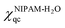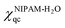Understanding the monomer deuteration effect on the transition temperature of poly(N-isopropylacrylamide) microgels in H2O†
Thomas
Nevolianis
 a,
Andrea
Scotti
a,
Andrea
Scotti
 *b,
Alexander V.
Petrunin
*b,
Alexander V.
Petrunin
 b,
Walter
Richtering
b,
Walter
Richtering
 b and
Kai
Leonhard
b and
Kai
Leonhard
 *a
*a
aInstitute of Technical Thermodynamics, RWTH Aachen University, 52062 Aachen, European Union, Germany. E-mail: kai.leonhard@ltt.rwth-aachen.de
bInstitute of Physical Chemistry, RWTH Aachen University, 52056 Aachen, European Union, Germany. E-mail: scotti@pc.rwth-aachen.de
First published on 6th February 2023
Abstract
Obtaining deuterated micro- and nanogels is essential to characterize their architecture and determine their response to crowding using neutron scattering with contrast variation. Experimental studies have reported that deuterated microgels have a higher volume phase transition temperature (VPTT) than hydrogenated microgels. However, to date, the effect of deuteration on the microgel properties has yet to be fully understood, questioning whether deuterated and hydrogenated microgels have comparable responsiveness to external stimuli, structure, and compressibility. To answer these questions, we both simulate and measure the effect of deuteration on the VPTT of N-isopropylacrylamide (NIPAM)-based microgels. Using quantum mechanical methods and considering only the zero-point energy difference, we study different NIPAM-(H2O)n complexes and calculate the changes in the value of the Flory interaction parameter as a consequence of deuteration. The resulting interaction parameter is used in the Flory–Rehner theory to predict microgels’ swelling behavior. Using multi-angle dynamic light scattering, we measure the hydrodynamic radius of non- and deuterated microgels as a function of temperature. Based on our model results, we obtain a VPTT shift of 4.9 K, only due to the zero-point energy difference between complexes containing H2O and NIPAM/D7NIPAM, which agrees with the 4.3 K shift of the VPTT observed in the experiments. In the future, our approach may be used to adjust effective parameters in molecular dynamics simulations studying deuterated microgels and providing insights into their properties, including VPTT, swelling ratio, and softness.
1 Introduction
The use of colloidal systems to study phase transitions and the flow properties of complex fluids is pivotal to both advance the understanding of fundamental problems1,2 and developing soft materials for industrial applications.3 In the last decades, the effects of softness on the self-assembly and macroscopic properties of soft materials have been largely investigated using micro- and nanogels.4 These particles consist of a crosslinked polymeric network, which contains up to >90% solvent in their swollen state.5–8 Consequently, these colloids are soft and deformable and can respond to increases of concentration in solution with both isotropic deswelling or faceting.9–12 Furthermore, if the polymer used in the synthesis is stimuli-responsive, the obtained particles are responsive to changes in the environments.3 For instance, poly(N-isopropylacrylamide) (NIPAM)-based microgels in H2O are thermoresponsive since the pNIPAM has a lower critical solution temperature (LCST).13,14 Below 305 K, H2O is a good solvent and the microgels are swollen and soft. Above this temperature, there is a volume phase transition and the H2O content decreases, making the microgel more compact and similar to a hard colloid.5 This property is appealing for both application and fundamental research. Microgels allow to realize responsive smart materials such as membranes for H2O filtration,15 responsive emulsions,16 and nano-carriers for drug delivery.17 The possibility of the microgels to change their volume in solution simply by changing the sample temperature also allows to explore the sample properties as a function of crowding in crystals, glasses, or jammed phases.18–23To appropriately tailor the microgel properties for applications and correctly interpret their behavior when used as models for atomic systems, one must adequately characterize their architecture and the changes in their structure due to the changes in their external environment. For these applications, the size of the microgels cannot exceed a few hundred nanometers. Therefore, scattering techniques are the only option to characterize such properties. In particular, small-angle neutron scattering with contrast variation is an advanced technique to investigate functional polymers as it allows us to selectively isolate and study the samples’ parts.6,9,24–29 This is because neutrons can distinguish between hydrogen and deuterium since they interact with the nuclei of the atoms composing the samples and not with their electron clouds.30 The use of deuterated monomers during the synthesis can result either in partially deuterated microgels or in individual microgels with regions composed of partially deuterated polymer. In both cases, a mixture of H2O and D2O can be used to selectively mask the deuterated or protonated part of the sample.31 This approach has been successfully used to characterize specific regions in the microgels6,28,32,33 or study the response of individual microgels to crowding.9,24–27
However, it has to be taken into account that deuteration can influence the reactivity of monomers and thus structure–property relationships of polymers might be affected. A recently reported example concerns the softest NIPAM-based microgels that can be realized by precipitation polymerization, which are the so-called ultra-low crosslinked microgels.4,34 These particles are synthesized without adding a crosslinker agent, and the polymeric network forms due to the NIPAM self-crosslinking.34 However, they cannot be synthesized using the usual deuterated monomer with seven deuterium atoms. This is because the isopropyl group of NIPAM is deuterated. A NIPAM deuterated monomer with only three deuterium atoms in the monomer backbone has been successfully used. We note that the deuterated and regular ultra-low crosslinked microgels have similar properties compared to regular microgels and microgels synthesized using the pD7NIPAM. For instance, they show a closer swelling ratio, i.e., softness,4 and the shift of the VPTT due to deuteration is negligible within the experimental error (see ESI†).
A key question is whether or not the use of a deuterated monomer changes the properties of the microgels. For instance, microgels synthesized using NIPAM with the seven hydrogen atoms of the isopropyl group substituted by deuterium show a higher VPTT compared to microgels synthesized using [C6H11NO].34,35
Furthermore, the use of this deuterated monomer suppresses the self-crosslinking34 and leads to obtaining microgels with a slightly different internal architecture compared to the one synthesized with the fully hydrogenated monomer.6 This means that selective deuteration might affect both the swelling behaviour and the internal structure and amount of crosslinking of the microgels. A possibility to compensate for the absence of self-crosslinking is to use a slightly different amount of crosslinker during the synthesis of deuterated and hydrogenated microgel to achieve a similar swelling ratio of the particles.26,27 However, this might as well affect the internal architecture of the microgel. As we have recently shown, the softness of a particle can be defined in various ways and depends on many aspects such as swelling degree, internal architecture, and elastic moduli.4 Therefore, the fact that deuterated and hydrogenated microgels have a comparable swelling ratio cannot guarantee per se that the two microgel have the same softness and a more detailed understanding of the deuteration effects on the microgel synthesis is needed.
Another aspect that must be considered is that selective deuteration changes the interaction between solvent and polymer.36 It is well known that both the use of deuterated solvents and selective deuteration change the VPTT of pNIPAM.37 The thermodynamics governing the microgel swelling has been described using the Flory–Huggins model.38–40 For neutral microgels, such as the pNIPAM ones, the swelling equilibrium is determined by the balance between the free energy due to the solvent–polymer mixing and the elasticity of the crosslinked polymeric network. Using this mean-field approach, microgels have been approximated by combining the Flory–Rehner theory of swelling of crosslinked polymer networks41–43 with the Hertz theory of pair interactions between elastic bodies.44 Many studies45–47 report values of Flory–Rehner theory's parameters in different ranges by describing successfully the swelling curve of microgels and thus, these parameter values vary and their choice is debatable.48
Since our main focus here is to study the deuteration effect of pNIPAM microgels at a fundamental level of theory and therefore, the choice of the parameters, as long as they still have a physical meaning, is not relevant. Approaches such as Monte Carlo simulations based on the Flory–Hertz model of microgels have been primarily used to describe the response of microgels to crowding.24,38,40 Different mechanistic models49–52 with quantum mechanical estimated parameters53–56 have been used to model microgel synthesis and tailor new microgel functionalities. New challenges appear for interpreting the swelling behavior of deuterated microgels due to interactions that the above-mentioned macroscopic models can not capture.
Thermoresponsivity of polymers in aqueous solution is governed by the interaction of the macromolecule with the solvent water. In this scenario, quantum mechanical calculations can provide unique information on the microscopic interactions between the solvent and deuterium substituted atoms and thus provides new insight in designing (partially) deuterated momomers to tailor properties of thermoresponsive polymers. In contrast to classical mechanics, nuclei show nuclear quantum effects such as zero-point energy, which are more pronounced for lighter isotopes.57 Detailed studies on isotope effects using different approaches can be found in the literature.58–60 Using quantum mechanical calculations, studies have successfully investigated equilibrium isotope effects of supramolecular host–guest systems61 and pyridine.62 In the case of microgels, quantum mechanic calculations can be used to study the effect of deuteration by considering only the zero-point energy difference and predicting the change of the Flory interaction parameter upon deuteration, which then can be used in the Flory–Rehner theory to model the swelling behavior of deuterated microgels.
Among the different deuterated monomers of NIPAM, the most commonly used one to realize partially or fully deuterated microgels is the one with seven deuterium atoms. Partially deuterated and fully deuterated pD7NIPAM microgels have mainly been used to determine the individual particle shape both in diluted,32,33,37 and concentrated suspensions.4,24–27,63 For these microgels, a single monomer is a promising model for studying the effect of deuteration. Given the large shifts in the VPTT, the different swelling ratios, and the large use of D7NIPAM, this monomer will be the main focus of this study. For another used NIPAM deuterated monomer, the D3NIPAM, such a single monomer approach seems less promising since the backbone atoms are in different hybridization states in the monomer (sp2) and the polymer (sp3), leading to different interactions with their environment. In addition, it is known that small changes in the precise backbone structure can have large effects on the VPTT of polymers, e.g., adding a methyl group to the backbone of pNIPAM counter-intuitively increases the VPTT.64 Therefore, a single monomer cannot be used as a model molecule, making quantum mechanical calculations orders of magnitude more expensive than for pD7NIPAM. However, as we mentioned above, the use of this monomer seems to have a negligible effect on the swelling properties, and VPTT shift of the obtained microgels compared with NIPAM-based ultra-low crosslinked microgels and, therefore, small changes on the quantum-mechanic scales are expected.
All quantum mechanical studies of the effect of deuteration we are aware of have studied the deuteration of solutes, not of the solvent, see, e.g., ref. 61 and 62. It turned out that an extension of their approach to the deuteration of the solvent is not easily possible (see ESI† for more details). Therefore, the changes in the VPTT of hydrogenated (C6H11NO) and partially deuterated (C6H4D7NO) NIPAM-based microgels suspended in H2O are investigated. In this study, the D7NIPAM microgel is used as an example to study the deuteration effect due to its large VPTT shift. We calculate the change of the Flory interaction parameter upon deuteration using quantum mechanical methods and considering only the zero-point energy difference. The results are used to model the swelling curve of the deuterated microgel, and the model predictions are validated against experimental observations. Different vibrational modes of NIPAM and D7NIPAM with H2O result in a difference in zero-point energy (ZPE). This difference influences the Flory interaction parameter and leads to the VPTT shift.
2 Materials and methods
2.1 Synthesis
All the microgels used in this study are obtained by standard precipitation polymerization. The main monomer used is N-isopropylacrylamide (NIPAM) ([C6H11NO]n) or deuterated monomers of NIPAM in which the seven hydrogen atoms of the isopropyl group have been substituted by deuterium. The ratio between the crosslinker N,N′-methylenebisacrylamide (BIS) and the monomer is 5 mol% in all the synthesis. The syntheses were performed according to standard precipitation polymerization.5,14 Surfactants have also been added during the synthesis to control the final microgel size and the size polydispersity.65 All samples were prepared using bi-distilled Milli-Q H2O as a solvent. A detailed description of the synthesis procedure for these microgels can be found in the literature.242.2 Dynamic light scattering
Multi-angle dynamic light scattering (DLS) was used to measure the intensity autocorrelation functions of diluted suspensions of microgels. The light source was a laser with a wavelength in vacuum λ0 = 633 nm. The refractive index of the H2O was n(λ0) = 1.33. The scattering vector is defined as q = 4πn/λ0sin(θ/2) and it was changed using scattering angles θ between 30° and 110° with steps of 10°. The solutions of microgels have been measured at a temperature between 293 K and 323 K in steps of 2 K. A thermal bath filled with toluene to match the refractive index of the glass was used to control the temperature during the measurements.From the analysis of the intensity autocorrelation functions with the second-order cumulant method,66 the decay rates, Γ, were obtained for each q-value.67,68 The errors on the values of D0 are computed using a linear regression.68 Finally, the value of the hydrodynamic radius, RH, was obtained using the Stokes–Einstein equation: RH = kbT/(6πηt(T)D0), where η(T) is the viscosity of the solvent used at the temperature T and kB is the Boltzmann's constant. The errors on the values of RH are obtained from error propagation of the uncertainty on D0 and T (±0.1 K).
2.3 Flory–Rehner theory
Flory–Rehner's theory has been applied in previous studies to describe the swelling behavior of microgels with temperature dependence.38,40,46,48 The theory takes into account the contributions to osmotic pressure as mixing thermodynamics of polymer–solvent and the elasticity of the polymer network, which are given by: | (1) |
 | (2) |
N C is the number of polymer chains in the network, φ is the polymer volume fraction occupied by the polymer within the microgel volume, V0 is the volume of the microgel in the reference state, and φ0 is the polymer volume fraction within the volume of the microgels in the reference state. Since the microgels are synthesized in bad solvent conditions, a natural choice for the reference state is the microgel in the collapsed state above the VPTT.4vS is the molar volume of the solvent, and χ is the Flory–Huggins interaction parameter. NA is the Avogadro's number. In thermodynamic equilibrium, the mixing and elastic contributions of the osmotic pressure are equal, which leads to eqn (3),46,69 in which for a given temperature, the hydrodynamic radius of the microgel can be calculated.
 | (3) |
N gel = (V0φ0NA)/(vSNC) is the number of segments between two crosslinking points and RH,0 is the hydrodynamic radius at the reference state.
2.4 Flory–Huggins interaction parameter
The Flory–Huggins interaction parameter χ can be expressed as a series expansion of temperature and volume fraction.70 We choose the original expression71 of χ as we obtain a reasonable agreement between the experiments of this study and the model. By introducing higher order terms in χ, additional free parameters are introduced, which makes the study of deuteration challenging. Therefore, in this study, the Flory–Huggins interaction parameter is defined as: | (4) |
The changes in the values of χiqc upon deuteration are calculated from quantum mechanical methods. The i = NIPAM-H2O, D7NIPAM-H2O stands for the quantum mechanically predicted Flory–Huggins interaction parameter describing the NIPAM- and D7NIPAM-based microgels in H2O, respectively. The interaction parameter χiqc for both NIPAM- and D7NIPAM-based microgels can be calculated as,
 | (5) |
The electronic structures of NIPAM and D7NIPAM remain the same, as the only difference is due to deuteration. To predict the change of χiqc from quantum mechanical methods, only the ZPEs are therefore considered. The change of the Flory–Huggins interaction parameter upon deuteration is given by (see ESI† for more details)
 | (6) |
It is known that the different C–H/D ZPEs result in different C–D and C–H bond lengths.72 We neglect these effects similar to literature studies,61,62 and thus, we assume that only the vibrational frequencies and ZPEs differ within isotopically substituted bonds.
2.5 Computational details
For each NIPAM-(H2O)n, (H2O)n, and NIPAM-NIPAM complexes, CREST73 is used to identify the most stable conformer at the GFN2-xTB level of theory. The identified complexes are used as input and further optimized at B3LYP74 method with TZVP75 basis set as well as D3BJ dispersion correction76 with the software GAUSSIAN. At the same level of theory, the vibrational frequency calculations are performed, and the rigid rotor harmonic oscillator (RRHO) model is used to obtain the ZPEs. The B3LYP method is known to give accurate equilibrium geometries77–79 and very accurate frequencies with a root mean square error of 31 cm−1 (ref. 80) at a low computational cost. We approximate the ΔGPP term of the NIPAM–NIPAM interactions by considering only two NIPAM monomers. For the ΔGSP and ΔGPP terms, we increase the number of H2O molecules until the Δχqc is converged. Electronic structures and geometries remain identical for the hydrogenated and deuterated complexes. Therefore, for the deuterated complexes, only vibrational frequency calculations are repeated using the force constants from the hydrogenated complexes’ calculations.3 Results and discussion
3.1 Study of NIPAM/D7NIPAM-(H2O)n complexes
The change of the Flory–Huggins interaction parameter upon deuteration in units of RT, where R is the gas constant and T is the temperature, against the number of H2O molecules for different NIPAM/D7NIPAM-(H2O)n complexes is shown in Fig. 1.For n = 1, a hydrogen bond occurs between the H of H2O and the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the NIPAM monomer due to oxygen's high electronegativity. To study the effect of individual hydrogen bonds on oxygen and nitrogen of NIPAM, we manually transfer the H2O molecule in the vicinity of nitrogen. We find that Δχqc takes the values −0.002(RT) (increase of VPTT) and 0.04(RT) (decrease of VPTT) for the hydrogen bonds H⋯O
C of the NIPAM monomer due to oxygen's high electronegativity. To study the effect of individual hydrogen bonds on oxygen and nitrogen of NIPAM, we manually transfer the H2O molecule in the vicinity of nitrogen. We find that Δχqc takes the values −0.002(RT) (increase of VPTT) and 0.04(RT) (decrease of VPTT) for the hydrogen bonds H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and H⋯N–H, respectively.
C and H⋯N–H, respectively.
Increasing the number of H2O molecules to take both hydrogen bonds with opposing effects into account (up to n = 8), Δχqc decreases till it reaches a local minimum for n = 6 and increases again till n = 8. For n = 6, the NIPAM/D7NIPAM (carbon atoms in green) with (H2O)6 (red and white) are shown in the left of Fig. 1. Two H2O molecules create hydrogen bonds with the oxygen atom of the NIPAM monomer. For n = 9, the most energetically stable NIPAM/D7NIPAM-(H2O)9 complex geometry that we obtain is shown in the top-right of Fig. 1 with Δχqc = −0.018t(RT). One of nine H2O molecules creates a hydrogen bond with the nitrogen atom of NIPAM. After oxygen, nitrogen is the most electronegative atom and thus, a hydrogen bond H⋯N–H is expected. For values larger than 9, the Δχqc decreases with the increase of n, which results in a higher shift of VPTT.
To further study the hydration of and the effect of deuteration, we add more H2O molecules to hydrate the isopropyl group. For n = 14, the isopropyl group is only partially hydrated. The value of Δχqc decreases to −0.13(RT), which indicates that the isotope effect is more substantially observable. Only for 25 H2O molecules, the isopropyl group is fully hydrated, which results in Δχqc = −0.29(RT). The most stable geometry of NIPAM-(H2O)25 complex is shown in bottom-right of Fig. 1. For this geometry, H2O molecules create hydrogen bonds with the oxygen and nitrogen atoms and have van der Waals interactions with the isopropyl group.
To fully characterize the isotope effect, the isopropyl group needs to be hydrated and this happens only when both oxygen and nitrogen atoms are hydrated. We perform calculations for n = 27 and n = 40, in order to check how Δχqc changes its value by adding more H2O molecules. Both for n = 27 and n = 40, Δχqc changes its value only by 2% which indicates that the result has converged. For this reason, we conclude that 25 H2O molecules are appropriate for studying the deuteration effect.
3.2 Swelling of microgels
In Fig. 2, the circles and triangles represent the values of the hydrodynamic radii obtained from multi-angle DLS, RH, as a function of the temperature T, for the pNIPAM and pD7NIPAM microgels, respectively. The calculated values for the hydrodynamic radius are plotted as lines.Eqn (3) and (4) (Flory–Huggins interaction parameter) are fitted (straight line and dashed line) against the experimental data (circles and triangles) of the pNIPAM and pD7NIPAM microgels, respectively. In general, a reasonable fit is obtained for the swelling curves of pNIPAM and pD7NIPAM microgels with a coefficient of determination (R2) of 0.99 and 0.96, respectively.
For the pNIPAM microgel, in the 20 °C ≤ T ≤ 25 °C and 48 °C ≤ T ≤ 50 °C range, the Flory–Huggins theory slightly overestimates and underestimates the hydrodynamic radius, respectively. This is known in the literature46 and it is due to the Flory–Huggins interaction parameter. Once more, it is important to note that the Flory–Rehner model can successfully describe the thermodynamic and mechanical parameters of pNIPAM microgels. However, studies have shown that unrealistic values for several fit parameters are required in order to obtain close agreement between the theory and experimental data for equilibrium swelling of microgels.48
Nonetheless, as the purpose of this work is to study the deuteration effect at a fundamental level, we conclude that the current choice of the Flory–Huggins interaction parameter given by eqn (4) leads to a reasonable fit and can describe the observed swelling behavior. A similar behavior is observed for the D7NIPAM microgel. In the 20 °C ≤ T ≤ 27 °C and 30 °C ≤ T ≤ 37 °C range, the Flory–Huggins theory overestimates and underestimates the hydrodynamic radius, respectively. During the fitting procedure, all four parameters (A, Θ, Ngel, and φ0) are free to change. The resulting values of the fitting parameters are shown in Table 1 for both microgels.
| Parameters | pNIPAM | pD7NIPAM | pD7NIPAM† |
|---|---|---|---|
| A | −30.9 | −29.5 | −30.9 (fixed) |
| Θ (°C) | 32.2 | 37.1 | 32.2 (fixed) |
| N gel | 19.9 | 59.7 | 57.4 |
| φ 0 | 0.88 | 0.82 | 0.84 |
After optimization, the dimensionless parameter A has a value of −30.9 and −29.5 for pNIPAM and pD7NIPAM microgels, respectively. Literature studies46,48,81–83 report A in the range of −43.3 ≤ A ≤ −2. The fitted temperature Θ is found = 32.2 °C and 37.1 °C in the case of pNIPAM and pD7NIPAM microgels, respectively, with a VPTT shift of 4.9 K. The calculated values of Θ deviate 1.9 K and 1.3 K from the experimental values with a VPTT shift of 4.3 K, which are determined by fitting the sigmoid function to the measurements (see ESI†). For the pNIPAM microgel, Ngel is found 19.9. Friesen et al.69 shows that Ngel = 10 for microgels with comparable but homogeneously distributed amount of crosslinks. Our values therefore indicate that that BIS is not homogeneously distributed inside the microgel. This is in agreement with the experimental structure of microgels probed using both scattering4,5,29,39 and microscopy techniques10,11,84 which shows a decreasing crosslinks concentration from the core to the microgel periphery.
For the pD7NIPAM microgel, Ngel is found 59.7. This larger value of Ngel compared to pNIPAM microgel value results by definition of Ngel into less amount of BIS incorporated into the microgel. The volume fraction of the polymer φ0 has a value of 0.88 and 0.82 for the pNIPAM and pD7NIPAM microgels, respectively. From the fitting, we obtain a standard error of 1σ = 0.06 for φ0. The φ0 values which we obtain in this work are consistent with measurements85–87 and theoretical studies46 of φ0 = 0.7 and φ0 = 0.84, respectively. However, this is still debated since, as reported by Lopez et al.48φ0 = 0.44 is also reasonable. The parameter φ0 is in the range of 0.44 ≤ φ0 ≤ 0.85 with an average value of 0.8. Using an approximation based on viscosimetry measurements and DLS (see ESI†), we estimate φ0 = 0.58 ± 0.06 and φ0 = 0.51 ± 0.03 for the pNIPAM and pD7NIPAM microgels, respectively. As a result, the simulated and approximated φ0 agrees only within the 4σ standard deviation. However, this derivation is based on an approximation and in this work, we obtain best fits for the parameter values shown in Table 1.
As described in detail in the previous section, the change of the Flory–Huggins interaction parameter upon deuteration Δχqc is obtained by eqn (6) with quantum chemical calculations. Next, Δχqc is added to χNIPAM-H2O to predict the swelling curve of pD7NIPAM microgel. During the calculation, the parameters A and Θ are fixed to the value determined from the pNIPAM microgel while the parameters φ0 and Ngel are set free for optimization. The resulting parameters are shown in Table 1. After optimization, we obtain a value of φ0 = 0.84. The value of the Ngel for the pD7NIPAM microgel is increased compared to the pNIPAM microgel. This might be explained by the absence of self-crosslinking for the D7NIPAM-based microgel,34 resulting to longer polymer chains between two crosslinking points. For both φ0 and Ngel parameters, similar values are obtained from the FR theory +Δχqc compared to FR theory in the case of pD7NIPAM microgel. The resulting swelling curve of the pD7NIPAM microgel is shown in Fig. 2 and agrees with the measured swelling curve with a coefficient of determination (R2) of 0.96. Based on our results, the VPTT shifts by 4.9 K, similar to the value which is obtained by just using the FR model. In the 20 °C ≤ T ≤ 25 °C and 28 °C ≤ T ≤ 36 °C range, our approach overestimates and underestimates the swelling behavior of the pD7NIPAM microgel.
In Fig. 3, the Flory interaction parameter χ in the case of pNIPAM (straight line) and pD7NIPAM (dashed line) microgels obtained by FR theory is shown, respectively. The Flory parameter of the pD7NIPAM microgel (dashdot line) calculated from eqn (4) and (6) for n = 25 is also shown in Fig. 3. For the temperature range between 20–50 °C, the χNIPAM-H2O of NIPAM-based microgel is always larger than the χD7NIPAM-H2O of D7NIPAM-based microgel with maximum difference (χD7NIPAM-H2O − χNIPAM-H2O) of −0.5 at T = 20 °C. That means that the Gibbs free energy difference is higher in the case of NIPAM-based microgel in H2O favoring the demixing compared to D7NIPAM-based microgel.
4 Conclusions
In this work, we studied experimentally and computationally the deuteration effect on pNIPAM microgels. To do so, pD7NIPAM microgel was used as a case study due to its large VPTT shift compared to pNIPAM microgel.Using quantum mechanical methods, we investigated different NIPAM-(H2O)n complexes with n being the number of H2O molecules and calculated the change in the value of the Flory–Huggins interaction parameter as a consequence of deuteration. We found that a complex of NIPAM with at least 25 H2O molecules is necessary to study the effect of deuteration. For this number of H2O molecules, the isopropyl group of NIPAM is fully hydrated.
The Flory–Rehner theory was used to model the swelling curve of pNIPAM and pD7NIPAM microgels. First, we fit the data of pNIPAM amd pD7NIPAM microgels, leaving all the parameters of the model free to change. In both cases, reasonable fits and values of the fitting parameters in agreement with the literature were obtained. From these fits, we also estimate the shift in the VPTT due to the deuteration is ≈4.3 K. We also note that this shift of the VPTT is consistent with the shift obtained starting from the value of χ for pNIPAM as obtained from the fit and adding the Δχqc we compute. Then, we modeled the swelling behavior of the pD7NIPAM microgel, by fixing the parameters A and Θ to the values found from the fitting of the pNIPAM microgel, and we optimized the parameters Ngel and φ0 based on the experimental results of pD7NIPAM microgel. During the optimization, the resulting change of the Flory–Huggins interaction parameter upon deuteration Δχqc from the ab initio calculations was incorporated in the Flory–Rehner theory. We found that Ngel is larger in the case of pD7NIPAM microgel, which may be due to the suppression of the self-crosslinking due to the use of D7NIPAM as monomer.34 The volume fraction of the polymer φ0 has a smaller value in the case of pD7NIPAM microgel.
In the future, our approach may be applied to the adjustment of effective parameters in molecular dynamics simulations for the study of deuterated microgels.8,39,88 This is will provide insights into properties such as VPTT, swelling ratio, and softness.
Data availability
All optimized molecular geometries and multi-angle dynamic light scattering measurements have been deposited in the RADAR4Chem database under doi (https://doi.org/10.22000/857).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the SFB 985 Functional Microgels and Microgel Systems of Deutsche Forschungsgemeinschaft (DFG, German Research Foundation). Simulations were performed with computing resources granted by RWTH Aachen University under project rwth0739. Molecular graphics in Fig. 1 performed with UCSF Chimera.References
- P. N. Pusey and W. Van Megen, Nature, 1986, 320, 340–342 CrossRef CAS.
- U. Gasser, E. R. Weeks, A. Schofield, P. Pusey and D. Weitz, Science, 2001, 292, 258–262 CrossRef CAS PubMed.
- M. Karg, A. Pich, T. Hellweg, T. Hoare, L. A. Lyon, J. Crassous, D. Suzuki, R. A. Gumerov, S. Schneider and I. I. Potemkin, et al. , Langmuir, 2019, 35, 6231–6255 CrossRef CAS PubMed.
- A. Scotti, M. F. Schulte, C. G. Lopez, J. J. Crassous, S. Bochenek and W. Richtering, Chem. Rev., 2022, 122, 11675–11700 CrossRef CAS PubMed.
- M. Stieger, W. Richtering, J. S. Pedersen and P. Lindner, J. Chem. Phys., 2004, 120, 6197–6206 CrossRef CAS PubMed.
- M. Cors, L. Wiehemeier, Y. Hertle, A. Feoktystov, F. Cousin, T. Hellweg and J. Oberdisse, Langmuir, 2018, 34, 15403–15415 CrossRef CAS PubMed.
- A. Scotti, J. Houston, M. Brugnoni, M. Schmidt, M. Schulte, S. Bochenek, R. Schweins, A. Feoktystov, A. Radulescu and W. Richtering, Phys. Rev. E, 2020, 102, 052602 CrossRef CAS PubMed.
- N. Gnan, L. Rovigatti, M. Bergman and E. Zaccarelli, Macromolecules, 2017, 50, 8777–8786 CrossRef CAS PubMed.
- A. Scotti, Soft Matter, 2021, 17, 5548–5559 RSC.
- G. M. Conley, P. Aebischer, S. Nöjd, P. Schurtenberger and F. Scheffold, Sci. Adv., 2017, 3, e1700969 CrossRef PubMed.
- G. M. Conley, C. Zhang, P. Aebischer, J. L. Harden and F. Scheffold, Nat. Commun., 2019, 10, 2436 CrossRef PubMed.
- A. Scotti, M. Pelaez-Fernandez, U. Gasser and A. Fernandez-Nieves, Phys. Rev. E, 2021, 103, 012609 CrossRef CAS PubMed.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Chem., 1968, 2, 1441–1455 CAS.
- R. Pelton and P. Chibante, Colloids Surf., 1986, 20, 247–256 CrossRef CAS.
- D. Menne, F. Pitsch, J. E. Wong, A. Pich and M. Wessling, Angew. Chem., Int. Ed., 2014, 53, 5706–5710 CrossRef CAS PubMed.
- W. Richtering, Langmuir, 2012, 28, 17218–17229 CrossRef CAS PubMed.
- F. A. Plamper and W. Richtering, Acc. Chem. Res., 2017, 50, 131–140 CrossRef CAS PubMed.
- J. Brijitta and P. Schurtenberger, Curr. Opin. Colloid Interface Sci., 2019, 40, 87–103 CrossRef CAS.
- H. Senff and W. Richtering, J. Chem. Phys., 1999, 111, 1705–1711 CrossRef CAS.
- J. Mattsson, H. M. Wyss, A. Fernandez-Nieves, K. Miyazaki, Z. Hu, D. R. Reichman and D. A. Weitz, Nature, 2009, 462, 83–86 CrossRef CAS PubMed.
- M. Deloney, K. Smart, B. A. Christiansen and A. Panitch, J. Controlled Release, 2020, 323, 47–58 CrossRef CAS PubMed.
- W. Richtering, I. Alberg and R. Zentel, Small, 2020, 16, 2002162 CrossRef CAS PubMed.
- E. Miceli, B. Kuropka, C. Rosenauer, E. R. Osorio Blanco, L. E. Theune, M. Kar, C. Weise, S. Morsbach, C. Freund and M. Calderón, Nanomedicine, 2018, 13, 2657–2668 CrossRef CAS PubMed.
- A. Scotti, A. R. Denton, M. Brugnoni, J. E. Houston, R. Schweins, I. I. Potemkin and W. Richtering, Macromolecules, 2019, 52, 3995–4007 CrossRef CAS.
- U. Gasser, J. Hyatt, J.-J. Lietor-Santos, E. Herman, L. A. Lyon and A. Fernandez-Nieves, J. Chem. Phys., 2014, 141, 034901 CrossRef CAS PubMed.
- P. S. Mohanty, S. Nöjd, K. v. Gruijthuijsen, J. J. Crassous, M. Obiols-Rabasa, R. Schweins, A. Stradner and P. Schurtenberger, Sci. Rep., 2017, 7, 1487 CrossRef PubMed.
- S. Nöjd, P. Holmqvist, N. Boon, M. Obiols-Rabasa, P. S. Mohanty, R. Schweins and P. Schurtenberger, Soft Matter, 2018, 14, 4150–4159 RSC.
- M. Cors, L. Wiehemeier, O. Wrede, A. Feoktystov, F. Cousin, T. Hellweg and J. Oberdisse, Soft Matter, 2020, 16, 1922–1930 RSC.
- J. E. Houston, L. Fruhner, A. de la Cotte, J. Rojo González, A. V. Petrunin, U. Gasser, R. Schweins, J. Allgaier, W. Richtering and A. Fernandez-Nieves, et al. , Sci. Adv., 2022, 8, eabn6129 CrossRef CAS PubMed.
- F. S. Varley, Neutron News, 1992, 3, 29–37 Search PubMed.
- A. Scotti, U. Gasser, B. Zhou, A. Arenas-Gullo, A. de la Cotte, J. R. González and A. Fernandez-Nieves, in Compressible Microgels in Concentrated Suspensions: Phase Behavior, Flow Properties, and Scattering Techniques to Probe Their Structure and Dynamics, John Wiley & Sons, Ltd, 2022, ch. 6, pp. 203–240 Search PubMed.
- M. Brugnoni, A. Scotti, A. A. Rudov, A. P. Gelissen, T. Caumanns, A. Radulescu, T. Eckert, A. Pich, I. I. Potemkin and W. Richtering, Macromolecules, 2018, 51, 2662–2671 CrossRef CAS.
- A. J. Schmid, J. Dubbert, A. A. Rudov, J. S. Pedersen, P. Lindner, M. Karg, I. I. Potemkin and W. Richtering, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- M. Brugnoni, A. C. Nickel, L. C. Kröger, A. Scotti, A. Pich, K. Leonhard and W. Richtering, Polym. Chem., 2019, 10, 2397–2405 RSC.
- T. Widmann, L. P. Kreuzer, N. Hohn, L. Bießmann, K. Wang, S. Rinner, J.-F. Moulin, A. J. Schmid, Y. Hannappel and O. Wrede, et al. , Langmuir, 2019, 35, 16341–16352 CrossRef CAS PubMed.
- H. Shirota, N. Kuwabara, K. Ohkawa and K. Horie, J. Phys. Chem. B, 1999, 103, 10400–10408 CrossRef CAS.
- M. Cors, L. Wiehemeier, J. Oberdisse and T. Hellweg, Polymers, 2019, 11(4), 620 CrossRef PubMed.
- M. Urich and A. R. Denton, Soft Matter, 2016, 12, 9086–9094 RSC.
- N. Boon and P. Schurtenberger, Phys. Chem. Chem. Phys., 2017, 19, 23740–23746 RSC.
- T. J. Weyer and A. R. Denton, Soft Matter, 2018, 14, 4530–4540 RSC.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, 1953 Search PubMed.
- P. J. Flory and J. Rehner, J. Chem. Phys., 1943, 11, 512–520 CrossRef CAS.
- P. J. Flory and J. Rehner, J. Chem. Phys., 1943, 11, 521–526 CrossRef CAS.
- L. D. Landau and E. M. Lifshitz, Theory of Elasticity, Elsevier, Amsterdam, 3rd edn, 1986 Search PubMed.
- J.-J. Lietor-Santos, B. Sierra-Martin, R. Vavrin, Z. Hu, U. Gasser and A. Fernandez-Nieves, Macromolecules, 2009, 42, 6225–6230 CrossRef CAS.
- S. Friesen, Y. Hannappel, S. Kakorin and T. Hellweg, Colloid Polym. Sci., 2022, 300, 1235–1245 CrossRef CAS.
- P. Voudouris, D. Florea, P. van der Schoot and H. M. Wyss, Soft Matter, 2013, 9, 7158–7166 RSC.
- C. G. Lopez and W. Richtering, Soft Matter, 2017, 13, 8271–8280 RSC.
- F. A. L. Janssen, M. Kather, L. C. Kröger, A. Mhamdi, K. Leonhard, A. Pich and A. Mitsos, Ind. Eng. Chem. Res., 2017, 56, 14545–14556 CrossRef CAS.
- F. A. Janssen, A. Ksiazkiewicz, M. Kather, L. C. Kröger, A. Mhamdi, K. Leonhard, A. Pich and A. Mitsos, 28th European Symposium on Computer Aided Process Engineering, Elsevier, 2018, vol. 43, pp. 109–114.
- F. Jung, F. A. L. Janssen, A. Ksiazkiewicz, A. Caspari, A. Mhamdi, A. Pich and A. Mitsos, Ind. Eng. Chem. Res., 2019, 58, 13675–13685 CrossRef CAS.
- S. Schneider, F. Jung, O. Mergel, J. Lammertz, A. C. Nickel, T. Caumanns, A. Mhamdi, J. Mayer, A. Mitsos and F. A. Plamper, Polym. Chem., 2020, 11, 315–325 RSC.
- L. C. Kröger, W. A. Kopp and K. Leonhard, J. Phys. Chem. B, 2017, 121, 2887–2895 CrossRef PubMed.
- P. Deglmann, I. Müller, F. Becker, A. Schäfer, K.-D. Hungenberg and H. Weiss, Macromol. React. Eng., 2009, 3, 496–515 CrossRef CAS.
- M. L. Coote, Macromol. Theory Simul., 2009, 18, 388–400 CrossRef CAS.
- T. Nevolianis, N. Wolter, L. F. Kaven, L. Krep, C. Huang, A. Mhamdi, A. Mitsos, A. Pich and K. Leonhard, Ind. Eng. Chem. Res., 2023, 62, 893–902 CrossRef CAS.
- M. Ceriotti and T. E. Markland, J. Chem. Phys., 2013, 138, 014112 CrossRef PubMed.
- M. Gómez-Gallego and M. A. Sierra, Chem. Rev., 2011, 111, 4857–4963 CrossRef PubMed.
- K. Świderek and P. Paneth, Chem. Rev., 2013, 113, 7851–7879 CrossRef PubMed.
- K. Karandashev, Z.-H. Xu, M. Meuwly, J. Vaníček and J. O. Richardson, Struct. Dyn., 2017, 4, 061501 CrossRef PubMed.
- J. S. Mugridge, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2012, 134, 2057–2066 CrossRef CAS PubMed.
- C. L. Perrin and P. Karri, J. Am. Chem. Soc., 2010, 132, 12145–12149 CrossRef CAS PubMed.
- A. Scotti, U. Gasser, E. S. Herman, M. Pelaez-Fernandez, J. Han, A. Menzel, L. A. Lyon and A. Fernández-Nieves, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 5576–5581 CrossRef CAS PubMed.
- S. Fujishige, K. Kubota and I. Ando, J. Phys. Chem., 1989, 93, 3311–3313 CrossRef CAS.
- M. Andersson and S. L. Maunu, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 3305–3314 CrossRef CAS.
- D. E. Koppel, J. Chem. Phys., 1972, 57, 4814–4820 CrossRef CAS.
- W. Burchard and W. Richtering, Prog. Colloid Polym. Sci., 1989, 80, 151–163 CAS.
- A. e. Scotti, W. Liu, J. Hyatt, E. Herman, H. Choi, J. Kim, L. Lyon, U. Gasser and A. Fernandez-Nieves, J. Chem. Phys., 2015, 142, 234905 CrossRef CAS PubMed.
- S. Friesen, Y. Hannappel, S. Kakorin and T. Hellweg, Gels, 2021, 7(2), 42 CrossRef CAS PubMed.
- B. Erman and P. J. Flory, Macromolecules, 1986, 19, 2342–2353 CrossRef CAS.
- P. J. Flory and J. Rehner, J. Chem. Phys., 1943, 11, 512–520 CrossRef CAS.
- J. Mugridge, R. Bergman and K. Raymond, Angew. Chem., Int. Ed., 2010, 49, 3635–3637 CrossRef CAS PubMed.
- P. Pracht, F. Bohle and S. Grimme, Phys. Chem. Chem. Phys., 2020, 22, 7169–7192 RSC.
- P. Stephens, F. Devlin, C. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- J. A. Montgomery, M. J. Frisch, J. W. Ochterski and G. A. Petersson, J. Chem. Phys., 1999, 110, 2822–2827 CrossRef CAS.
- L. A. Curtiss, P. C. Redfern, K. Raghavachari and J. A. Pople, J. Chem. Phys., 2001, 114, 108–117 CrossRef CAS.
- J. Zheng, Y. Zhao and D. G. Truhlar, J. Chem. Theory Comput., 2009, 5, 808–821 CrossRef CAS PubMed.
- J. P. Merrick, D. Moran and L. Radom, J. Phys. Chem. A, 2007, 111, 11683–11700 CrossRef CAS PubMed.
- Y. Hertle, M. Zeiser, C. Hasenöhrl, P. Busch and T. Hellweg, Colloid Polym. Sci., 2010, 288, 1047–1059 CrossRef CAS.
- V. Nigro, R. Angelini, M. Bertoldo and B. Ruzicka, Colloids Surf., A, 2017, 532, 389–396 CrossRef CAS.
- T. López-León and A. Fernández-Nieves, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 011801 CrossRef PubMed.
- M. F. Schulte, S. Bochenek, M. Brugnoni, A. Scotti, A. Mourran and W. Richtering, Angew. Chem., Int. Ed., 2021, 60, 2280–2287 CrossRef CAS PubMed.
- G. Romeo, L. Imperiali, J.-W. Kim, A. Fernández-Nieves and D. A. Weitz, J. Chem. Phys., 2012, 136, 124905 CrossRef PubMed.
- M. Reufer, P. Dıaz-Leyva, I. Lynch and F. Scheffold, Eur. Phys. J. E: Soft Matter Biol. Phys., 2009, 28, 165–171 CrossRef CAS PubMed.
- A. K. Lele, M. M. Hirve, M. V. Badiger and R. A. Mashelkar, Macromolecules, 1997, 30, 157–159 CrossRef CAS.
- S. V. Nikolov, A. Fernandez-Nieves and A. Alexeev, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 27096–27103 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01511k |
| This journal is © The Royal Society of Chemistry 2023 |