Effect of amino group modification at allyl position of methacrylamides on polymerization and polymer pH-/thermo-responsiveness†
Yasuhiro
Kohsaka
 *ab,
Nyamdolgor
Chinbat
b,
Kei-ichiro
Ito
b and
Yosuke
Akae
bc
*ab,
Nyamdolgor
Chinbat
b,
Kei-ichiro
Ito
b and
Yosuke
Akae
bc
aResearch Initiative for Supra-Materials (RISM), Interdisciplinary Cluster for Cutting Edge Research (ICCER), Shinshu University, Japan. E-mail: kohsaka@shinshu-u.ac.jp
bFaculty of Textile Science and Technology, Shinshu University, Nagano386-8567, Japan
cJapan Society for the Promotion of Science, Tokyo, Japan
First published on 1st February 2023
Abstract
With an interest in β-amino acid derivative polymers containing a carbon backbone, the polymerization of N-n-propyl α-(aminomethyl)acrylamide (4b) and N-isopropyl α-(aminomethyl)acrylamide (4b) were investigated. The synthesis of 4 was achieved within many fewer steps than reported by using selective acylation and subsequent conjugate substitution of α-(chloromethyl)acryloyl chloride. 4 hardly homopolymerized, but copolymerization with N-substituted acrylamides afforded polymers with various compositions. Surprisingly, substitution with amino groups increased the hydrophobicity of the polymers and lowered the cloud point (Tc). Nevertheless, polymers containing the amino monomers as major components exhibited significantly higher Tc in 1 M HCl aq than those in water. Thus, in order to give clear pH responsiveness, it is necessary to use 4 as a major monomer.
Introduction
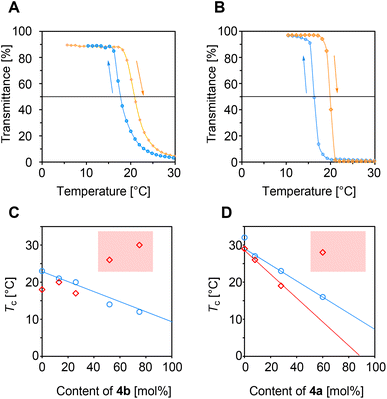
Vinyl polymers containing repeating units derived from α-amino acids have long attracted attention as macromolecules that exhibit properties different from those of polypeptides.1–5 These vinyl polymers were often investigated as biocompatible materials similar to polypeptides,6–8 while they were also studied as polymer materials utilizing carboxy and amino groups for pH- and temperature-responsive materials7,9–14 and polymer electrolytes.15 In typical examples, amino or carboxy groups of α-amino acids were used as reactive sites to link the carbon backbones.1–11 On the other hand, poly(α-dehydroalanine) [poly(1)] is a vinyl polymer with a carbon backbone composed of two sp3 carbons in alanine units, of which amino and carboxy pendants remain active (Scheme 1A). Because of its structural features, poly(1) has long been a target for synthesis.10,16–19 However, poly(1) cannot be synthesized directly from 1, as the monomer isomerizes to an imine form by tautomerism. Therefore, the synthesis has only recently been achieved.15 In contrast, 3a, an unsaturated naturally occurring β-amino acid, is stable enough to be handled.20–22 Thus, we investigated the radical polymerization of the corresponding ester 3b and found that the polymer poly(3b) was pH- and temperature-responsive (Scheme 1B).23 Unfortunately, this polymerization suffered from a side reaction between the amino pendants of the polymer and the ester of the unreacted monomer. For this reason, the structures of the obtained polymers were sensitively dependent on the polymerization conditions. To avoid the side reaction of ester–amide exchange, we have newly designed 4, which is a derivative of 3a but has an acrylamide structure (Scheme 1C). As is well known, acrylamides with appropriate N-substituents exhibit temperature responsiveness.24 Furthermore, the polymer of 4 was expected to be pH responsive due to the amino pendants. Herein, we describe the synthesis and polymerization of 4 and the stimulus responsiveness of the polymer.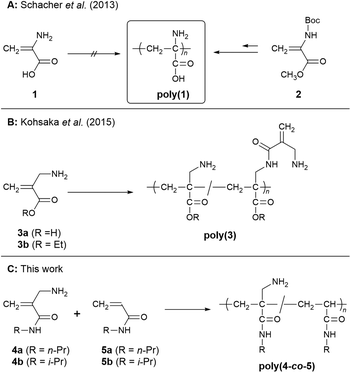 | ||
| Scheme 1 Examples of polymers of amino acid derivatives composed of a vinyl polymer backbone. A: Poly(α-dehydroalanine) reported in ref. 14. B: Our previous work in ref. 22. C: This work. | ||
Results and discussion
Monomer synthesis
In our previous report,223 was synthesized in a three-step reaction starting with the Morita–Baylis–Hillman (MBH) reaction of ethyl acrylate and formaldehyde. A similar reaction was performed with N,N-diethyl acrylamide, but the resulting monomer, 9, exhibited poor polymerizability (Scheme 2).25 In analogy with common N,N-dialkyl methacrylamides, the acrylic skeleton of 9 cannot maintain a planar structure due to the steric repulsion between the N-substituents and the α-substituent, resulting in a twisted conjugated system with poor reactivity.26 Therefore, the methacrylamide with one less N-substituent, 4, was interesting, although its synthesis was not achieved due to the low reactivity of 5 in the MBH reaction (Scheme 2: Route A). For this reason, Zhuang et al. synthesized 6 in six steps starting from 9 (Route B);27 if 4 is synthesized through this route, the required overall reaction steps are eight. Since Route B was too long, an alternative route consisting of four steps from tert-butyl acrylate (11) was investigated (Route C).One of the key reactions of Route C was the conversion of 12 to 13, because 12 is active in both acyl and conjugate substitution reactions. Although our previous work showed a preference for acyl substitution,28 conjugate substitution with residual primary amines affording 14 was also expected. Therefore, in our first examination, conjugate substitution was promoted as a reaction to be aimed at rather than a side reaction to be avoided. 12 was added neat at 25 °C over 1 min into a solution of a large excess (10 molar equiv.) of n-propylamine (Table 1, entry 1). The 1H NMR spectrum of the products suggested the production of 14a, although a series of signals assignable to 15a were also observed (Fig. S1A†). This implied the conjugate substitution of 13a and 14a as a side reaction. To suppress this reaction, a solution of 12 in CHCl3 (0.50 M) was added dropwise to a solution of n-propylamine (2.0 M) over 100 min. Furthermore, in order to increase the selectivity of the acyl substitution reactions, the temperature was lowered to −20 °C (entry 2, Fig. S1B†) or −40 °C (entry 3, Fig. S1C†), resulting in a suppressed yield of 15a (ca. 5%). However, the selective synthesis of 14a or purification were not achieved. Thus, isopropylamine (entry 4, Fig. S2B†) and tert-butylamine (entry 5, Fig. S2A†) were examined as bulky amines to reduce the nucleophilicity of the secondary amino group of 14. Notably, tert-butylamine was effective, and 14c was obtained with high selectivity (99.5%, entry 5). Unfortunately, the radical polymerization of 14c using 2,2′-azoisobutyronitrile (AIBN) in 1,4-dioxane (0.5 M) at 60 °C did not afford a polymeric product. The poor polymerizability of 14c was anticipated due to the large steric hindrance of the tert-butyl group. Thus, 4 was synthesized as a monomer with less steric hindrance.
| Entrya | Amine | (Equimol.) | Temp. [°C] | Time [h] | Compositionb [%] | ||
|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | |||||
| a A solution of 12 in CHCl3 (0.50 M) was added dropwise to a solution of n-propylamine (2.0 M) over 100 min. b Estimated from 1H NMR spectra. c 12 was added to a 6.4 M solution of n-propylamine in CH2Cl2. d Et3N (1.0 equimol.) was added. | |||||||
| 1c | n-PrNH2 | 10 | 25 | 18 | 79 | 21 | |
| 2 | n-PrNH2 | 10 | −20 | 18 | 94 | 6 | |
| 3 | n-PrNH2 | 10 | −40 | 18 | 95 | 5 | |
| 4 | i-PrNH2 | 10 | −20 | 18 | 97 | 3 | |
| 5 | t-BuNH2 | 10 | −20 | 18 | >99 | Trace | |
| 6 | n-PrNH2 | 1.0d | −20 | 22 | 100 | ||
| 7 | i-PrNH2 | 2.0 | −40 | 23 | 100 | ||
As mentioned above, for the synthesis of 4, 12 must undergo only an acyl substitution reaction with a primary amine and be selectively converted to 13. The key was found unexpectedly during a study of the synthesis of 14d. We first attempted to synthesize 14d with methylamine. Since methylamine was commercially available only in aqueous solution, it was extracted in advance with chloroform, and 12 was added dropwise to the organic layer at −40 °C. The 1H NMR spectrum of the reaction mixture confirmed only signals of unreacted 12 and 13d, suggesting no formation of 14d. This was probably due to the incomplete extraction of methylamine, which made the feeding ratio of methylamine lower than planned. In other words, it was suggested that only the acyl substitution reaction proceeded selectively when 12 reacted with a primary amine at less than the stoichiometric ratio in a chloroform solution at −40 °C. Therefore, n-propylamine was selected as a primary amine that can be accurately weighed, and two molar equivalents to 12 were reacted (Table 1, entry 6). In addition to one molar equivalent of triethylamine required for the acyl substitution reaction, an excess of one molar equivalent was used to capture the liberated hydrogen chloride. The 1H NMR spectrum of the product suggested that 13a was produced selectively (yield: 63%). Similarly, 13b was synthesized from isopropylamine (entry 7, yield: 77%).
Next, the synthesis of 4a was investigated by adding an excess amount of aqueous ammonia to the 1,4-dioxane solution of 13a at 25 °C. In the 1H NMR spectrum of the products (Fig. S4A†), a series of signals different from that of the main product 4a was observed. From the chemical shift value and integrated intensity ratio, this suggested the formation of 8a. 8a is generated by the conjugate substitution reaction of 4a and 13a. A similar side reaction occurred in the synthesis of 14a, although the formation of 15a was suppressed at a lower temperature. Therefore, the reaction between 13a and aqueous ammonia was carried out at 0 °C. Since unreacted 13a remained after 5 h (Fig. S4B†), the reaction time was extended to 22 h, resulting in the selective synthesis of 4a (Fig. S4C†). 14b was also synthesized in the same way.
In the 1H NMR spectrum of 14b, three signals were observed at 6.6, 3.4 and 1.6 ppm, each with an integrated intensity of one hydrogen atom (Fig. 1A). The signal at 6.6 ppm showed a COSY correlation with the methine hydrogen signal at 4.1 ppm, suggesting assignment to the amide protons (Fig. S5†). The signals at 3.4 and 1.6 ppm showed a COSY correlation with each other and were assigned to protons of the amino group. The two separated signals for the amino protons suggest slow bond rotation. In order to understand this explanation, the stable conformation of 14b was simulated using molecular mechanics and density functional theory (DFT). The results suggested that the hydrogen atom of the amide group and the nitrogen atom of the amino group formed a hydrogen bond in the most stable conformation (Fig. S6†).
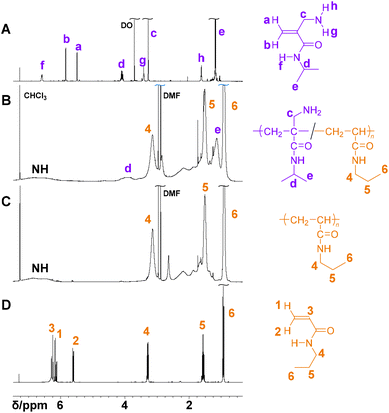 | ||
| Fig. 1 1H NMR spectra of 4b (A), poly(4b-co-5a) (Table 2, entry 10, B), poly(5a) (entry 12, C) and 5a (D) (400 MHz, CDCl3, 298 K). | ||
Polymerization
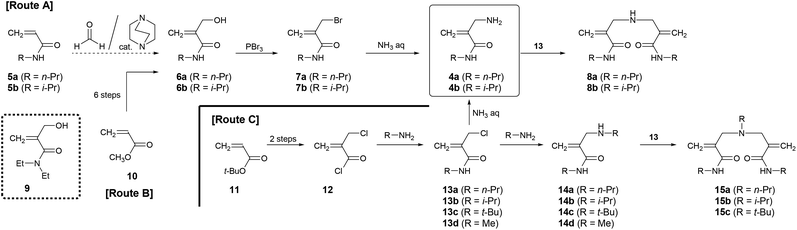
The homopolymerization of 4 was conducted using AIBN in N,N-dimethyl formamide (DMF) at 65 °C for 24 h. However, neither 4a (Table 2, entry 13) nor 4b (entry 1) yielded a polymer.29 As a control experiment, N-isopropyl acrylamide (5b, entry 7) and N-n-propyl acrylamide (5a, entry 12) were polymerized, respectively, resulting in polymer formation as expected. In other words, the lack of progress of the homopolymerization of 4 was not attributed to a technical cause. Therefore, the copolymerization of 4b and 5b was investigated at various feeding ratios (entries 2–6). Although all entries yielded polymers, it was difficult to evaluate the polymer composition from the 1H NMR spectrum due to the structural similarity of 4b and 5b (Fig. S7†). Therefore, combinations of monomers with different N-substituents were investigated. For example, an equimolar mixture of 4b and 5a was used for copolymerization (entries 8–12). In the 1H NMR spectrum of the product, clearly separated signals originating from the two N-substituents were observed (Fig. 1B). The signal of the N-methine proton of the isopropyl group around 4 ppm and that of the methylene proton of the n-propyl group around 3.1 ppm were typical examples. However, these signals were so broad that accurate calculation of the integrated intensity was difficult. Therefore, the composition was estimated from the signals of the terminal methyl group of the n-propyl group at 0.8–1 ppm and that of the two methyl groups of the isopropyl group at 1–1.4 ppm. As a result, the molar fraction of 4b units was estimated to be 52%, roughly matching the feeding ratio of 50%. Fig. 2A shows the growth of monomer conversions at each reaction time. Both monomers were consumed at approximately the same rate until 5 h when the conversion of 5a reached 50%. Since 4b was not homopolymerized, the monomer conversions implied alternating copolymerization. The tertiary carbon radical derived from 4b is more stable than the secondary carbon radical derived from 5a due to the large hyperconjugation effect. Therefore, propagating radicals preferentially attack 4b. However, it is thermodynamically difficult for the 4b-derived radical to attack 4b to form a homo-sequence, probably due to the steric hindrance of the substituents. Therefore, 4b hardly homopolymerizes. On the other hand, 5a, the sterically less hindered monomer, can be attacked, resulting in an alternating sequence of 4b–5a. This interpretation alone cannot explain the fact that 4b continues to be consumed in the later stages of the reaction while the conversion of 5a remains almost unchanged. A possible scenario is as follows: In the later stages of the reaction when the monomer concentration becomes lower, the termination reaction occurs before the 4b-derived radical propagates to 5b. However, it is difficult to draw definitive conclusions from this result alone. As the feeding ratio of 4b increased, the number average molecular weight tended to decrease. This is an expected result because 4b does not homopolymerize. In entry 6, the feed ratio and conversion of 5a were 20% and 2%, respectively, but the molar fraction of 5a units in the copolymer was 25%. As the yield implies, not all products were recovered in this experiment. Considering that 4b hardly homopolymerized, it could be understood that the product recovered as a precipitate was the polymeric component containing a large amount of 5a units.| Entry | M1 (4) | M2 (5) | Feed [mol%] | Conv.a [mol%] | Yield [%] | Comp.a [mol%] |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
Đ |
T
c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [°C] c [°C] |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 4 | 5 | 4 | 5 | H2O | 1 M HCl aq | ||||||
| a Determined by 1H NMR spectrometry. b Determined by size-exclusion chromatography [poly(methyl methacrylate) standards, 0.5 wt% Libr solution in DMF, 40 °C]. c Cloud points defined as the temperature at which the transmittance at 589 nm became 50% in the heating process. d Ref. 30. | |||||||||||||
| 1 | 4b | 5b | 100 | 0 | 0 | ||||||||
| 2 | 4b | 5b | 75 | 25 | 98 | 92 | 57 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.40 | 21 | 31 | ||
| 3 | 4b | 5b | 20 | 80 | >99 | >99 | 68 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.72 | 23 | 25 | ||
| 4 | 4b | 5b | 15 | 85 | >99 | >99 | 53 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.48 | 49 | 26 | ||
| 5 | 4b | 5b | 10 | 90 | >99 | 94 | 26 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.48 | 33 | 29 | ||
| 6 | 4b | 5b | 5 | 95 | >99 | 94 | 40 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.36 | 30 | 30 | ||
| 7 | 4b | 5b | 0 | 100 | >99 | 30 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.49 | (32)d | 29 | |||
| 8 | 4b | 5a | 80 | 20 | 51 | 2.3 | 11 | 75 | 25 | 4700 | 1.61 | 12 | 30 |
| 9 | 4b | 5a | 50 | 50 | 93 | 66 | 8.7 | 52 | 48 | 6200 | 1.73 | 14 | 26 |
| 10 | 4b | 5a | 20 | 80 | >99 | 90 | 74 | 26 | 74 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.85 | 20 | 17 |
| 11 | 4b | 5a | 10 | 90 | >99 | 78 | 64 | 13 | 87 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.43 | 21 | 20 |
| 12 | 4b | 5a | 0 | 100 | >99 | 35 | 0 | 100 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.48 | 23 | 18 | |
| 13 | 4a | 5b | 100 | 0 | 0 | ||||||||
| 14 | 4a | 5b | 50 | 50 | 95 | 74 | 7.8 | 60 | 40 | 4480 | 1.63 | 16 | 28 |
| 15 | 4a | 5b | 20 | 80 | >99 | >99 | 9.4 | 28 | 72 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.43 | 23 | 19 |
| 16 | 4a | 5b | 10 | 90 | >99 | >99 | 39 | 8 | 92 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.28 | 27 | 26 |
Similar copolymerization was performed for 4a and 5b. Monomer conversion monitoring in copolymerization with equimolar mixtures suggested preferential consumption of 4a (Fig. 2B). 4a is less sterically hindered by the N-substituent than 4b, while 5b has greater bulkiness than 5a. Consequently, the order of polymerization rates was expected to be as follows: 4a > 4b > 5a > 5b. In fact, the difference in consumption rate between 4a and 5b was significant compared to the combination of 4b and 5a.
pH-/thermo-responsiveness
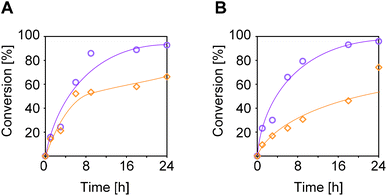
The transmittances at 589 nm (sodium D-line) of the aqueous solution of the prepared polymers were measured with rising temperature. The cloud point (Tc) was evaluated as the point at which the transmittance of the polymer aqueous solution became half before and after the change in the heating process (Fig. 3A). The homopolymer of 5a exhibited a thermo-response with a lower critical separation temperature (LCST), and the Tc was 23 °C (entry 12). Tc decreased linearly with an increasing mole fraction of 4b in the polymer (entries 8–12, Fig. 3C). Our previous study using an N,N-disubstituted acrylamide with a hydroxy pendant showed a linear trend of increasing Tc with increasing hydroxy content.25 Since the amino group is a hydrophilic group as well as the hydroxy group, the decrease in Tc by copolymerization was contrary to our expectations. Amino groups have stronger properties as hydrogen-bond acceptors than hydroxy groups. In fact, as mentioned above, conformational analysis of 4b by 1H NMR spectroscopy and DFT calculations suggested that the nitrogen atom of the amino group forms a hydrogen bond with the hydrogen atom of the amide bond. Therefore, it was speculated that the amino group functioned as an acceptor for intramolecular hydrogen bonds and had the effect of lowering Tc.A similar experiment was performed in 1 M HCl aq (Fig. 3B). For the copolymers with 0–26 mol% 4b content, the Tc in 1 M HCl aq were lower than those in pure water (entries 10–12, Fig. 3C). In contrast, the Tc of the copolymers with 52 and 75 mol% 4b content were drastically increased from those in pure water. In these copolymers, the ammonium pendant groups function as a strong hydrophilic group and inhibit the association of polymer chains by electric repulsion, resulting in a much higher Tc than expected. On the other hand, in order to actualize the effect of such an amino group, it is necessary to prepare a copolymer with 4a content of at least 52 mol%. A similar trend was observed for copolymers of 4a and 5b (Fig. 3D).
Conclusions
The polymerization of 4 was investigated from an interest in polymers of β-amino acid derivatives. 4 was synthesized in a four-step reaction starting from tert-butyl acrylate. Although optimizations of reaction conditions were required, the reaction steps were significantly reduced from the conventional synthesis method using a protection/deprotection protocol. The intermediate, 13, is also attractive as a precursor of various allylic-modified methacrylamides because it exhibits activity in conjugate substitution reactions. Although 4 is an N-monosubstituted methacrylamide, the homopolymerization did not afford a polymeric product. Since the reactivity of (meth)acrylamide is known to vary greatly depending on temperature, solvent, and catalyst,31–33 the polymerization of 4 still needs further investigation. For the copolymer of 5, the incorporation of 4 was effective at increasing the hydrophilicity in acidic solution, but it was necessary to use 4 as the main component. Rather, copolymers with low 4 content were more hydrophobic than the homopolymer of 5. This result was in contrast to copolymers bearing hydroxy groups. These facts suggest that the hydrogen-bond acceptability of the amino groups affected the hydrophobicity of the polymer.Although various functional polymers have been reported for α-functionalized acrylates,34 acrylamides have hardly been studied. As shown here, modification at the α-position does not simply reflect the properties of the functional group, such as hydrophilicity and hydrophobicity, in the polymer. Therefore, the chemistry of α-functionalized acrylamide has the potential to produce unexpected materials and functions.
Author contributions
Y. K. supervised this study and wrote a draft of this article. N. C. conducted all experiments on 4a, 4b and the polymers. K. I. examined the synthesis of 14, 15 and 13d. Y. A. helped all experiments as a post-doc researcher.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by JSPS KAKENHI (No. 19H02763) for Y. K. 5b was a kind gift from KJ Chemicals Co. 11 was a kind gift from Osaka Organic Chemical Industry Ltd.References
- H. Mori and T. Endo, Macromol. Rapid Commun., 2012, 33, 1090 CrossRef CAS PubMed.
- A. C. Evans, J. Skey, M. Wright, W. Qu, C. Ondeck, D. A. Longbottom and R. K. O'Reilly, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6814 CrossRef CAS.
- G. B. Thomas, C. E. Lipscomb and M. K. Mahanthappa, Polym. Chem., 2012, 3, 741 RSC.
- Z. Zhu, J. Cui, J. Zhang and X. Wan, Polym. Chem., 2012, 3, 668 RSC.
- M. Casolaro, Macromolecules, 1995, 28, 2351 CrossRef CAS.
- H. Sun and C. Gao, Biomacromolecules, 2010, 11, 3609 CrossRef CAS PubMed.
- S. Kumar, R. Acharya, U. Chatterji and P. De, Langmuir, 2013, 29, 15375 CrossRef CAS PubMed.
- A. Bentolia, I. Vlodavsky, R. Ishai-Michaeli, O. Kovalchuk, C. Haloun and A. J. Domb, J. Med. Chem., 2000, 43, 2591 CrossRef PubMed.
- S. Ghosh and P. De, Polym. Chem., 2014, 5, 6365 RSC.
- H. Mori, H. Iwaya, A. Nagai and T. Endo, Chem. Commun., 2005, 41, 4872 RSC.
- J. Du and R. K. O'Reilly, Macromol. Chem. Phys., 2010, 211, 1530 CrossRef CAS.
- T. Mori, M. Hamada, T. Kobayashi, H. Okamura, K. Minagawa, S. Masuda and M. Tanaka, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4942 CrossRef CAS.
- H. Okamura, T. Mori, K. Minagawa, S. Masuda and M. Tanaka, Polymer, 2002, 43, 3825 CrossRef CAS.
- K. Bauri, M. Nandi and P. De, Polym. Chem., 2018, 9, 1257 RSC.
- U. Günther, J. V. Sigolaeva, D. V. Pergushov and F. H. Schacher, Macromol. Chem. Phys., 2013, 214, 2202 CrossRef.
- S. Sakakibara, Bull. Chem. Soc. Jpn., 1959, 33, 814 CrossRef.
- S. Sakakibara, Bull. Chem. Soc. Jpn., 1960, 34, 174 CrossRef.
- R. S. Asquith, K. L. Gardner and K. W. Yeung, J. Polym. Sci., Polym. Chem. Ed., 1978, 16, 3275 CrossRef CAS.
- P. Fábián, V. S. Chauhan and S. Pongor, Biochem. Acta, 1994, 1208, 89 Search PubMed.
- Y. Kashman, L. Fishelson and I. Ne'eman, Tetrahedron, 1973, 29, 3655 CrossRef CAS.
- M. B. Yunker and P. J. Scheuer, Tetrahedron Lett., 1978, 19, 4651 CrossRef.
- A. Holm and P. J. Sceuer, Tetrahedron Lett., 1980, 21, 1125 CrossRef CAS.
- Y. Kohsaka, Y. Matsumoto and T. Kitayama, Polym. Chem., 2015, 6, 5026 RSC.
- A. Narumi, S. Sato, X. Shen and T. Kakuchi, Polym. Chem., 2022, 13, 1293 RSC.
- Y. Kohsaka and Y. Tanimoto, Polymers, 2016, 8, 374 CrossRef PubMed.
- M. Kobayashi, T. Ishizone and S. Nakahama, Macromolecules, 2000, 33, 4411 CrossRef CAS.
- J. Zhuang, B. Zhao, X. Meng, J. D. Schiffman, S. L. Perry, R. W. Vachet and S. Thayumanavan, Chem. Sci., 2020, 11, 2103 RSC.
- Y. Kohsaka and K. Nagai, Macromol. Rapid Commun., 2021, 42, 2000570 CrossRef CAS PubMed.
- Although other monomers (14a–d) have low purity, polymerizations using AIBN were also investigated. Some phenomena implying polymerization, e.g. the increase of viscosity of the reaction mixture and the broad 1H NMR signals with a small diffusion coefficient in DOSY spectra, were observed. However, no peak in high molecular weight region (>103) was observed by size exclusion chromatography (SEC) using chloroform as an eluent.
- K. Jain, R. Vedarajan, M. Watanabe, M. Ishikiriyama and N. Matsumi, Polym. Chem., 2015, 6, 6819 RSC.
- T. Hirano, Y. Fujita, M. Shinomiya, Y. Arakawa, F. Yagishita, A. Emoto, M. Oshimura and K. Ute, Polymer, 2021, 226, 123823 CrossRef CAS.
- T. Hirano, T. Segata, J. Hashimoto, Y. Miwa, M. Oshimura and K. Ute, Polym. Chem., 2015, 6, 4927 RSC.
- S. Ishii, A. Sezai, Y. Kohsaka, S. Deguchi and M. Osada, Ind. Eng. Chem. Res., 2022, 61, 17012 CrossRef CAS.
- Y. Kohsaka, Y. Akae, R. Kawatani and A. Kazama, J. Macromol. Sci., Part A: Pure Appl.Chem., 2022, 59, 83 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01611g |
| This journal is © The Royal Society of Chemistry 2023 |