Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The sustainability impact of Nobel Prize Chemistry: life cycle assessment of C–C cross-coupling reactions†
Jose Luis
Osorio-Tejada
 ad,
Francesco
Ferlin
ad,
Francesco
Ferlin
 b,
Luigi
Vaccaro
b,
Luigi
Vaccaro
 b and
Volker
Hessel
b and
Volker
Hessel
 *ac
*ac
aDepartment of Chemical Engineering, School of Engineering, University of Warwick, Coventry, UK
bLaboratory of Green S.O.C. – Dipartimento di Chimica, biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, 06123 – Perugia, Italy. E-mail: luigi.vaccaro@unipg.it
cSchool of Chemical Engineering, University of Adelaide, Adelaide, Australia. E-mail: volker.hessel@adelaide.edu.au
dFaculty of Environmental Sciences, Universidad Tecnológica de Pereira, Pereira, Colombia
First published on 17th October 2023
Abstract
Carbon-to-carbon (C–C) cross-coupling reaction (CCR) protocols represent a major breakthrough in synthetic chemistry. These innovative CCRs are widely applied in medicinal chemistry to produce a multitude of drugs, as well as in many other applied sciences. Despite strong efforts to implement green chemistry principles in synthetic chemistry, only individual outcomes for (many) single reactions with single synthesis protocols have been reported, and a comprehensive view of the environmental value of major chemical breakthroughs is lacking in the literature. This study concerns the life cycle assessment (LCA) of CCRs, which were awarded the Nobel Prize in 2010. The required materials were obtained from datasets based on industrial data, while the analysed compounds and target products from C–C coupling reaction protocols were modelled based on laboratory-scale experimental data (the only data available), and the use of electrical power was assumed. We discovered that it is essential to separate intrinsic and extrinsic impacts to allow focus on delivering a generic sustainability view. The former are the genuine generalizable innovations of the novel chemical process method, and are worth improving via LCA. The latter are outcomes of individual laboratory chemical protocols, which are aimed at achieving chemical innovation but typically not at holistically optimising the environmental profile, with a limited scope for LCA prediction. In terms of the intrinsic value of the Nobel Prize CCRs, we determined the environmental profile for variations of the (1) CCR catalyst, (2) CCR halobenzene, and (3) CCR ligand, all of which are constitutional innovations of the chemical protocols of the 2010 Nobel Prize in Chemistry. The findings indicate that (1) the catalyst should be recycled, as its footprint is far too dominating; (2) the halobenzene should be chosen carefully to balance its intrinsic (‘backpack’) and extrinsic (yield, catalyst choice) impacts; and (3) the ligand should be recycled in the context of the catalyst recycling. As demonstrated herein, the value of LCA is to provide predictions for future improvements (‘anticipatory or ex-ante LCA’) and to assure the best leverage of environmental gains that can readily be achieved for major chemical innovations, as exemplified by the 2010 Nobel Prize in Chemistry.
1. Introduction
Cross-coupling reactions (CCR) are among the most important reactions for the synthesis of building blocks, and their great significance led to them being awarded the Nobel Prize in 2010.1 Their major advantages are providing access to structural diversity2,3 to give molecules equipped with a number of functional groups, and selectivity, including enantiomeric excess when creating chiral molecules. The major challenge is always reactivity, which strongly depends on the combination the catalyst, aryl halide (I > Br > Cl), and possibly, the ligand. Variations of these three fundamental features have created an enormous amount of literature, which can be classified into ‘three waves’ of innovations: a first (catalyst), second (halide), and third (ligand) wave.4 Since their discovery, CCR strategies have been therefore improved in terms of reactivity along the lines of the research innovation ‘waves’.CCR reactions have also undoubtedly contributed to the health of mankind. Aromatic groups are by far the most essential pharmacophores for medicinal chemistry and drug development. Most recent phase III and marketed pharmaceuticals contain at least one aryl or heteroaryl group. These functionalities, especially phenyls, are incorporated into pre-assembled active pharmaceutical ingredients (APIs).5 Recent advances in metal-catalysed CCR have greatly facilitated the versatility of incorporating aryls for medicinal chemistry. However, most of these reactions rely on the availability of aryl halides.
It is unsurprising but remarkable that CCR innovations have readily reached industrial translation4 with many relevant examples worldwide. The CCR synthesis of the pharmaceutical drug Losartan has been conducted by BMS Company (originally Dupont–Merck);6 the Merck Singulair process for the synthesis of Montelukast is based on a Heck coupling involving isomerization of an allylic alcohol;7,8 the total synthesis of discodermolide was achieved a Negishi and Suzuki coupling;9–13 large-scale Corriu–Kumada and Negishi couplings yielded PDE472, a potential drug for the treatment of asthma;14 the production of a hepatitis C polymerase inhibitor by Pfizer Company incorporates a Heck reaction carried out on a 40 kg scale;15 the use of a Suzuki–Miyaura coupling was reported for the synthesis of Crizotinib, a potent anti-cancer agent, on a similar industrial scale (50 kg).16
As mentioned, CCR innovations have been made in three steps, termed ‘waves’. Step 1 is catalysts, step 2 is halobenzenes, and step 3 is ligands (for the catalysts). As shown in the first wave, these innovations may lead to co-innovation. Better catalysts allow the use of better reagents, giving better reactions. Step 1 caused a change from ‘hard’ Li and MgBr towards ‘soft’ Al and Zn-based reactive catalysts, which have better compatibility with functional groups. The second wave innovations involving the coupling partners are expected to have a major impact. Better reactivity of halobenzenes in CCRs improves atom economy by reducing reaction times, temperatures, and material usage (reagents, bases, catalyst, and solvents). Additionally, the safety issues associated with the halobenzene must be considered, for example, replacing toxic and flammable halobenzenes with safer reagents. In the third wave, the findings in the CCR literature revealed that the ligands are as important as the metals. The steric and electronic properties of the CCR ligands, in particular phosphines, serve to provide a well-defined steric volume to foster CCR reactions. Therefore, ligands have been included in this evaluation, despite their life cycle assessment (LCA) being challenging.
Reports about improving the sustainability of CCR, as a whole, are largely missing in literature, with only a few contributions, mainly regarding the possibility of using safer reaction media or heterogeneous catalysts.5,17,18 CCR are indeed still largely defined by (homogeneous) catalysts and their ligands. As homogeneous catalysts are typically complex molecules, they are associated with large ecological backpack due to the required multi-step synthesis. Their cost, the huge chemical and environmental impact of their manufacture, their leaching, and the consequent cleaning of the products are concerning. Approximately only half the metals that enter wastewater are retained in the sludge of wastewater treatment plants; the rest is released in the effluent into rivers, mainly generating freshwater ecotoxicity and human health impacts.19
Halobenzenes should also have similarly great environmental impacts, as they are mostly produced from fossil resources. Moreover, the “halo” atoms are released into the reaction mixture in different ways, increasing their environmental complexity. The same is true for ligands, but their LCA impact is more difficult to quantify, as they are complex molecules with great individual differences and the documentation needed to determine their sustainability is lacking in the literature. The conclusion is that there is a gap requiring a comprehensive study based on LCA methodology to evaluate the environmental profiles of CCRs in terms of the materials involved and their energy consumption.20,21
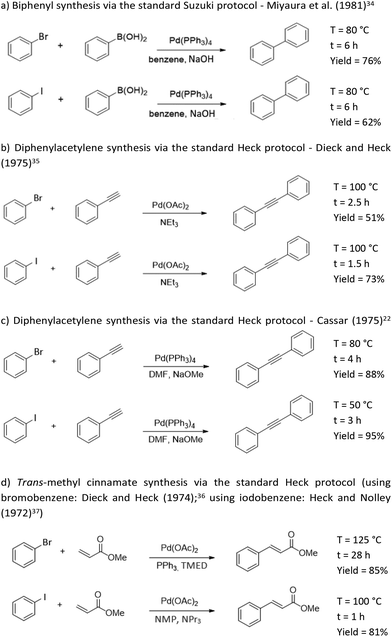
Environmental assessments are complex (multi-criteria) in nature and require a balanced viewpoint to rate innovations in chemical processes. To exemplify the demand for a holistic view of innovations relevant to CCRs, the Cassar (1975)22 experiments to synthesize diphenylacetylene demonstrated that using iodobenzene as halide instead of bromobenzene improved the yield (from 88% to 95%) and the reaction conditions (from 4 h at 80 °C to 3 h at 50 °C). However, as the present study has the aim of evaluating the overall environmental parameters behind these mere experimental observations, we report here the environmental assessment of upstream and downstream processing for CCRs, which are decisive in terms of the overall impact.
From the viewpoint of comprehensive analysis, LCA is valuable in the early stages of product and process design to pinpoint ‘hot spots’ that contribute to impacts on humans and the environment.23 LCA also helps to identify burden-shifting, in which products claiming to be ‘green’ or ‘sustainable’ based on a single indicator, such as greenhouse gas emissions or toxicity, may actually have greater impacts than conventional alternatives in terms of other environmental issues. This is where LCA serves as a crucial tool for comparing products and processes, characterizing multiple environmental impact categories, and preventing ‘greenwashing’ and unsupported sustainability claims.23
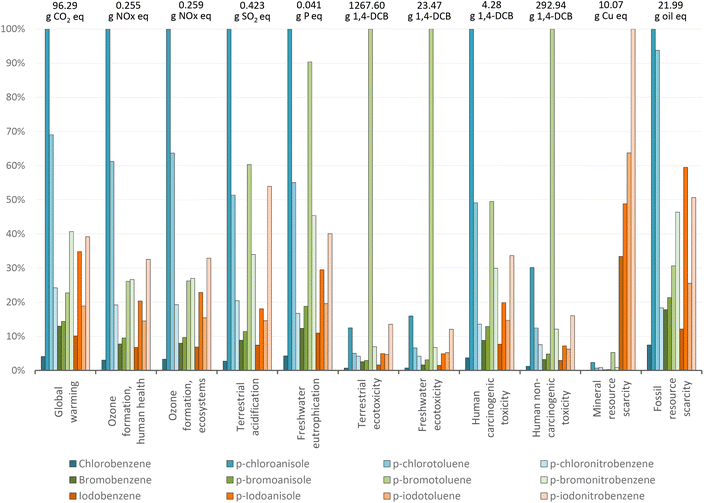
To cover the upstream processing issue of halobenzene complexity, we report LCA of the production of different starting materials based on chlorine (chlorobenzene, 4-chloroanisole, 4-chlorotoluene, and 4-chloronitrobenzene), bromine (bromobenzene, 4-bromoanisole, 4-bromotoluene, and 4-bromonitrobenzene) and iodine (iodobenzene, 4-iodoanisole, 4-iodotoluene, and 4-iodonitrobenzene). Moreover, we perform LCA for the production of catalysts and ancillary materials involved in selected CCRs. Finally, we perform LCA of standard Suzuki and Heck protocols to provide a comprehensive view on all innovations to assess the context of real-life innovations. This can only be done with reference to specific chemical products, such as biphenyl, diphenylacetylene, and trans-methyl cinnamate, which determines the range of predictability of our assessment. It is worth mentioning that although the Negishi coupling plays a central role in the 2010 Nobel Prize CCRs, in this study we have decided to exclude this reaction for the following reasons: (1) the overall sustainability as well as the endpoint score analysis of the Negishi coupling is profoundly influenced by the stoichiometric use of arylzinc. These molecular entities add an extra parameter that must be considered for the inventories, and therefore will not allow for a linear comparison with the other methodologies for the CCR discussed herein. (2) The Negishi coupling has historically been used to smoothly couple arylzinc with alkylhalides in a stereospecific manner, and its utilization for the synthesis of biaryls has only been reported to prove the scope of the process; therefore, its discussion could be misleading for the actual aim of the present work.
This manuscript compensates for the above-mentioned limitations, with the need to be specific in terms of products and reaction ingredients, by presenting the LCA assessment in a narrative along the lines of the main 2010 Nobel Prize in Chemistry innovations, which are classified as the first through third waves. In this way, the focus is kept on the intrinsic value rather than all the individual process variations (extrinsic). This evaluation demonstrates its usefulness when hypothesising improvements for one of the major chemical innovations of a century.
2. Methods
This study was performed by following the phases for LCA described by the ISO 14044 standard.20 Firstly, for the goal and scope definition, the specific chemical compounds and processes, as well as the typology of the study, are described. For the inventory analysis phase, the procedures, methods, and assumptions for the elaboration of the materials and energy balances are detailed. The phases of the impact assessments and the interpretation of the results are described at the end of this section.2.1. Goal and scope definition
Different experimental procedures for the preparation of common aryl halides and palladium-based catalysts used in standard protocols for CCRs were analysed to evaluate their environmental profiles. From these results, their role in the overall environmental impact of the selected compounds was analysed through a comparative assessment of the CCR processes using different starting aryl halides to obtain the same compound under similar experimental conditions. The functional unit was 1 g of the target compound. A cut-off system model based on mass allocation and a cradle-to-gate scope were defined. The study considered the impacts of the extraction, production, and transportation of all materials and energy, and the emissions of the procedure itself, but excluded the impacts of the manufacture of tools and lab equipment.Both palladium(II) acetate (formula Pd(O2CCH3)2, often abbreviated Pd(OAc)2) and palladium tetrakis (or tetrakis(triphenylphosphine)palladium(0)), formula Pd(P(C6H5)3)4, often abbreviated Pd(PPh3)4 are used in CCRs as homogeneous catalysts in powder form. The experimental procedures for the preparation of palladium(II) acetate were based on Heck (1985) and Zang et al. (2017)24,25 and those for palladium tetrakis were based on Cotton (1972).26
| Halobenzene | Preparation process | Ref. |
|---|---|---|
| Chlorobenzene | Benzene chlorination | 27 |
| 4-Chloroanisole | Anisole chlorination | 28 |
| 4-Chlorotoluene | Toluene chlorination | 29 |
| 4-Chloronitrobenzene | Chlorobenzene nitration | 30 and 31 |
| Bromobenzene | Benzene bromination | 32 |
| 4-Bromoanisole | Anisole bromination | 33 |
| 4-Bromotoluene | 4-Toluidine bromination | 32 |
| 4-Bromonitrobenzene | Bromobenzene nitration | 32 |
| Iodobenzene | Benzene iodination | 32 |
| 4-Iodoanisole | Anisole iodination | 33 |
| 4-Iodotoluene | 4-Toluidine iodination | 32 |
| 4-Iodonitrobenzene | Nitroaniline iodination | 32 |
2.2. Inventory analysis
Material and energy balances were developed based on experimental data from the literature by considering the most common or conventional method to produce the selected compounds. The data sources are referenced in the previous section next to each compound. Given that only the quantities of input materials used in the reactions are detailed, various assumptions and calculations were applied to obtain the energy inputs and the outputs (air and water emissions) of the synthesis and the wastewater processes.Inventory datasets for the upstream processes to produce the input materials and energy were based on the life cycle inventory database Ecoinvent 3.8,38 using mostly datasets for the European market. Inventory datasets for the preparation of target compounds were analysed under lab conditions. Therefore, energy requirements were thermodynamically estimated and adjusted according to the efficiency of electric lab devices for heating and stirring based on our own measurements.39 Waste heat is also included in the inventory as a function of the electricity consumed in the process, which is released to the air with a rate of 3.6 MJ per kWh.40 This is calculated based on the gross calorific value of energy carriers. Datasets in Ecoinvent for final energy supply, e.g., ‘heat, light fuel oil, at industrial boiler’ or ‘light fuel oil, burned in industrial boiler’, already include waste heat emissions. However, for electricity consumption, the corresponding waste heat must be recorded in the process that utilizes the electricity.41 Moreover, 0.2% of the volatile materials were estimated as air emissions.42 Cooling requirements were calculated by considering an electric chiller consumption of 0.046 kWh per MJ of cooling energy.43 Since most of the synthesis reactions are multi-output processes, all the inputs required during the whole process were proportionally allocated according to the mass of the resulting valuable co-products. The final separation steps for the analysed products via CCR were not considered because no detailed quantities of materials or procedures were reported. These steps mostly consist of simple processes such as filtering, washing, drying, or crystallization. These processes are generally not energy-intensive, except for drying (solvent evaporation), which was addressed by including a distillation step.
Regarding downstream processes, we modelled the impacts of the wastes generated after experiments in labs. Except for solvents, the remaining mixtures of unreacted reagents, bases, ligands, and homogeneous catalysts are disposed of into the lab sinks. This is because the recovery of these compounds is complex and usually more expensive than disposal and purchase of new materials, while the recovery of solvents via distillation is straightforward. An average solvent recovery rate (SRR) of 71%44 for the different solvents was considered. This SSR was defined because the specific figures for most of the solvents were unknown. The energy required for the distillation of solvents was also thermodynamically estimated and included in the process electricity consumption, as it is usually performed in modern labs using fully electricity-powered devices. The emissions generated due to the wastes (including the unrecovered fraction of solvents) that reach sewage were estimated based on transfer coefficients for each organic and inorganic compound in wastewater treatment plants (WWTP). A removal efficiency of 90% for organic matter was assumed; this matter is mostly released later in the form of CO2 to the air.40 The remaining 10% of the organic compounds are released in their original form in the effluent. It is important to note that of the total removed organic matter, approximately 71.1% is converted into CO2 in the WWTP (27.1% during aerobic treatment and 43.9% during sludge digestion). The remaining 28.9% is retained in the sludge and mainly transformed into CO2 at a later stage, with the extent of transformation varying depending on whether the sludge is used for soil improvement or incinerated.19 Assuming complete incineration, minor fractions of the carbon contained in the sludge (<2%)45 can be emitted as CH4, CO, and NMVOCs, and another fraction contained in the slag residue (0.76%),45 which goes to landfills, can be transferred to groundwater. However, these minor emissions were neglected in the analysis, in line with the Ecoinvent report for the life cycle inventories of chemicals,40 in which the removed organic matter is assumed to be entirely emitted as CO2. The values for the chemical oxygen demand (COD) were calculated from the amount of organic compound in the treated wastewater assuming a carbon conversion of 96% for COD.46 The COD was obtained from the general equation CxHyOz + (1/4)(4x + 4 − 2z)O2 = xCO2 + (y/2)H2O; then, per gram of CxHyOz, the theoretical COD = 8(4x + y − 2z)/(12x + y + 16z) [g]. Similarly, the values for total organic carbon (TOC) per gram of CxHyOz were estimated using the equation TOC = 12x/(12x + y + 16z) [g]. The values for biochemical oxygen demand (BOD) and dissolved organic carbon (DOC) were calculated by assuming the worst-case scenario in which BOD = COD and TOC = DOC, as used in the Ecoinvent report.40 The transfer coefficients of inorganic compounds to effluent were based on elimination rates for each chemical.19,45,47,48 The transport of the resulting sludge was not included in the datasets, because its contribution to the total environmental impacts was negligible.39
For the reagents and other supplies required to prepare the analysed products in this study, i.e., chemicals sourced from the market to labs, that were not available in the Ecoinvent database, their inventories were created based on specific publications, such as articles and patents (referenced in the ESI-1†). The quantities of precursors utilized and yields from these publications were considered. However, for the energy consumption, generic data to produce organic chemicals were utilized, and transport averages were used to emulate the production of these chemicals at industrial scales. A total of 2 kJ of steam and 0.333 W h of electricity per gram of compound was considered.49,50 For the transport of materials to the factory, average transport in Europe by lorry and train for distances of 100 km and 600 km were considered.42,51
Regarding the impacts of the facilities, since all the inventory datasets of chemicals taken from Ecoinvent consider the impacts of the plant construction process, we also included this process for the analysed compounds to maintain consistency in the graphs and impact assessments. For this process, a generic chemicals factory with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonne per year production capacity and 50 years of useful life was considered for all the prepared products.
000 tonne per year production capacity and 50 years of useful life was considered for all the prepared products.
In brief, the data for the materials used to produce the reagents and ancillary compounds were obtained from LCI databases, which are derived from industrial data. For materials not available in such databases, we relied on data from publications and general assumptions to emulate their industrial production. On the other hand, due to limited data, the main compounds and target products from the CCR protocols were modelled at lab scale to allow for the comparison of the different processes under similar experimental conditions.
The prepared inventories for the analysed catalysts, halobenzenes and the products obtained via standard CCR protocols, as well as for precursor compounds not available in the database, such as palladium chloride and triphenylphosphine for catalysts; anisole, t-butyl hypochlorite, copper(I) bromide, 4-toluidine, potassium iodide, 4-nitroaniline, and 4-nitroacetanilide for halobenzenes; and phenylboronic acid, tetramethylethylenediamine, tripropylamine, tributylamine, phenylacetylene, and styrene dibromide for the CCR, are detailed in the ESI-1.† Details of the impacts assessment method and interpretation phase are described in the ESI-2.†
3. Results and discussion
3.1. Impact of palladium catalyst – ‘first wave’ of innovation
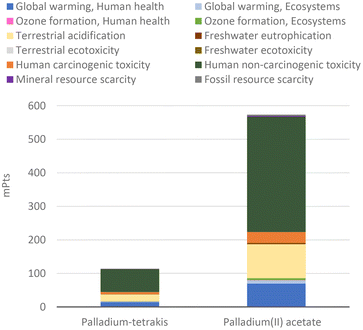
The ‘first wave’ of innovation for C–C couplings was concerned with improving the catalyst. We compared two of the initial ‘pioneering’ catalysts as a constitutional part of the chemical protocol of the Nobel Prize innovations, which are palladium(II) acetate and palladium tetrakis.Per gram of catalyst, the production of palladium(II) acetate shows five times greater environmental impact than the preparation of palladium tetrakis (Fig. 1). This is because the incorporation of triphenylphosphine in the catalyst means that palladium tetrakis contains much less metal than palladium(II) acetate, with ca. 0.10 g Pd vs. 0.52 g Pd per gram of catalyst, respectively. This strategy proportionally reduced the environmental impacts per unit mass of catalyst because the impacts of triphenylphosphine and the additional materials required in the production of palladium tetrakis are insignificant compared to those of palladium (see Fig. 2).
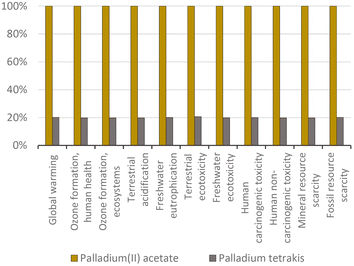 | ||
| Fig. 1 Palladium(II) acetate vs. palladium tetrakis: proportional comparison against the highest value per impact category (normalized to 100%); ReCiPe 2016 midpoints. | ||
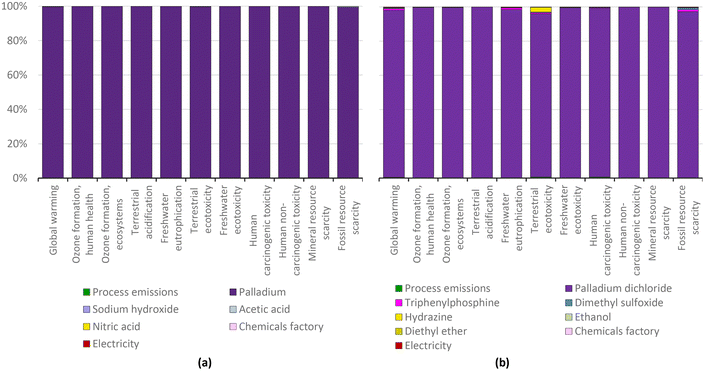 | ||
| Fig. 2 Palladium acetate (a) and palladium tetrakis (b) production: characterization shares; ReCiPe 2016 midpoints. | ||
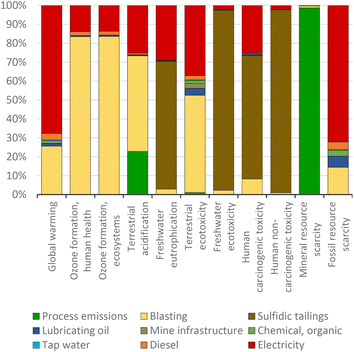
Palladium is extracted globally from various mines, most of which are located in Russia (45%) and South Africa (41%).38 The palladium extracted from mines from South Africa, along with other metals including platinum, contributes most of the environmental impact due to the blasting activities and sulfidic tailings (see Fig. 3), which results in a severe impact on freshwater ecotoxicity and human health toxicity. This is particularly concerning due to the increased probability of humans suffering from cancer or non-cancer diseases caused by inhalation or ingestion of pollutants in the environment.52
The large environmental impact of the catalyst is a matter of concern. The obvious solution of reducing the noble metal content is not necessarily effective. In terms of the aggregate environmental profile, the most severe impacts of these catalysts are on human toxicity and terrestrial acidification, as shown in Fig. 4.
In general terms, it can be also seen that the ecological backpack of palladium causes these catalysts to have much greater impacts than the analysed aryl halides per mass unit (see next section). However, it is important to note that the quantity of catalysts consumed in CCRs is much lower than the quantity of reagents. For this reason, the actual contribution of catalysts in CCRs is analysed at the end in section 3.4.
3.2. Impact of the halobenzene coupling partner – ‘second wave’ of innovation
The ‘second wave’ considered the choice of coupling partner for the C–C cross couplings. We investigated the role of the halobenzene, as the initial ‘pioneering’ innovation and a constitutional part of the chemical protocol for CCRs. In this context, the choice is among the chloro-, bromo- or iodo-derivative of a given aromatic core as the coupling agent.The motivation is to determine the environmental backpack stemming from the different processes used to generate these halobenzenes. This documentation is missing in the literature, despite the fact that mono-halobenzenes are ‘platform chemicals’ for major chemical transformations, including the Nobel Prize reactions considered in this manuscript and the Appel and Wittig reactions,53,54 and are also commonly investigated in emission analyses.
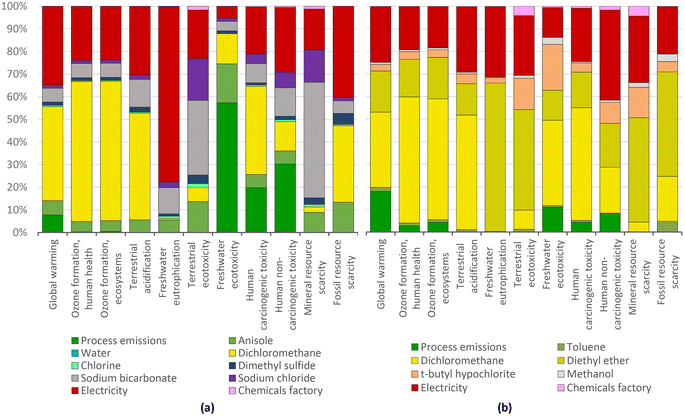 | ||
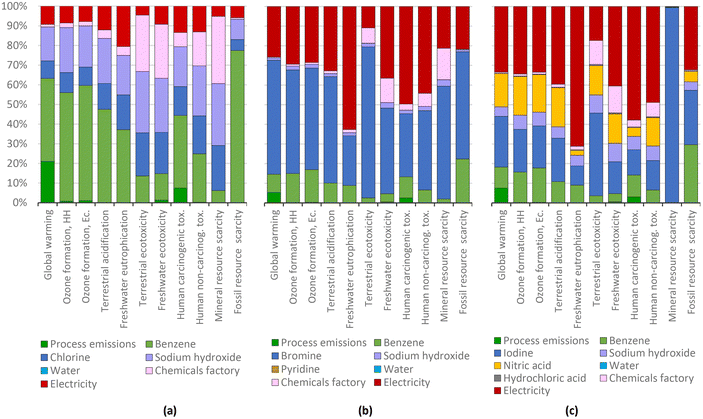
| Fig. 6 4-Chloroanisole (a) and 4-chlorotoluene (b) production: characterization shares; ReCiPe 2016 midpoints. | ||
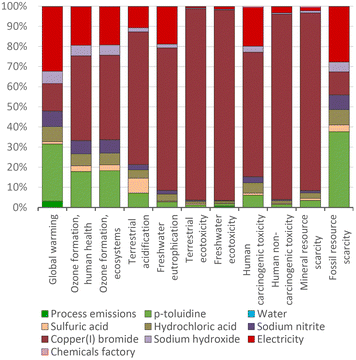
In contrast to the dominating impact of the solvents in the production of the halobenzenes, the dominant environmental impact in the manufacture of 4-bromotoluene arises from the starting material copper(I) bromide being unrecovered. In the three LCA categories of terrestrial ecotoxicity, freshwater ecotoxicity and human non-carcinogenic toxicity, the impact is up to 100 times higher than that for chlorobenzene. This is caused by the copper extraction for the manufacture of copper(I) bromide (Fig. 7).
The LCA impact of the manufacturing of the halogens has been determined (Fig. 5), as they are important as reactants for the halobenzenes. A relevant aspect is that iodine-based compounds have higher impacts than chloride- and bromide-based compounds in the category of mineral resource scarcity, because iodine is mainly extracted from ores. This can be seen in Fig. 8 in the comparison of the LCA impacts of the starting materials, in which chlorine and bromine do not have contributions due to mineral resource scarcity.
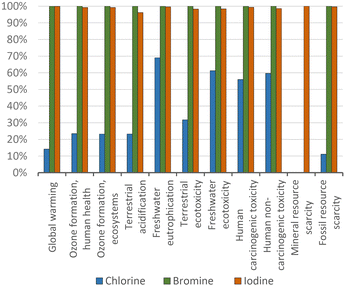 | ||
| Fig. 8 Chlorine, bromine, and iodine production: proportional comparison against the highest value per impact category (normalized to 100%); ReCiPe 2016 midpoints. | ||
From Fig. 8 demonstrates the reason that chlorobenzene has the lowest environmental impacts among the compared halobenzenes in Fig. 5, in which the impacts of the production of bromine and iodine are shown to be higher than those of chlorine production. The impacts of bromine and iodine production are similar, but as shown in Fig. 5, the impacts of bromobenzene are relatively higher than iodobenzene in all the impact categories except for mineral resource scarcity.
This is basically due to the higher yield of the iodination process compared to that the bromination process (87% and 49% respectively), which means that a greater quantity of bromine must be used to obtain bromobenzene. For this reason, the share of bromine manufacture in the total impact of bromobenzene production is higher (Fig. 9). Benzene makes a more important contribution in chlorobenzene production because the lower the impacts of chlorine, electricity, and other inputs, the greater the impact of the starting material. The electrical efficiencies of the halogen processes differ greatly, and might represent an area for optimization.
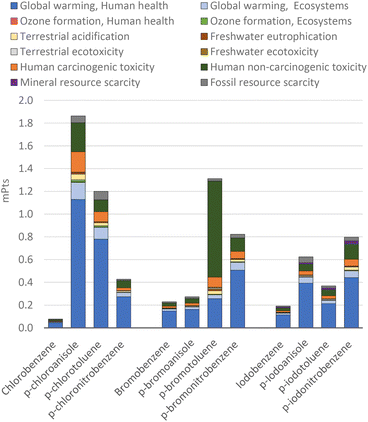
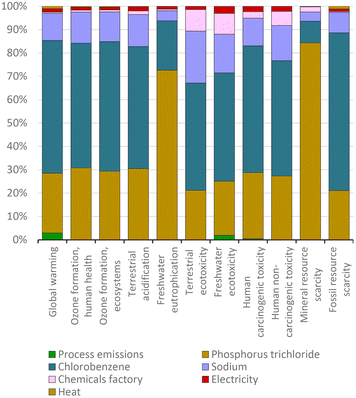
For an aggregate analysis of the obtained results, the LCA impacts were weighted and normalized with regards to endpoint damage areas to compare the halobenzenes using a single indicator as a benchmark of the global environmental impact through dimensionless metrics, i.e., in millipoints (mPts), which represent the relative impact of the results according to their severity in the global context39 (Fig. 10).
As expected from the analysis of the results given above, the preparation of 4-chloroanisole and of 4-chlorotoluene are among the most polluting processes due to their high impact on global warming. This is due to the manufacturing of solvents and the emission of these organic solvents into the environment, which are later emitted into the air in the form of CO2.
The main environmental concern for the more complex substituted halobenzenes is their global warming potential (indicated in dark and light blue), exempting derivatives of 4-bromotoluene. While for 4-chloroanisole, 4-chlorotoluene, and 4-iodoanisole, the global warming potential is related to the use of solvents, other factors apply for the other halo- or substituted halobenzenes. For 4-bromoanisole, the energy consumption dominates; while for 4-bromonitrobenzene, it is the bromobenzene production, for 4-iodotoluene, the potassium iodide production, for 4-chloronitrobenzene, the nitric acid production, and for 4-iodonitrobenzene, the 4-nitroaniline production. These detailed contributions are presented in the ESI-1.†
The impact of the “second wave” of innovation (the choice of aryl halide) can be therefore summarized in terms of the direct comparison between the halobenzenes analysed (Fig. 10).
Considering the simple halobenzenes, the endpoint total magnitudes follow the order Br > I > Cl. The same order is observed for NO2 substitution at the para position. Methoxy substitution at the para position with respect to the halogen atom results in an inversion of the trend (Cl > I > Br), with the iodine-based halobenzene still in the middle, while 4-methyl substitution gave Br > Cl > I, with the iodo-based derivative having the lowest impact. Based on this evidence, it possible to highlight that even considering the mineral resources exploited by iodine-based compounds, they represent a good overall choice in terms of environmental scores. It should be also noted that most frequently, CCRs are used to build variously functionalized target molecular architectures, and therefore, substituted aryl halides are used rather than simple halobenzenes.
3.3. Impact of ligand – ‘third wave’ of innovation
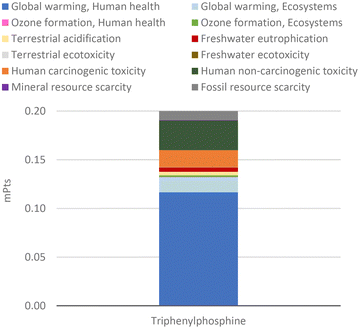
This wave of innovation used ligands, particularly phosphines, to provide a well-defined steric volume to promote CCRs. Among these compounds, in standard CCR protocols, triphenylphosphine is a typical ligand in homogeneous catalysis reactions. Triphenylphosphine is usually not recovered after these reactions, and thus, it is necessary to evaluate the environmental impacts of its production. Industrially, it is produced from chlorobenzene, phosphorus trichloride, and sodium. Each of these materials has relevant contributions in each impact category (Fig. 11).In the category of freshwater eutrophication, around 71% of the impact of triphenylphosphine production is due to the use phosphorus trichloride. The high impact of this material in this category is mainly due to the release of phosphates into water during its production. In the other impact categories, the relevant impact of phosphorus trichloride production is mainly due to the process of obtaining phosphorus, which in turn is mainly due to the extraction of phosphate rocks and the electricity required in this process.
However, since most of the impacts of the production of triphenylphosphine are due to the use of chlorobenzene, we can affirm that the origins of this ligand and the halobenzenes used in CCR are connected. As shown in Fig. 9(a), benzene is one of the main materials affecting the environment in the production of chlorobenzene, with this reagent contributing 78% of the impact on fossil resource scarcity, and consequently, it is also responsible for the high impacts of chlorobenzene in triphenylphosphine production.
In terms of overall environmental impact, the production of this ligand has a similar impact to the production of unsubstituted halobenzenes. These generate less than 0.2 mPts per gram of compound, with over 60% of the environmental impact related to global warming, as shown in Fig. 10 for different halobenzenes and in Fig. 12 for triphenylphosphine. Nevertheless, as the amount of ligands used in CCRs is typically a fraction of the amount of halobenzenes, their actual environmental impact in these protocols is assessed in the next section.
3.4. Impact of the Nobel Prize concepts for cross-coupling and combined effect on innovations
The above-discussed ‘waves’ of innovations are dominated by the environmental history of the chemical constituents, before these constituents are used in the Nobel Prize cross-coupling reactions. The aggregate of the environmental impact of the various concepts (Suzuki, Heck) of the cross-coupling reactions, including some of their variants, has been determined. This allows us to compare the intrinsic potential these concepts have in terms of environmental merit, and how much they can be improved by the individual innovations of the ‘first wave’ of catalysts, the ‘second wave’ of coupling partners, and ‘third wave’ of ligands.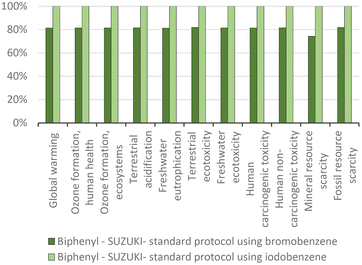 | ||
| Fig. 13 Biphenyl production via the standard Suzuki protocol for CCR: proportional comparison against the highest value per impact category (normalized to 100%). | ||
The improved environmental impacts due to the yield increase are mainly ascribed to the lower catalyst consumption, as the catalyst has the highest impact, unless it is recovered (Fig. 14). While the quantity of benzene used is 15 times higher than that of the catalyst, the catalyst contributes over 75% of the impact in all categories.
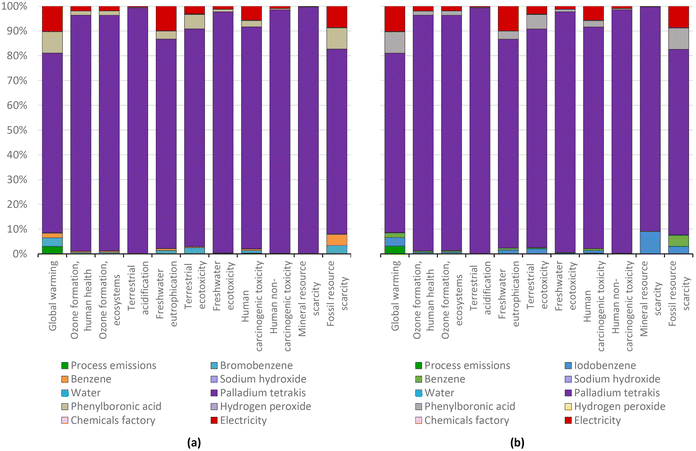 | ||
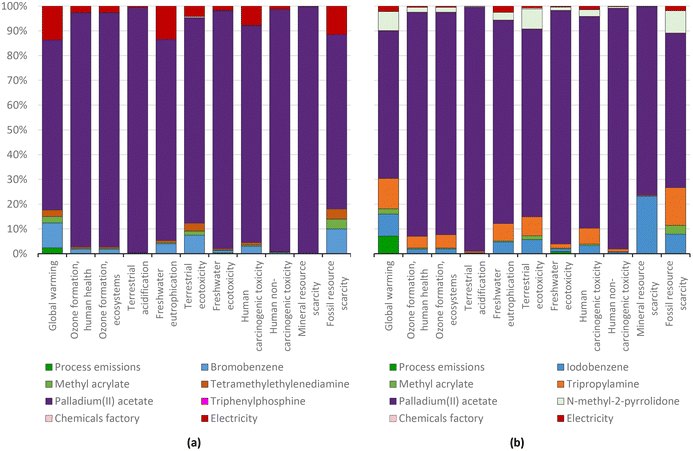
| Fig. 14 Biphenyl production via the standard Suzuki protocol for CCR using bromobenzene (a) and iodobenzene (b): characterization shares; ReCiPe 2016 midpoints. | ||
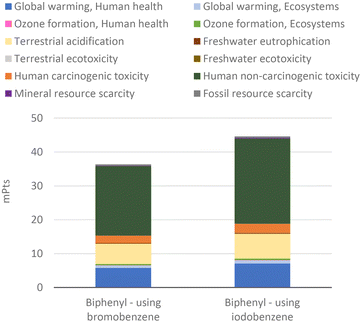
For this reason, the total aggregate environmental impact of this process is only 23% higher than the process using bromobenzene, because the process using iodobenzene consumed 20% more catalyst (Fig. 15). The impacts of biphenyl production, which are mainly human toxicity and terrestrial acidification, are due to the use of the catalysts, whose production mostly affects the same impact categories (see Fig. 4). Nevertheless, it can be seen that the impact on global warming is also relevant in biphenyl production, due to the respective halobenzene and phenylboronic acid consumption. It is noteworthy that, in accordance with the above-mentioned aggregate environmental impact and the intrinsic parameters that affected it, a higher yield CCR process generally corresponds to a lower environmental impact. This situation is even more in favour of the iodoarenes, as much as the degree of substitution increases and therefore, the environmental impact of the considered halobenzene increases.
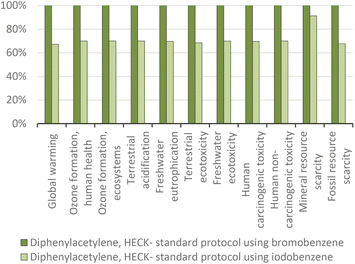 | ||
| Fig. 16 Diphenylacetylene production via the standard Heck protocol for CCR (variant Dieck and Heck, 1975): proportional comparison against the highest value per impact category (normalized to 100%). | ||
As found for the Suzuki reaction, the use of the palladium catalyst is most relevant in terms of the environmental impact (Fig. 17). In this case, the environmental impacts were reduced to an extent similar to the reduction of the quantity of catalyst from 0.025 g to 0.017 g per gram of target product.
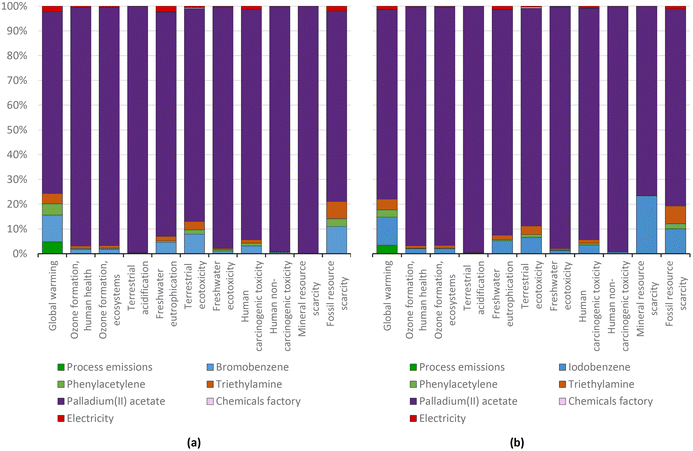 | ||
| Fig. 17 Diphenylacetylene production via the standard Heck protocol for CCR (variant Dieck and Heck, 1975) using bromobenzene (a) and iodobenzene (b): characterization shares; ReCiPe 2016 midpoints. | ||
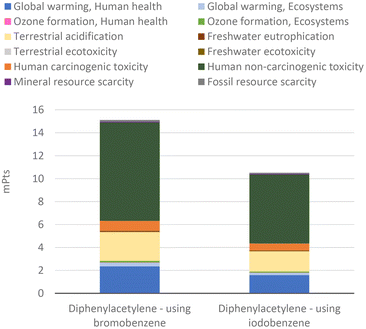
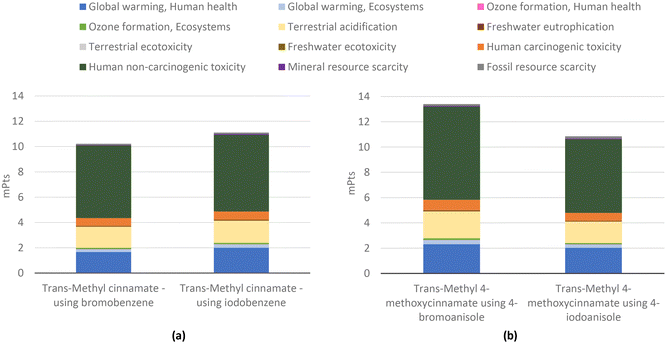
For the above-mentioned reason, the aggregate environmental score of producing diphenylacetylene from iodobenzene was also reduced by around 30%, i.e., from 15.11 to 10.51 mPts, as presented in Fig. 18.
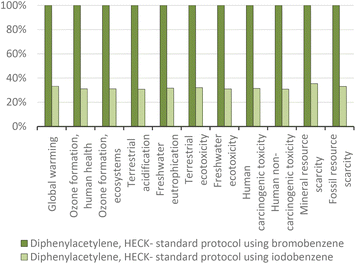 | ||
| Fig. 19 Diphenylacetylene production via the standard Heck protocol for CCR (variant Cassar 1975): proportional comparison against the highest value per impact category (normalized to 100%). | ||
Here, as found for the Heck–Dieck reaction, the use of the palladium catalyst generates the greatest environmental impacts (Fig. 20). However, the manufacturing of palladium tetrakis has about 20% of the impact of palladium(II) acetate, but experiments fail to take full advantage because of too-low yields. To match the performance of palladium(II) acetate, the amount of palladium tetrakis used in the Cassar (1975) synthesis must be increased about 18 times when using bromobenzene or about 8 times when using iodobenzene, compared to the amount of catalysts used for the experiments of Dieck and Heck (1975). As the net outcome of the manufacturing history and yield impact, the Cassar reaction with palladium tetrakis as the catalyst (Fig. 21) generates more than three times the impact of the Heck–Dieck reaction with palladium(II) acetate (Fig. 18) when using bromobenzene. These results corroborate the important role of the catalyst, which overshadows any improvement in process time and temperature in the analysis of environmental profiles of the target products.
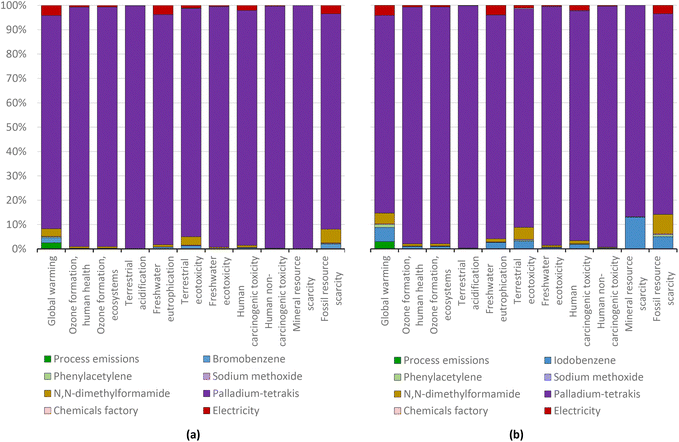 | ||
| Fig. 20 Diphenylacetylene production via the standard Heck protocol for CCR (variant Cassar, 1975) using bromobenzene (a) and iodobenzene (b): characterization shares; ReCiPe 2016 midpoints. | ||
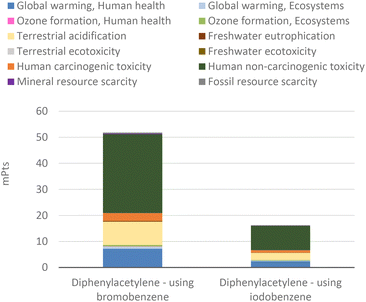 | ||
| Fig. 21 Endpoint-single score for 1 g of diphenylacetylene production via Heck standard protocols (variant Cassar, 1975). | ||
Nevertheless, beyond the relevance of the palladium catalyst and the similarity in the distribution of impacts by categories in the experiments presented by both Cassar (1975) and Dieck and Heck (1975), this comparison revealed the importance of the environmental assessment based on LCA, which showed that similar synthesis processes using the same reagents and slight modification of the structure of catalysts based on the same metal to improve the reaction conditions, can produce worse results from an environmental perspective. More importantly, the selection of a more reactive (iodoarene) substrate is preferred to lower the overall environmental impact.
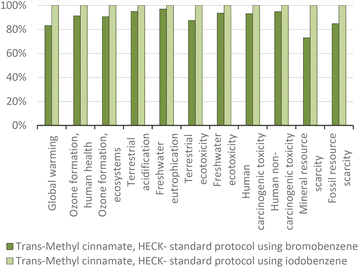
Overall, the aggregate environmental score of the faster process using iodobenzene in Fig. 24(a) is 8.6% higher. The increase in the total environmental impacts increased to the extent of the increase in the amount of catalyst (4.9% more palladium(II) acetate). The 8.6% increase is mainly due to the utilization of a different base (tripropylamine) and solvent (N-methylpyrrolidone) which slightly increased the impacts on global warming, as seen in Fig. 24 in the blue bars. In these terms, and for better comparison, we also considered a case study using substituted haloarenes to obtain trans-methyl 4-methoxycinnamate (Fig. 24(b)). In these examples, the yield is highly differentiated (54% using 4-bromoanisole and 64% using 4-iodoanisole), and neither protocol involves solvents. As a result, there is a 19% reduction in total environmental impacts when using the iodine-based reagent. Although 4-iodoanisole generates an three-fold higher impact than iodobenzene per gram of compound (as shown in Fig. 10), its use results in a higher yield for the target product, therefore reducing the need for ancillary materials and ultimately lowering the overall environmental impact.
In an endpoint aggregate analysis of the overall environmental impacts, it can be concluded that the main environmental impact category affected by the production of aryl halides is global warming due to the use of organic compounds such as benzene and solvents. On the other hand, the production of building blocks using standard protocols for CCRs mostly affects the human non-carcinogenic toxicity and terrestrial acidification categories due to the use of palladium-based homogeneous catalysts. More generally, the yield and, therefore the selection of more-reactive coupling partners, predominates for the final environmental assessment.
3.5. Sensitivity analysis: impact of circularity of synthesis processes
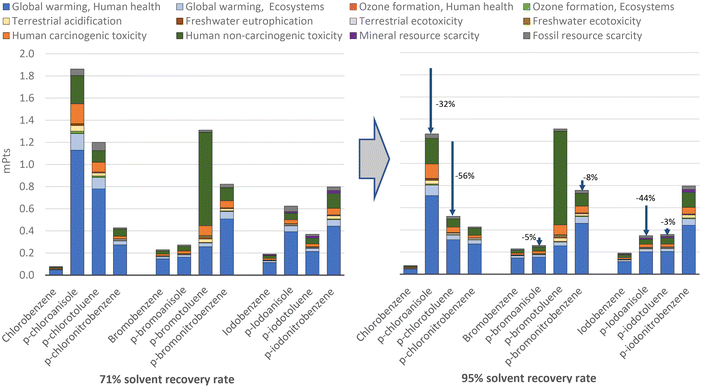
Given the relevance of some solvents to the environmental impacts of halobenzene production, such as 4-chloroanisole and 4-chlorotoluene, as well as given the fact that the assumed 71% SRR can be improved to around 97% in the best cases,44 a sensitivity analysis was performed in this regard.In this study, one of the solvents presenting the greatest environmental impacts was dichloromethane. The results for the halobenzenes produced using this solvent could be greatly affected, because a higher SRR than 71% for this commonly used material can be assumed. Geisler et al. (2004),55 based on their knowledge and industry data, determined a 95% SRR for a theoretical solvent consisting of equimolar quantities of toluene, acetone, and dichloromethane. This 95% SRR is also in line with commercial equipment providers56 and patents57 for the fractional distillation process of dichloromethane. In this context, the scenario assuming a 95% SRR to produce all the analysed halobenzenes is presented in Fig. 25.
From this sensitivity analysis, significant environmental impact reductions as a result of increasing the SRR can be seen, especially for the production 4-chloroanisole and 4-chlorotoluene, for which obtaining dichloromethane had the biggest contribution to the total impacts, as previously shown in Fig. 6. Likewise, the impacts on the production of 4-iodoanisole were significantly reduced by 44% in this scenario given the high impacts of obtaining diethyl ether and ethanol, as well as the related carbon emissions due to the use and disposal of the unrecovered fraction (see Fig. S10 in ESI SM2†). As expected, the reduction in the quantity of solvent that must be added in each run of the halobenzene production processes in this scenario mainly improved the profile of the products heavily affected by the use of solvents. Furthermore, in Fig. 25, it can be seen that the variations in the aggregate scores were mainly due to the reduction of impacts related to global warming, an impact category in which organic solvents play an important role. However, despite the lower amount of new solvent added in each run, the energy required to distil the used solvent after each run is still the same in this high SRR scenario. For this reason, impact on global warming is still high due to the electricity input, which suggests the need to optimize the amount of solvent utilized to further reduce the aggregate environmental profiles. Nevertheless, beyond the amount of solvent, the relevance of the SRR in the search for more circular chemical processes was demonstrated. For this reason, in this 95% SRR scenario, the halobenzene showing the worst environmental profile is no longer the solvent-intensive process for 4-chloroanisole, and the most polluting process is now the production of 4-bromotoluene. The environmental impacts of 4-bromotoluene remained relatively unchanged in this scenario, because no solvents other than water are used during its production and most of its impacts are on human toxicity due to the use of copper compounds, as shown in Fig. 7.
To the same extent, and regarding the circularity of the analysed products obtained via CCRs, the main strategy by far would be the use of heterogenous catalysts58–60 or other more easily recyclable catalysts, preferably derived from a circularity context.61 In a scenario in which almost 100% of the catalyst is recovered, the impacts on human toxicity and terrestrial acidification would be very low, and then the environmental profile would be similar to that of the halobenzenes with important reductions in the endpoint single score.
4. Conclusions
This contribution shows that Nobel Prize innovations can open exceptional opportunities to achieve impacts on environmental issues. They cover a large scientific scope and potential for societal impact. In particular, CCRs have opened opportunities for improving human health as a strategy to readily synthesize pharmaceutically relevant building blocks. This cannot be covered by the present manuscript, which focuses on life cycle assessment (LCA). Human toxicity potential is a sub-class of LCA, but data regarding the medicinal impact of pharmaceutical manufacturing and general medicinal benefits are not easily accessible for one specific class of reactions (CCRs).The scientific “waves” highlighted herein have been governed by curiosity, rather than by real-life impacts. Accordingly, the creation and choice of catalysts and ligands has dominated the past CCR literature. In contrast, this manuscript demonstrates that environmentally, catalysts and ligands have the dominant effects on the application of the Nobel Prize CCR innovations, which is closely linked to the economic impact. In this context, a major impact would have been obtained from investigation of halobenzenes. These reactants, as major constituents of the material-weighted (kg) mixture, are decisive in terms of the environmental impact of CCR. This study, therefore, focuses on the crucial role of halobenzenes. Recycling and recovery are solutions to the problem of the environmental impacts of catalysts, ligands, and other auxiliary agents. This is relevant for promoting the development of separation processes based on sustainability metrics at industrial scales, where the decision to separate, reuse, or dispose of specific chemicals is frequently determined by the cost of recycling versus the cost of purchasing new material. This typically results in the disposal of chemicals, given the high costs of separation, because, unlike chemical reactions, which can be exothermic or endothermic, separation processes are primarily endothermic, leading to significant energy expenditure.
Finally, the awareness of researchers working at the lab-scale of sustainability implications needs to be improved. The outcomes reported in the chemical literature could be improved by such awareness, e.g., by dismissing chemical reaction pathways that are clearly not environmental or economical.
As the aim of this manuscript is to synchronize achievements in chemistry, chemical engineering, and sustainability, the objectives and value of this study were to evaluate the intrinsic potential of CCR protocols through LCA-based environmental assessment and to demonstrate that previous large-scale developments in the literature have had an impact. It is hoped that this study will contribute to the improvement and optimization of future CCR research.
4.1. Outlook
In layman's terms, a great deal remains to be done in the area of transforming innovations into industrial practices and creating societal value. Current academic curricula do not support this, and current research faces difficulties in implementing sustainability assessments and expertise in chemical engineering and chemistry, despite the urgent global need to move towards circularity. Researchers are motivated by this, which is the ‘good news’, yet they are limited in their ability to genuinely determine what is environmentally benign.This paper is intended to provide this perspective in the context of a centennial achievement, the innovations in CCRs that were awarded the Nobel Prize. This is a true challenge, and the authors realized when writing how much care is needed when reporting the sustainability assessment of this eminent achievement. Appreciation is given to the ‘fathers of this innovation’, and the world has changed since then, counting on five decades. It is very important that the scientific community learns to handle past major breakthroughs under the new directives of the global economy and society in this moment (the year 2023).
This study aims to give advice and guidance on the complex matter of the transformation of academic innovations into practice. This study is aware of the time scale, requiring half a century to be fully delivered. This study is a computational and methodological effort to assist the delivery of long-lasting innovations.
Author contributions
J. Osorio-Tejada: writing – original draft, investigation, conceptualization, methodology. F. Ferlin: visualization, investigation. L. Vaccaro: writing – review & editing. V. Hessel: writing – reviewing and editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been funded by the European Union – NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem grant ECS00000041 – VITALITY. We also acknowledge Università degli Studi di Perugia for ‘Fondo di ricerca di Base 2021’.References
- Nobel Prize Outreach, NobelPrize.org, https://www.nobelprize.org/prizes/chemistry/2010/summary/, (accessed 15 April 2023).
- C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS PubMed.
- M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237–4266 RSC.
- R. D. Larsen, A. O. King, C. Y. Chen, E. G. Corley, B. S. Foster, F. E. Roberts, C. Yang, D. R. Lieberman, R. A. Reamer, D. M. Tschaen, T. R. Verhoeven, P. J. Reider, Y. S. Lo, L. T. Rossano, A. S. Brookes, D. Meloni, J. R. Moore and J. F. Arnett, J. Org. Chem., 1994, 59, 6391–6394 CrossRef CAS.
- I. Shinkai, A. O. King and R. D. Larsen, Pure & Appl. Chem., 1994, 66, 1551–1556 CrossRef CAS.
- A. O. King, E. G. Corley, R. K. Anderson, R. D. Larsen, T. R. Verhoeven, P. J. Reider, Y. B. Xiang, M. Belley and Y. Leblanc, J. Org. Chem., 2002, 58, 3731–3735 CrossRef.
- A. B. Smith, T. J. Beauchamp, M. J. LaMarche, M. D. Kaufman, Y. Qiu, H. Arimoto, D. R. Jones and K. Kobayashi, J. Am. Chem. Soc., 2000, 122, 8654–8664 CrossRef CAS.
- A. B. Smith, M. D. Kaufman, T. J. Beauchamp, M. J. LaMarche and H. Arimoto, Org. Lett., 1999, 1, 1823–1826 CrossRef CAS PubMed.
- S. J. Mickel, G. H. Sedelmeier, D. Niederer, R. Daeffler, A. Osmani, K. Schreiner, M. Seeger-Weibel, B. Bérod, K. Schaer, R. Gamboni, S. Chen, W. Chen, C. T. Jagoe, F. R. Kinder, M. Loo, K. Prasad, O. Repič, W.-C. Shieh, R.-M. Wang, L. Waykole, D. D. Xu and S. Xue, Org. Process Res. Dev., 2003, 8, 92–100 CrossRef.
- S. J. Mickel, G. H. Sedelmeier, D. Niederer, F. Schuerch, D. Grimler, G. Koch, R. Daeffler, A. Osmani, A. Hirni, K. Schaer, R. Gamboni, A. Bach, A. Chaudhary, S. Chen, W. Chen, B. Hu, C. T. Jagoe, H.-Y. Kim, F. R. Kinder, Y. Liu, Y. Lu, J. McKenna, M. Prashad, T. M. Ramsey, O. Repič, L. Rogers, W.-C. Shieh, R.-M. Wang and L. Waykole, Org. Process Res. Dev., 2003, 8, 101–106 CrossRef.
- S. J. Mickel, G. H. Sedelmeier, D. Niederer, F. Schuerch, G. Koch, E. Kuesters, R. Daeffler, A. Osmani, M. Seeger-Weibel, E. Schmid, A. Hirni, K. Schaer, R. Gamboni, A. Bach, S. Chen, W. Chen, P. Geng, C. T. Jagoe, F. R. Kinder, G. T. Lee, J. McKenna, T. M. Ramsey, O. Repič, L. Rogers, W.-C. Shieh, R.-M. Wang and L. Waykole, Org. Process Res. Dev., 2003, 8, 107–112 CrossRef.
- P. W. Manley, M. Acemoglu, W. Marterer and W. Pachinger, Org. Process Res. Dev., 2003, 7, 436–445 CrossRef CAS.
- D. Camp, C. F. Matthews, S. T. Neville, M. Rouns, R. W. Scott and Y. Truong, Org. Process Res. Dev., 2006, 10, 814–821 CrossRef CAS.
- P. D. De Koning, D. McAndrew, R. Moore, I. B. Moses, D. C. Boyles, K. Kissick, C. L. Stanchina, T. Cuthbertson, A. Kamatani, L. Rahman, R. Rodriguez, A. Urbina, A. Sandoval and P. R. Rose, Org. Process Res. Dev., 2011, 15, 1018–1026 CrossRef CAS.
- S. Santoro, F. Ferlin, L. Luciani, L. Ackermann and L. Vaccaro, Green Chem., 2017, 19, 1601–1612 RSC.
- S. Vásquez-Céspedes, R. C. Betori, M. A. Cismesia, J. K. Kirsch and Q. Yang, Org. Process Res. Dev., 2021, 25, 740–753 CrossRef.
- J. L. Osorio-Tejada, M. Varón-Hoyos and T. Morales-Pinzón, Water, Air, Soil Pollut., 2022, 233, 174 CrossRef CAS PubMed.
- ISO, ISO 14040:2006. Environmental management—Life cycle assessment—Principles and framework, ISO, Geneva, 2nd edn, 2006, vol. 2006 Search PubMed.
- A. Sivo, T. K. Kim, V. Ruta, R. Luisi, J. Osorio-Tejada, M. Escriba-Gelonch, V. Hessel, M. Sponchioni and G. Vilé, React. Chem. Eng., 2022, 7, 2650–2658 RSC.
- L. Cassar, J. Organomet. Chem., 1975, 93, 253–257 CrossRef CAS.
- P. Fantke and A. Ernstoff, in Life Cycle Assessment, ed. M. Z. Hauschild, R. K. Rosenbaum and S. I. Olsen, Springer International Publishing, Cham, 2018, pp. 783–815 Search PubMed.
- Y. Da Zang, C. J. Li, X. Y. Song, J. Ma, J. Z. Yang, N. H. Chen and D. M. Zhang, J. Asian Nat. Prod. Res., 2017, 19, 623–629 CrossRef PubMed.
- R. F. Heck, Palladium Reagents in Organic Synthesis, Academic Press, 1985 Search PubMed.
- F. A. Cotton, Inorganic syntheses, McGraw-Hill, Inc., 1972, vol. XIII Search PubMed.
- H. E. Fierz-David and L. Blangley, The Fundamental Processes Of Dye Chemistry, Interscience Publishers, Inc., New York, 1949 Search PubMed.
- G. A. Olah, L. Ohannesian and M. Arvanaghi, Synthesis, 1986, 868–870 CrossRef CAS.
- K. Smith and M. Butters, Synthesis, 1985, 1157–1158 CrossRef CAS.
- M. Howe-Grant, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, New York, NY, 4th edn, 1993, vol. 17 Search PubMed.
- IARC, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 65, International Agency for Research on Cancer, Lyon, 1996 Search PubMed.
- A. I. Vogel, Vogel's textbook of practical organic chemistry, Longman Scientific & Technical, London, 5th edn, 1989, vol. 14 Search PubMed.
- G. Hilgetag and A. Martini, Weygand/Hilgetag -Preparative Organic Chemistry, John Wiley & Sons, New York, 1972 Search PubMed.
- N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513–519 CrossRef CAS.
- H. A. Dieck and F. R. Heck, J. Organomet. Chem., 1975, 93, 259–263 CrossRef CAS.
- H. A. Dieck and R. F. Heck, J. Am. Chem. Soc., 1974, 96, 1133–1136 CrossRef CAS.
- R. F. Heck and J. P. J. Nolley, J. Org. Chem., 1972, 37, 2320–2322 CrossRef CAS.
- ETH, Ecoinvent LCA database, https://www.ecoinvent.org, (accessed 22 May 2021).
- J. Osorio-Tejada, F. Ferlin, L. Vaccaro and V. Hessel, Green Chem., 2022, 24, 325–337 RSC.
- H.-J. Althaus, M. Chudacoff, R. Hischier, N. Jungbluth, M. Osses and A. Primas, Life Cycle Inventories of Chemicals, Ecoinvent Report No. 8, v2.0., Dübendorf, CH., 2007 Search PubMed.
- R. Frischknecht, N. Jungbluth, H.-J. Althaus, G. Doka, T. Heck, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer, M. Spielmann and G. Wernet, Overview and Methodology, Ecoinvent Report No. 1, Swiss Centre for Life Cycle Inventories, Dübendorf, 2007 Search PubMed.
- R. Hischier, S. Hellweg, C. Capello and A. Primas, Int. J. Life Cycle Assess., 2005, 10, 59–67 CrossRef CAS.
- J. Osorio-Tejada, N. N. Tran and V. Hessel, Sci. Total Environ., 2022, 826, 154162 CrossRef CAS PubMed.
- C. Capello, S. Hellweg, B. Badertscher and K. Hungerbühler, Environ. Sci. Technol., 2005, 39, 5885–5892 CrossRef CAS PubMed.
- G. Doka, Life Cycle Inventories of Waste Treatment Services, Ecoinvent Report No. 13, Swiss Centre for Life Cycle Inventories, Dübendorf, 2009, vol. 1 Search PubMed.
- J. Sutter, Life Cycle Inventories of Petrochemical Solvents, Final Report Ecoinvent Data v2.0 No. 22, Dübendorf, CH, 2007 Search PubMed.
- P. Koppe and A. Stotzek, Kommunales Abwasser, Vulkan Verlag, Essen, Germany, 1993 Search PubMed.
- M. Boller and M. Hafliger, Verbleib von Schwermetallen bei unterschiedlicher Meteorwasserentsorgung, Gas Wasser Abwasser, No. 1/1996, Zurich, Switzerland, 1996 Search PubMed.
- Gendorf, Environmental declaration of the chemical plants at the Gendorf site in Burgkirchen, Germany, 2000 Search PubMed.
- G. Wernet, S. Hellweg and K. Hungerbühler, Int. J. Life Cycle Assess., 2012, 17, 720–728 CrossRef CAS.
- R. Frischknecht, N. Jungbluth, H.-J. H.-J. Althaus, G. Doka, R. Dones, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer, M. Spielmann, T. Heck and Anonymous, Int. J. Life Cycle Assess., 2005, 10, 112–122 CrossRef.
- Y. Zhang, S. Guo, Y. Gong and L. Wang, Water Res., 2022, 215, 118222 CrossRef CAS PubMed.
- R. Appel, Angew. Chem., Int. Ed. Engl., 1975, 14, 801–811 CrossRef.
- G. Wittig and U. Schöllkopf, Chem. Ber., 1954, 87, 1318–1330 CrossRef.
- G. Geisler, T. B. Hofstetter and K. Hungerbühler, Int. J. Life Cycle Assess., 2004, 9, 101–113 CrossRef CAS.
- B/R Instrument, Recycling dichloromethane, https://brinstrument.com/fractional-distillation/9600-high-purity-solvent-recycling-dichloromethane, (accessed 8 February 2023).
- C. J. Kaemmerlen Jr., C. M. Oualline Jr. and A. G. Williams, US3152968A, 1958.
- G. Strappaveccia, E. Ismalaj, C. Petrucci, D. Lanari, A. Marrocchi, M. Drees, A. Facchetti and L. Vaccaro, Green Chem., 2015, 17, 365–372 RSC.
- F. Valentini, L. Carpisassi, A. Comès, C. Aprile and L. Vaccaro, ACS Sustainable Chem. Eng., 2022, 10, 12386–12393 CrossRef CAS.
- F. Valentini, F. Ferlin, E. Tomarelli, H. Mahmoudi, M. Bagherzadeh, M. Calamante and L. Vaccaro, ChemSusChem, 2021, 14, 3359–3366 CrossRef CAS PubMed.
- F. Valentini, F. Ferlin, S. Lilli, A. Marrocchi, L. Ping, Y. Gu and L. Vaccaro, Green Chem., 2021, 23, 5887–5895 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: LCA inventories dataset (ESI-1); LCA impacts assessment results (ESI-2). See DOI: https://doi.org/10.1039/d3gc01896b |
| This journal is © The Royal Society of Chemistry 2023 |