to ensure that desired bioactivity and mechanical properties remain unaffected. For instance, Koffler et al. used a microscale continuous projection printing method (μCPP) to create a complex neural structures for spinal cord regenerative medicine applications (Fig. 8B).229 This study showed that μCPP can print 3D biomimetic hydrogel scaffolds tailored to the dimensions of the rodent spinal cord in 1.6 seconds and is scalable to human spinal cord sizes and lesion geometries.229 They depicted 3D biomimetic scaffolds fabricated from polyethylene glycol–gelatine methacrylate (PEG-GelMA) loaded with neural progenitor cells (NPCs) using DLP-based printing to construct a complex scaffold.229 This 3D biomimetic scaffolds supported injured host axon regeneration and significantly improved functional outcomes, highlighting the potential therapeutic efficacy of 3D PEG-GelMA scaffolds in enhancing CNS regeneration through precision medicine.229
All in all, the remarkable capabilities of 3D printing technologies have revolutionized the landscape of tissue engineering, particularly in the creation of highly customized and biomimetic constructs for neural tissue regeneration.230,231 3D bioprinting emerges as an appealing technology for fabricating intricate neural grafts, allowing for the seamless integration of diverse bioactive factors and cells to synergistically promote advanced neural regeneration.232 The advancements underscore the transformative impact of 3D printing in regenerative medicine and neuroscience, supporting the development of advanced therapies and treatments tailored to individual patients. As research in this field continues to progress, the integration of biochemical, mechanical, physical, and electrical cues within printed constructs holds promise for addressing complex neurological disorders and advancing our understanding of the nervous system.233
The key advantage of mould-casting is the potential for high-resolution architectures without compromising the application-intended properties of the hydrogel substrate, while ensuring uniformity and consistency.236Fig. 9 shows representative technologies of mould casting for neural tissue engineering. By carefully controlling component ratios, curing time and curing parameters, hydrogel architectures with consistent properties can be achieved seamlessly.188 This process facilitates the integration of bioactive molecules or conductive materials into the hydrogel structure, enhancing its functionality without detracting from its intended properties.237 Furthermore, this method ensures the uniform distribution of additives, thereby maintaining the overall integrity of the hydrogel substrate.236 As depicted in Fig. 9A, this versatility is exemplified by An et al., where a simple and efficient method for fabricating 3D structures was proposed without resorting to complex processes such as soft lithography and 3D bioprinting.238 Utilizing a direct tissue casting method, a complex U-shaped tissue structure was reconstructed using catechol-conjugated alginate-based hydrogels, rendering a non-toxic substrate with suitable stiffness biocompatibility for H9c2 cells.238 Furthermore, the stable attachment of model drugs to the surface of the 3D film structure via the catechol group supports the potential of direct tissue casting as an alternative approach for implementing multi-scale tissue mimetic strategies.238 This method can be further applied for neural tissue fabrication.
 | ||
| Fig. 9 Mould-casting technologies for neural tissue engineering. (A) A direct tissue casting method to fabricate catechol-conjugated alginate based hydrogel for reproducing complex-shaped target tissues.238 Reproduced from ref. 238 with permission from Bioengineering, copyright 2021. (B) A new strategy for damage-free demolding is based on using the 3D printed soft ultrafine fibers as the molds and peeling off these fibers from the hydrogel softly.240 Reproduced from ref. 240 with permission from IOP Publishing, copyright 2020. (C) A simple 3D mold fabrication technology to prepare tubular tissue grafts of different sizes by combination of 3D printing and mould-casting.244 Reproduced from ref. 244 with permission from Colloids and Surfaces A: Physicochemical and Engineering Aspects, copyright 2023. | ||
The primary challenge lies in devising non-toxic, easy-to-remove templates capable of accurately replicating the desired tissue geometry and topography. Common limitations to mould-casting are inherent to the moulding materials and to the complexity of the intended structure. First, moulding materials should not interact with the hydrogel solution to facilitate mould removal. For example, acrylic resin is highly versatile but it can stick to hydrogel materials like PVA,239 which challenges removal and can damage the intended hydrogel structure.240 Recently, there are some methods emerging to avoid brittle hydrogels damage while demolding. As shown in Fig. 9B, making soft removable moulds to decrease local contact between the moulds and the hydrogel, which can induce significantly less adhesive and frictional force.240 Second, the size and geometry of the mould can impact on the intended final structure. For instance, simple nerve conduit models are commonly achieved using tubular structures like needles, rods, or by first creating sheets and rolling them on a mandrel with a variety of materials.235,241 However, this approach is limited when aiming to create nerve guiding conduits with channel size in the range of tens to few hundreds of microns, which are dimensions suggested to supporting axonal regeneration.239 While there are non-toxic moulding materials available, such as stainless steel wires that meet this dimensional criteria, handling them to accommodate intended structures presents a challenge.242 Stainless steel wires are inherently rigid, often thin and small in diameter which make it difficult to manipulate into complex shapes or configurations.242
Mould-casting is further limited when aiming to create non-regular 3D structures. While mould-casting can create 3D structures with specific patterns, channels, or gradients at the micro- and nanometre scale,240,243 these are of moulds that only interface the hydrogels on the surface, ultimately not feasible for creating enclosed nerve conduits. Developing complex, three dimensionally irregular tissue structures via mould casting requires support of additive manufacturing technologies. For instance, a model of the scala tympani with characteristic free-form, non-uniform channels of cochlear structures, have been made possible by creating flexible silicone moulds enabled by 3D printing negative templates.235 As shown in Fig. 9C, Zhou et al. developed an accessible, simple 3D mold fabrication technology to prepare tubular tissue grafts of different sizes by combination of 3D printing and mould-casting.244 First, they used computer-aided design for additive manufacturing of 3D molds to fabricate PLA molds with different sizes and styles.244 Then, the hydrogel precursor was poured into the PLA mold with a syringe to obtain hydrogel tubes with different wall thicknesses and layers.244 Morever, this device can be used to prepare a hydrogel tube with a diameter less than 6 mm, which could be used as an artificial blood vessel for biological transplantation.244 Other technologies like pre-cooling can be incorporated with mould-casting to advance the conventional methods and create higher resolution, more complicated structure. For example, He et al. found that pre-cooling before illumination made gelatin-based hydrogels resilient due to the partial regain of triple-helix structures, which could be demolded successfully and stably from a mold with a resolution of a feature size of 6–80 μm.245 These methods can advance and expand the application scope of mould-casting from surface structure fabrication to inner or 3D architecture development.
In conclusion, mould-casting stands out as a versatile and cost-effective method for producing hydrogels with well-defined and precisely controlled structure, size, and mechanical properties. Its compatibility with cells, biochemical, and electrical cues make it suitable for a wide range of applications, spanning from in vivo transplantation and drug delivery to in vitro modeling of neural tissue.
3.3. The challenges of hydrogel-based neural tissue models
While hydrogels with their highly tuneable properties have emerged as promising tools for engineering 3D neural tissues, current models fall short of fully recapitulating the intricate complexity and organization of neural tissue at the human scale. There remain limitations in replicating intricate cell–cell and cell–ECM interactions inherent in biological tissue.246 Achieving a high level of biomimicry in hydrogel-based neural models is a formidable challenge. It entails creating a dynamic physiological niche that replicates the normal in vivo environments produced through developmental processes. Challenges persist in integrating multiple cell types, ECM components, and growth factors with spatial and temporal control.247 Addressing these complexities becomes more intricate when considering the diverse nutrient requirements of different cell types within a permeable hydrogel substrate.Additionally, the absence of a vascular network is a key challenge for developing viable, long-term tissue cultures. Although hydrogels are inherently permeable to nutrients, the diffusion control is challenging and may lead to gradients of nutrients, oxygen, and signalling molecules that are not representative of the target tissue biology.248 Moreover, growing cells over extended periods can bring issues like substrate degradation, cell overgrowth, and altered neural behaviours, challenging tissue culture stability and functionality.249 This necessitates the development of hydrogels capable of mirroring the intricate mechanical properties of neural tissue while ensuring prolonged cell functionality.33 Addressing these challenges demands the utilization of cutting-edge hydrogel biomaterials and fabrication methodologies.
As tissue models are scaled-up to meet human dimensions, the characterisation of tissue viability and function presents added technical challenges and may need customised solutions. While there are established imaging and biochemical techniques for assessing viability in cell cultures they have not been translated and validated for large-scale tissue models. Also, imaging becomes more challenging as hydrogels could be opaque requiring clearing techniques,235 and may require more sophisticated approaches such as light-sheet microscopy or microtomography.250 Where electrophysiological characterisation is needed, interfacing with external experimental systems, like microelectrode arrays is required and the tissue model needs to be sufficiently robust to withstand contact with stiff electrode materials.251,252
Finally, the scalability and reproducibility of neural tissue engineering approaches must be addressed for clinical translation. Developing consistent and reliable methods for fabricating hydrogel-based neural tissue models is essential, and validating findings against in vivo data remains elusive.253 This task is further challenged by the lack of standardisation in the fabrication of hydrogel substrates, especially those with biological components. With cell-supporting biomolecules introducing natural variability and a multitude of hydrogel substrates, each with different formulations and fabrication approaches, the potential of hydrogels for engineering nerve tissue is ultimately hindered. Overall, the development of engineered neural tissue models and hydrogel systems for neural tissue engineering is a complex and challenging task.254 However, significant progress has been made in recent years, and the field holds great promise for the development of new therapies for neural injuries and diseases.252 Through meticulous design and optimization, engineered tissues offer the potential to navigate these obstacles effectively, ensuring improved outcomes in tissue engineering and regenerative medicine endeavours.255
4. Conclusions and perspective
Neural tissues are characterised by environments with highly networked architectures that support rapid and efficient bi-directional flow of electrical and biochemical signals from the brain to the extremities. With our current technologies and techniques, it is not yet possible to replicate the exquisite organisation of human neural networks or represent the high complexity of neural pathways. This knowledge gap underscores the importance of accelerating research and developing innovative, advanced models for future advancement of the field.256The growing field of neural tissue engineering has witnessed remarkable progress in advancing our comprehension of neural development and disease therapy15 through the use of both self-assembled and engineered hydrogel-based approaches.257 Such models have provided invaluable tools for investigating complex cellular behaviours and interactions within a context that, to an extent, mimics the native neural microenvironment.258 The unique capability of self-assembled models to emulate the spontaneous formation of neural cells and tissue-like structures serves as a fundamental asset, enabling the recapitulation of some in vivo processes.7 However, as shown in Fig. 10, challenges such as poor reproducibility, prolonged developmental times, uncontrolled organization, and inefficient biological maturation emphasize the need to refine protocols and methodologies.69,70 Engineered models, particularly those utilizing hydrogels, while not ideal, offer controlled organization, a defined microenvironment, and tuneable properties that allow for the incorporation of crucial biochemical, mechanical, physical, and electrical cues.22 Advanced fabrication techniques, like 3D printing and mould casting further enhance the precision and reproducibility of these models.212,230,235
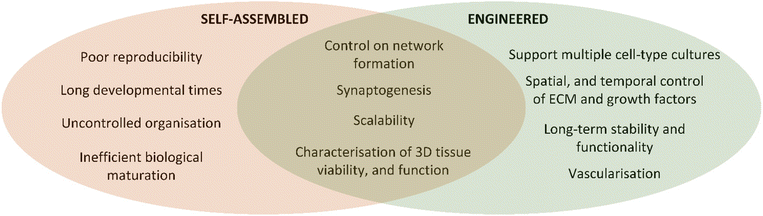 | ||
| Fig. 10 Challenges of self-assembled and engineered 3D tissue models to recreate neural tissue in laboratory settings. | ||
The key challenges limiting development of any 3D tissue engineered models at the human scale are fabrication approaches, vascularisation, and long-term stability on the bench.7,235,259,260 The fabrication of engineered tissues capable of supporting multiple cell types both structurally and functionally, while accurately reproducing the scale and extracellular environment in terms of its spatial and temporal dynamics, has not yet been achieved. The absence of a vascular network poses important challenges, highlighting the need for innovative solutions to deliver oxygen and nutrients and remove waste from tissue constructs. Additionally, maintaining stability and functionality over prolonged periods presents another obstacle, which necessitates ongoing research efforts to overcome these temporal limitations.261 Additionally, maintaining stability and functionality over prolonged periods presents another obstacle, which necessitates ongoing research efforts to overcome these temporal limitations.261
In neural tissue engineering, alongside generic tissue engineering challenges, specific barriers remain unaddressed. These encompass guiding long axonal outgrowth, controlling network formation and synaptogenesis, and ensuring proper nerve fascicle development, which involves successful integration of glial and accessory cells. The persistent challenge of promoting long axonal outgrowth reflects the complexity of replicating in vitro the extensive connections formed by nerve cells in vivo, necessitating precise biochemical and topographical cues within engineered scaffolds. Similarly, achieving controlled network formation and synaptogenesis within an engineered construct demands meticulous regulation of cellular interactions and signalling pathways. Moreover, ensuring proper nerve fascicle development requires fine-tuning biochemical and mechanical cues within the hydrogel matrix. This requires integrating and develop glial and accessory cells, which adds another layer of complexity and demands a more comprehensive understanding of their interactions and roles in neural tissue physiology. Addressing these challenges is vital for advancing neural tissue engineering and developing effective therapies for neurological disorders and injuries.
In the short term, integrating the strengths of self-assembled and engineered models is a promising strategy for mitigating individual limitations and enhancing overall model robustness.262,263 The evolution of more advanced fabrication techniques with higher resolution and adaptability to cater to different materials is imperative for creating intricate tissue models with a structurally organised, biologically functional tissue.264 This will contribute to achieving a higher degree of biomimicry and, subsequently, enhance the translational potential of these 3D models.
Of note, engineering neural tissue through hydrogel substrates necessitates alternative approaches, especially for the development or regeneration of long nerve fascicles. As discussed in section 3.1.1, the traditional approach involves presenting cells with a supporting substrate containing relevant growth factors for promoting axonal outgrowth. However, in biological tissue, this only represents the behaviour of cells of tissues in close proximity,265 occurring during either developmental stages or through regeneration of injured tissue. Axonal outgrowth and innervation of tissue, such as skeletal muscle, can start developing in fetal stages and expands as the body grows. Tissue engineering such cellular behaviour would require a clear understanding of the environment of the developing tissue, as well as a substrate that can expand in parallel with cell growth. While stretching hydrogels have been proposed to support enhanced neurite extension and axon elongation,151 the elasticity of current hydrogel matrices falls short of supporting the formation of a large nerve fascicle from cell cultures at the micro- and millimetre scale.
To effectively address such a multifaceted task, it is crucial to continue exploring hydrogel-based biomaterials, fabrication techniques, and cellular interactions. These efforts are essential for overcoming current limitations and unlocking the full potential of 3D models in understanding neural physiology and pathology and developing transformative applications for prevention, diagnosis, and treatment of neurological disorders. Moreover, it is vital to advance our understanding of neural tissue development processes. This understanding is crucial for guiding the precise delivery of physical and soluble factors necessary for growth and differentiation.
Given the extensive array of variables involved, leveraging bioinformatics, proteomics, and machine learning techniques may be indispensable in unravelling some of these challenges. The integration of these multidisciplinary approaches can help gaining deeper insights into the intricate mechanisms governing neural tissue development and enhance our ability to design tailored strategies for tissue engineering applications. Finally, to attain the ultimate goal of translating advanced 3D models into clinical applications requires not only scientific advancements but also collaboration with medical professionals, regulatory bodies and industry partners to revolutionise drug and device testing, and develop innovative, personalised interventions for neurological disorders.266,267
Author contributions
Shuqian Wan: conceptualization, writing – original draft, writing – review & editing, investigation. Ulises Aregueta Robles: conceptualization, supervision, investigation, writing – original draft, writing – review & editing. Laura Poole-Warren: conceptualization, supervision, project administration, writing – original draft, writing – review & editing. Dorna Esrafilzadeh: conceptualization, writing – original draft, supervision, writing – review & editing, project administration.Conflicts of interest
There are no conflicts to declare.References
- Y. Zhao, Z. Xiao, B. Chen and J. Dai, Organogenesis, 2017, 13, 63–70 CrossRef CAS PubMed.
- K. R. Long and W. B. Huttner, Open Biol., 2019, 9, 180216 CrossRef CAS PubMed.
- P. M. Bradley, C. K. Denecke, A. Aljovic, A. Schmalz, M. Kerschensteiner and F. M. Bareyre, J. Exp. Med., 2019, 216, 2503–2514 CrossRef CAS PubMed.
- C. T. Chu, J. Neuropathol. Exp. Neurol., 2006, 65, 423–432 CrossRef PubMed.
- R. M. Eglen and T. Reisine, SLAS Technol., 2019, 24, 18–27 CrossRef CAS PubMed.
- F. Schutgens and H. Clevers, Annu. Rev. Pathol.: Mech. Dis., 2020, 15, 211–234 CrossRef CAS PubMed.
- N. Rouleau, N. J. Murugan and D. L. Kaplan, Nat. Rev. Bioeng., 2023, 1, 252–270 CrossRef PubMed.
- I. Yurchenko, M. Farwell, D. D. Brady and C. Staii, Biomimetics, 2021, 6, 41 CrossRef CAS PubMed.
- K. M. Brown, T. A. Gillette and G. A. Ascoli, Semin. Cell Dev. Biol., 2008, 19, 485–493 CrossRef PubMed.
- S. Das, W. J. Gordián-Vélez, H. C. Ledebur, F. Mourkioti, P. Rompolas, H. I. Chen, M. D. Serruya and D. K. Cullen, npj Regener. Med., 2020, 5, 11 CrossRef PubMed.
- K. Franze, J. Gerdelmann, M. Weick, T. Betz, S. Pawlizak, M. Lakadamyali, J. Bayer, K. Rillich, M. Gögler, Y.-B. Lu, A. Reichenbach, P. Janmey and J. Käs, Biophys. J., 2009, 97, 1883–1890 CrossRef CAS PubMed.
- B. K. Gu, D. J. Choi, S. J. Park, Y. J. Kim and C. H. Kim, Adv. Exp. Med. Biol., 2018, 1078, 15–28 CrossRef CAS PubMed.
- J. H. Choi, H. Y. Cho and J. W. Choi, Bioengineering, 2017, 4, 77 CrossRef PubMed.
- A. A. Panoutsopoulos, Neuroscientist, 2021, 27, 463–472 CrossRef CAS PubMed.
- P. Zhuang, A. X. Sun, J. An, C. K. Chua and S. Y. Chew, Biomaterials, 2018, 154, 113–133 CrossRef CAS PubMed.
- L. G. Zhang and D. L. Kaplan, Neural Engineering: From Advanced Biomaterials to 3D Fabrication Techniques, Springer International Publishing AG, Cham, Cham, 2016 Search PubMed.
- X. Qian, H. Song and G.-L. Ming, Development, 2019, 146, dev166074 CrossRef CAS PubMed.
- J. Gopalakrishnan, Bioessays, 2019, 41, e1900011 CrossRef PubMed.
- X.-Y. Tang, S. Wu, D. Wang, C. Chu, Y. Hong, M. Tao, H. Hu, M. Xu, X. Guo and Y. Liu, Signal Transduction Targeted Ther., 2022, 7, 168 CrossRef PubMed.
- A. Fatehullah, S. H. Tan and N. Barker, Nat. Cell Biol., 2016, 18, 246–254 CrossRef PubMed.
- G. Chakrapani, M. Zare and S. Ramakrishna, Mater. Adv., 2022, 3, 7757–7772 RSC.
- J. George, C.-C. Hsu, L. T. B. Nguyen, H. Ye and Z. Cui, Biotechnol. Adv., 2020, 42, 107370 CrossRef CAS PubMed.
- U. A. Aregueta-Robles, P. J. Martens, L. A. Poole-Warren and R. A. Green, Acta Biomater., 2019, 95, 269–284 CrossRef CAS PubMed.
- N. Chaicharoenaudomrung, P. Kunhorm and P. Noisa, World J. Stem. Cells, 2019, 11, 1065–1083 CrossRef PubMed.
- S. Naahidi, M. Jafari, M. Logan, Y. Wang, Y. Yuan, H. Bae, B. Dixon and P. Chen, Biotechnol. Adv., 2017, 35, 530–544 CrossRef CAS PubMed.
- A. R. Murphy, A. Laslett, C. M. O'Brien and N. R. Cameron, Acta Biomater., 2017, 54, 1–20 CrossRef CAS PubMed.
- Y. Ma, M. Lin, G. Huang, Y. Li, S. Wang, G. Bai, T. J. Lu and F. Xu, Adv. Mater., 2018, 30, e1705911 CrossRef PubMed.
- H. N. Kim and N. Choi, BioChip J., 2019, 13, 8–19 CrossRef CAS.
- S. J. Franco and U. Müller, Dev. Neurobiol., 2011, 71, 889–900 CrossRef CAS PubMed.
- W. Ma, T. Tavakoli, E. Derby, Y. Serebryakova, M. S. Rao and M. P. Mattson, BMC Dev. Biol., 2008, 8, 90 CrossRef PubMed.
- S. E. Stabenfeldt, G. Munglani, A. J. García and M. C. LaPlaca, Tissue Eng., Part A, 2010, 16, 3747–3758 CrossRef CAS PubMed.
- B. Mammadov, M. O. Guler and A. B. Tekinay, Methods Mol. Biol., 2014, 1202, 131–148 CrossRef CAS PubMed.
- P. Madhusudanan, G. Raju and S. Shankarappa, J. R. Soc., Interface, 2020, 17, 20190505 CrossRef CAS PubMed.
- H. Liu, Y. Wang, K. Cui, Y. Guo, X. Zhang and J. Qin, Adv. Mater., 2019, 31, e1902042 CrossRef PubMed.
- D. Seliktar, Science, 2012, 336, 1124–1128 CrossRef CAS PubMed.
- L. R. Doblado, C. Martínez-Ramos and M. M. Pradas, Front. Nanotechnol., 2021, 3, 640537 Search PubMed.
- C. D’Antoni, L. Mautone, C. Sanchini, L. Tondo, G. Grassmann, G. Cidonio, P. Bezzi, F. Cordella and S. Di Angelantonio, Int. J. Mol. Sci., 2023, 24, 10762 CrossRef PubMed.
- S. M. Kang, D. Kim, J. H. Lee, S. Takayama and J. Y. Park, Adv. Healthc. Mater., 2021, 10, e2001284 CrossRef PubMed.
- G. Costamagna, G. P. Comi and S. Corti, Int. J. Mol. Sci., 2021, 22, 2659 CrossRef CAS PubMed.
- A. Shankaran, K. Prasad, S. Chaudhari, A. Brand and K. Satyamoorthy, 3 Biotech, 2021, 11, 257 CrossRef PubMed.
- E. R. Shamir and A. J. Ewald, Nat. Rev. Mol. Cell Biol., 2014, 15, 647–664 CrossRef CAS PubMed.
- M. C. Decarli, R. Amaral, D. P. D. Santos, L. B. Tofani, E. Katayama, R. A. Rezende, J. Silva, K. Swiech, C. A. T. Suazo, C. Mota, L. Moroni and Â. M. Moraes, Biofabrication, 2021, 13, 032002 CrossRef CAS PubMed.
- N. E. Ryu, S. H. Lee and H. Park, Cells, 2019, 8, 1620 CrossRef CAS PubMed.
- S. Gunti, A. T. K. Hoke, K. P. Vu and N. R. London Jr., Cancers, 2021, 13, 874 CrossRef CAS PubMed.
- J. Friedrich, C. Seidel, R. Ebner and L. A. Kunz-Schughart, Nat. Protoc., 2009, 4, 309–324 CrossRef CAS PubMed.
- S. Knowlton, Y. Cho, X. J. Li, A. Khademhosseini and S. Tasoglu, Biomater. Sci., 2016, 4, 768–784 RSC.
- Y. T. Dingle, M. E. Boutin, A. M. Chirila, L. L. Livi, N. R. Labriola, L. M. Jakubek, J. R. Morgan, E. M. Darling, J. A. Kauer and D. Hoffman-Kim, Tissue Eng., Part C, 2015, 21, 1274–1283 CrossRef CAS PubMed.
- C. Bock, M. Boutros, J. G. Camp, L. Clarke, H. Clevers, J. A. Knoblich, P. Liberali, A. Regev, A. C. Rios, O. Stegle, H. G. Stunnenberg, S. A. Teichmann, B. Treutlein, R. G. J. Vries and O. Human Cell Atlas ‘Biological Network, Nat. Biotechnol., 2021, 39, 13–17 CrossRef CAS PubMed.
- K. Białkowska, P. Komorowski, M. Bryszewska and K. Miłowska, Int. J. Mol. Sci., 2020, 21, 6225 CrossRef PubMed.
- D. Seidel, D. Krinke, H. G. Jahnke, A. Hirche, D. Kloß, T. G. Mack, F. Striggow and A. Robitzki, PLoS One, 2012, 7, e49150 CrossRef CAS PubMed.
- L. Song, A. C. Tsai, X. Yuan, J. Bejoy, S. Sart, T. Ma and Y. Li, Tissue Eng., Part A, 2018, 24, 915–929 CrossRef CAS PubMed.
- M. Hofer and M. P. Lutolf, Nat. Rev. Mater., 2021, 1–19, DOI:10.1038/s41578-021-00279-y.
- N. de Souza, Nat. Methods, 2018, 15, 23–23 CrossRef CAS.
- E. Gabriel and J. Gopalakrishnan, J. Visualized Exp., 2017, 122, 55372 Search PubMed.
- Y. L. Latour, R. Yoon, S. E. Thomas, C. Grant, C. Li, M. Sena-Esteves, M. L. Allende, R. L. Proia and C. J. Tifft, Mol. Genet. Metab. Rep., 2019, 21, 100513 CAS.
- M. Mukhopadhyay, Nat. Methods, 2021, 18, 119–119 CrossRef CAS PubMed.
- M. A. Lancaster and J. A. Knoblich, Science, 2014, 345, 1247125 CrossRef PubMed.
- P. Worsdorfer, N. Dalda, A. Kern, S. Kruger, N. Wagner, C. K. Kwok, E. Henke and S. Ergun, Sci. Rep., 2019, 9, 15663 CrossRef PubMed.
- E. A. Makrygianni and G. P. Chrousos, Front. Physiol., 2021, 12, 621970 CrossRef PubMed.
- N. Vogt, Nat. Methods, 2021, 18, 27–27 CrossRef CAS PubMed.
- A. Castello, Berkeley Sci., 2021, 25, 64–67 Search PubMed.
- Y. Miura, M.-Y. Li, F. Birey, K. Ikeda, O. Revah, M. V. Thete, J.-Y. Park, A. Puno, S. H. Lee, M. H. Porteus and S. P. Paşca, Nat. Biotechnol., 2020, 38, 1421–1430 CrossRef CAS PubMed.
- Y. Miura, M.-Y. Li, O. Revah, S.-J. Yoon, G. Narazaki and S. P. Paşca, Nat. Protoc., 2022, 17, 15–35 CrossRef CAS PubMed.
- S. Kanton and S. P. Paşca, Development, 2022, 149, dev201120 CrossRef CAS PubMed.
- S. Wu, D. Wang and Y. Liu, J. Life Med., 2023, 2, lnad031 CrossRef.
- J. G. Roth, L. G. Brunel, M. S. Huang, Y. Liu, B. Cai, S. Sinha, F. Yang, S. P. Paşca, S. Shin and S. C. Heilshorn, Nat. Commun., 2023, 14, 4346 CrossRef CAS PubMed.
- C. Schmidt, Nature, 2021, 597, S22–S23 CrossRef CAS.
- S. Kofman, X. Sun, V. C. Ogbolu, L. Ibric and L. Qiang, bioRxiv, 2023, preprint, DOI:10.1101/2023.06.30.547293.
- R. J. Muckom, R. G. Sampayo, H. J. Johnson and D. V. Schaffer, Adv. Funct. Mater., 2020, 30, 2002931 CrossRef CAS PubMed.
- M. J. Kratochvil, A. J. Seymour, T. L. Li, S. P. Pasca, C. J. Kuo and S. C. Heilshorn, Nat. Rev. Mater., 2019, 4, 606–622 CrossRef CAS PubMed.
- M. G. Andrews and A. R. Kriegstein, Annu. Rev. Neurosci., 2022, 45, 23–39 CrossRef CAS PubMed.
- S. L. Giandomenico, M. Sutcliffe and M. A. Lancaster, Nat. Protoc., 2021, 16, 579–602 CrossRef CAS PubMed.
- J. Andersen, O. Revah, Y. Miura, N. Thom, N. D. Amin, K. W. Kelley, M. Singh, X. Chen, M. V. Thete, E. M. Walczak, H. Vogel, H. C. Fan and S. P. Pasca, Cell, 2020, 183, 1913–1929 CrossRef CAS PubMed.
- J. M. Faustino Martins, C. Fischer, A. Urzi, R. Vidal, S. Kunz, P. L. Ruffault, L. Kabuss, I. Hube, E. Gazzerro, C. Birchmeier, S. Spuler, S. Sauer and M. Gouti, Cell Stem Cell, 2020, 26, 172–186 CrossRef CAS PubMed.
- S. Velasco, A. J. Kedaigle, S. K. Simmons, A. Nash, M. Rocha, G. Quadrato, B. Paulsen, L. Nguyen, X. Adiconis, A. Regev, J. Z. Levin and P. Arlotta, Nature, 2019, 570, 523–527 CrossRef CAS PubMed.
- A. C. Duarte, E. C. Costa, H. A. L. Filipe, S. M. Saraiva, T. Jacinto, S. P. Miguel, M. P. Ribeiro and P. Coutinho, Biomater. Adv., 2023, 151, 213428 CrossRef CAS PubMed.
- A. Passaniti, H. K. Kleinman and G. R. Martin, J. Cell Commun. Signaling, 2022, 16, 621–626 CrossRef CAS PubMed.
- E. A. Aisenbrey and W. L. Murphy, Nat. Rev. Mater., 2020, 5, 539–551 CrossRef CAS PubMed.
- M. T. Kozlowski, C. J. Crook and H. T. Ku, Commun. Biol., 2021, 4, 1387 CrossRef PubMed.
- K. H. Vining and D. J. Mooney, Nat. Rev. Mol. Cell Biol., 2017, 18, 728–742 CrossRef CAS PubMed.
- K. A. Jansen, D. M. Donato, H. E. Balcioglu, T. Schmidt, E. H. J. Danen and G. H. Koenderink, Biochim. Biophys. Acta, Mol. Cell Res., 2015, 1853, 3043–3052 CrossRef CAS PubMed.
- M. Madhavan, Z. S. Nevin, H. E. Shick, E. Garrison, C. Clarkson-Paredes, M. Karl, B. L. L. Clayton, D. C. Factor, K. C. Allan, L. Barbar, T. Jain, P. Douvaras, V. Fossati, R. H. Miller and P. J. Tesar, Nat. Methods, 2018, 15, 700–706 CrossRef CAS PubMed.
- S. L. Giandomenico, S. B. Mierau, G. M. Gibbons, L. M. D. Wenger, L. Masullo, T. Sit, M. Sutcliffe, J. Boulanger, M. Tripodi, E. Derivery, O. Paulsen, A. Lakatos and M. A. Lancaster, Nat. Neurosci., 2019, 22, 669–679 CrossRef CAS PubMed.
- A. Bhaduri, M. G. Andrews, W. Mancia Leon, D. Jung, D. Shin, D. Allen, D. Jung, G. Schmunk, M. Haeussler, J. Salma, A. A. Pollen, T. J. Nowakowski and A. R. Kriegstein, Nature, 2020, 578, 142–148 CrossRef CAS PubMed.
- Z. Zhao, X. Chen, A. M. Dowbaj, A. Sljukic, K. Bratlie, L. Lin, E. L. S. Fong, G. M. Balachander, Z. Chen, A. Soragni, M. Huch, Y. A. Zeng, Q. Wang and H. Yu, Nat. Rev. Methods Primers, 2022, 2, 94 CrossRef CAS PubMed.
- N. Shin, Y. Kim, J. Ko, S. W. Choi, S. Hyung, S. E. Lee, S. Park, J. Song, N. L. Jeon and K. S. Kang, Biotechnol. Bioeng., 2022, 119, 566–574 CrossRef CAS PubMed.
- S. Grebenyuk and A. Ranga, Front. Bioeng. Biotechnol., 2019, 7, 39 CrossRef PubMed.
- X. Yin, B. E. Mead, H. Safaee, R. Langer, J. M. Karp and O. Levy, Cell Stem Cell, 2016, 18, 25–38 CrossRef CAS PubMed.
- R. D. Bierman-Duquette, G. Safarians, J. Huang, B. Rajput, J. Y. Chen, Z. Z. Wang and S. K. Seidlits, Adv. Healthc. Mater., 2022, 11, e2101577 CrossRef PubMed.
- A. L. R. Costa, S. M. Willerth, L. G. de la Torre and S. W. Han, Mater. Today Bio, 2022, 13, 100221 CrossRef CAS PubMed.
- Y. Wang, E. R. Zoneff, J. W. Thomas, N. Hong, L. L. Tan, D. J. McGillivray, A. W. Perriman, K. C. L. Law, L. H. Thompson, N. Moriarty, C. L. Parish, R. J. Williams, C. J. Jackson and D. R. Nisbet, Nat. Commun., 2023, 14, 457 CrossRef CAS PubMed.
- I. M. Tayler and R. S. Stowers, Acta Biomater., 2021, 132, 4–22 CrossRef CAS PubMed.
- M. S. Vieira, A. K. Santos, R. Vasconcellos, V. A. M. Goulart, R. C. Parreira, A. H. Kihara, H. Ulrich and R. R. Resende, Biotechnol. Adv., 2018, 36, 1946–1970 CrossRef CAS PubMed.
- M. G. Tupone, M. d'Angelo, V. Castelli, M. Catanesi, E. Benedetti and A. Cimini, Front. Bioeng. Biotechnol., 2021, 9, 639765 CrossRef PubMed.
- V. D. L. Putra, K. A. Kilian and M. L. Knothe Tate, Commun. Biol., 2023, 6, 75 CrossRef PubMed.
- H. Cao, L. Duan, Y. Zhang, J. Cao and K. Zhang, Signal Transduction Targeted Ther., 2021, 6, 426 CrossRef CAS PubMed.
- T. C. Ho, C. C. Chang, H. P. Chan, T. W. Chung, C. W. Shu, K. P. Chuang, T. H. Duh, M. H. Yang and Y. C. Tyan, Molecules, 2022, 27, 2902 CrossRef CAS PubMed.
- S. Mantha, S. Pillai, P. Khayambashi, A. Upadhyay, Y. Zhang, O. Tao, H. M. Pham and S. D. Tran, Materials, 2019, 12, 3323 CrossRef CAS PubMed.
- R. Hama, A. Ulziibayar, J. W. Reinhardt, T. Watanabe, J. Kelly and T. Shinoka, Biomolecules, 2023, 13, 280 CrossRef CAS PubMed.
- B. Ozcelik, in Biosynthetic Polymers for Medical Applications, ed. L. Poole-Warren, P. Martens and R. Green, Woodhead Publishing, 2016 Search PubMed.
- S. Bashir, M. Hina, J. Iqbal, A. H. Rajpar, M. A. Mujtaba, N. A. Alghamdi, S. Wageh, K. Ramesh and S. Ramesh, Polymers, 2020, 12, 2702 CrossRef CAS PubMed.
- S. M. Willerth, K. J. Arendas, D. I. Gottlieb and S. E. Sakiyama-Elbert, Biomaterials, 2006, 27, 5990–6003 CrossRef CAS PubMed.
- C. R. Kothapalli and R. D. Kamm, Biomaterials, 2013, 34, 5995–6007 CrossRef CAS PubMed.
- S. M. de Leeuw, S. Davaz, D. Wanner, V. Milleret, M. Ehrbar, A. Gietl and C. Tackenberg, J. Neurosci. Methods, 2021, 360, 109254 CrossRef CAS PubMed.
- A. Stanzione, A. Polini, V. La Pesa, A. Quattrini, A. Romano, G. Gigli, L. Moroni and F. Gervaso, Biomater. Sci., 2021, 9, 7492–7503 RSC.
- Y. Lei and D. V. Schaffer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, E5039–E5048 CAS.
- D. D. McKinnon, A. M. Kloxin and K. S. Anseth, Biomater. Sci., 2013, 1, 460–469 RSC.
- S. Ma, Z. Cong, H. Chen, H. Wen, L. Cao, C. Liu, F. Yang and Y. Liao, Eur. J. Pharm. Sci., 2021, 167, 106003 CrossRef CAS PubMed.
- H. Samadian, H. Maleki, Z. Allahyari and M. Jaymand, Coord. Chem. Rev., 2020, 420, 213432 CrossRef CAS.
- G. Tang, B. Zhou, F. Li, W. Wang, Y. Liu, X. Wang, C. Liu and X. Ye, Front. Bioeng. Biotechnol., 2020, 8, 745 CrossRef PubMed.
- J. Arulmoli, H. J. Wright, D. T. T. Phan, U. Sheth, R. A. Que, G. A. Botten, M. Keating, E. L. Botvinick, M. M. Pathak, T. I. Zarembinski, D. S. Yanni, O. V. Razorenova, C. C. W. Hughes and L. A. Flanagan, Acta Biomater., 2016, 43, 122–138 CrossRef CAS PubMed.
- Y. Yang, Y. Fan, H. Zhang, Q. Zhang, Y. Zhao, Z. Xiao, W. Liu, B. Chen, L. Gao, Z. Sun, X. Xue, M. Shu and J. Dai, Biomaterials, 2021, 269, 120479 CrossRef CAS PubMed.
- E. M. Cruz, L. S. Machado, L. N. Zamproni, L. V. Bim, P. S. Ferreira, L. A. Pinto, L. A. Pessan, E. H. Backes and M. A. Porcionatto, Pharmaceutics, 2023, 15, 627 CrossRef CAS PubMed.
- J. Hartmann, I. Lauria, F. Bendt, S. Rütten, K. Koch, A. Blaeser and E. Fritsche, Adv. Mater. Interfaces, 2022, 10, 2200580 CrossRef.
- J. Ryu, P. F. Y. Vincent, N. K. Ziogas, L. Xu, S. Sadeghpour, J. Curtin, A. S. Alexandris, N. Stewart, R. Sima, S. du Lac, E. Glowatzki and V. E. Koliatsos, PLoS One, 2019, 14, e0224846 CrossRef CAS PubMed.
- J. Kapr, L. Petersilie, T. Distler, I. Lauria, F. Bendt, C. M. Sauter, A. R. Boccaccini, C. R. Rose and E. Fritsche, Adv. Healthc. Mater., 2021, 10, e2100131 CrossRef PubMed.
- P. Zhou, P. Xu, J. Guan, C. Zhang, J. Chang, F. Yang, H. Xiao, H. Sun, Z. Zhang, M. Wang, J. Hu and Y. Mao, Colloids Surf., B, 2020, 194, 111214 CrossRef CAS PubMed.
- M. Darvishi, H. Ghasemi Hamidabadi, S. Sahab Negah, A. Moayeri, T. Tiraihi, J. Mirnajafi-Zadeh, A. Jahanbazi Jahan-Abad and A. Shojaei, Iran. J. Basic Med. Sci., 2020, 23, 431–438 Search PubMed.
- G. Akcay and R. Luttge, Micromachines, 2021, 12, 165 CrossRef PubMed.
- I. Raimondi, M. Tunesi, G. Forloni, D. Albani and C. Giordano, J. Tissue Eng., 2020, 11, 2041731420963981 Search PubMed.
- C. Wu, A. Liu, S. Chen, X. Zhang, L. Chen, Y. Zhu, Z. Xiao, J. Sun, H. Luo and H. Fan, ACS Appl. Mater. Interfaces, 2019, 11, 22152–22163 CrossRef CAS PubMed.
- L. Wang, D. Song, X. Zhang, Z. Ding, X. Kong, Q. Lu and D. L. Kaplan, ACS Biomater. Sci. Eng., 2019, 5, 613–622 CrossRef CAS PubMed.
- F. Seven, T. Gölcez, Z. B. Yaralı, G. Onak, O. Karaman and M. Şen, RSC Adv., 2020, 10, 26120–26125 RSC.
- G. Morello, G. De Iaco, G. Gigli, A. Polini and F. Gervaso, Gels, 2023, 9, 132 CrossRef CAS PubMed.
- J. Li, Y. Liu, Y. Zhang, B. Yao, Enhejirigala, Z. Li, W. Song, Y. Wang, X. Duan, X. Yuan, X. Fu and S. Huang, Front. Cell Dev. Biol., 2021, 9, 640388 CrossRef PubMed.
- S. Yang, J. Zhu, C. Lu, Y. Chai, Z. Cao, J. Lu, Z. Zhang, H. Zhao, Y. Y. Huang, S. Yao, X. Kong, P. Zhang and X. Wang, Bioact. Mater., 2022, 8, 529–544 CAS.
- J. A. Burdick, M. Ward, E. Liang, M. J. Young and R. Langer, Biomaterials, 2006, 27, 452–459 CrossRef CAS PubMed.
- X. Gao, W. Cheng, X. Zhang, Z. Zhou, Z. Ding, X. Zhou, Q. Lu and D. L. Kaplan, ACS Appl. Mater. Interfaces, 2022, 14, 3701–3715 CrossRef CAS PubMed.
- X. Zhang, X. Chen, H. Hong, R. Hu, J. Liu and C. Liu, Bioact. Mater., 2022, 10, 15–31 CAS.
- X. T. Meng, Y. S. Du, Z. Y. Dong, G. Q. Wang, B. Dong, X. W. Guan, Y. Z. Yuan, H. Pan and F. Wang, J. Neural Eng., 2020, 17, 056048 Search PubMed.
- M. C. Cramer and S. F. Badylak, Ann. Biomed. Eng., 2020, 48, 2132–2153 CrossRef PubMed.
- L. Luckenbill-Edds, Brain Res. Rev., 1997, 23, 1–27 CrossRef CAS PubMed.
- L. K. Wareham, R. O. Baratta, B. J. Del Buono, E. Schlumpf and D. J. Calkins, Mol. Neurodegener., 2024, 19, 11 CrossRef CAS PubMed.
- J. Li, R. Xing, S. Bai and X. Yan, Soft Matter, 2019, 15, 1704–1715 RSC.
- Z. Balion, V. Cepla, N. Svirskiene, G. Svirskis, K. Druceikaite, H. Inokaitis, J. Rusteikaite, I. Masilionis, G. Stankeviciene, T. Jelinskas, A. Ulcinas, A. Samanta, R. Valiokas and A. Jekabsone, Biomolecules, 2020, 10, 754 CrossRef CAS PubMed.
- N. Mahmoodi, J. Ai, Z. Hassannejad, S. Ebrahimi-Barough, E. Hasanzadeh, H. Nekounam, A. R. Vaccaro and V. Rahimi-Movaghar, Sci. Rep., 2021, 11, 21722 CrossRef CAS PubMed.
- L. Huang, J. Gao, H. Wang, B. Xia, Y. Yang, F. Xu, X. Zheng, J. Huang and Z. Luo, ACS Appl. Mater. Interfaces, 2020, 12, 48380–48394 CrossRef CAS PubMed.
- T. B. Ngo, B. S. Spearman, N. Hlavac and C. E. Schmidt, ACS Biomater. Sci. Eng., 2020, 6, 6819–6830 CrossRef CAS PubMed.
- E. R. Aurand, K. J. Lampe and K. B. Bjugstad, Neurosci. Res., 2012, 72, 199–213 CrossRef CAS PubMed.
- E. A. Cavalcanti-Adam, T. Volberg, A. Micoulet, H. Kessler, B. Geiger and J. P. Spatz, Biophys. J., 2007, 92, 2964–2974 CrossRef CAS PubMed.
- N. J. Gardiner, Dev. Neurobiol., 2011, 71, 1054–1072 CrossRef CAS PubMed.
- J. Lou and D. J. Mooney, Nat. Rev. Chem., 2022, 6, 726–744 CrossRef CAS PubMed.
- S. B. Shah and A. Singh, Acta Biomater., 2017, 53, 29–45 CrossRef CAS PubMed.
- J. Gao, X. Yu, X. Wang, Y. He and J. Ding, Engineering, 2022, 13, 31–45 CrossRef CAS.
- W. Man, S. Yang, Z. Cao, J. Lu, X. Kong, X. Sun, L. Zhao, Y. Guo, S. Yao, G. Wang and X. Wang, Biomaterials, 2021, 276, 120971 CrossRef CAS PubMed.
- K. J. Lampe, A. L. Antaris and S. C. Heilshorn, Acta Biomater., 2013, 9, 5590–5599 CrossRef CAS PubMed.
- S. Song, J. Zhou, J. Wan, X. Zhao, K. Li, C. Yang, C. Zheng, L. Wang, Y. Tang, C. Wang and J. Liu, Int. J. Bioprint., 2023, 9, 692 CrossRef PubMed.
- Y. Yin, W. Wang, Q. Shao, B. Li, D. Yu, X. Zhou, J. Parajuli, H. Xu, T. Qiu, A. K. Yetisen and N. Jiang, Biomater. Sci., 2021, 9, 2887–2892 RSC.
- X. Zhao, X. Lu, K. Li, S. Song, Z. Luo, C. Zheng, C. Yang, X. Wang, L. Wang, Y. Tang, C. Wang and J. Liu, Bioact. Mater., 2023, 24, 331–345 CAS.
- C. Y. Yang, W. Y. Huang, L. H. Chen, N. W. Liang, H. C. Wang, J. Lu, X. Wang and T. W. Wang, J. Mater. Chem. B, 2021, 9, 567–584 RSC.
- Q. Mei, H.-Y. Yuen and X. Zhao, Bio-Des. Manuf., 2022, 5, 714–728 CrossRef CAS.
- C. F. Guimarães, L. Gasperini, A. P. Marques and R. L. Reis, Nat. Rev. Mater., 2020, 5, 351–370 CrossRef.
- P. C. Georges, W. J. Miller, D. F. Meaney, E. S. Sawyer and P. A. Janmey, Biophys. J., 2006, 90, 3012–3018 CrossRef CAS PubMed.
- B. Niemczyk, P. Sajkiewicz and D. Kolbuk, J. Neural Eng., 2018, 15, 051002 CrossRef CAS PubMed.
- D. Joung, V. Truong, C. C. Neitzke, S. Z. Guo, P. J. Walsh, J. R. Monat, F. Meng, S. H. Park, J. R. Dutton, A. M. Parr and M. C. McAlpine, Adv. Funct. Mater., 2018, 28, 1801850 CrossRef PubMed.
- L. D. Laporte, Y. Yang, M. L. Zelivyanskaya, B. J. Cummings, A. J. Anderson and L. D. Shea, Mol. Ther., 2009, 17, 318–326 CrossRef PubMed.
- F. Johansson, P. Carlberg, N. Danielsen, L. Montelius and M. Kanje, Biomaterials, 2006, 27, 1251–1258 CrossRef CAS PubMed.
- W. Chen, S. Han, W. Qian, S. Weng, H. Yang, Y. Sun, L. G. Villa-Diaz, P. H. Krebsbach and J. Fu, Nanoscale, 2018, 10, 3556–3565 RSC.
- M. C. Murphy, J. Huston 3rd, C. R. Jack Jr., K. J. Glaser, A. Manduca, J. P. Felmlee and R. L. Ehman, J. Magn. Reson. Imaging, 2011, 34, 494–498 CrossRef PubMed.
- M. C. Murphy, D. T. Jones, C. R. Jack Jr., K. J. Glaser, M. L. Senjem, A. Manduca, J. P. Felmlee, R. E. Carter, R. L. Ehman and J. Huston 3rd, Neuroimage. Clin., 2016, 10, 283–290 CrossRef PubMed.
- T. Pizzute, K. Lynch and M. Pei, Stem Cell Rev. Rep., 2015, 11, 119–132 CrossRef CAS PubMed.
- H. De Belly, E. K. Paluch and K. J. Chalut, Nat. Rev. Mol. Cell Biol., 2022, 23, 465–480 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed.
- N. D. Leipzig and M. S. Shoichet, Biomaterials, 2009, 30, 6867–6878 CrossRef CAS PubMed.
- S. K. Seidlits, Z. Z. Khaing, R. R. Petersen, J. D. Nickels, J. E. Vanscoy, J. B. Shear and C. E. Schmidt, Biomaterials, 2010, 31, 3930–3940 CrossRef CAS PubMed.
- H. Long, B. E. Vos, T. Betz, B. M. Baker and B. Trappmann, Adv. Sci., 2022, 9, e2105325 CrossRef PubMed.
- C. Cassel de Camps, S. Aslani, N. Stylianesis, H. Nami, N.-V. Mohamed, T. M. Durcan and C. Moraes, ACS Appl. Bio Mater., 2022, 5, 214–224 CrossRef CAS PubMed.
- H. Ge, M. Tian, Q. Pei, F. Tan and H. Pei, Front. Oncol., 2021, 11, 631991 CrossRef CAS PubMed.
- P. Manganas, P. Kavatzikidou, A. Kordas, E. Babaliari, E. Stratakis and A. Ranella, Front. Cell. Neurosci., 2022, 16, 948454 CrossRef PubMed.
- K. Man, J. Liu, K. M. Phan, K. Wang, J. Y. Lee, X. Sun, M. Story, D. Saha, J. Liao, H. Sadat and Y. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 17081–17092 CrossRef CAS PubMed.
- F. Liu, J. Xu, L. Wu, T. Zheng, Q. Han, Y. Liang, L. Zhang, G. Li and Y. Yang, Stem Cells Int., 2021, 2021, 8124444 Search PubMed.
- Y. Luo and M. S. Shoichet, Nat. Mater., 2004, 3, 249–253 CrossRef CAS PubMed.
- R. Midha, C. A. Munro, P. D. Dalton, C. H. Tator and M. S. Shoichet, J. Neurosurg., 2003, 99, 555–565 Search PubMed.
- W. Daly, L. Yao, D. Zeugolis, A. Windebank and A. Pandit, J. R. Soc., Interface, 2012, 9, 202–221 CrossRef CAS PubMed.
- J. L. Curley and M. J. Moore, J. Biomed. Mater. Res., Part A, 2011, 99, 532–543 CrossRef PubMed.
- J. S. Belkas, M. S. Shoichet and R. Midha, Neurol. Res., 2004, 26, 151–160 CrossRef PubMed.
- D. H. Smith, J. C. Burrell, K. D. Browne, K. S. Katiyar, M. I. Ezra, J. L. Dutton, J. P. Morand, L. A. Struzyna, F. A. Laimo, H. I. Chen, J. A. Wolf, H. M. Kaplan, J. M. Rosen, H. C. Ledebur, E. L. Zager, Z. S. Ali and D. K. Cullen, Sci. Adv., 2022, 8, eabm3291 CrossRef PubMed.
- Y. Berkovitch and D. Seliktar, Int. J. Pharm., 2017, 523, 545–555 CrossRef CAS PubMed.
- G. Li, S. Li, L. Zhang, S. Chen, Z. Sun, S. Li, L. Zhang and Y. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 37397–37410 CrossRef CAS PubMed.
- J. S. Goldner, J. M. Bruder, G. Li, D. Gazzola and D. Hoffman-Kim, Biomaterials, 2006, 27, 460–472 CrossRef CAS PubMed.
- Y. Hou, L. Yu, W. Xie, L. C. Camacho, M. Zhang, Z. Chu, Q. Wei and R. Haag, Nano Lett., 2020, 20, 748–757 CrossRef CAS PubMed.
- S. Musah, S. A. Morin, P. J. Wrighton, D. B. Zwick, S. Jin and L. L. Kiessling, ACS Nano, 2012, 6, 10168–10177 CrossRef CAS PubMed.
- S. Musah, P. J. Wrighton, Y. Zaltsman, X. Zhong, S. Zorn, M. B. Parlato, C. Hsiao, S. P. Palecek, Q. Chang, W. L. Murphy and L. L. Kiessling, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 13805–13810 CrossRef CAS PubMed.
- U. Blache, E. M. Ford, B. Ha, L. Rijns, O. Chaudhuri, P. Y. W. Dankers, A. M. Kloxin, J. G. Snedeker and E. Gentleman, Nat. Rev. Methods Primers, 2022, 2, 98 CrossRef CAS PubMed.
- S. Babu, I. Chen, S. Vedaraman, J. Gerardo-Nava, C. Licht, Y. Kittel, T. Haraszti, J. Di Russo and L. De Laporte, Adv. Funct. Mater., 2022, 32, 2202468 CrossRef CAS.
- A. Banerjee, M. Arha, S. Choudhary, R. S. Ashton, S. R. Bhatia, D. V. Schaffer and R. S. Kane, Biomaterials, 2009, 30, 4695–4699 CrossRef CAS PubMed.
- D. D. Dewitt, S. N. Kaszuba, D. M. Thompson and J. P. Stegemann, Tissue Eng., Part A, 2009, 15, 2785–2793 CrossRef CAS PubMed.
- J. Lantoine, T. Grevesse, A. Villers, G. Delhaye, C. Mestdagh, M. Versaevel, D. Mohammed, C. Bruyère, L. Alaimo, S. P. Lacour, L. Ris and S. Gabriele, Biomaterials, 2016, 89, 14–24 CrossRef CAS PubMed.
- D. E. Koser, A. J. Thompson, S. K. Foster, A. Dwivedy, E. K. Pillai, G. K. Sheridan, H. Svoboda, M. Viana, L. D. Costa, J. Guck, C. E. Holt and K. Franze, Nat. Neurosci., 2016, 19, 1592–1598 CrossRef CAS PubMed.
- A. Tanaka, Y. Fujii, N. Kasai, T. Okajima and H. Nakashima, PLoS One, 2018, 13, e0191928 CrossRef PubMed.
- M. Uz and S. K. Mallapragada, J. Indian Inst. Sci., 2019, 99, 489–510 CrossRef.
- J. Huang, X. Hu, L. Lu, Z. Ye, Q. Zhang and Z. Luo, J. Biomed. Mater. Res., Part A, 2010, 93, 164–174 CrossRef PubMed.
- L. Ghasemi-Mobarakeh, M. P. Prabhakaran, M. Morshed, M. H. Nasr-Esfahani, H. Baharvand, S. Kiani, S. S. Al-Deyab and S. Ramakrishna, J. Tissue Eng. Regener. Med., 2011, 5, e17–e35 CrossRef CAS PubMed.
- E. Tomaskovic-Crook, Q. Gu, S. N. A. Rahim, G. G. Wallace and J. M. Crook, Cells, 2020, 9, 658 CrossRef CAS PubMed.
- Y. Lu, Y. Wang, J. Zhang, X. Hu, Z. Yang, Y. Guo and Y. Wang, Acta Biomater., 2019, 89, 217–226 CrossRef CAS PubMed.
- M. Imaninezhad, K. Pemberton, F. Xu, K. Kalinowski, R. Bera and S. P. Zustiak, J. Neural Eng., 2018, 15, 056034 CrossRef PubMed.
- S. J. Lee, W. Zhu, M. Nowicki, G. Lee, D. N. Heo, J. Kim, Y. Y. Zuo and L. G. Zhang, J. Neural Eng., 2018, 15, 016018 CrossRef PubMed.
- X. Chen, V. D. Ranjan, S. Liu, Y. N. Liang, J. S. K. Lim, H. Chen, X. Hu and Y. Zhang, Macromol. Biosci., 2021, 21, e2000374 CrossRef PubMed.
- G. Agarwal, N. Kumar and A. Srivastava, Mater. Sci. Eng., C, 2021, 118, 111518 CrossRef CAS PubMed.
- B. Guo and P. X. Ma, Biomacromolecules, 2018, 19, 1764–1782 CrossRef CAS PubMed.
- L. He, Q. Xiao, Y. Zhao, J. Li, S. Reddy, X. Shi, X. Su, K. Chiu and S. Ramakrishna, ACS Appl. Mater. Interfaces, 2020, 12, 53150–53163 CrossRef CAS PubMed.
- P. J. Arthur-Farraj, M. Latouche, D. K. Wilton, S. Quintes, E. Chabrol, A. Banerjee, A. Woodhoo, B. Jenkins, M. Rahman, M. Turmaine, G. K. Wicher, R. Mitter, L. Greensmith, A. Behrens, G. Raivich, R. Mirsky and K. R. Jessen, Neuron, 2012, 75, 633–647 CrossRef CAS PubMed.
- M. D. Tang-Schomer, Brain Res., 2018, 1678, 288–296 CrossRef CAS PubMed.
- W. Liu, Y. Luo, C. Ning, W. Zhang, Q. Zhang, H. Zou and C. Fu, J. Nanobiotechnol., 2021, 19, 286 CrossRef CAS PubMed.
- A. R. Del Bakhshayesh, N. Asadi, A. Alihemmati, H. Tayefi Nasrabadi, A. Montaseri, S. Davaran, S. Saghati, A. Akbarzadeh and A. Abedelahi, J. Biol. Eng., 2019, 13, 85 CrossRef PubMed.
- Y. Zhao, J. Liu, Y. Gao, Z. Xu, C. Dai, G. Li, C. Sun, Y. Yang and K. Zhang, J. Mater. Chem. B, 2022, 10, 1582–1590 RSC.
- C. C. Lin, J. J. Chang, M. C. Yung, W. C. Huang and S. Y. Chen, ACS Biomater. Sci. Eng., 2020, 6, 1144–1153 CrossRef CAS PubMed.
- H. Y. Gong, J. Park, W. Kim, J. Kim, J. Y. Lee and W. G. Koh, ACS Appl. Mater. Interfaces, 2019, 11, 47695–47706 CrossRef CAS PubMed.
- M. Han, E. Yildiz, H. N. Kaleli, S. Karaz, G. O. Eren, I. B. Dogru-Yuksel, E. Senses, A. Şahin and S. Nizamoglu, Adv. Healthc. Mater., 2022, 11, e2102160 CrossRef PubMed.
- X. Meng, X. Yu, Y. Lu, Z. Pei, G. Wang, M. Qi, R. Liu, J. Zhou, X. Guo, Z. Zhou and F. Wang, J. Neural Eng., 2023, 20, 046009 CrossRef PubMed.
- D. Joung, N. S. Lavoie, S. Z. Guo, S. H. Park, A. M. Parr and M. C. McAlpine, Adv. Funct. Mater., 2020, 30, 1906237 CrossRef CAS PubMed.
- L. de la Vega, C. Lee, R. Sharma, M. Amereh and S. M. Willerth, Brain Res. Bull., 2019, 150, 240–249 CrossRef PubMed.
- L. Moroni, J. A. Burdick, C. Highley, S. J. Lee, Y. Morimoto, S. Takeuchi and J. J. Yoo, Nat. Rev. Mater., 2018, 3, 21–37 CrossRef CAS PubMed.
- Q. Gu, E. Tomaskovic-Crook, R. Lozano, Y. Chen, R. M. Kapsa, Q. Zhou, G. G. Wallace and J. M. Crook, Adv. Healthc. Mater., 2016, 5, 1429–1438 CrossRef CAS PubMed.
- L. Y. Daikuara, Z. Yue, D. Skropeta and G. G. Wallace, Acta Biomater., 2021, 123, 286–297 CrossRef CAS PubMed.
- J. S. Kong, X. Huang, Y. J. Choi, H. G. Yi, J. Kang, S. Kim, J. Kim, H. Lee, Y. A. Rim, J. H. Ju, W. K. Chung, C. J. Woolf, J. Jang and D. W. Cho, Adv. Healthc. Mater., 2021, 10, e2100581 CrossRef PubMed.
- D. Joung, N. S. Lavoie, S. Z. Guo, S. H. Park, A. M. Parr and M. C. McAlpine, Adv. Funct. Mater., 2020, 30, 1906237 CrossRef CAS PubMed.
- M. Hospodiuk, M. Dey, D. Sosnoski and I. T. Ozbolat, Biotechnol. Adv., 2017, 35, 217–239 CrossRef CAS PubMed.
- A. Jandyal, I. Chaturvedi, I. Wazir, A. Raina and M. I. Ul Haq, Sustainable Oper. Comput., 2022, 3, 33–42 CrossRef.
- S. Raees, F. Ullah, F. Javed, H. M. Akil, M. Jadoon Khan, M. Safdar, I. U. Din, M. A. Alotaibi, A. I. Alharthi, M. A. Bakht, A. Ahmad and A. A. Nassar, Int. J. Biol. Macromol., 2023, 232, 123476 CrossRef CAS PubMed.
- A. K. Nguyen, P. L. Goering, V. Reipa and R. J. Narayan, Biointerphases, 2019, 14, 021007 CrossRef CAS PubMed.
- K. Elkhoury, J. Zuazola and S. Vijayavenkataraman, SLAS Technol., 2023, 28, 142–151 CrossRef CAS PubMed.
- J. M. Crook, 3D Bioprinting: Principles and Protocols, Springer US, New York, NY, Imprint: Humana, New York, NY, 1st ed 2020 edn., 2020 Search PubMed.
- E. Lepowsky, M. Muradoglu and S. Tasoglu, Bioprinting, 2018, 11, e00034 CrossRef.
- N. Paxton, W. Smolan, T. Böck, F. Melchels, J. Groll and T. Jungst, Biofabrication, 2017, 9, 044107 CrossRef PubMed.
- K. A. Homan, D. B. Kolesky, M. A. Skylar-Scott, J. Herrmann, H. Obuobi, A. Moisan and J. A. Lewis, Sci. Rep., 2016, 6, 34845 CrossRef CAS PubMed.
- S. E. Doyle, S. Duchi, C. Onofrillo, A. Quigley, C. Di Bella, E. Pirogova and C. D. O'Connell, Adv. Mater. Technol., 2021, 6, 2100189 CrossRef CAS.
- C. Yu, J. Schimelman, P. Wang, K. L. Miller, X. Ma, S. You, J. Guan, B. Sun, W. Zhu and S. Chen, Chem. Rev., 2020, 120, 10695–10743 CrossRef CAS PubMed.
- J. Koffler, W. Zhu, X. Qu, O. Platoshyn, J. N. Dulin, J. Brock, L. Graham, P. Lu, J. Sakamoto, M. Marsala, S. Chen and M. H. Tuszynski, Nat. Med., 2019, 25, 263–269 CrossRef CAS PubMed.
- M. Cadena, L. Ning, A. King, B. Hwang, L. Jin, V. Serpooshan and S. A. Sloan, Adv. Healthc. Mater., 2021, 10, e2001600 CrossRef PubMed.
- Y. Fang, Y. Guo, T. Liu, R. Xu, S. Mao, X. Mo, T. Zhang, L. Ouyang, Z. Xiong and W. Sun, Chin. J. Mech. Eng., 2022, 1, 100011 Search PubMed.
- T. Bedir, S. Ulag, C. B. Ustundag and O. Gunduz, Mater. Sci. Eng., C, 2020, 110, 110741 CrossRef CAS PubMed.
- X. B. Chen, A. Fazel Anvari-Yazdi, X. Duan, A. Zimmerling, R. Gharraei, N. K. Sharma, S. Sweilem and L. Ning, Bioact. Mater., 2023, 28, 511–536 CAS.
- F. Yanagawa, S. Sugiura and T. Kanamori, Regener. Ther., 2016, 3, 45–57 CrossRef PubMed.
- U. A. Aregueta Robles, F. Bartlett-Tomasetig and L. A. Poole-Warren, Biofabrication, 2023, 15, 035014 CrossRef PubMed.
- F. Ali, I. Khan, J. Chen, K. Akhtar, E. M. Bakhsh and S. B. Khan, Gels, 2022, 8, 205 CrossRef CAS PubMed.
- X. Lin, X. Zhao, C. Xu, L. Wang and Y. Xia, J. Polym. Sci., 2022, 60, 2525–2542 CrossRef CAS.
- Y.-H. An and S.-H. Kim, Bioengineering, 2021, 8, 164 CrossRef CAS PubMed.
- N. Sakai, S. Yarimitsu, Y. Sawae, M. Komori and T. Murakami, Biosurf. Biotribol., 2018, 4, 24–33 CrossRef.
- S. Lv, J. Nie, Q. Gao, C. Xie, L. Zhou, J. Qiu, J. Fu, X. Zhao and Y. He, Biofabrication, 2020, 12, 025015 CrossRef CAS PubMed.
- L. A. Struzyna, D. O. Adewole, W. J. Gordián-Vélez, M. R. Grovola, J. C. Burrell, K. S. Katiyar, D. Petrov, J. P. Harris and D. K. Cullen, J. Visualized Exp., 2017, e55609, DOI:10.3791/55609.
- S. Fu, D. Yu, Y. Chen, K. An and X. Chen, Mater. Sci. Eng., A, 2020, 790, 139686 CrossRef CAS.
- M. R. Lee, K. W. Kwon, H. Jung, H. N. Kim, K. Y. Suh, K. Kim and K. S. Kim, Biomaterials, 2010, 31, 4360–4366 CrossRef CAS PubMed.
- L. Zhou, Y. Li, Q. Tu and J. Wang, Colloids Surf., A, 2023, 662, 130952 CrossRef CAS.
- C. He, X. Chen, Y. Sun, M. Xie, K. Yu, J. He, J. Lu, Q. Gao, J. Nie, Y. Wang and Y. He, Bio-Des. Manuf., 2022, 5, 641–659 CrossRef CAS.
- P. S. Barhouse, M. J. Andrade and Q. Smith, Front. Chem. Eng., 2022, 4, 832754 CrossRef.
- J. G. Roth, M. S. Huang, T. L. Li, V. R. Feig, Y. Jiang, B. Cui, H. T. Greely, Z. Bao, S. P. Pasca and S. C. Heilshorn, Nat. Rev. Neurosci., 2021, 22, 593–615 CrossRef CAS PubMed.
- L. Kaplan, B. W. Chow and C. Gu, Nat. Rev. Neurosci., 2020, 21, 416–432 CrossRef CAS PubMed.
- E. Knight and S. A. Przyborski, J. Anat., 2014, 227, 746–756 CrossRef PubMed.
- S. Daetwyler and R. P. Fiolka, Commun. Biol., 2023, 6, 502 CrossRef PubMed.
- A. Erofeev, I. Antifeev, A. Bolshakova, I. Bezprozvanny and O. Vlasova, Sensors, 2022, 22, 9085 CrossRef CAS PubMed.
- M. N. Collins, F. Zamboni, A. Serafin, A. Escobar, R. Stepanian, M. Culebras, R. L. Reis and J. M. Oliveira, In Vitro Models, 2022, 1, 129–150 CrossRef.
- L. A. Struzyna, J. P. Harris, K. S. Katiyar, H. I. Chen and D. K. Cullen, Neural Regener. Res., 2015, 10, 679–685 CrossRef PubMed.
- Y. Ikada, J. R. Soc., Interface, 2006, 3, 589–601 CrossRef CAS PubMed.
- S. Pina, V. P. Ribeiro, C. F. Marques, F. R. Maia, T. H. Silva, R. L. Reis and J. M. Oliveira, Materials, 2019, 12, 1824 CrossRef CAS PubMed.
- H.-Y. Tan, H. Cho and L. P. Lee, Nat. Biomed. Eng., 2021, 5, 11–25 CrossRef PubMed.
- L. N. Zamproni, M. Mundim and M. A. Porcionatto, Front. Cell Dev. Biol., 2021, 9, 649891 CrossRef PubMed.
- T. J. Bechtel, T. Reyes-Robles, O. O. Fadeyi and R. C. Oslund, Nat. Chem. Biol., 2021, 17, 641–652 CrossRef CAS PubMed.
- P. K. Chandra, S. Soker and A. Atala, in Principles of Tissue Engineering, ed. R. Lanza, R. Langer, J. P. Vacanti and A. Atala, Academic Press, 5th edn, 2020, pp. 1–35 Search PubMed.
- R. Boni, A. Ali, A. Shavandi and A. N. Clarkson, J. Biomed. Sci., 2018, 25, 90 CrossRef CAS PubMed.
- A. Shakeel and P. R. Corridon, Front. Physiol., 2022, 13, 1079421 CrossRef PubMed.
- Y. Li, Q. Saiding, Z. Wang and W. Cui, Prog. Mater. Sci., 2024, 141, 101216 CrossRef CAS.
- K. Hillion and M. M. Mahe, Nat. Methods, 2022, 19, 1347–1348 CrossRef CAS PubMed.
- M. Dong, D. Jiao, Q. Zheng and Z. L. Wu, J. Polym. Sci., 2023, 61, 1026–1039 CrossRef CAS.
- T. Lϕmo and J. K. S. Jansen, in Current Topics in Developmental Biology, ed. A. A. Moscona and A. Monroy, Academic Press, 1980, vol. 16, pp. 253–281 Search PubMed.
- J. M. Bliley, D. J. Shiwarski and A. W. Feinberg, Sci. Transl. Med., 2022, 14, eabo7047 CrossRef PubMed.
- J. A. Wagner and D. L. Kroetz, Clin. Transl. Sci., 2016, 9, 3–5 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |




