Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolomics-based analysis in Daphnia magna after exposure to low environmental concentrations of polystyrene nanoparticles†
Egle
Kelpsiene
 ab,
Tommy
Cedervall
ab,
Tommy
Cedervall
 *ab and
Anders
Malmendal
*ab and
Anders
Malmendal
 c
c
aDepartment of Biochemistry and Structural Biology, Lund University, P.O. Box 124, SE-221 00 Lund, Sweden. E-mail: tommy.cedervall@biochemistry.lu.se
bNanoLund, Lund University, P.O. Box 118, SE-221 00, Lund, Sweden
cDepartment of Science and Environment, Roskilde University, P.O. Box 260, DK-4000 Roskilde, Denmark
First published on 30th May 2023
Abstract
Larger plastic pieces break down into micro- and eventually nano-sized plastics. This makes nanoplastics ubiquitous in the environment, giving rise to great concern for its effect on biota. Many studies use polystyrene nanoparticles (PS-NPs) as a model for nanoplastics, showing a negative impact on various organisms, but the molecular effects are yet not fully explored. Here we applied 1H nuclear magnetic resonance (NMR) metabolomics to characterize the metabolic changes in Daphnia magna during long-term (37 days) exposure to low concentrations of positively and negatively charged (aminated and carboxylated) PS-NPs. We show that exposure to PS-NPs at concentrations down to 3.2 μg L−1 affected amino acid metabolism and the bacterial metabolite isopropanol in D. magna. These effects were largely independent of particle concentration and surface charge. The results highlight the importance of (1) performing chronic exposures under low concentrations and (2) further investigation of particles with different surface charges.
Environmental significanceNano-sized particles are ubiquitous; therefore, adversary effects of nanoparticles (NPs) have attracted both societal and scientific attention. Polystyrene (PS) is one of the most used plastics, wherefore PS NPs as model particles have been widely used in the toxicity studies in various organisms. Yet molecular mechanism behind these particles is not fully understood. Metabolomics-based studies allow identification of physiological changes in the organisms in response to pollutants even at low concentrations. The present study shows that daphnids metabolism is affected by PS NPs at 3.2 μg L−1 after two days exposure. The effect remains throughout the whole experiment (37 days). Therefore, chronic exposures at low concentrations should be priority when it comes to the toxicity studies to get broader understanding about nanoplastics effects to aquatic biota. The differences between different particle concentrations and between positive and negative surface charges were limited. |
Introduction
The global plastic production increased from 1.5 million metric tons to 367 million metric tons between 1950 and 2020.1 Most plastic is non-biodegradable and, therefore, remains as a waste for many years in the environment.2 It has been calculated that almost 300 million tons of plastic are consumed each year,3 of which 60 to 100 million tons are mismanaged and around 90% ends up in waterways, potentially reaching the oceans.4 An increased levels of plastic production, improper disposal, poor waste management, and low recovery rate, leads to hazardous plastic waste being thrown out into the environment, which has attracted public attention.5Depending on size, plastic debris is mainly classified as macro- (>5 mm), micro- (MPs, <5 mm, >1 μm), and nano-plastic (NPs, <1 μm).6 NPs can be further divided into primary or intentionally manufactured and secondary or generated by fragmentation of larger plastic pieces.7 The presence and chemical composition of NPs in seawater samples have been found in the North Atlantic Subtropical Gyre.8 Additionally, NPs were found and quantified in the surface water samples from lakes and streams in Siberian Arctic tundra (mean 51 μg L−1), a forest landscape in southern Sweden (mean 563 μg L−1)9 and snow in the remote high-altitude Alps, Austria (mean 46.5 μg L−1).10 There was no PS found in the southern Sweden, however the highest concentrations were observed for polyethylene (PE), followed by polypropylene (PP), polyvinylchloride (PVC) and polyethylene terephthalate (PET).9 Similarly, no PS was found in high-altitude Alps, however the main polymers were PP and PET.10 Whereas, in PS, PE, PP and PVC were found in Siberian Arctic tundra9 and the surface waters of Italian Subalpine.11 Breakdown of PS is also evident due to the presence of styrene oligomers in oceans, beaches, and waterways.8,12,13 The concentration of oligomers was shown to be between 0.17 μg L−1 and 4.26 μg L−1 in surface waters and 0.31 μg L−1 and 4.31 μg L−1 in deep waters.12
The small size and high surface to volume ratio allow NPs to enter cells and interact with biological molecules more efficiently than larger size particles.14 Besides the size, particle surface charge also affects the toxicity.15 Nano-sized (25 nm to 60 nm) carboxylated surface charged PS NPs that were shown not to be toxic in the acute (24 h) exposure to D. magna,16 appeared to be toxic in the life-time (103 days) exposure.17 In general, 24–48 h acute toxicity exposure scenarios are the most commonly used tests to evaluate the adverse effects on organism's survival.18 However, acute toxicity tests often use high concentrations of toxicants, whereas pollutants are present at sub-lethal levels in the natural environment.19 Thus, acute toxicity tests fail to provide insight into how toxicity manifests at sub-lethal levels and gives little information regarding the biochemical mode of action. Therefore, other endpoints are needed to get broader understanding in terms of responses to pollutants at the molecular level.
Omics approaches such as transcriptomics, proteomics, and metabolomics provide a broad overview of the molecular changes underlying physiological processes affected by toxicants.20 Metabolomics may be defined as the quantitative measurement of the dynamic multiparametric response of a living system to a stimuli or genetic modification.21 Nuclear magnetic resonance (NMR) metabolomics is a highly reproducible high-throughput approach to metabolome analysis that require minimal sample preparation.22,23 It is an untargeted technique, that allows detection of many metabolites, and therefore suitable for studies where no prior assumptions have been made.24 Here we apply NMR metabolomics to study abnormalities in the metabolism associated with toxicity of environmental pollutants.
Metabolomics have previously been used to study the metabolic responses of D. magna induced by various toxicants, such as silver (Ag) nitrate and Ag NPs,25 PE MPs,26 pharmaceuticals,27 cadmium,28 arsenic, copper, lithium,29 insecticides, or industrial chemicals.30,31 It has been shown that released Ag+ induced disturbance in energy metabolism and oxidative stress,25 PE MPs downregulated phosphatidylcholine and upregulated phosphatidylethanolamine, as well as induced the degradation of amino acids,26 copper and lithium altered production of neurotransmitters and impaired energy metabolism29 to D. magna.
D. magna, used in the present study as a model organism, are small (<1–5 mm) freshwater filter-feeder crustaceans which play a key role in food webs.32 Under laboratory conditions the lifespan of most Daphnia species is approximately 60 days.32 During that time, a daphnid neonate goes through four to six juvenile instars before it produces eggs for the first time after approximately 5–10 days.32Daphnia species have become important model organisms in both ecology and toxicology studies due to their sensitivity to environmental contaminants.32 Various adverse effects, such as increased mortality, inhibited reproduction, induced abnormal embryonic development, alterations in swimming pattern, increase in superoxide dismutase activity, caused by PS NPs to D. magna have previously been shown.16,17,33–38 Most of the studies performed acute (24–48 h) toxicity test using high PS NPs concentrations, ranging from 500 μg L−1 to 1.5 × 105 μg L−1.16,33,36,38 Unfortunately, there are not many studies that focus on a long-term exposure at lower concentrations of PS NPs.
Despite the well-known adverse effects caused by NPs, the molecular mechanisms behind PS NPs are largely unknown and, therefore, need to be further investigated. Therefore, this study focuses on the metabolic responses on D. magna after a long-term exposure to low environmental concentrations of PS NPs with different surface charge but similar size, by using 1H NMR metabolomics. The study aims to analyze how the metabolome is affected by PS NP type, concentration, and daphnid aging.
Materials and methods
Preparation and characterization of polystyrene nanoparticles
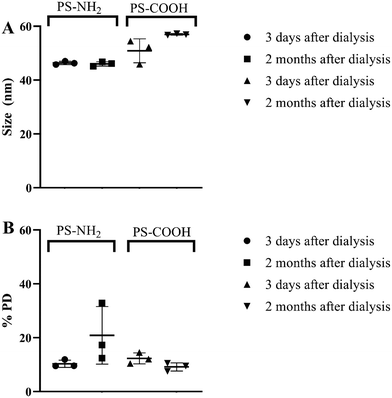
Positively (aminated, PS-NH2, diameter size of 53 nm, catalog number: PA02N, 9.1% solids) and negatively (carboxylated, PS-COOH, diameter size of 62 nm, catalog number: PC 02003, 10.1% solids) surface charged PS NPs were purchased from Bangs Laboratories Inc. (https://www.bangslabs.com). These particles suspension might contain sodium azide, therefore before the experiment, particles were diluted to 10 mg mL−1 and dialyzed in Standard RC Tubing, dialysis membrane (MWCO: 3.5 kD) for 72 h in 10 L of MilliQ water. The water was changed after 4 h the first day and once a day on the following days. Dialysis was performed to remove additives from the NPs and to create a stock solution suitable for toxicity testing of NPs. The particle sizes were measured in triplicated using DLS on DynaPro Plate Reader II (Wyatt instruments, USA) 3 days and 2 months after dialysis to ensure that particle aggregation after dialysis did not occur. Zeta potential measurements were performed in MilliQ water (100 mg L−1 of NPs) and in tap water at 25 °C using a Zetasizer Nano ZS instrument (Malvern Instruments, Worcestershire, UK). Measurements were repeated three times and averaged for three consecutive analyses of the same sample.Study organisms
The filter feeder D. magna culture used in the present study originates from Lake Bysjön, Southern Sweden (55°40′31.3′′N, 13°32′41.9′′E) and has been kept in the laboratory for several hundred generations. The culture was fed ad libitum 2–3 times per week with an algae diet mainly composed of the green algae Scenedesmus sp. Additionally, there might be blue-green algae in the culture. Before the feeding, the algal culture was filtered through 20 μm mesh filter to remove larger algal species, such as cyanobacteria, from the culture. The algal culture was fed with 250 μL of liquid plant nutrient, containing 5.1 g nitrogen, 1.0 g phosphorus, and microelements per 100 mL. Both Daphnia and algal cultures, as well as experimental groups were maintained at 18 °C at an 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 h light/dark photoperiod.
16 h light/dark photoperiod.
Exposure to polystyrene nanoparticles
A long-term (37 days) experiment on D. magna was performed to analyze the effects on metabolites after exposure to three different environmentally relevant concentrations (320, 32 and 3.2 μg L−1, or 3.91 × 109 particles per mL and 2.44 × 109 for PS-NH2 and PS-COOH, respectively at concentrations of 320 μg L−1) of PS particles of different surface charge with similar sizes. Two-five days old D. magna individuals from the same population were randomly assigned to the different groups. Ten individuals were put into a 100 mL uncovered glass beaker with 80 mL total exposure volume (5 replicates for each treatment, 10 individuals in each replicate). The first batch of samples were fixed after 2 days exposure, whereas the following samples were fixed every 7 days on a similar time of the day. D. magna individuals were transferred into Eppendorf tubes and immediately after placed in a mixture of dry ice and 99% acetic acid to quench the metabolism. D. magna samples were subsequently lyophilized and stored at −80 °C before further analysis. The remaining D. magna individuals were gently transferred to the fresh tap water, containing 5 mL (∼500 μg L−1) of food (algae), with (treatment) or without (control) NPs, by using a 1 mL plastic pipette with a removed tip to reduce handling stress. The fresh medium was changed once a week after samples were collected and fixed. The pH was measured for all treatments and remained stable throughout the exposure period (pH 7.19 ± 0.75). Offspring were removed once a week. The reproduction rate was not followed in the present study.Sample preparation
Immediately before NMR measurements, the samples were rehydrated in 200 μL of 37.5 mM phosphate buffer (pD 6.95) in heavy water (D2O) by shaking at 800 rpm at 22 °C for 45 min. The buffer contained 0.747 mM of the chemical shift reference (trimethylsilyl)propionic-2,2,3,3-d4 acid, sodium salt (TSPd4), and 0.05% w/v of sodium azide to prevent bacterial growth.NMR spectroscopy
The NMR measurements were carried out at 25 °C on a Bruker Avance-III 700 spectrometer (Bruker Biospin, Germany) operating at a 1H frequency of 700.20 MHz and equipped with a and 5 mm QCI cryoprobe. The 1H NMR spectra were acquired using a noesygppr1d experiment. The water signal was suppressed by presaturation and a total of 64k data points spanning a spectral width of 30 ppm were collected in 128 transients. The spectra were processed using Topspin (Bruker). An exponential line broadening of 0.5 Hz was applied to the free induction decay prior to Fourier transformation. All spectra were referenced to the TSPd4 signal at 0 ppm, phased, and baseline corrected. The spectra were aligned using icoshift,39 and the region around the residual water signal (4.84–4.74 ppm) was removed. The spectra were normalized by probabilistic quotient area normalization,40 and the data were scaled using Pareto scaling41 and centered.NMR data analysis
Initially, the whole dataset was subjected to principal component analysis (PCA).42 Multivariate analysis of variance (MANOVA) was used to determine whether (1) there were significant effects of daphnid aging, as well as the presence, concentration, and type of PS NPs, and (2) to identify the lowest PS NPs concentration that caused a significant metabolite response. For all NP-related properties, the analyses were made at each day. For an effect to be judged as significant the median p-value across ages needed to be <0.001. Then, orthogonal projection to latent structures discriminant analysis (OPLS-DA) models were created to separate the different daphnid aging days for control (without PS NPs) and treatment (D. magna exposed to PS-NH2 or PS-COOH NPs) groups. OPLS-DA models are multivariate models that predict group membership based on multivariate input, in this case, the NMR spectra. The model separates variations due to group membership from other (orthogonal) variations.43 This allows us to focus on the spectral changes between different types of samples. The OPLS-DA scores used for further analysis were calculated using cross validation, where models were made with randomly chosen groups of samples left out one at a time, after which the scores were calculated for the left-out samples to avoid overfitting. Significant spectral correlations were identified by applying sequential Bonferroni correction (p < 0.05) for an assumed total number of 50 metabolites. The correlations were performed in MATLAB (The MathWorks, Natick, MA). Signal assignments were based on chemical shifts using earlier assignments and spectral databases.44,45 All multivariate analyses were performed using the Simca-P software (Umetrics, Sweden). Aging and PS NP type effects for individual metabolites were calculated using 2-way ANOVA.Results
Characterization of polystyrene nanoparticles
PS NPs were measured both 3 days and 2 months after dialysis to ensure that NPs did not aggregate during the exposure period. DLS measurements showed the sizes to be slightly lower compared to the information provided by a supplier (46.37 ± 0.49 nm and 50.85 ± 8.74 nm for PS-NH2 and PS-COOH, respectively), however particle sizes remained stable during the exposure period (Fig. 1). Zeta potential analysis showed that PS-NH2 and PS-COOH NPs (100 mg L−1) in MilliQ water had positive (+26 ± 3 mV) and negative (−37 ± 4 mV) charges, respectively. The Z-potential measured in tap water showed similar values (ESI† Fig. S1).Identification of significant effects of daphnid aging, and the presence, concentration, and type of polystyrene nanoparticles
First, we used MANOVA on the scores from principal component analysis (PCA) to test if there was an effect of the daphnid aging in the control group (without PS NPs). The data shows that there was a significant effect of daphnid age (p = 7.9 × 10−11). Secondly, we tested if there was an effect of presence, concentration, and type of NPs by using MANOVA on the PCA scores at different daphnid ages (Table S1†). This analysis shows an effect of the presence of NPs (pmedian = 1.9 × 10−6) and this effect is significant even at the lowest (3.2 μg L−1) PS-NP concentration (pmedian = 3.8 × 10−4). This effect is significant already after 2 days (p = 6.5 × 10−9). On the other hand, there were no significant effects of PS NP concentration or type of PS NPs. Yet, we chose to show data for PS-NH2 and PS-COOH NPs separately.Overall changes in the D. magna metabolome as a function of daphnid aging
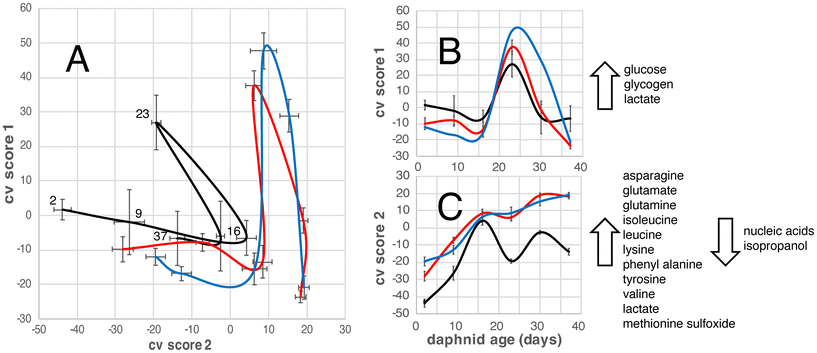
The OPLS-DA model allows us to focus on the effects of daphnid age and exposure to PS NPs on the D. magna metabolome (Fig. 2). For untreated (control group) the metabolome changes in one direction until an age of 16 days, displaying an increase in alanine, asparagine, glutamate, glutamine, isoleucine, leucine, lysine, phenyl alanine, tyrosine, valine, lactate, and methionine sulfoxide; and a decrease in glucose, glycogen, nucleic acids, and isopropanol. Then they change in another direction, with increases in glucose, glycogen, and lactate, and at 23 days they turn back again meaning that the metabolome of the 37-day old D. magna individuals is quite similar to that of the 16-day old (Fig. 2). The metabolome of the D. magna exposed to PS NPs with different surface charges follows a similar pattern as the control group in that it changes in one direction from day 2 to 16 and then in another direction, until day 23, after which they change again, however the starting point and directions of the changes are slightly different (Fig. 2).Effects on individual metabolites
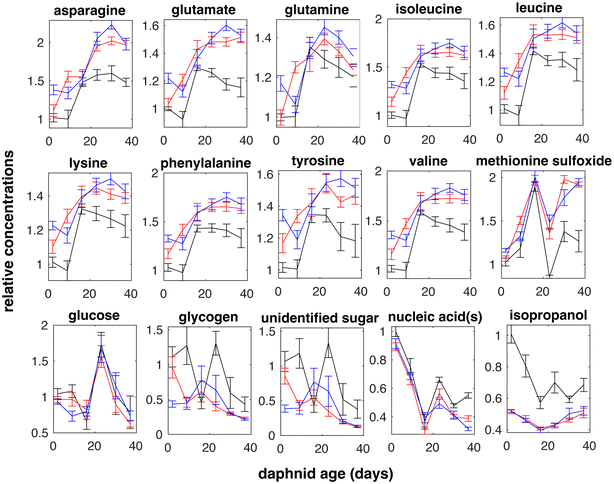
Metabolite effects due to daphnid aging and exposure to surface charged PS NPs were identified using 2-way ANOVA. Metabolite variation between D. magna at different aging in the absence (control group) and in the presence of PS NPs (treatment) are shown in Fig. 3 and significant metabolites both overall and for the lowest metabolite concentration (3.2 μg L−1) are listed in Table 1. In total, 15 significantly affected metabolites were identified from the 1H NMR spectra (Fig. 3).| Metabolite | Aging | PS NPs | 3.2 μg L−1 of PS NPs |
|---|---|---|---|
| Asparagine | 3.6 × 10−32 | 3.1 × 10−8 | 8.5 × 10−7 |
| Glutamate | 4.2 × 10−26 | 4.2 × 10−9 | 1.4 × 10−7 |
| Glutamine | 4.8 × 10−18 | 0.014 | 0.13 |
| Isoleucine | 3.5 × 10−22 | 2.9 × 10−7 | 4.1 × 10−5 |
| Leucine | 6.9 × 10−20 | 3.2 × 10−6 | 2.2 × 10−4 |
| Lysine | 4.1 × 10−17 | 3.1 × 10−6 | 3.2 × 10−4 |
| Phenylalanine | 5.0 × 10−19 | 1.7 × 10−7 | 3.5 × 10−5 |
| Tyrosine | 1.5 × 10−9 | 6.3 × 10−6 | 2.1 × 10−4 |
| Valine | 2.1 × 10−24 | 6.2 × 10−8 | 4.3 × 10−6 |
| Methionine sulfoxide | 1.7 × 10−35 | 1.8 × 10−9 | 2.3 × 10−8 |
| Glucose | 2.8 × 10−15 | 0.25 | 0.15 |
| Glycogen | 7.9 × 10−5 | 1.4 × 10−4 | 9.2 × 10−6 |
| Unidentified sugar | 1.2 × 10−6 | 4.8 × 10−5 | 1.7 × 10−6 |
| Nucleic acid(s) | 1.1 × 10−31 | 0.097 | 0.38 |
| Isopropanol | 1.4 × 10−9 | 1.7 × 10−28 | 8.6 × 10−17 |
Discussion
In the present study, D. magna were exposed to low concentrations of differently surface charged PS-NPs for 37 days. Interestingly, positively and negatively charged particles induced similar metabolic changes. In general, positively surface charged PS NPs often exhibit greater effects compared to negatively surface charged NPs.35 However, no difference in the toxicity, as similar to the results observed in the present study, have been seen in the toxicity towards D. magna after a life-time exposure to differently surface charged PS-NPs.17 There are no clear answers regarding why positive functionalization shows higher toxicity compared to negative one. However, one of the explanations to the lack of differences in the toxicity can be that in the long-term experiment the particle's hydrophobic regions are more important than functional groups. Positively surface charged particles are often seen as a model for cationic NPs, whereas negatively surface charged particles as anionic particles.46 The role of hydrophobic regions for the toxicity can be enhanced by reducing the number of amino groups on the surface of the particle, by cationic groups binding with certain components to shield the positive charge, which consequently decreases the interaction between cell membrane and NPs.46 Furthermore, it has been shown that the affinity of biomolecules binding to the surface of the particle with a carboxylic group is weaker at pH 7, which can further affect the presence of other molecules and ions and the toxicity.47 The differences in biomolecules binding to PS NPs with amino and carboxylic groups have previously been shown for PS NPs after being filtrated by D. magna.48Already at the lowest PS NP concentration (3.2 μg L−1) there was a significant effect on the metabolome. This is far below the concentrations of nanoplastics found in different environments, such as Siberian Arctic tundra (50 μg L−1), a forest landscape in southern Sweden (560 μg L−1),9 and snow in the remote high-altitude Alps, Austria (46 μg L−1).10 Furthermore, this concentration (3.2 μg L−1) is within the concentration range of breakdown styrene oligomers in surface waters (between 0.17 μg L−1 and 4.26 μg L−1) and in deep waters (0.31 μg L−1 and 4.31 μg L−1) in the Pacific Ocean.12 It is hundred times lower than previously measured toxic concentrations for life-time (103 days) exposure of D. magna to 62 nm PS-COOH and 53 nm PS-NH2.17 A low-dose stimulation and a high-dose inhibition is a common phenomenon, called hormesis, observed in biology:49 at the lowest dose, organisms have a maximum stimulatory response in comparison with a higher concentration of toxicants. This can partly explain why we see effects already at the lowest concentrations used here.
The NP concentration of 3.2 μg L−1 is not only very low compared to concentrations used in other NP studies but is also low compared to a variety of pollutant concentrations used in ecotoxicity studies. For example, zebrafish Danio rerio has been exposed for 30 days to 44 nm PS NPs (1, 10, and 100 μg L−1)50 and for 96 h to ∼190 nm PE NPs (5 × 105 μg L−1).51 Additionally, D. magna has previously been exposed to MPs and NPs, for example, 24 h exposure to 20 μm and 30 μm PE MPs (2–6 × 104 μg L−1),26 5 days exposure to 52 nm PS NPs (5 × 103 μg L−1),34 and 21 days exposure to ∼71 nm PS NPs (500–2 × 103 μg L−1).52
We observed that upon aging, the metabolome in both untreated control and PS NP exposed D. magna follow the same pattern (Fig. 1A). This suggests an unaffected timing of the daphnids' developmental stages (e.g., juvenile, first and/or second egg development). Previously, Zhang and co-authors53 showed that D. similis start to develop the first eggs in the brood chamber already after 5–7 days, whereas the second egg development might start after another 5–6 days.
A total of 15 significantly affected metabolites were identified (Table 1), 12 of which were affected in response to PS NPs. Many of these were amino acids that changed in the same way with daphnid aging and between control and treatment groups (Fig. 3). Amino acids have been shown to be intimately linked to most biochemical pathways related to stress.30,54 For example, lysine is among amino acids that are stored by crustaceans as energy reserves during molting cycles.55 Temporary periods of starvation are experienced during the molting process in crustaceans, such as prawns, crabs, or shrimps, during which individuals use reservoirs of amino acids.55 An increased stress level could make the daphnid less resilient to other stress factors such as predators, lack of food, temperature, and ultraviolet (UV) radiation.
If we look at individual amino acids, an increase in lysine has been associated with alterations in molt frequency and disruption of normal hormone signaling.56 The increase of frequency of molt has previously been shown in D. magna after exposure to 2 ± 1 μm PVC MPs.57 Furthermore, the present data shows that glucose levels varied significantly due to daphnid aging but were not affected by PS NPs (Table 1). It is known that Daphnia species are able to maintain minimal levels of the energy molecule glucose for survival,58 however the main change in glucose was observed at exactly day 23 (Fig. 3), which might be explained due to daphnid aging. Disturbed glucose metabolism has previously been observed in aged zebrafish.59,60 Additionally, a short (24 h) exposure to 20 μm and 30 μm PE MPs have been shown to significantly interfere with energy metabolism in D. magna.26
Changes in aromatic amino acids such as phenylalanine and tyrosine have been associated with disruptions in catecholamine synthesis,28 where elevated catecholamine levels as a response to environmental stressors.61 Phenylalanine is the precursor to tyrosine, which is used to produce neurotransmitters such as octopamine and dopamine62 and pigment compound melanin.63 The increase in phenylalanine in response to PS NPs might lead to lower amount of pigmentation.64,65 This is an important factor for daphnids as several Daphnia species have ability to maintain their pigmentation by coping and responding to UV radiation.66
The most significant effect of PS NP exposure was a decrease in isopropanol while similar changes with time are observed both control and treatment groups (Fig. 3). Microorganisms, for example Lactobacillus brevis, Clostridium beijerinckii, C. aurantibutyricum, C. ragsdalei, and Acetobacterium woodii, are known to produce isopropanol from acetone.67–69 The decrease detected here might indicate that the bacterial conversion of acetone to isopropanol through isopropanol dehydrogenase is affected.70
Conclusions
In the present study, we aimed to answer how the D. magna metabolome is affected by PS NPs and their charge and concentration, as well as by daphnid aging, using 1H NMR-based metabolomics. First, we wanted to see if there is an effect of PS NPs, and at which concentration such an effect occurs. Our results show that significant effects on amino acids metabolism and the bacterial metabolite isopropanol were already observed at the lowest concentration (3.2 μg L−1) used here. These effects appeared already after two days and remained throughout the experiment (37 days). Secondly, we wanted to see if PS NPs with different surface charges affect the metabolome differently. The results show that exposure to 53 nm PS-NH2 and 62 nm PS-COOH NPs gave rise to very similar effects. This is an important observation, as PS-COOH NPs have previously been shown to be non-toxic after acute (24 h) exposure. Additionally, daphnids aging also had significant effects on amino acids metabolism and the bacterial metabolite isopropanol.Metabolomics-based studies allow us to better understand the physiological state of an organism and its response to different types of stimuli, including pollutants. The obtained results highlight that daphnids' metabolism can be affected significantly regardless of the surface charge of PS NPs after a long-term exposure even at low concentrations. The present study shows the effect of PS NPs on the D. magna endometabolome, the metabolites kept by the D. magna, therefore future studies may also focus in the exometabolome, the metabolites that are excreted into the exposure medium. Here we used model PS particles with defined sizes and shapes. Future studies may evaluate the metabolic response after exposure to more environmentally realistic nanoplastics with greater diversity in shape and size.
Author contributions
Egle Kelpsiene: investigation, methodology, writing – original draft, review & editing. Tommy Cedervall: conceptualization, funding acquisition, methodology, supervision, writing – review & editing supervised the work. Anders Malmendal: NMR data analysis, methodology, writing – review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Funding for the present study was provided by Mistra Environmental Nanosafety Program. We also would like to thank Dr. Anders Bay Nord at Swedish NMR Centre, University of Gothenburg, for his help with NMR samples analysis. We also would like to thank Dr. Martin Lundqvist for helping with zeta potential measurements.References
- Plastics the Facts 2021, https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf, 2021, Accessed 9 Feb 2023.
- R. C. Thompson, C. J. Moore, F. S. Vom Saal and S. H. Swan, Plastics, the environment and human health: current consensus and future trends, Philos. Trans. R. Soc., B, 2009, 364, 2153–2166 CrossRef CAS PubMed.
- M. Rujnić-Sokele and A. Pilipović, Challenges and opportunities of biodegradable plastics: A mini review, Waste Manage. Res., 2017, 35, 132–140 CrossRef PubMed.
- L. Lebreton and A. Andrady, Future scenarios of global plastic waste generation and disposal, Palgrave Communications, 2019, 5, 6 CrossRef.
- J. Soares, I. Miguel, C. Venâncio, I. Lopes and M. Oliveira, Perspectives on Micro (Nano) Plastics in the marine environment: biological and societal considerations, Water, 2020, 12, 3208 CrossRef CAS.
- SAPEA, Science Advice for Policy by European Academies, A Scientific Perspective on Microplastics in Nature and Society, SAPEA, Berlin, 2019, DOI:10.26356/microplastics.
- J. Gigault, A. Ter Halle, M. Baudrimont, P.-Y. Pascal, F. Gauffre, T.-L. Phi, H. El Hadri, B. Grassl and S. Reynaud, Current opinion: What is a nanoplastic?, Environ. Pollut., 2018, 235, 1030–1034 CrossRef CAS PubMed.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono, L. Brach and J. Gigault, Nanoplastic in the North Atlantic Subtropical Gyre, Environ. Sci. Technol., 2017, 51, 13689–13697 CrossRef CAS PubMed.
- D. Materić, M. Peacock, J. Dean, M. Futter, T. Maximov, F. Moldan, T. Röckmann and R. Holzinger, Presence of nanoplastics in rural and remote surface waters, Environ. Res. Lett., 2022, 17, 054036 CrossRef.
- D. Materić, E. Ludewig, D. Brunner, T. Röckmann and R. Holzinger, Nanoplastics transport to the remote, high-altitude Alps, Environ. Pollut., 2021, 288, 117697 CrossRef PubMed.
- M. Sighicelli, L. Pietrelli, F. Lecce, V. Iannilli, M. Falconieri, L. Coscia, S. Di Vito, S. Nuglio and G. Zampetti, Microplastic pollution in the surface waters of Italian Subalpine Lakes, Environ. Pollut., 2018, 236, 645–651 CrossRef CAS PubMed.
- B. G. Kwon, K. Amamiya, H. Sato, S.-Y. Chung, Y. Kodera, S.-K. Kim, E. J. Lee and K. Saido, Monitoring of styrene oligomers as indicators of polystyrene plastic pollution in the North-West Pacific Ocean, Chemosphere, 2017, 180, 500–505 CrossRef CAS PubMed.
- K. Amamiya, K. Saido, S.-Y. Chung, T. Hiaki, D. S. Lee and B. G. Kwon, Evidence of transport of styrene oligomers originated from polystyrene plastic to oceans by runoff, Sci. Total Environ., 2019, 667, 57–63 CrossRef CAS PubMed.
- K. Tallec, O. Blard, C. González-Fernández, G. Brotons, M. Berchel, P. Soudant, A. Huvet and I. Paul-Pont, Surface functionalization determines behavior of nanoplastic solutions in model aquatic environments, Chemosphere, 2019, 225, 639–646 CrossRef CAS PubMed.
- A. Sukhanova, S. Bozrova, P. Sokolov, M. Berestovoy, A. Karaulov and I. Nabiev, Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties, Nanoscale Res. Lett., 2018, 13, 44–44 CrossRef PubMed.
- K. Mattsson, E. V. Johnson, A. Malmendal, S. Linse, L.-A. Hansson and T. Cedervall, Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain, Sci. Rep., 2017, 7, 11452 CrossRef PubMed.
- E. Kelpsiene, O. Torstensson, M. T. Ekvall, L.-A. Hansson and T. Cedervall, Long-term exposure to nanoplastics reduces life-time in Daphnia magna, Sci. Rep., 2020, 10, 5979 CrossRef CAS PubMed.
- OECD, Test No. 202: Daphnia sp. Acute Immobilisation Test, 2004.
- R. Lenz, K. Enders and T. G. Nielsen, Microplastic exposure studies should be environmentally realistic, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4121–E4122 CAS.
- H. C. Poynton, H. Wintz and C. D. Vulpe, Progress in ecotoxicogenomics for environmental monitoring, mode of action, and toxicant identification, Adv. Exp. Biol., 2008, 2, 21–323 Search PubMed.
- J. K. Nicholson, J. C. Lindon and E. Holmes, 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data, Xenobiotica, 1999, 29, 1181–1189 CrossRef CAS PubMed.
- C. J. Clarke and J. N. Haselden, Metabolic profiling as a tool for understanding mechanisms of toxicity, Toxicol. Pathol., 2008, 36, 140–147 CrossRef CAS PubMed.
- E. G. Nagato, B. P. Lankadurai, R. Soong, A. J. Simpson and M. J. Simpson, Development of an NMR microprobe procedure for high-throughput environmental metabolomics of Daphnia magna, Magn. Reson. Chem., 2015, 53, 745–753 CrossRef CAS PubMed.
- G. A. Nagana Gowda and D. Raftery, NMR-Based Metabolomics, Adv. Exp. Med. Biol., 2021, 1280, 19–37 CrossRef CAS PubMed.
- L. Li, H. Wu, C. Ji, C. A. van Gestel, H. E. Allen and W. J. Peijnenburg, A metabolomic study on the responses of Daphnia magna exposed to silver nitrate and coated silver nanoparticles, Ecotoxicol. Environ. Saf., 2015, 119, 66–73 CrossRef CAS PubMed.
- P. Wang, Q.-Q. Li, J. Hui, Q.-Q. Xiang, H. Yan and L.-Q. Chen, Metabolomics reveals the mechanism of polyethylene microplastic toxicity to Daphnia magna, Chemosphere, 2022, 307, 135887 CrossRef CAS PubMed.
- T.-Y. Jeong and M. J. Simpson, Daphnia magna metabolic profiling as a promising water quality parameter for the biological early warning system, Water Res., 2019, 166, 115033 CrossRef CAS PubMed.
- H. C. Poynton, N. S. Taylor, J. Hicks, K. Colson, S. Chan, C. Clark, L. Scanlan, A. V. Loguinov, C. Vulpe and M. R. Viant, Metabolomics of microliter hemolymph samples enables an improved understanding of the combined metabolic and transcriptional responses of Daphnia magna to cadmium, Environ. Sci. Technol., 2011, 45, 3710–3717 CrossRef CAS PubMed.
- E. G. Nagato, C. Jessica, B. P. Lankadurai, D. G. Poirier, E. J. Reiner, A. J. Simpson and M. J. Simpson, 1H NMR-based metabolomics investigation of Daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium, Chemosphere, 2013, 93, 331–337 CrossRef CAS PubMed.
- E. G. Nagato, A. J. Simpson and M. J. Simpson, Metabolomics reveals energetic impairments in Daphnia magna exposed to diazinon, malathion and bisphenol-A, Aquat. Toxicol., 2016, 170, 175–186 CrossRef CAS PubMed.
- T. Vandenbrouck, O. A. H. Jones, N. Dom, J. L. Griffin and W. De Coen, Mixtures of similarly acting compounds in Daphnia magna: From gene to metabolite and beyond, Environ. Int., 2010, 36, 254–268 CrossRef CAS PubMed.
- D. Ebert, Introduction to Daphnia biology, Ecology, Epidemiology and Evolution of Parasitism in Daphnia, 2005, pp. 5–18 Search PubMed.
- R. Frankel, M. T. Ekvall, E. Kelpsiene, L.-A. Hansson and T. Cedervall, Controlled protein mediated aggregation of polystyrene nanoplastics does not reduce toxicity towards Daphnia magna, Environ. Sci.: Nano, 2020, 7, 1518–1524 RSC.
- R. Cui, S. W. Kim and Y.-J. An, Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata, Sci. Rep., 2017, 7, 12095 CrossRef PubMed.
- F. Nasser and I. Lynch, Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna, J. Proteomics, 2016, 137, 45–51 CrossRef CAS PubMed.
- A. Pochelon, S. Stoll and V. I. Slaveykova, Polystyrene nanoplastic behavior and toxicity on crustacean Daphnia magna: media composition, size, and surface charge effects, Environments, 2021, 8, 101 CrossRef.
- V. P. Vaz, D. J. Nogueira, D. S. Vicentini and W. G. Matias, Can the sonication of polystyrene nanoparticles alter the acute toxicity and swimming behavior results for Daphnia magna?, Environ. Sci. Pollut. Res., 2021, 28, 14192–14198 CrossRef CAS PubMed.
- W. Lin, R. Jiang, S. Hu, X. Xiao, J. Wu, S. Wei, Y. Xiong and G. Ouyang, Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna, Ecotoxicol. Environ. Saf., 2019, 180, 509–516 CrossRef CAS PubMed.
- F. Savorani, G. Tomasi and S. B. Engelsen, icoshift: A versatile tool for the rapid alignment of 1D NMR spectra, J. Magn. Reson., 2010, 202, 190–202 CrossRef CAS PubMed.
- F. Dieterle, A. Ross, G. Schlotterbeck and H. Senn, Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics, Anal. Chem., 2006, 78, 4281–4290 CrossRef CAS PubMed.
- A. Craig, O. Cloarec, E. Holmes, J. K. Nicholson and J. C. Lindon, Scaling and normalization effects in NMR spectroscopic metabonomic data sets, Anal. Chem., 2006, 78, 2262–2267 CrossRef CAS PubMed.
- C. Spearman, “General Intelligence” Objectively Determined and Measured, Am. J. Psychol., 1904, 15, 201–293 CrossRef.
- M. Bylesjö, A. Sjödin, D. Eriksson, H. Antti, T. Moritz, S. Jansson and J. Trygg, MASQOT-GUI: spot quality assessment for the two-channel microarray platform, Bioinformatics, 2006, 22, 2554–2555 CrossRef PubMed.
- Q. Cui, I. A. Lewis, A. D. Hegeman, M. E. Anderson, J. Li, C. F. Schulte, W. M. Westler, H. R. Eghbalnia, M. R. Sussman and J. L. Markley, Metabolite identification via the madison metabolomics consortium database, Nat. Biotechnol., 2008, 26, 162–164 CrossRef CAS PubMed.
- D. S. Wishart, D. Tzur, C. Knox, R. Eisner, A. C. Guo, N. Young, D. Cheng, K. Jewell, D. Arndt and S. Sawhney, HMDB: the human metabolome database, Nucleic Acids Res., 2007, 35, D521–D526 CrossRef CAS PubMed.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Understanding biophysicochemical interactions at the nano–bio interface, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- P. Rama, J. A. G. Urréa and Z. Abbas, Interfacial Interactions of Humic Acids with Polystyrene Nano-plastics in Aqueous/Ionic Environments: A Molecular dynamics exploration, Environ. Sci.: Nano, 2023, 10, 1385–1393 RSC.
- E. Kelpsiene, I. Brandts, K. Bernfur, M. T. Ekvall, M. Lundqvist, M. Teles and T. Cedervall, Protein binding on acutely toxic and non-toxic polystyrene nanoparticles during filtration by Daphnia magna, Environ. Sci.: Nano, 2022, 9, 2500–2509 RSC.
- E. J. Calabrese, Hormesis: why it is important to toxicology and toxicologists, Environ. Toxicol. Chem., 2008, 27, 1451–1474 CrossRef CAS PubMed.
- M. Teng, X. Zhao, C. Wang, C. Wang, J. C. White, W. Zhao, L. Zhou, M. Duan and F. Wu, Polystyrene nanoplastics toxicity to zebrafish: dysregulation of the brain–intestine–microbiota axis, ACS Nano, 2022, 16, 8190–8204 CrossRef CAS PubMed.
- M. Sun, R. Ding, Y. Ma, Q. Sun, X. Ren, Z. Sun and J. Duan, Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos, Chemosphere, 2021, 282, 131124 CrossRef CAS PubMed.
- Z. Liu, Y. Li, M. S. Sepúlveda, Q. Jiang, Y. Jiao, Q. Chen, Y. Huang, J. Tian and Y. Zhao, Development of an adverse outcome pathway for nanoplastic toxicity in Daphnia pulex using proteomics, Sci. Total Environ., 2020, 144249 Search PubMed.
- B. Zhang, H. Zhang, C. Du, Q. X. Ng, C. Hu, Y. He and C. N. Ong, Metabolic responses of the growing Daphnia similis to chronic AgNPs exposure as revealed by GC-Q-TOF/MS and LC-Q-TOF/MS, Water Res., 2017, 114, 135–143 CrossRef CAS PubMed.
- D. Lane, R. Soong, W. Bermel, P. Ning, R. Dutta Majumdar, M. Tabatabaei-Anaraki, H. Heumann, M. Gundy, H. Bönisch, Y. Liaghati Mobarhan, M. J. Simpson and A. J. Simpson, Selective amino acid-only in vivo NMR: a powerful tool to follow stress processes, ACS Omega, 2019, 4, 9017–9028 CrossRef CAS PubMed.
- S. Maity, A. Jannasch, J. Adamec, M. Gribskov, T. Nalepa, T. O. Höök and M. S. Sepúlveda, Metabolite profiles in starved Diporeia spp. using liquid chromatography-mass spectrometry (LC-MS) based metabolomics, J. Crustacean Biol., 2012, 32, 239–248 CrossRef.
- G. A. LeBlanc, Crustacean endocrine toxicology: a review, Ecotoxicology, 2007, 16, 61–81 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, H. Zhao, J. Cai, Y. Sultan, H. Fang, B. Zhang and J. Ma, Effects of polyvinyl chloride microplastics on reproduction, oxidative stress and reproduction and detoxification-related genes in Daphnia magna, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2022, 254, 109269 CAS.
- N. N. Smirnov, Physiology of the Cladocera, Academic Press, 2017 Search PubMed.
- M. Liu, B. Sun, X. Zhou and L. Chen, Disturbed glucose metabolism by perfluorobutanesulfonate pollutant and benefit of young fecal transplantation in aged zebrafish, Ecotoxicol. Environ. Saf., 2022, 241, 113721 CrossRef CAS PubMed.
- A. M. Houbrechts, A. Beckers, P. Vancamp, J. Sergeys, C. Gysemans, C. Mathieu and V. M. Darras, Age-dependent changes in glucose homeostasis in male deiodinase type 2 knockout zebrafish, Endocrinology, 2019, 160, 2759–2772 CrossRef CAS PubMed.
- S. G. Reid, N. J. Bernier and S. F. Perry, The adrenergic stress response in fish: control of catecholamine storage and release1Communicated by Dr P.W. Hochachka, Editor. 1, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1998, 120, 1–27 CAS.
- M. D. McCoole, N. J. Atkinson, D. I. Graham, E. B. Grasser, A. L. Joselow, N. M. McCall, A. M. Welker, E. J. Wilsterman Jr, K. N. Baer and A. R. Tilden, Genomic analyses of aminergic signaling systems (dopamine, octopamine and serotonin) in Daphnia pulex, Comp. Biochem. Physiol., Part D: Genomics Proteomics, 2012, 7, 35–58 CAS.
- A. G. Scoville and M. E. Pfrender, Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4260–4263 CrossRef CAS PubMed.
- S. Fuchs, V. Behrends, J. G. Bundy, A. Crisanti and T. Nolan, Phenylalanine Metabolism Regulates Reproduction and Parasite Melanization in the Malaria Mosquito, PLoS One, 2014, 9, e84865 CrossRef PubMed.
- Y. Nagasaki, Y. Matsubara, H. Takano, K. Fujii, M. Senoo, J. Akanuma, K. Takahashi, S. Kure, M. Hara, Y. Kanegae, I. Saito and K. Narisawa, Reversal of Hypopigmentation in Phenylketonuria Mice by Adenovirus-Mediated Gene Transfer, Pediatr. Res., 1999, 45, 465–473 CrossRef CAS PubMed.
- L.-A. Hansson and S. Hylander, Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behavior, and community ecology of zooplankton, Photochem. Photobiol. Sci., 2009, 8, 1266–1275 CrossRef CAS PubMed.
- J.-S. Chen, Alcohol dehydrogenase: multiplicity and relatedness in the solvent-producing clostridia, FEMS Microbiol. Rev., 1995, 17, 263–273 CrossRef CAS PubMed.
- K. D. Ramachandriya, M. R. Wilkins, M. J. M. Delorme, X. Zhu, D. K. Kundiyana, H. K. Atiyeh and R. L. Huhnke, Reduction of acetone to isopropanol using producer gas fermenting microbes, Biotechnol. Bioeng., 2011, 108, 2330–2338 CrossRef CAS PubMed.
- K. Arslan, T. Schoch, F. Höfele, S. Herrschaft, C. Oberlies, F. Bengelsdorf, M. C. Veiga, P. Dürre and C. Kennes, Engineering Acetobacterium woodii for the production of isopropanol and acetone from carbon dioxide and hydrogen, Biotechnol. J., 2022, 17, 2100515 CrossRef CAS PubMed.
- D. T. Jones and D. Woods, Acetone-butanol fermentation revisited, Microbiol. Rev., 1986, 50, 484–524 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00142c |
| This journal is © The Royal Society of Chemistry 2023 |