Open Access Article
Open Access ArticleCommercial photocatalyst changes the behavior of Formica pratensis and Formica polyctena†
Zsolt
Czekes
*ab,
Dóra
Bai
a,
Judit
Vincze
a,
Emese
Gál
c,
Zsuzsanna
Réthi-Nagy
ade,
Lucian
Baia
 bfg and
Zsolt
Pap
bfg and
Zsolt
Pap
 *bfh
*bfh
aHungarian Department of Biology and Ecology, Babeş-Bolyai University, Cluj-Napoca, Romania
bNanostructured Materials and Bio-Nano-Interfaces Center, Institute for Interdisciplinary Research on Bio-Nano-Sciences, Babeş – Bolyai University, Cluj-Napoca, Romania. E-mail: zsolt.pap@ubbcluj.ro
cFaculty of Chemistry and Chemical Engineering, Babeş-Bolyai University, Cluj-Napoca, Romania
dBiological Research Centre, Institute of Biochemistry, MTA Lendület Laboratory of Cell Cycle Regulation, University of Szeged, Szeged, Hungary
eDoctoral School of Biology, University of Szeged, Szeged, Hungary
fInstitute of Research-Development-Innovation in Applied Natural Sciences, “Babes-Bolyai” University, Cluj-Napoca, Romania
gEmil. G. Racoviţă Institute, Babeş-Bolyai University, Cluj-Napoca, Romania
hDepartment of Applied and Environmental Chemistry, University of Szeged, Szeged, Hungary. E-mail: pzsolt@chem.u-szeged.hu
First published on 24th November 2022
Abstract
Nanosized materials are currently applied nearly everywhere and nanotechnology is part of our everyday lives. For important challenges such as those relating to clean air, water and energy, semiconductive nanoparticles show the most promising solutions. TiO2 is among those semiconductors which are widely applied and studied in water purification processes by photocatalysis. Despite its abundant applications, its ecotoxicological investigations are relatively rare and none of these address insect behaviour. Therefore, in the present study the impact of a well-known commercial TiO2 (Evonik Aeroxide P25) on the interspecific behaviour of two territorial ant species (Formica polyctena and Formica pratensis) was investigated. Changes in the behaviour of the ants were observed using aggressivity assays. We found that the results of these tests can be linked to the changes observed in the cuticular hydrocarbon (CHC) profile of the ants, as the applied semiconductor demonstrated its photocatalytic activity and oxidized the hydrocarbons to corresponding alcohols, aldehydes and carboxylic acids, altering the CHC profile, and thus interfering with species recognition. The results are relevant in terms of the potential uses of nanoparticles in more technologies. A significant proportion of these nanoparticles are photoactive materials and can interact with insects using chemical communication channels (e.g. ants and possibly bees).
Environmental significancePhotocatalytic materials are currently used in everyday life in self-cleaning paints, sensors, and surface disinfectants, as well as in other fields not directly related to photocatalysis, such as electronics, sensors, etc. these nanomaterials are intruding into the environment slowly but surely and are already present in water and surface soil samples. Therefore, the effects of these nanomaterials on the environment should be investigated. While classical ecotoxicological investigations on animals or model organisms investigate mortality, fertility and evolutionary aspects, behavioural ecotoxicology shows the changes in behaviour of the organism. This is a more long-term effect which should be considered, as behaviour within an environmental matrix influences whole ecologic chains, hence influencing the whole environment. The investigated material, titania, is one of the most abundant semiconductors used, covering the full application spectrum mentioned above, and it can be found nearly everywhere in our everyday life. Additionally, it is currently the most efficient photocatalyst, generating strongly oxidizing holes and OH radicals when excited. Therefore, the question posed is whether such a material can intervene and influence the behaviour of animals. For this ants were chosen, as an omnipresent model organism that communicates with pheromones and cuticular hydrocarbons (CHCs, as identifier compounds). It was found that titania modified the composition of the CHCs and influenced the behaviour of the investigated ants. As this was found to be a significant interaction, we should ask serious questions about how we affect animal life through the use of nanoparticles. The effects of such a behavioural modification are not immediate; hence further work needs to be dedicated to this area, as bees and other insects are also dependent on chemical communication. |
Current concerns regarding the safety of nanomaterials need to be urgently addressed. Safety concerns are focused mainly on humans. However, nanomaterial safety is evaluated by using animal-based experiments1,2 including other model organisms.3–5 It is crucial to understand the effect of nanomaterials on the environment. One of the most quickly developing research fields in nanotechnology and materials science is photocatalysis. This research area focuses on the exploitation of the photoinduced semiconductive properties of several materials. The functioning of photocatalysts starts with photon absorption by the semiconductive material, which (if the energy of the photon corresponds to the band-gap energy of the semiconductor) can generate charge carrier species (electrons and holes). Holes can participate in oxidation, while electrons can participate in reduction reactions, making possible the degradation of organic pollutants by direct oxidation or by the generation of reactive radical species, such as ˙OH.
In this way, a low-cost, low-energy input method is available to degrade organic pollutants, such as dyes,6 pesticides,7 pharmaceuticals,8 hormones9 and other potentially dangerous organic pollutants.10
The most important photocatalyst is titanium dioxide, which is currently applied in self-cleaning surfaces,11 paints,12 antimicrobial coatings,13 solar photocatalytic reactors,14,15 and for water cleaning. Additionally, it has potential applications in artificial photosynthesis16 and H2 generation.17 As these applications are already established and commercial products are readily available to purchase, it is necessary to consider the erosion and degradation of these products, which contain photoactive titania nanoparticles. Ecotoxicological investigations on titania nanoparticles are scarce compared to the huge range of applications of the materials, as detailed above, which is worrying. Studies on specific organisms are already available for different microalgae,18,19 and combined terrestrial and aquatic organisms20 including investigations at the microenvironment level. In other cases the effects of nanosized titania have been investigated on red clover and its rhizobial symbiont.21 In each of the cases of growth inhibition, changes in reproduction or impact on the microenvironment's nitrification bacteria number were revealed. These investigations demonstrated direct ecotoxicological issues with immediate effects, which can be directly interpreted. There is, however, a void of critical knowledge in the scientific literature on long-term effects. Among these effects are those which are not immediately visible by spontaneous observation, which include changes in behaviour, communication, food harvesting, pathfinding, etc. of different species. All these factors can influence the whole structure and composition of ecosystems, which are multivariable systems, at all organisational levels.22 Therefore, in the present work, social insects (ants) were considered. The impact of titania nanoparticles on the behaviour of two ant species was investigated, considering the chemical background of the observed changes and the possible long-term effects.
Ants are ideal organisms for monitoring environmental changes,23 and therefore they can also be used to observe toxic effects. They are also considered to be ideal indicators of pollution due to the facts that they are predators and their nesting behaviour makes them less mobile compared to other insects.24 Most ant species, such as red wood ants, are situated high in the food chain, and an eventual biomagnification effect makes them susceptible to long-term but lower-level pollution, making them ideal for observing the toxic effect of materials which are considered to have no or only a short-term effect, but which might cause problems in the long term.
Ants are also easy to collect in large numbers, repeating of tests is possible, the tested effect can be observed at an individual, colony and community level, and the collection of ants can be conducted in a relatively non-invasive way and at low cost.23,25–27
Some species groups appear to be resistant to pollutants; therefore, we have chosen red wood ants (Formica s str.) for our study, due to the fact that they have already been proved to be sensitive to pollution.28
Behavioral changes in invertebrates caused by toxic compounds have been observed in the case of pesticides,29,30 but few studies have been conducted concerning the effect of industrial pollutants.31,32 To assess the behavioural effects of different compounds, ants are ideal study organisms as they can accumulate pollutants,28,33 and their aggressive behaviour towards other ant species is well documented.34–37 Ants recognise, and can distinguish between, other ants at different levels, from nestmates to other species based on their cuticular hydrocarbon profile,35,38,39 and any misidentification could lead to an altered hierarchy between ant species, altering the ant community and implicitly the whole ecosystem. Ants show a complex hierarchical system based on dominance, with species ranging from submissive to territorial.40 Red wood ants, as territorial species, show a greater aggressive interspecific behaviour; therefore, studying the effect of foreign material on their behaviour is valuable.
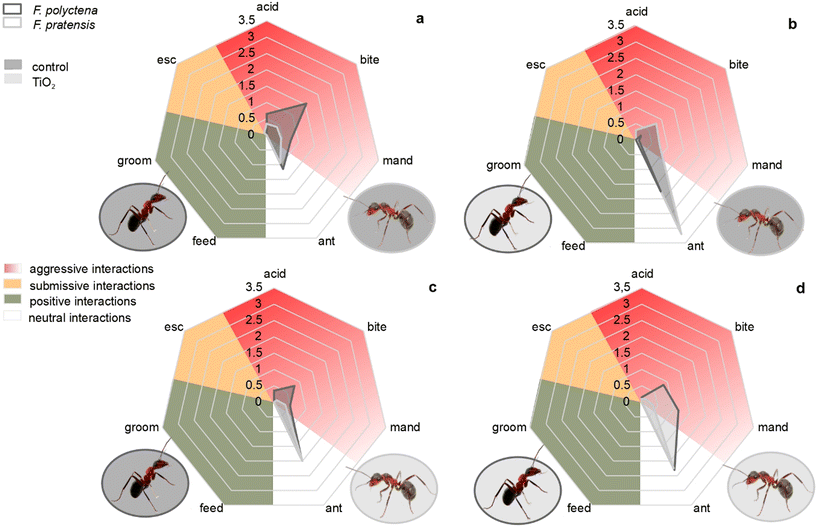
In order to assess the behavioral patterns of workers, we observed the number of interactions with the rival ant workers in all the possible scenarios. The behavioral assays showed interesting insights into the behavioral changes caused by the treatment with TiO2. The behavioral profile of F. polyctena during an aggressivity test using both species under normal conditions (without the application of titania for both species) showed highly aggressive behaviour towards its rival F. pratensis – the gaping of mandibles, biting and even formic acid squirting were frequent. Although in these circumstances F. pratensis also showed a slightly aggressive behavioral profile, the neutral interactions used for recognition (antennating) were the most characteristic (Fig. 1a).
When workers of both species were treated with P25, the more aggressive behavioral profile of F. polyctena was maintained, although no acid squirting occurred, while the behavior of F. pratensis became even less aggressive. In addition, the recognition behavior became much more pronounced in both species, suggesting a hardened recognition (Fig. 1d). During testing in which only F. pratensis was treated with TiO2, F. polyctena again showed an enhanced level of aggressiveness, displaying not just mandible gaping and biting, but also quite frequent acid squirts, while F. pratensis still showed few signs of aggression (Fig. 1c). Surprisingly, in the test in which only F. polyctena was the subject of titania treatment, the roles reversed. F. pratensis showed a high number of aggressive interactions, while the behavioral profile of F. polyctena became much less aggressive. Antennating, however, was much more pronounced for F. pratensis (Fig. 1b).
In general, the tests showed a dominance of F. polyctena when both species were untreated, or when the rivals were both treated with titania, which seems to lower the level of aggression of the treated individual, but enhance the antennation of the rival ant, suggesting difficulties in the recognition process.
The effect of the photocatalyst on the aggressive and neutral interactions of the ants
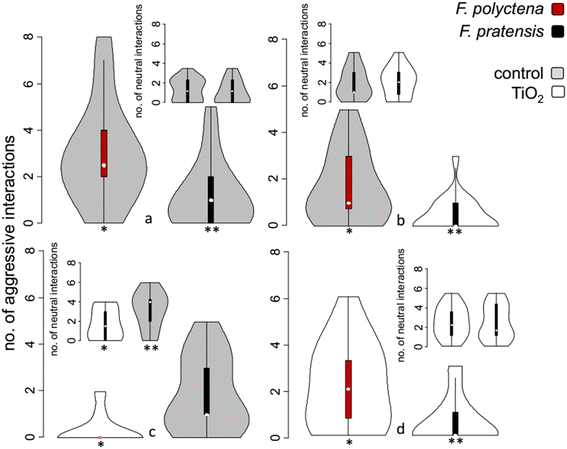
When none of the ant species were treated with the photocatalyst suspension, F. polyctena proved to be dominant, showing significantly more aggressive interactions towards F. pratensis. (GLM, z = −2.303, p = 0.021, n = 20; Fig. 2a). When only F. pratensis was treated, this dominance persisted (GLM, z = −2.280, p = 0.022, n = 20; Fig. 2b); however, the number of interactions between the two seemed to get slightly lower.This scenario was repeated when ants of both species were treated with titania (GLM, z = −2.138, p = 0.032, n = 20; Fig. 2d). Despite F. pratensis being the less aggressive species in every other scenario, when only F. polyctena was treated, F. pratensis became clearly dominant, and the number of aggressive interactions displayed by F. polyctena was much lower (GLM, z = 2.128, p = 0.033, n = 20; Fig. 2c).
The number of neutral interactions displayed by workers of the two species did not differ in most cases; however, F. pratensis showed a higher number of neutral interactions (antennating) when only its rival was treated with TiO2 (GLM, z = 2.501, p = 0.012, n = 20; Fig. 2c – insert).
The number of submissive interactions was extremely low, while no positive interaction was observed; therefore, no differences were found between the numbers of these interactions. It is worth mentioning, however, that all submissive interactions (escaping) were displayed by F. pratensis workers in the situation in which none of the ants were treated with TiO2.
In general, we can state that the photocatalyst did not decrease the aggressivity of the workers when the rival species was also treated, but significantly decreased the aggressive behaviour when the rival species was not treated, in the case of both species. This effect can be attributed to the change of the CHC profile, but also hints at a toxic effect, which can decrease the overall activity of the workers.
The interesting changes in the behaviour of the ants can be understood by analysing their cuticular hydrocarbon profile. Observations were made concerning the fact that the normal behaviour of each of the ant species studied changes when in contact with TiO2 under irradiation. Additionally, the reference measurements proved that the observed effects originated from the photocatalytic activity of P25. It should be mentioned that normal UV-A radiation does not affect the behavior of any ant species (they encounter this type of electromagnetic irradiation in nature in much higher flux during the day than in our experiments).
Therefore, the main question still remains – what is the real reason for such a change in behavior? The test duration is in the order of minutes and aggressivity related issues are directly linked with recognition and therefore with cuticular hydrocarbons. This suggests that it is necessary to investigate the CHC profile of the ants before and after the aggressivity tests.
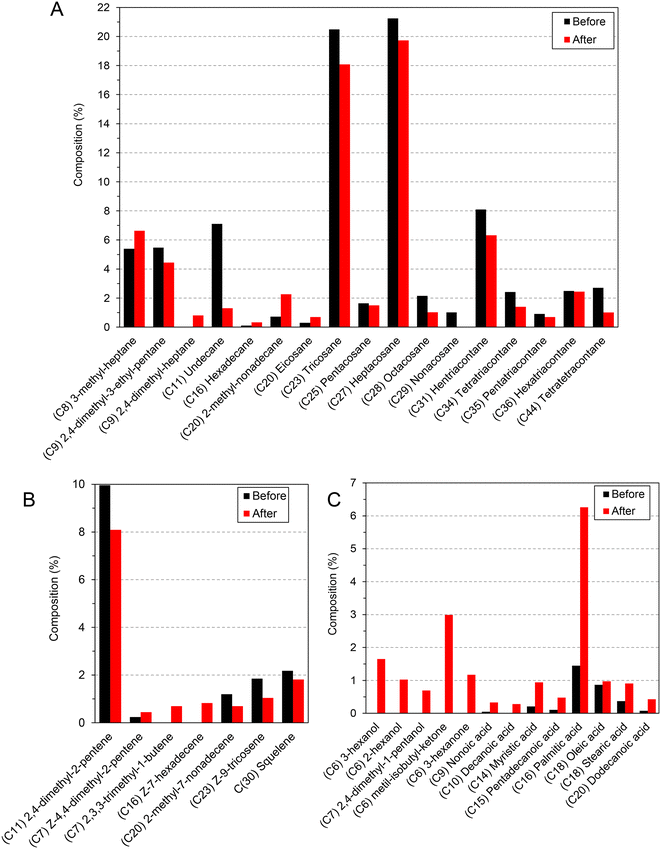
The analysis of the CHC profile was carried out as described in the experimental section. In the case of Formica pratensis the analysis demonstrated that the expected hydrocarbons were present in the samples. C25, C27, C29, C31, C33 and C35 alkanes, among others (Fig. 3), were identified as the major fingerprints, which is in good accordance with the data available in the literature.41 These were identified in the mass spectra of the reference sample, which contained untreated ants. As discussed above, any changes in this fingerprint could lead to drastic behavioral changes, as it changes the identity of the ant within its community. It is already known that Evonik Aeroxide P25 can efficiently degrade hydrocarbons42,43 under UV light irradiation; therefore, the outcome of the contact between titania and the CHC is keenly awaited.
 | ||
| Fig. 3 The composition of the CHC profile of Formica pratensis (A – alkanes, B – alkenes) before and after the aggressivity tests, showing the intensive degradation of the specific hydrocarbon fingerprints. The degradation products were identified afterwards (C).‡ | ||
The relatively short contact time (including irradiation) was enough to degrade nearly all the hydrocarbons (mostly alkanes, but some of the alkenes) that were identified as fingerprint compounds. These hydrocarbons are very stable and they are secreted continuously by the ant, so the possibility of sublimation and spontaneous degradation can be ruled out. Moreover, the appearance of oxidation products, such as alcohols, carboxylic acids and aldehydes, was detected. The process was so fast that most probably the more volatile/gaseous degradation intermediates (such as low carbon number aldehydes, alcohols, carboxylic acids and CO2 (ref. 44)) disappeared immediately. The appearance of the products mentioned previously is not surprising as titania photocatalysts produce a large number of hydroxyl radicals,45 which are quite aggressive and non-selective oxidizers. Carbon chain fragmentation was also observed, as C11–C15 hydrocarbons started to appear as well. Interestingly, shorter chained, branched C8–C9 hydrocarbons were also observed in the starting samples for Formica pratensis, meaning they might be part of the CHC profile.
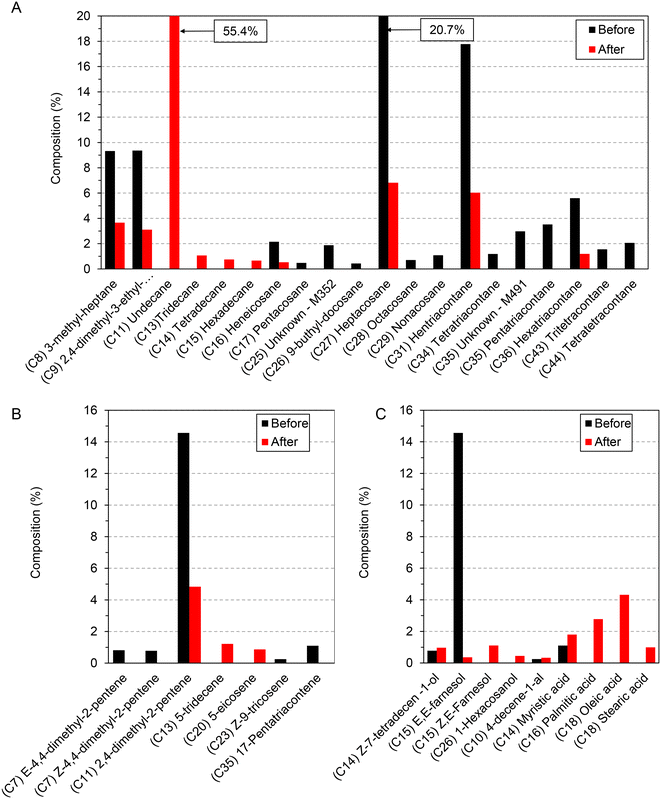
The situation is quite similar in the case of Formica polyctena (Fig. 4), as fingerprint hydrocarbons were successfully detected, such as those from C23–C32,41 although other hydrocarbons were also observed (smaller than C23 and greater than C32). The major difference was that the alkane ratio did not change significantly after degradation, just the overall quantity, meaning that each of the CHC components degraded. In the case of alkenes, a more diverse set of compounds was observed, pointing to a more intensive carbon chain break. Additionally, the appearance of alcohols, ketones and carboxylic acids as degradation products was observed.
Conclusions
The contact of titania nanoparticles with ants seems to have a significant effect on their behavior. This issue was investigated by putting two ant species (Formica pratensis and Formica polyctena) in contact with Evonik Aeroxide P25 nanoparticles. The impact of the nanostructures was explored by investigating the reciprocal behavioral patterns of the ants. The observed effects were explained by following the changes in the CHC profiles of the ants using GC-MS.The effect of these particles seemed to be to decrease the aggressivity of the treated ants, as well as increase the antennating, which suggests an increase in the difficulty of species recognition. This change can be mainly attributed to the change in the CHC profile of both the investigated ant species. As a result of the active photocatalyst, the hydrocarbon structure was modified or suffered degradation. Therefore, carbon chain fragmentation occurred, and the formation of alcohols, ketones, aldehydes and carboxylic acids as oxidation products was detected. As these compounds are not naturally part of the CHC profile, the finely tuned and highly important social behavior of the affected ants can change. The impact of such a change is long-term and of high importance, because these organisms interact dynamically with their environment, being a crucial element of their ecosystem. In a stable terrestrial habitat, any modification occurring in the interspecific behavior of these predators can alter the balance of the ecosystem, resulting in long-term unpredictable effects. If this issue is not assigned enough importance, it may be too late by the time nanostructures (which are already in our surrounding environment) induce irreversible and most probably unpredictable effects on our environment.
Materials and methods§
Aggressivity tests
For testing the interspecific differences in the ants' aggressivity caused by the nano-sized titania, we used two territorial red wood ant species (Formica polyctena Nylander, 1848 and F. pratensis Retzius, 1783). A small number of ant workers was collected from large colonies, with minimal disturbance, from the nest surface in order to ensure that the collected workers belonged to the same cast. The ants were kept in an artificial nest for acclimatization to laboratory conditions for 2 weeks prior to the experiments, during which time they were fed by artificial food widely used for rearing ants,46 and their soil was kept moist by adding clear water. 24 hours before testing we transferred 70 ants in each of two open-top boxes for each species, the internal vertical walls of which were treated with a PTFE suspension to prevent the ants from escaping. For each species, the workers in one of the boxes were treated with 5 mL of 1 g L−1 Evonik Aeroxide P25 aqueous suspension, while those in the other box (used as a control) were treated with the same amount of distilled water applied using an atomizer. The ants were kept under UV-A light (6 W blacklight, 365 nm emission maximum) for 24 hours before testing, although real situations could include much longer contact times. The UV-A or P25 without UV-A light did not change the structure of the hydrocarbons. It is important to mention that environmental UV is several orders of magnitude higher (the catalyst can be activated by any wavelength of UV below 400 nm). The energy of the Sun is ∼1400 Wh m−2 (general data, no light obstruction) of which ∼140 Wh m−2 is UV. In our case just a 6 W lamp was used (higher power could not be considered due to the excessive emitted heat). The ants were marked on the abdomen using different coloured acrylic paint for each group. Behavioral tests were performed for each possible pairing of the four groups (treated and untreated Formica pratensis and F. polyctena), each test type being replicated 20 times.The tests were performed in polystyrene Petri dishes (d = 90 mm), each time using one worker of each group. Before the start of the test, the ants were separated until they settled down, after which the separator was removed. The duration of the tests was 1 min, counted from the first interaction. During the tests the following interactions were noted for each ant: antennating, feeding, grooming (neutral interactions), biting, ant squirting, mandible gaping (aggressive interactions), and escaping (submissive interaction). The effect of TiO2 on the behaviour of the ants was tested using GLM models, with the number of interactions as the dependent variable and the treatment as the independent variable.
For the GC/MS analysis, a Shimadzu GC-2010 and MS QP 2010 Plus system were used. The measurements were performed on a (5%-phenyl)-methylpolysiloxane stationary phase (30 m × 0.25 mm i.d. × 0.25 mm film thickness) GC column. The samples were injected using the splitless injection mode through an inlet liner with glass wool. The injection volume was 2 mL, while the injector temperature was 220 °C. Helium (99.999% purity) was used as the carrier gas. The oven temperature program was as follows: 50 °C for 5 minutes, increasing up to 200 °C at 20 °C min−1, increasing up to 300 °C at 5 °C min−1, and then maintaining this temperature for 10 min. The total run time was 42 min. The temperature of the MS source was 200 °C, and the interface temperature was 250 °C. For the identification of compounds the NIST database was used. All the ants were used in the hexane extraction and GC/MS analysis to obtain accurate results. For 5 ants 1 mL of hexane was used to extract the CHCs.
P25 was used as the photoactive material, as stated in the previous section. The purchased commercial powder was analysed to verify the main properties of the nanopowder and to validate its quality. The structural analysis carried out by XRD indicated clearly that 89 wt% of anatase and 11 wt% of rutile was the crystal phase composition, while a smaller amount of an amorphous phase has also been suggested in some publications.47 The mean primary crystallite size was calculated to be 25.4 nm for anatase and 45.4 nm for rutile, which is also in agreement with the already available data, and this was confirmed by transmission electron microscopy. The specific surface area was also measured, and the usual 49.5 m2 g−1 value was obtained. Optical data was also collected, and the band-gap was calculated by the Kubelka–Munk/Tauc plot48 approach, which indicated that the powder is photoactive under UV-A irradiation (3.12 eV was the calculated band-gap energy). All the basic characterisation showed that the nanopowder used had the necessary quality for our experiments. Two degradation experiments were also carried out with monuron and phenol to check the photoactivity, and the same activity was obtained as in our previous publications.49,50 All the basic data and analysis is summarized in the Supplementary Information. Based on these results, the P25 was validated and ecotoxicological investigations were carried out.
Author contributions
Zs. C., D. B., J. V. performed experiments, analysis, and writing the manuscript. E. G., Zs. R.-N., L. B. performed conceptualization, analysis, and editing. Zs. P. completed funding acquisition, conceptualization, review, editing, and supervision. Zs. C. and Zs. P. performed conceptualization, investigation, analysis, writing, review, editing, data curation, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude for the financial support provided by the PN-III-P1-1.1-TE-2019-1318 and GTC Nr. 35276/18.11.2020 projects and the Bolyai János research scholarship is also thanked for supporting this researchReferences
- E. Kelpsiene, I. Brandts, K. Bernfur, M. T. Ekvall, M. Lundqvist, M. Teles and T. Cedervall, Environ. Sci.: Nano, 2022, 9, 2500–2509 RSC.
- D. Gökçe, S. Köytepe and İ. Özcan, Chem. Ecol., 2018, 34, 301–323 CrossRef.
- J. Bahamonde, B. Brenseke, M. Y. Chan, R. D. Kent, P. J. Vikesland and M. R. Prater, Toxicol. Pathol., 2018, 46, 431–443 CrossRef CAS PubMed.
- J. Cazenave, A. Ale, C. Bacchetta and A. S. Rossi, Curr. Pharm. Des., 2019, 25, 3927–3942 CrossRef CAS PubMed.
- S. Rana and A. Kumar, Algal Res., 2022, 62, 102641 CrossRef.
- A. Rafiq, M. Ikram, S. Ali, F. Niaz, M. Khan, Q. Khan and M. Maqbool, J. Ind. Eng. Chem., 2021, 97, 111–128 CrossRef CAS.
- M. H. El-Saeid, A. Baqais and M. Alshabanat, Molecules, 2022, 27, 634 CrossRef CAS PubMed.
- L. Lin, H. Wang, W. Jiang, A. R. Mkaouar and P. Xu, J. Hazard. Mater., 2017, 333, 162–168 CrossRef CAS PubMed.
- I. Ramírez-Sánchez and E. Bandala, Catalysts, 2018, 8, 625 CrossRef.
- G. K. Prasad, T. H. Mahato, B. Singh, K. Ganesan, A. R. Srivastava, M. P. Kaushik and R. Vijayraghavan, AIChE J., 2008, 54, 2957–2963 CrossRef CAS.
- N. T. Padmanabhan and H. John, J. Environ. Chem. Eng., 2020, 8, 104211 CrossRef CAS.
- M.-Z. Guo, A. Maury-Ramirez and C. S. Poon, J. Cleaner Prod., 2016, 112, 3583–3588 CrossRef CAS.
- F. Heidenau, W. Mittelmeier, R. Detsch, M. Haenle, F. Stenzel, G. Ziegler and H. Gollwitzer, J. Mater. Sci.: Mater. Med., 2005, 16, 883–888 CrossRef CAS PubMed.
- F. Mazille, T. Schoettl, N. Klamerth, S. Malato and C. Pulgarin, Water Res., 2010, 44, 3029–3038 CrossRef CAS PubMed.
- P. E. Zaruma-Arias, C. M. Núñez-Núñez, L. A. González-Burciaga and J. B. Proal-Nájera, Catalysts, 2022, 12, 132 CrossRef CAS.
- S. Bai, K. Zhang, R. Luo, D. Li, A. Chen and C. C. Liu, J. Mater. Chem., 2012, 22, 12643 RSC.
- C. I. Fort, Z. Pap, E. Indrea, L. Baia, V. Danciu and M. Popa, Catal. Lett., 2014, 144, 1955–1961 CrossRef CAS.
- I. M. Sadiq, S. Dalai, N. Chandrasekaran and A. Mukherjee, Ecotoxicol. Environ. Saf., 2011, 74, 1180–1187 CrossRef CAS PubMed.
- V. Aruoja, H. C. Dubourguier, K. Kasemets and A. Kahru, Sci. Total Environ., 2009, 407, 1461–1468 CrossRef CAS PubMed.
- V. Vijayaraj, C. Liné, S. Cadarsi, C. Salvagnac, D. Baqué, A. Elger, M. Barret, F. Mouchet and C. Larue, Environ. Sci. Technol., 2018, 52, 12757–12764 CrossRef CAS PubMed.
- J. Moll, A. Okupnik, A. Gogos, K. Knauer, T. D. Bucheli, M. G. A. van der Heijden and F. Widmer, PLoS One, 2016, 11, e0155111 CrossRef PubMed.
- M. Anand, A. Gonzalez, F. Guichard, J. Kolasa and L. Parrott, Diversity, 2010, 2, 395–410 CrossRef.
- J. Majer and M. Kaspari, Ants: standard methods for measuring and monitoring biodiversity, Smithsonian Institution Press, Washington D.C., 2000 Search PubMed.
- G. Bengtsson and S. Rundgren, Ambio, 1984, 13, 29–33 CAS.
- P. Skórka, M. Witek and M. Woyciechowski, Insectes Soc., 2006, 53, 97–100 CrossRef.
- I. M. Grześ, Eur. J. Soil Biol., 2010, 46, 350–355 CrossRef.
- W. Czechowski, A. Radchenko, W. Czechowska and K. Vepsäläinen, The ants of Poland : with reference to the myrmecofauna of Europe, Natura optima dux Foundation, Warszawa, 2012 Search PubMed.
- T. Eeva, J. Sorvari and V. Koivunen, Environ. Pollut., 2004, 132, 533–539 CrossRef CAS PubMed.
- K. Heliövaara and R. Väisänen, Ann. Entomol. Fenn., 1983, 49, 103–109 Search PubMed.
- R. H. Parkinson, S. Zhang and J. R. Gray, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 5510–5515 CrossRef CAS PubMed.
- C. Y. Pan, J. C. Mo and M. L. Cheng, Behav. Ecol. Sociobiol., 2006, 48, 841–848 Search PubMed.
- J. Sorvari and T. Eeva, Sci. Total Environ., 2010, 408, 3189–3192 CrossRef CAS PubMed.
- I. M. Grześ, Pedobiologia, 2009, 53, 65–73 CrossRef.
- A. Katzerke, P. Neumann, C. W. W. Pirk, P. Bliss and R. F. A. Moritz, Behav. Ecol. Sociobiol., 2006, 61, 143–150 CrossRef.
- S. Martin and F. Drijfhout, J. Chem. Ecol., 2009, 35, 1151–1161 CrossRef CAS PubMed.
- S. E. Wittman, D. J. O'Dowd and P. T. Green, Ecosphere, 2018, 9, e02403 CrossRef.
- W. Chen, Á. O'sullivan and E. S. Adams, Ecol. Entomol., 2018, 43, 263–272 CrossRef.
- A. Lenoir, S. Depickère, S. Devers, J. P. Christidès and C. Detrain, J. Chem. Ecol., 2009, 35, 913–921 CrossRef CAS PubMed.
- P. P. Sprenger and F. Menzel, Myrmecol. News, 2020, 30, 1–26 Search PubMed.
- R. Savolainen and K. Vepsalainen, Oikos, 1988, 51, 135–155 CrossRef.
- S. J. Martin, H. Helanterä and F. P. Drijfhout, Biol. J. Linn. Soc., 2008, 95, 131–140 CrossRef.
- E. K. Tetteh, S. Rathilal and D. B. Naidoo, Sci. Rep., 2020, 10, 1–12 CrossRef PubMed.
- I. U. Haq, W. Ahmad, I. Ahmad and M. Yaseen, Water Environ. Res., 2020, 92, 2086–2094 CrossRef PubMed.
- I. Izumi, W. W. Dunn, K. O. Wilbourn, F. R. F. Fan and A. J. Bard, J. Phys. Chem., 2002, 84, 3207–3210 CrossRef.
- C. Buck, N. Skillen, J. Robertson and P. K. J. Robertson, Chin. Chem. Lett., 2018, 29, 773–777 CrossRef CAS.
- A. Bhatkar and W. H. Whitcomb, Fla. Entomol., 1970, 53, 229–232 CrossRef.
- B. Ohtani, O. O. Prieto-Mahaney, D. Li and R. Abe, J. Photochem. Photobiol., A, 2010, 216, 179–182 CrossRef CAS.
- P. Kubelka and F. Munk, Z. Phys. Chem., 1931, 12, 593–601 Search PubMed.
- G. Kovács, S. Fodor, A. Vulpoi, K. Schrantz, A. Dombi, K. Hernádi, V. Danciu, Z. Pap and L. Baia, J. Catal., 2015, 325, 156–167 CrossRef.
- B. Hampel, G. Kovács, Z. Czekes, K. Hernádi, V. Danciu, O. Ersen, M. Girleanu, M. Focşan, L. Baia and Z. Pap, ACS Sustainable Chem. Eng., 2018, 6, 12993–13006 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d1en01119g |
| ‡ A sample MS spectra is provided in the ESI. |
| § The methods used for the basic characterization of titania are detailed in the ESI. |
| This journal is © The Royal Society of Chemistry 2023 |