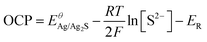Potentiometric nanosensor for real-time measurement of hydrogen sulfide in single cell†
Shuwen
Cheng
,
Shuai
Zhang
,
Rantong
Liu
,
Hui
Zeng
,
Yongyue
Yin
and
Meining
Zhang
 *
*
Department of Chemistry, Renmin University of China, Beijing, 100872, China. E-mail: mnzhang@ruc.edu.cn
First published on 19th January 2023
Abstract
One potentiometric nanosensor for monitoring intracellular hydrogen sulfide (H2S) with fast potential response, high selectivity and excellent antifouling properties was developed. This study constructs a powerful tool to real-time track the changes of intracellular H2S in situ, promoting the future studies of physiologically relevant processes.
Hydrogen sulfide (H2S), an important neuromodulator, is one of the three major gas signaling molecules.1,2 Neurodegenerative diseases including Alzheimer's disease, Parkinson's disease and Huntington's disease are associated with the level of H2S.3–5 Endogenous H2S plays an indispensable role in vivo by expanding blood vessels, regulating metabolism and resisting inflammation.6–9 H2S is involved in cellular regulatory processes including regulating intracellular pH, promoting calcium influx of neurons and facilitating N-methyl-D-aspartic acid (NMDA) receptors.9 To the best of our knowledge, most of the sulfur stored in cells with different kinds of forms like acid-labile sulfur and bound sulfur, which will release H2S under external stimulation.10 Although chromatography,11,12 fluorescence,13–16 colorimetric assays17–19 and electrochemical methods19–21 have been used to detect H2S in the brain or single cells, it is still challenging to real-time track intracellular H2S in situ with fast response and selectivity.
Electrochemistry with high spatiotemporal resolution has been widely applied in monitoring neurochemicals released in biological and pathological systems continuously and rapidly.22–25 To date, electrochemical sensing of H2S can be realized through both amperometry and potentiometry. For example, Hu et al. developed a 3D porous structure to detect H2S released from single HeLa cells with amperometric methods.26 Dong et al. developed a biosensor for the monitoring of endogenous H2Sn and H2S with differential pulse voltammetry in hippocampal slices and living rats based on specific reaction.27 However, the amperometric method faces the issue of contamination from sulfur in continuous measurement, resulting in low stability and sensitivity decrease. To address this problem, Pan et al. synthesized one single-atom catalyst for electrocatalytic H2S at very low potential, which enables the construction of one stable H2S sensor with galvanic redox potentiometry for real-time H2S sensing in the living brain.28 We developed one potential method to selectively track the dynamics of H2S in the brain with Ag2S coated Ag nanoparticles (AgNPs) on a microelectrode based on a potential method.29 Compared to amperometric methods, potentiometry guided by the Nernst equation is independent of electrode area and has very small current flow, which will be beneficial for cell analysis. Therefore, we anticipate that our potentiometric sensor could be pushed to the nano-size and evaluate its feasibility in cell sensing.
Herein, we demonstrate one potentiometric method using a AgNP-modified carbon fiber nanoelectrode treated with Na2S, forming Ag2S/AgNPs/CFNE for the real-time monitoring of H2S in a single cell with high selectivity and stability. We find that potentiometry is easily controlled for intracellular electrochemistry analysis comparing with amperometry. Moreover, Ag2S/AgNPs/CFNE exhibits a highly selective and stable potential response to H2S. Because Ag2S/AgNPs/CFNE can resist acid and base interference, Ag2S/AgNPs/CFNE is exploited as a powerful tool to achieve the continuous monitoring of H2S in single cells with different pH environments. Beyond all that, we found that intracellular H2S will be released upon the external stimulation of potassium. This real-time and in situ H2S measurement method with high selectivity will present a new perspective in the study of physiology and pathophysiology associated with H2S.
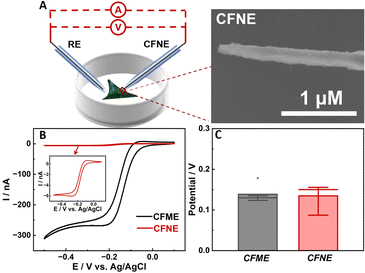
To demonstrate that the potential response is independent of electrode area, which is different from amperometric methods, cyclic voltammograms (CV) and the open-circuit potentials of the CFME and CFNE in HEPES buffer containing 1 mM Ru(NH3)63+ were compared (Fig. 1B and C). As shown in Fig. 1B, the current response of the CFME is 50 times larger than that of the CFNE. In contrast, both the CFME and CFNE have close potential responses towards 1 mM Ru(NH3)63+ (ca. 150 mV) (Fig. 1C). For intracellular electrochemical analysis using the amperometric method, non-membrane permeable Ru(NH3)63+ is normally used to evaluate the insertion depth of the CFNE into the cell.30 With the increase of the insertion depth of the CFNE into the cells incubated with HEPES buffer containing 1 mM Ru(NH3)63+, the reduction current decreased correspondingly. The OCP response did not change until the nanoelectrode inserted into the cell completely (Fig. S1, ESI†). These results indicate that open-circuit potential is easier to control for intracellular analysis because it is not necessary to ensure that the whole CFNE is inserted into the cell.
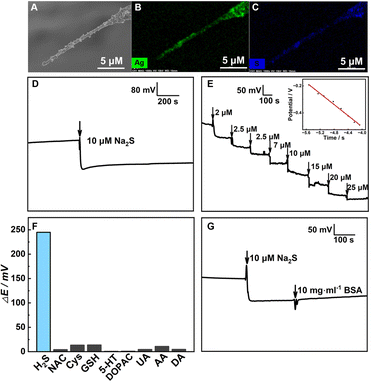
To develop one H2S nanosensor, Ag nanoparticles (NPs) were electrodeposited on the CFNE building up a layer with a reversible redox equilibrium at the interface of AgNPs/CFNE. And the AgNPs/CFNE was further pre-treated with 5 μM Na2S at open-circuit potential until the potential balanced for 300 s to form the Ag2S/AgNPs/CFNE. The SEM image (Fig. 2A) and the corresponding energy dispersive spectrometer (EDS) elemental mapping (Fig. 2B and C) of the local Ag2S/AgNPs/CFNE suggests the homogeneous decoration of Ag2S/AgNPs on the surface of the CFNE.
To output OCP, Ag/AgCl was used as a reference electrode and the Ag2S/AgNPs/CFNE as a working electrode. In this work, we mainly utilized Na2S as the source of dissolved H2S in the bulk electrolyte because there is a transformation between them in the solution:
| H2S ⇌ HS− + H+ ⇌ S2− + 2H+ |
Considering an insoluble salt electrode, the output potential OCP equals the potential difference between the indicated electrode and the reference electrode according to the Nernest equation, and the change of OCP response is only related to the concentration of S2−:
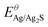 , R, T and F are the formal potential of Ag/Ag2S, the universal gas constant, the temperature, and the Faraday constant, respectively. As we can see from Fig. 2D, the Ag2S/AgNPs/CFNE exhibited an excellent steep potential response for 10 μM Na2S in HEPES (pH = 7.4) and remained stable for 1000 s. The good stability is ascribed to there being no current flow in the OCP, resulting in no sulfur formation, which is ensured by no sulfur characteristic peak in the X-Ray photoelectron spectra of the electrode after detection (Fig. S2, ESI†). This nanoelectrode also exhibited a linear relationship (Fig. 2E) between the potential decrease and the logarithmic concentration gradient of H2S from 2 μM to 84 μM (OCP (mV) = −205.32
, R, T and F are the formal potential of Ag/Ag2S, the universal gas constant, the temperature, and the Faraday constant, respectively. As we can see from Fig. 2D, the Ag2S/AgNPs/CFNE exhibited an excellent steep potential response for 10 μM Na2S in HEPES (pH = 7.4) and remained stable for 1000 s. The good stability is ascribed to there being no current flow in the OCP, resulting in no sulfur formation, which is ensured by no sulfur characteristic peak in the X-Ray photoelectron spectra of the electrode after detection (Fig. S2, ESI†). This nanoelectrode also exhibited a linear relationship (Fig. 2E) between the potential decrease and the logarithmic concentration gradient of H2S from 2 μM to 84 μM (OCP (mV) = −205.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg[S2−] (μM) − 1324.14 (mV), R2 = 0.9903). The detection limit for the nanoelectrode was estimated to be ca. 0.6 μM (S/N = 3). We also investigated the interference from the electroactive species and sulfur-containing species coexisting in the cell. The potential response of 20 μM dopamine (DA), 200 μM ascorbic acid (AA), 50 μM uric acid (UA), 20 μM dihydroxy-phenyl acetic acid (DOPAC), 10 μM 5-hydroxytryptamine (5-HT), 200 μM glutathione (GSH), 20 μM cysteine (Cys), and 20 μM N-acetyl-L-cysteine (NAC) shows a negligible response compared with that of 20 μM Na2S at the Ag2S/AgNPs/CFNE in HEPES buffer (Fig. 2F). These results indicate that Ag2S/AgNPs/CFNE possesses good selectivity towards H2S against these interferents. Furthermore, with the addition of bull serum albumin (BSA) (10 mg mL−1), the output potential (Fig. 2G) did not evidently change and was retained well resulting from the effective layer of Ag2S/AgNPs and the inherent property of potentiometry. In summary, the properties of fast response, good stability, and high selectivity make the Ag2S/AgNPs/CFNE greatly promising for real-time detection of H2S.
lg[S2−] (μM) − 1324.14 (mV), R2 = 0.9903). The detection limit for the nanoelectrode was estimated to be ca. 0.6 μM (S/N = 3). We also investigated the interference from the electroactive species and sulfur-containing species coexisting in the cell. The potential response of 20 μM dopamine (DA), 200 μM ascorbic acid (AA), 50 μM uric acid (UA), 20 μM dihydroxy-phenyl acetic acid (DOPAC), 10 μM 5-hydroxytryptamine (5-HT), 200 μM glutathione (GSH), 20 μM cysteine (Cys), and 20 μM N-acetyl-L-cysteine (NAC) shows a negligible response compared with that of 20 μM Na2S at the Ag2S/AgNPs/CFNE in HEPES buffer (Fig. 2F). These results indicate that Ag2S/AgNPs/CFNE possesses good selectivity towards H2S against these interferents. Furthermore, with the addition of bull serum albumin (BSA) (10 mg mL−1), the output potential (Fig. 2G) did not evidently change and was retained well resulting from the effective layer of Ag2S/AgNPs and the inherent property of potentiometry. In summary, the properties of fast response, good stability, and high selectivity make the Ag2S/AgNPs/CFNE greatly promising for real-time detection of H2S.
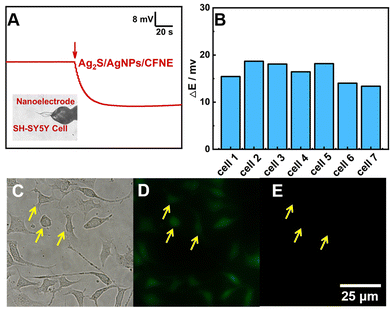
Therefore, we applied this nanoelectrode to detect intercellular H2S. As shown in Fig. 3A, the OCP response of the Ag2S/AgNPs/CFNE presents an obvious decrease for about 18 mV after penetrating into one SH-SY5Y cell immediately, which is calculated to be 1.21 μM H2S according to the calibration. In order to ensure the accuracy of this result, we investigated seven cells under the same experimental conditions and the same step, thus achieving the change of potential statistics for 16.32 ± 1.95 mV, with the corresponding intracellular H2S being 1.20 ± 0.03 μM (Fig. 3B). Fig. 3C shows an image of a cell after inserting the Ag2S/AgNPs/CFNE. The morphology of the cell didn’t change dramatically after penetration by the Ag2S/AgNPs/CFNE. The penetrated cells were stained by fluorescent Calcein-AM (green, live cell, Fig. 3D) and propidium iodide (PI) (red, dead cell, Fig. 3E) indicating negligible damage to the cells by the Ag2S/AgNPs/CFNE. These results demonstrate that the Ag2S/AgNPs/CFNE can measure the intracellular H2S without affecting the cell activity.
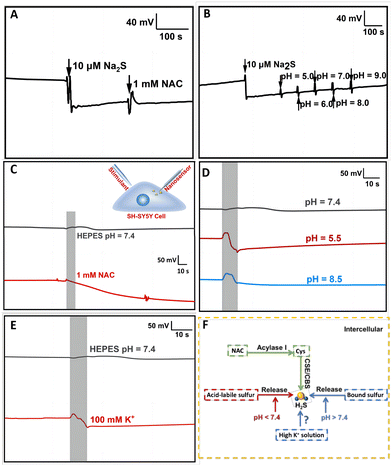
H2S in mammalian cells is produced by both enzymatic and nonenzymatic pathways, with the nonenzymatic pathway accounting for only a small fraction of synthesis. As studied previously, among the enzymatic pathways, two pyridoxal-5′-phosphate (PLP)-dependent enzymes including cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), can catalyse Cys to produce H2S. Cys is abundant in nerve cells and can be transformed from NAC.31 In addition, a large amount of H2S exists in different kinds of compounds in the cell. The critical pH value is one of the most essential factors impacting the release of stored sulfur in cells. Acid-labile released H2S in acid conditions below pH 5.4 and bound sulfur released H2S in alkaline conditions above pH 8.4 have been reported in previous articles.10 First of all, we investigated whether the physiological concentration of NAC and the fluctuation of the pH would have interference, thus affecting the nanosensor selectivity. The result is shown in Fig. 4A and B. Compared with the evident potential decrease in the response to Na2S, the output potential did not change evidently toward 1 mM NAC and with the change of pH by adding HCl and NaOH from 5.0 to 9.0. These results indicate that the Ag2S/AgNPs/CFNE is stable enough to be suitable for tracking H2S under the stimulation of NAC, acid and alkaline solutions. To further explore the physiologic stimuli that might enhance the stored sulfur to transform into free H2S, we measured the change of OCP by the Ag2S/AgNPs/CFNE inserted in a cell and stimulated by 1 mM NAC (2 μL s−1, for 10 s) after the nanoelectrode achieved stability. As shown in Fig. 4C (red curve), after the addition of 1 mM NAC, the potential decreased slowly. In contrast, the addition of HEPES (pH = 7.4, grey curve) did not cause the decrease of the OCP. These results indicate that NAC has a positive influence on promoting the cell to release H2S, which is consistent with reported literature,30 suggesting that the Ag2S/AgNPs/CFNE can monitor the release of H2S. To illustrate the effects of different pH values on the release of H2S, the solution (pH = 5.5, pH = 7.4 and pH = 8.5) was used to simulate the cell. The solution of pH 5.5 (Fig. 4D, red curve) and pH 8.5 (Fig. 4D, blue curve) leads to a quick OCP decrease. In contrast, injecting HEPES (pH = 7.4) as the control group, the output OCP potential response remained constant in the same time scale (Fig. 4D, grey curve). All these results demonstrate that the stored sulfur in cells will be released quickly in a short time when the pH value of the surroundings dramatically changes.
When neurons are excited, Na+ enters and K+ exits from cells, resulting in high K+ concentrations in the extracellular environment and the depolarization of surrounding cells.10 Inspired by this phenomenon, we applied this nanoelectrode to investigate whether stimulation with neuronal excitation (i.e. high concentration of K+) will cause the release of H2S. We found that an apparent potential decrease (Fig. 4E, red curve) was recorded after injecting 100 mM K+ compared to that with HEPES at pH = 7.4 (Fig. 4E, grey curve). This result demonstrates that high K+ concentrations can stimulate a fast release of H2S. This release might result from the activation of the Na+/HCO3− transporter by high K+ concentration. However, the exact mechanism still needs to be studied further. Taken together, the overall results indicate that the Ag2S/AgNPs/CFNE has the strong capability to real-time monitor H2S release under different kinds of pathways (Fig. 4F) in living single cells with a quick response.
In summary, we have fabricated an Ag2S/AgNPs/CFNE with high selectivity and stability for detecting H2S continuously. The combined characteristics of the Ag2S/AgNPs/CFNE with tiny dimensions, fast response and high sensitivity enable this nanoelectrode to real-time and in situ monitor the changes of endogenous H2S in a single-cell under different conditions. We believe that this functional nanoelectrode will open up a new window for the understanding of H2S in physiological and pathological processes.
We greatly acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21874152, 22174162).
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. Mustafa, W. Mu, S. Zhang, S. Snyder and R. Wang, Science, 2008, 322, 587–590 CrossRef CAS PubMed.
- H. Kimura, Neurochem. Int., 2013, 63, 492–497 CrossRef CAS PubMed.
- M. Wang, J. Tang, L. Wang, J. Yu, L. Zhang and C. Qiao, Neural Regener. Res., 2021, 16, 1353–1358 CrossRef CAS PubMed.
- J. Zhang, H. Shan, L. Tao and M. Zhang, J. Mol. Neurosci., 2020, 70, 2020–2030 CrossRef CAS PubMed.
- A. Ramya, M. Joseph, V. Karunakaran, C. Ahammed, A. Samanta and K. K. Maiti, Sens. Actuators, B, 2022, 355, 131118 CrossRef CAS.
- C. Xu, F. Wu, P. Yu and L. Mao, ACS Sens., 2019, 4, 3102–3118 CrossRef CAS PubMed.
- H. Kimura, Amino Acids, 2011, 41, 113–121 CrossRef CAS PubMed.
- Y. Wang, J. Li, Y. Qin, Q. Liu, Z. Liao and X. Xiao, Exp. Clin. Endocrinol. Diabetes, 2020, 128, 137–143 CrossRef CAS PubMed.
- J. Wallace and R. Wang, Nat. Rev. Drug Discovery, 2015, 14, 329–345 CrossRef CAS PubMed.
- M. Ishigami, K. Hiraki, K. Umemura, Y. Ogasawara, K. Ishii and H. Kimura, Antioxid. Redox Signaling, 2009, 11, 205–214 CrossRef CAS PubMed.
- S. Koike, K. Kawamura, Y. Kimura, N. Shibuya, H. Kimura and Y. Ogasawara, Free Radical Biol. Med., 2017, 113, 355–362 CrossRef CAS PubMed.
- C. Hine, Y. Zhu, A. Hollenberg and J. Mitchell, Antioxid. Redox Signaling, 2018, 28, 1483–1502 CrossRef CAS PubMed.
- S. Chen, Z. Chen, W. Ren and H. Ai, J. Am. Chem. Soc., 2012, 134, 9589–9592 CrossRef CAS PubMed.
- A. Lippert, E. New and C. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS PubMed.
- Q. Zhong, R. Zhang, B. Yang, T. Tian, K. Zhang and B. Liu, ACS Sens., 2022, 7, 893–899 CrossRef CAS PubMed.
- L. Yan, Q. Gu, W. Jiang, M. Tan, Z. Tan, G. Mao, F. Xu and C. Li, Anal. Chem., 2022, 94, 5514–5520 CrossRef CAS PubMed.
- Z. Chen, C. Chen, H. Huang, F. Luo, L. Guo, L. Zhang, Z. Lin and G. Chen, Anal. Chem., 2018, 90, 6222–6228 CrossRef CAS PubMed.
- P. Zhang, Y. Hong, H. Wang, M. Yu, Y. Gao, R. Zeng, Y. Long and J. Chen, Polym. Chem., 2017, 8, 7271–7278 RSC.
- C. Wang, M. Wang, W. Zhang, J. Liu, M. Lu, K. Li and Y. Lin, Anal. Chem., 2020, 92, 662–667 CrossRef CAS PubMed.
- T. Xu, N. Scafa, L. Xu, S. Zhou, K. Abdullah Al-Ghanem, S. Mahboob, B. Fugetsu and X. Zhang, Analyst, 2016, 141, 1185–1195 RSC.
- Y. Zhao, Y. Yang, L. Cui, F. Zheng and Q. Song, Biosens. Bioelectron., 2018, 117, 53–59 CrossRef CAS PubMed.
- M. Aref, E. Ranjbari, J. García-Guzmán, K. Hu, A. Lork, G. Crespo, A. Ewing and M. Cuartero, Anal. Chem., 2021, 93, 15744–15751 CrossRef CAS PubMed.
- X. Zhang, A. Hatamie and A. Ewing, Curr. Opin. Electrochem., 2020, 22, 94–101 CrossRef CAS.
- Y. Qi, H. Jiang, W. Wu, F. Zhang, S. Tian, W. Fan, Y. Liu, C. Amatore and W. Huang, J. Am. Chem. Soc., 2022, 144, 9723–9733 CrossRef CAS PubMed.
- R. Pan, D. Wang, K. Liu, H. Chen and D. Jiang, J. Am. Chem. Soc., 2022, 144, 17558–17566 CrossRef CAS PubMed.
- X. Hu, Y. Liu, H. Zhang, C. Xiao, Y. Qin, H. Duo, J. Xu, S. Guo, D. Pang and W. Huang, ChemElectroChem, 2016, 3, 1998–2002 CrossRef CAS.
- H. Dong, Q. Zhou, L. Zhang and Y. Tian, Angew. Chem., Int. Ed., 2019, 58, 13948–13953 CrossRef CAS PubMed.
- C. Pan, F. Wu, J. Mao, W. Wu, G. Zhao, W. Ji, W. Ma, P. Yu and L. Mao, J. Am. Chem. Soc., 2022, 144, 14678–14686 CrossRef CAS PubMed.
- L. Zhang, T. Xu, W. Ji, X. Wang, S. Cheng, S. Zhang, Y. Zhang and M. Zhang, Anal. Chem., 2021, 93, 7063–7070 CrossRef CAS PubMed.
- W. Wu, X. Chen, Y. Jiao, W. Fan, Y. Liu and W. Huang, Angew. Chem., Int. Ed., 2022, 61, e202115820 CAS.
- D. Ezerina, Y. Takano, K. Hanaoka, Y. Urano and T. Dick, Cell Chem. Biol., 2018, 25, 447–459 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experiment details, two tables and eleven figures. See DOI: https://doi.org/10.1039/d2cc06557f |
| This journal is © The Royal Society of Chemistry 2023 |