Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-induced stabilization of microtubules by photo-crosslinking of a Tau-derived peptide†
Soei
Watari
a,
Hiroshi
Inaba
 *ab,
Tomonori
Tamura
*ab,
Tomonori
Tamura
 c,
Arif Md. Rashedul
Kabir
c,
Arif Md. Rashedul
Kabir
 d,
Akira
Kakugo
d,
Akira
Kakugo
 de,
Kazuki
Sada
de,
Itaru
Hamachi
de,
Kazuki
Sada
de,
Itaru
Hamachi
 cf and
Kazunori
Matsuura
cf and
Kazunori
Matsuura
 *ab
*ab
aDepartment of Chemistry and Biotechnology, Graduate School of Engineering, Tottori University, Tottori 680-8552, Japan. E-mail: hinaba@tottori-u.ac.jp; ma2ra-k@tottori-u.ac.jp
bCentre for Research on Green Sustainable Chemistry, Tottori University, Tottori 680-8552, Japan
cDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan
dFaculty of Science, Hokkaido University, Sapporo 060-0810, Japan
eGraduate School of Chemical Sciences and Engineering, Hokkaido University, Sapporo 060-0810, Japan
fJST-ERATO, Hamachi Innovative Molecular Technology for Neuroscience, Nishikyo-ku, Kyoto 615-8530, Japan
First published on 5th August 2022
Abstract
For light-induced stabilization of microtubules (MTs) to manipulate cells, a photo-reactive diazirine group was conjugated to a Tau-derived peptide, a motif binding on the inside of MTs. Ultraviolet (UV) light irradiation induced significant stabilization of MTs via the formation of a covalent bond of the peptide and showed toxicity.
Microtubules (MTs) are tubular cytoskeletal elements with a 15 nm inner diameter that are formed by the polymerization of tubulin dimers. MTs play important roles in various cell functions such as cell division and shape and intracellular transport, being associated with motor proteins (kinesin and dynein).1–5 Malfunction of MTs is linked to various pathological conditions, including neurodegenerative diseases, while controlling MT stabilization/destabilization is effective for the treatment of MT-related diseases and cancers.6–9 Taxol (paclitaxel) is a well-known anticancer drug, which stabilizes MTs by binding to a hydrophobic pocket of β-tubulin inside of the MTs and inhibiting their dynamic instability. However, Taxol has several limitations including a complex synthesis due to its complex structure, low water solubility and side effects resulting from its adverse outcomes on normal cells.10,11 Gaining spatiotemporal control of the stability and structures of MTs using external stimuli is a promising strategy to manipulate specific cell populations with reduced side effects.12–19 For example, Thorn-Seshold et al. have developed Taxol-azobenzene conjugates that allow control of the structures of intracellular MTs by photoisomerization of the azobenzene moiety.15 Although photoisomerization is useful for modulating the stabilization of MTs, its reversible property might result in moderate MT stabilization. Thus, we focused on using a photoaffinity labelling technique.20,21 In this technique, light irradiation converts photoaffinity probes to highly reactive species such as carbene that form covalent bonds with nearby residues among the target proteins. Horwitz et al. have developed Taxol-photoaffinity labelling agents to identify the binding site of Taxol and its analogues.22,23 The Taxol-photoaffinity labelling agents have not been used to control the stability and structures of MTs by light irradiation, probably because of the stabilization of MTs even without light irradiation.
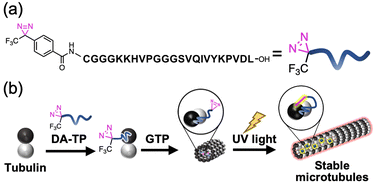
We have previously designed a Tau-derived peptide (TP) that binds to the interior hydrophobic pocket of MTs like Taxol.24 Its sequence (CGGGKKHVPGGGSVQIVYKPVDL) was based on the repeat domain of the MT-associated protein Tau. Using TP, we have encapsulated various nanomaterials such as proteins25,26 and magnetic nanoparticles27 to modulate the structure and function of MTs.28 We have also confirmed binding of red fluorescent tetramethylrhodamine (TMR)-labelled TP to MTs in living cells.29 Cyclic TP bound to tubulin strongly (Kd = 0.94 μM), promoting the stabilization of MTs compared to TP (Kd = 6.0 μM).30 It is considered that the TP-photoaffinity labelling agent could increase the binding affinity of TP to tubulin and stabilize MTs by light-induced covalent bond formation. In this study, we have conjugated a photo-reactive diazirine (DA), which forms a carbene upon UV light irradiation,20,21 at the N-terminus of TP (DA-TP). This strategy allowed stabilization of MTs via covalent bond formation upon UV light irradiation in vitro and in living cells (Fig. 1). Compared to Taxol-photoaffinity labelling agents22,23 and Taxol-azobenzene conjugates,15DA-TP has high water solubility and allows large structural changes of MTs by photoaffinity labeling of DA.
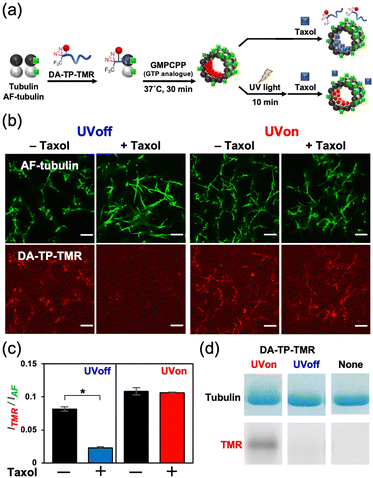
This DA-TP was synthesised by Fmoc solid-phase chemistry followed by the introduction of a DA moiety at its N-terminus on the resin (Fig. S1a and S2a, ESI†). The UV-visible spectrum of DA-TP changed upon UV light irradiation (Fig. S3, ESI†), confirming the generation of carbene as reported previously.31 The dissociation constant (Kd) of DA-TP to tubulin was estimated as 6.1 μM (Fig. S4, ESI†), by measuring the fluorescence change of the intrinsic tryptophan of tubulin as reported previously.32 The affinity is similar to that of TMR-labelled TP (Kd = 6.0 μM). To evaluate the binding of DA-TP to MTs using confocal laser scanning microscopy (CLSM), the cysteine residue of DA-TP was modified with TMR (DA-TP-TMR) (Scheme S1 and Fig. S1d, S2d, ESI†). The MTs were prepared by preincubation with tubulin, Alexa Fluor 488-labelled tubulin (AF-tubulin) and DA-TP-TMR, and subsequent polymerization by guanosine-5′-[(α,β)-methyleno]triphosphate (GMPCPP), a GTP analogue used to form stable MTs (Fig. 2a). The binding of DA-TP-TMR to MTs was confirmed by measuring the co-localisation of TMR and AF (Fig. 2b). When Taxol was added to the DA-TP-TMR-incorporated MTs, the TMR fluorescence on the MTs was significantly reduced, indicating binding of DA-TP-TMR to the interior pocket of the MTs like Taxol. When UV light was applied to the DA-TP-TMR-incorporated MTs for 10 min, the TMR fluorescence was not reduced by the addition of Taxol. The amount of DA-TP-TMR bound to the MTs was estimated by analysing the fluorescence intensity ratio (ITMR/IAF) from the CLSM images (Fig. 2c). The ITMR/IAF ratio was significantly reduced upon Taxol treatment in the absence of UV light irradiation, while this ratio remained unchanged when UV light irradiation was applied for 10 min. These results indicate a covalent bond formation of DA-TP-TMR to the Taxol-binding pocket of the MTs upon UV light irradiation, remaining unaffected by Taxol administration. To further confirm a covalent bond formation between DA-TP-TMR and tubulin, SDS-PAGE with fluorescence scanning was performed (Fig. 2d and Fig. S18, ESI†). Following UV light irradiation of the DA-TP-TMR and tubulin mixture, TMR fluorescence was observed at the band corresponding to tubulin, contrary to non-UV-irradiated controls. These results confirm a covalent bond formation between DA-TP-TMR and tubulin upon UV light irradiation. Molecular mechanics calculations by a MacroModel module predicted the potential binding sites of DA of DA-TP-TMR to tubulin (Supporting text and Fig. S5, ESI†).
The effect of DA-TP on the formation efficiency of MTs was estimated using MTs prepared with GTP, which are generally unstable. DA-TP-encapsulated MTs were prepared with GTP and the supernatant (tubulin) and pellet (MTs) were separated by ultracentrifugation and analysed by SDS-PAGE to estimate the formation efficiency of MTs (Fig. S6, ESI†). Treatment of DA-TP increased the MT formation efficiency (62%) compared to controls without any additive (39%). Following UV light irradiation to the DA-TP-encapsulated MTs, the MT formation efficiency further increased (74%) reaching levels similar to Taxol treatment. These results indicate that DA-TP stabilizes MTs, while UV light irradiation further enhances its stabilization effect.
Stabilization of GTP MTs by DA-TP was further evaluated using CLSM. Although GTP MTs were unstable, with aggregates only observed without additive, DA-TP induced the formation of MTs similar to Taxol (Fig. 3a). Conversely, the formation of MTs was not observed when UV light was applied to the mixture of tubulin and DA-TP after the addition of GTP (Fig. S7, ESI†). Interestingly, UV light irradiation to DA-TP-encapsulated MTs induced the formation of longer and more rigid MTs. Analysis of MTs using the CLSM images revealed that applying UV light irradiation to the DA-TP-encapsulated MTs increased both their contour length (1.6-fold) and persistence length (1.4-fold), compared to controls without UV irradiation (Fig. S8, ESI†). Under depolymerization conditions of MTs (4 °C), DA-TP-encapsulated MTs without UV light irradiation were also completely depolymerized and formed dot-like aggregates (Fig. 3b). By contrast, DA-TP-encapsulated MTs exposed to UV light irradiation remained even at 4 °C, while their contour length and persistence length were similar to those of MTs treated with Taxol (Fig. S9, ESI†). Thus, the covalent bond formation between DA-TP and MTs induced by UV light irradiation resulted in long, rigid and stable MTs. The increased amount of DA-TP triggered by UV light also generated longer and more rigid MTs (Fig. S10, ESI†). Two control peptides (i.e., TP modified with DA at different positions and actin-binding peptide, Lifeact, modified with DA) were not bound to the Taxol-binding pocket and showed no effects on the stabilization of MTs with and without UV light irradiation (Supporting text and Fig. S11, S12, ESI†). Thus, the TP sequence with a DA at the N-terminus is important for the light-induced stabilization of MTs by covalent bond formation.
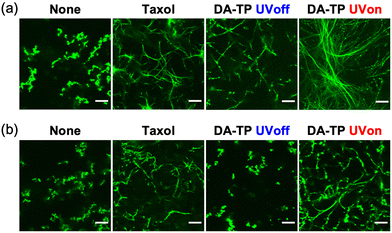 | ||
| Fig. 3 CLSM images of GTP MTs prepared with DA-TP, Taxol or without any additives (a) after polymerization and (b) further incubation at 4 °C for 15 min. UV light was applied for 5 min. Contour and persistence lengths of MTs were determined from CLSM images (Fig. S8g and S9e, ESI†). Preparation concentrations: 2 μM tubulin, 2 μM AF-tubulin, 8 μM DA-TP or Taxol. Scale bars, 10 μm. | ||
The motile properties of DA-TP-encapsulated GMPCPP MTs driven by ATP on kinesin-coated substrates were next analysed by fluorescence microscopy (Fig. S13, ESI†). The velocity of DA-TP-encapsulated GMPCPP MTs increased 1.3 and 1.7-fold, respectively, before and after UV light irradiation compared to control MTs. The persistence length of GMPCPP MTs was also increased by the treatment of DA-TP and subsequent UV light irradiation. The increased velocity of GMPCPP MTs was presumably due to an increased rigidity of the MTs as we have reported previously.23–25
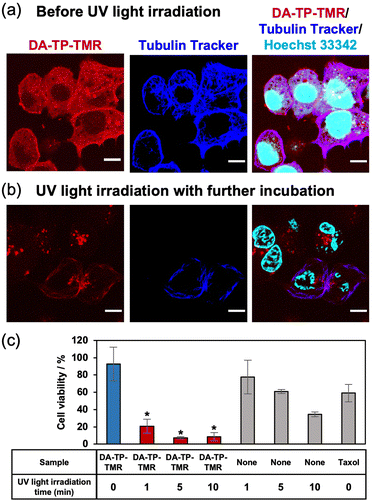
After confirming the UV light-induced stabilization of the DA-TP-encapsulated MTs in vitro, the binding of DA-TP-TMR to human hepatoma HepG2 cells and the resulting effects on the cells were evaluated. Before introducing DA-TP-TMR, demecolcine was applied to the cells to depolymerize intracellular MTs,33,34 thus allowing for the binding of DA-TP-TMR to tubulin and subsequent repolymerization. We confirmed that intracellular MTs were depolymerized by demecolcine and were repolymerized following washing and further incubation (Fig. S14, ESI†). Then, DA-TP-TMR was introduced to the demecolcine-treated cells using a protein transfection reagent (ProteoCarry). After incubation for 30 min, co-localisation of DA-TP-TMR and Tubulin Tracker was observed, indicating binding of DA-TP-TMR to the intracellular MTs (Fig. 4a). When UV light was applied for 5 min to the DA-TP-TMR-treated cells, there was no apparent change immediately after the irradiation (Fig. S15, ESI†). However, after a subsequent incubation for 15 h, many cells displayed abnormal shapes with nuclear defects (Fig. 4b). These nuclear defects are hallmarks of cells treated with MT stabilizers.15,35 However, the cell abnormalities were not observed when DA-TP-TMR was incubated without UV light irradiation (Fig. S16, ESI†). These findings indicate that the binding of DA-TP-TMR to intracellular MTs and the stabilization of MTs by UV light irradiation induced the observed cellular abnormalities. The toxicity of DA-TP-TMR was further evaluated using the WST assay (Fig. 4c). Viability was greatly reduced in cells treated with DA-TP-TMR and UV light irradiation, whereas DA-TP-TMR without UV light irradiation had no apparent effect. Although UV light irradiation without DA-TP-TMR also showed some cytotoxicity, the combination of DA-TP-TMR and UV light irradiation had greater effects. It is suggested that DA-TP-TMR bound to MTs in cells combined with UV light irradiation stabilized MTs, thus inhibiting cell proliferation. DA-TP and Taxol reduced cell viability in a concentration-dependent manner; however, the tendency was not completely matched (Fig. S17, ESI†). Since the diffusion of DA-TP-TMR into the cytoplasm was also observed in the CLSM image (Fig. 4a), it may be possible that parts of DA-TP-TMR bind intracellular molecules non-specifically and inhibit their activity by photoaffinity labelling.
In conclusion, we showed that DA-conjugated TP formed covalent bonds to MTs and stabilized their structure upon UV light irradiation. Binding of DA-TP-TMR to intracellular MTs together with a strong toxicity was observed upon UV light irradiation, indicating that DA-TP-TMR can induce MT stabilization within cells. Although Taxol has low water solubility and is difficult to synthesize, DA-TP has the advantages of good water solubility and being relatively easy to synthesize. In addition, DA-TP can stabilize MTs only when it forms a covalent bond to MTs by photoaffinity labelling. Thus, it is expected that MTs will be stabilized only at the light-exposed areas. This technique will lead to various applications, such as the development of MT-stabilizing drugs with minimal side effects and local cell manipulation. Because prolonged UV light irradiation causes damage to cellular tissues, stabilization of MTs by visible light irradiation is required in the future.
This work was supported by KAKENHI (No. 19K15699 for H. I.) from the Japan Society for the Promotion of Science (JSPS), ACT-X (JPMJAX2012 for H. I.) and FOREST Program (JPMJFR2034 for H. I.) from the Japan Science and Technology Agency (JST).
Conflicts of interest
There are no conflicts to declare.References
- A. Desai and T. J. Mitchison, Annu. Rev. Cell Dev. Biol., 1997, 13, 83–117 CrossRef CAS PubMed.
- E. Karsenti, Nat. Rev. Mol. Cell Biol., 2008, 9, 255–262 CrossRef CAS PubMed.
- C. Conde and A. Cáceres, Nat. Rev. Neurosci., 2009, 10, 319–332 CrossRef CAS PubMed.
- A. Akhmanova and M. O. Steinmetz, Nat. Rev. Mol. Cell Biol., 2015, 16, 711–726 CrossRef CAS PubMed.
- G. J. Brouhard and L. M. Rice, Nat. Rev. Mol. Cell Biol., 2018, 19, 451–463 CrossRef CAS PubMed.
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253–265 CrossRef CAS PubMed.
- The Role of Microtubules in Cell Biology, Neurobiology, and Oncology, ed. T. Fojo, Humana Press, Totowa, NJ, 2008 Search PubMed.
- S. Manzoor, A. Bilal, S. Khan, R. Ullah, S. Iftikhar, A.-H. Emwas, M. Alazmi, X. Gao, A. Jawaid, R. S. Z. Saleem and A. Faisal, Sci. Rep., 2018, 8, 3305 CrossRef PubMed.
- M. O. Steinmetz and A. E. Prota, Trends Cell Biol., 2018, 28, 776–792 CrossRef CAS PubMed.
- C. Dumontet and M. A. Jordan, Nat. Rev. Drug Discovery, 2010, 9, 790–803 CrossRef CAS PubMed.
- C. Janke and M. O. Steinmetz, EMBO J., 2015, 34, 2114–2116 CrossRef CAS PubMed.
- M. Borowiak, W. Nahaboo, M. Reynders, K. Nekolla, P. Jalinot, J. Hasserodt, M. Rehberg, M. Delattre, S. Zahler, A. Vollmar, D. Trauner and O. Thorn-Seshold, Cell, 2015, 162, 403–411 CrossRef CAS PubMed.
- J. van Haren, R. A. Charafeddine, A. Ettinger, H. Wang, K. M. Hahn and T. Wittmann, Nat. Cell Biol., 2018, 20, 252–261 CrossRef CAS PubMed.
- R. C. Adikes, R. A. Hallett, B. F. Saway, B. Kuhlman and K. C. Slep, J. Cell Biol., 2018, 217, 779–793 CrossRef CAS PubMed.
- A. Müller-Deku, J. C. M. Meiring, K. Loy, Y. Kraus, C. Heise, R. Bingham, K. I. Jansen, X. Qu, F. Bartolini, L. C. Kapitein, A. Akhmanova, J. Ahlfeld, D. Trauner and O. Thorn-Seshold, Nat. Commun., 2020, 11, 4640 CrossRef PubMed.
- L. Gao, J. C. M. Meiring, Y. Kraus, M. Wranik, T. Weinert, S. D. Pritzl, R. Bingham, E. Ntouliou, K. I. Jansen, N. Olieric, J. Standfuss, L. C. Kapitein, T. Lohmüller, J. Ahlfeld, A. Akhmanova, M. O. Steinmetz and O. Thorn-Seshold, Cell Chem. Biol., 2021, 28(228-241), e6 Search PubMed.
- A. Sailer, J. C. M. Meiring, C. Heise, L. N. Pettersson, A. Akhmanova, J. Thorn-Seshold and O. Thorn-Seshold, Angew. Chem., Int. Ed., 2021, 60, 23695–23704 CrossRef CAS PubMed.
- L. Gao, J. C. M. Meiring, C. Heise, A. Rai, A. Müller-Deku, A. Akhmanova, J. Thorn-Seshold and O. Thorn-Seshold, Angew. Chem., Int. Ed., 2022, 61, e202114614 CAS.
- L. Gao, J. C. M. Meiring, A. Varady, I. E. Ruider, C. Heise, M. Wranik, C. D. Velasco, J. A. Taylor, B. Terni, T. Weinert, J. Standfuss, C. C. Cabernard, A. Llobet, M. O. Steinmetz, A. R. Bausch, M. Distel, J. Thorn-Seshold, A. Akhmanova and O. Thorn-Seshold, J. Am. Chem. Soc., 2022, 144, 5614–5628 CrossRef CAS PubMed.
- F. Kotzyba-Hibert, I. Kapfer and M. Goeldner, Angew. Chem., Int. Ed. Engl., 1995, 34, 1296–1312 CrossRef CAS.
- J. Das, Chem. Rev., 2011, 111, 4405–4417 CrossRef CAS PubMed.
- S. Rao, L. He, S. Chakravarty, I. Ojima, G. A. Orr and S. B. Horwitz, J. Biol. Chem., 1999, 274, 37990–37994 CrossRef CAS PubMed.
- C.-P. H. Yang, C. Wang, I. Ojima and S. B. Horwitz, J. Nat. Prod., 2018, 81, 600–606 CrossRef CAS PubMed.
- H. Inaba, T. Yamamoto, A. Md., R. Kabir, A. Kakugo, K. Sada and K. Matsuura, Chem. – Eur. J., 2018, 24, 14958–14967 CrossRef CAS PubMed.
- H. Inaba, T. Yamamoto, T. Iwasaki, A. M. R. Kabir, A. Kakugo, K. Sada and K. Matsuura, Chem. Commun., 2019, 55, 9072–9075 RSC.
- H. Inaba, Y. Sueki, M. Ichikawa, A. M. R. Kabir, T. Iwasaki, H. Shigematsu, A. Kakugo, K. Sada and K. Matsuura, bioRxiv, 2022 DOI:10.1101/2022.01.27.476107.
- H. Inaba, M. Yamada, Mst R. Rashid, A. Md. R. Kabir, A. Kakugo, K. Sada and K. Matsuura, Nano Lett., 2020, 20, 5251–5258 CrossRef CAS PubMed.
- H. Inaba and K. Matsuura, Bull. Chem. Soc. Jpn., 2021, 94, 2100–2112 CrossRef CAS.
- H. Inaba, T. Yamamoto, T. Iwasaki, A. Md., R. Kabir, A. Kakugo, K. Sada and K. Matsuura, ACS Omega, 2019, 4, 11245–11250 CrossRef CAS PubMed.
- H. Inaba, M. Nagata, K. Juliano-Miyake, A. M. R. Kabir, A. Kakugo, K. Sada and K. Matsuura, Polym. J., 2020, 52, 1143–1151 CrossRef CAS.
- T. Seifert, M. Malo, J. Lengqvist, C. Sihlbom, E. M. Jarho and K. Luthman, J. Med. Chem., 2016, 59, 10794–10799 CrossRef CAS PubMed.
- P. Mondal, G. Das, J. Khan, K. Pradhan, R. Mallesh, A. Saha, B. Jana and S. Ghosh, ACS Chem. Neurosci., 2019, 10, 2609–2620 CrossRef CAS PubMed.
- S. Dutertre, M. Ababou, R. Onclercq, J. Delic, B. Chatton, C. Jaulin and M. Amor-Guéret, Oncogene, 2000, 19, 2731–2738 CrossRef CAS PubMed.
- G. E. Davey, P. Murmann, M. Hoechli, T. Tanaka and C. W. Heizmann, Biochim. Biophys. Acta, 2000, 1498, 220–232 CrossRef CAS.
- T. J. Mitchison, Mol. Biol. Cell, 2012, 23, 1–6 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting figures. See DOI: https://doi.org/10.1039/d2cc01890j |
| This journal is © The Royal Society of Chemistry 2022 |