Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amyloid fibril-UiO-66-NH2 aerogels for environmental remediation†
Mohammad
Peydayesh
 a,
Xiulin
Chen
a,
Julia
Vogt
a,
Felix
Donat
a,
Xiulin
Chen
a,
Julia
Vogt
a,
Felix
Donat
 b,
Christoph R.
Müller
b,
Christoph R.
Müller
 b and
Raffaele
Mezzenga
b and
Raffaele
Mezzenga
 *ac
*ac
aDepartment of Health Sciences and Technology, ETH Zurich, Schmelzbergstrasse 9, 8092 Zurich, Switzerland. E-mail: raffaele.mezzenga@hest.ethz.ch; Tel: +41 44 632 9140
bDepartment of Mechanical and Process Engineering, ETH Zurich, Leonhardstrasse 21, 8092 Zurich, Switzerland
cDepartment of Materials, ETH Zurich, Wolfgang-Pauli-Strasse 10, 8093 Zurich, Switzerland
First published on 31st March 2022
Abstract
A sustainable hybrid aerogel based on β-lactoglobulin amyloid fibril/UiO-66-NH2 is developed for environmental remediation. The hybrid aerogel's CO2 capture and water purification performances were investigated. The hybrid aerogel can achieve CO2 capture and possesses excellent adsorption capacities for several heavy metals, dyes, and organic solvents.
Toxic pollutants, such as heavy metals and organic compounds, impart deleterious effects on human health and thus trigger global concerns.1,2 In addition, the planetary boundary of climate change has already been exceeded, and is causing irreversible damage to the Earth.3 Therefore, several water purification and CO2 capture approaches have been introduced.4,5 Although these technologies are both reliable and efficient, they are not sustainable due to high energy demands and costs.6 Therefore, developing sustainable and environmentally-friendly technologies is crucial. Metal–organic frameworks (MOFs) are highly porous nanostructures comprising metal ions/clusters and organic linkers7 with exceptional characteristics, such as high porosity and surface area, diversity, and flexibility.8 These properties enable MOFs to possess superior potential in adsorption,9 gas capture,10 and separation,11 as well as environmental remediation.12 Zirconium-based MOFs, UiO-66, and UiO-66-NH2 have high hydrothermal stability,13 which is beneficial for water applications. Furthermore, amino groups in UiO-66-NH2 allow for CO2 adsorption properties.14 However, certain limitations exist for direct application of powdered MOFs, such as poor processability due to fragile and crystalline structures,12 as well as aggregation issues.7 We hereby design hybrid MOF-amyloid fibril materials capable to meet the above challenges.
Amyloid fibrils can be prepared by self-assembly of various proteins under proper denaturation and hydrolysis conditions.15 Different advanced materials based on amyloid fibrils have been developed, such as aerogels, hydrogels, and membranes.2,16,17 Indeed, it has been shown that amyloid fibrils can remove microplastics,18 heavy metals,1 dyes, and organic matter2 from water. In this study, β-lactoglobulin (β-Lg) was used to produce amyloid fibrils, since it is not only the most abundant protein in whey (a by-product of cheese manufacturing), but it is also a globular protein that can easily self-assemble into amyloid fibrils.19 The hybrid UiO-66-NH2/β-Lg amyloid fibrils aerogel is subsequently studied for two critical environmental applications: CO2 capture and water purification.
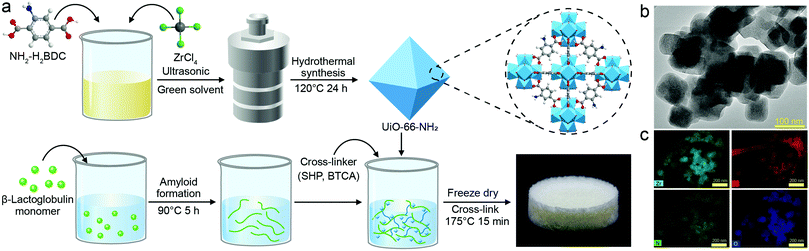
The hybrid aerogel was prepared by a green and straightforward protocol, schematically described in Fig. 1a, with the details elaborated in the experimental section. The morphology and structure of UiO-66-NH2 were assessed by TEM analysis (Fig. 1b), showing the octahedral shape of UiO-66-NH2 with a particle size in the range of 80–100 nm. The element mapping images of UiO-66-NH2 in Fig. 1c indicate that the elements Zr, C, N, and O were distributed uniformly in the composite. As shown in Fig. S1a (ESI†), the N2 sorption isotherm of UiO-66-NH2 was type IV, indicating a microporous structure and more than 60% of the pore volume is linked with the pores smaller than 2 nm. The N2 sorption results are presented in Table S1 (ESI†). The N2 sorption isotherm and the surface area, pore radius, and pore volume of the hybrid aerogels are shown in Fig. S1b and Table S1 (ESI†). As shown in Fig. S2 (ESI†), the isoelectric point for UiO-66-NH2 was reached when the pH value was approximately 3.4. UiO-66-NH2 had positive charges at the condition of pH 2 amyloid fibrils solution, which is beneficial for the adsorption of negatively-charged molecules and compounds.
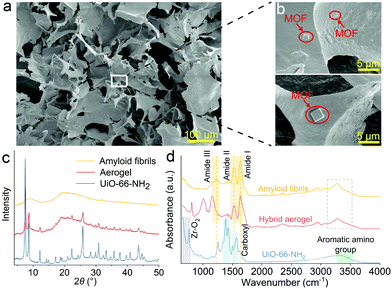
In Fig. 2a, the hybrid aerogel's homogenous and highly porous structure can be observed by SEM. Fig. 2b and Fig. S3 (ESI†) demonstrate the distribution of the regular-shaped UiO-66-NH2 particles on the amyloid network surface. The hybrid aerogels were ultralight and robust, the density was approximately 40 mg cm−3, and the Young's modulus was approximately E = 2.0 × 10−2 MPa (Table S2, ESI†). The compressive strain and stress curve of the hybrid aerogel is shown in Fig. S4 (ESI†). XRD patterns presented in Fig. 2c reveal the purity and crystallinity of the materials. The pattern of amyloid fibrils had no diffraction peaks and exhibited a broad amorphous peak due to β-Lg.20 The pattern of UiO-66-NH2 was in accordance with previous literature,21,22 indicating that pure and crystalline UiO-66-NH2 was successfully synthesized. The pattern contains the characteristic main peaks of UiO-66 at 2θ = 7.34° and 8.48°.23 Moreover, the presence of the –NH2 group in UiO-66 did not affect the XRD pattern due to the MOF structure.24 In the pattern of the hybrid aerogel, the same characteristic peaks of UiO-66-NH2 can be seen, suggesting that the hybridization with amyloid fibrils did not influence the crystal structure of UiO-66-NH2. Given the amorphous nature of the MOF/amyloid interface, high-resolution techniques such as gel-state NMR spectroscopy can be considered for future studies to provide additional insights regarding local structures and interactions.25–27 The thermal stability of the aerogel was evaluated by thermogravimetric analysis (TGA) and shown in Fig. S5 (ESI†). No significant weight change was observed in N2 or air up to 250 °C, indicating a good thermal stability of the hybrid aerogel. The interaction between amyloid fibrils and UiO-66-NH2 was also investigated using FTIR spectroscopy (Fig. 2d). For UiO-66-NH2, the bands at 3475 and 3363 cm−1 can be assigned to N–H symmetric and asymmetric vibrations, respectively.28 A wide and medium absorbance feature at approximately 3300 cm−1 proves the existence of H-bonded hydroxyls. The absorption band at 1570 cm−1 indicates the presence of the reaction of –COOH with Zr.21 The doublet at 1421 and 1387 cm−1 are attributed to the stretching modes of the carboxylic groups in the organic linker.29 The bands at 1337 and 1258 cm−1 are due to Caromatic–N vibration.30 The observed peaks between 600–800 cm−1 represent Zr–O2 as longitudinal and transverse modes.31 The FTIR spectrum of amyloid fibrils exhibits the amide I region at 1630 cm−1, reflecting C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and the amide II region at 1525 cm−1, due to N–H bending vibration and C–N stretching vibration, and the amide III region at 1230 cm−1 in the protein.32 The broad peak at approximately 3270 cm−1 can be ascribed to the –OH vibration.33 The slight shifts of peaks in the hybrid aerogel pattern can be attributed to the formation of hydrogen bonds between amyloid fibrils and UiO-66-NH2 at the interface.24 Furthermore, two new peaks in the region between 900–1200 cm−1 appear which are tentatively attributed to the shift of carboxyl stretch of UiO-66-NH2, caused by the interaction of amyloid fibrils and UiO-66-NH2.34
O stretching vibration and the amide II region at 1525 cm−1, due to N–H bending vibration and C–N stretching vibration, and the amide III region at 1230 cm−1 in the protein.32 The broad peak at approximately 3270 cm−1 can be ascribed to the –OH vibration.33 The slight shifts of peaks in the hybrid aerogel pattern can be attributed to the formation of hydrogen bonds between amyloid fibrils and UiO-66-NH2 at the interface.24 Furthermore, two new peaks in the region between 900–1200 cm−1 appear which are tentatively attributed to the shift of carboxyl stretch of UiO-66-NH2, caused by the interaction of amyloid fibrils and UiO-66-NH2.34
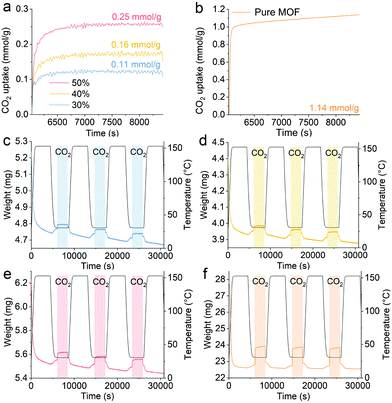
Fig. 3 presents the CO2 capture performance of hybrid aerogels for different loadings of UiO-66-NH2 near ambient temperature. The CO2 uptake of the pure UiO-66-NH2 and the hybrid aerogels containing 30%, 40%, and 50% UiO-66-NH2 (weight percentage) were 1.14, 0.11, 0.16, and 0.25 mmol g−1, respectively. The performance of pure UiO-66-NH2 was comparable with published data of approximately 1.6 mmol g−1.35 Measurements of pure amyloid aerogel revealed that amyloid fibrils make no significant contribution to CO2 capture (Fig. S6, ESI†), in contrast with previous findings on designed amyloids.36,37 Therefore, the hybrid aerogels possessed a larger capacity for CO2 uptake with higher content of UiO-66-NH2. In the hybrid aerogel, while amyloid fibrils act as a robust platform for the materials, UiO-66-NH2 endowed the materials with binding sites for CO2 capture. The micropores in UiO-66-NH2 favored the transport of CO2 to binding sites and the retention of CO2, which is related to physical adsorption. In addition, the interactions of amino groups in UiO-66-NH2 with CO2 molecules were responsible for CO2 capture performance.14 The results demonstrated that the adsorption capacity of the materials was maintained after three consecutive cycles of CO2 sorption–desorption, confirming the high durability and reversibility of the hybrid aerogels.
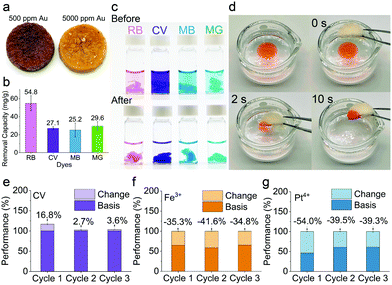
Fig. S7 and S8 (ESI†) show the removal performances of the hybrid aerogels for different heavy metals. The mechanism for heavy metal adsorption is mainly attributed to the chemical chelation between amino acids on the amyloid surface and the heavy metal ions.38 The results revealed that the hybrid aerogels had a much higher capacity for Au3+ (547 mg g−1) and Pt4+ (186 mg g−1) than the pure amyloid fibrils aerogel. While the hybrid aerogels demonstrated excellent capacity for Cr6+ (308 mg g−1) and Fe3+ (226 mg g−1), amyloid fibril aerogel showed no capacity for these metals. Additionally, the hybrid aerogels exhibited high removal efficiency for Au3+, Fe3+, and Pt4+. The improved performance of the hybrid aerogel can be ascribed to UiO-66-NH2. The adsorption mechanism of UiO-66-NH2 is expected to be the ion exchange and electrostatic effect of –NH2 and –COOH groups, combined with the material's nano-porous structure.39,40 To investigate the competitive adsorption, the hybrid aerogel was immersed in a mixture solution containing 10 heavy metals, the results of which are presented in Fig. S9 (ESI†). The data demonstrate that the aerogel had high adsorption efficiency for Au3+, Fe3+, and Ag+. The efficiency for Pt4+ was much lower compared with the separately conducted adsorption experiment, which might be because Ag+ adsorbed faster than Pt4+, and they share the same binding sites. After the adsorption of Ag+, there were probably fewer binding sites available for Pt4+. Compared with other type of adsorbents (see Table S3, ESI†), the hybrid aerogels in this work exhibited excellent adsorption capacity for Au3+, which could be attributed to the synergies of UiO-66-NH2 and amyloid fibrils. Fig. S10 (ESI†) shows the fitted binding isotherm of Au3+. The hybrid aerogel reduced Au3+ into Au nanoparticles and pellets when the concentration of Au3+ in solution was 500 ppm and 5000 ppm, respectively (Fig. 4a). The XRD patterns (Fig. S11, ESI†) revealed the characteristic peaks of Au(0),41 indicating the redox reaction between the hybrid aerogels and Au3+.
The capability of hybrid aerogels for removing organic dyes was also investigated. The results shown in Fig. 4b and c indicate that the removal capacity for Rhodamine B, Crystal violet, Methylene blue, and Malachite green were 54.8, 27.1, 25.2, and 29.6 mg g−1, respectively. The adsorption can be explained mainly by the hydrophobic interactions between the benzene ring of dyes and the hydrophobic domains of the amyloid fibrils.38 Moreover, Fig. 4d shows the rapid adsorption of n-hexane stained with Oil Red O within 10 s by the hybrid aerogel (ESI† Movie available). The reason for the excellent oil adsorption performance might be that the highly porous structure of the hybrid aerogel provides abundant capillary tunnels that drive the oil to pass through by capillary force rapidly,42 and the oil remained trapped within interconnected micropores.43
To evaluate the reusability of the hybrid aerogels, three subsequent adsorption-regeneration experiments were conducted and the results are presented in Fig. 4e–g. The basis adsorption performances, which means 100%, were obtained from fresh aerogels. The changes in adsorption performance are illustrated by light color in each panel. For Crystal violet, the performance for cycle 1, 2, and 3 increased by 16.8%, 2.7%, and 3.6%, respectively. The performance improvement could be explained by introducing new hydrophobic binding sites by methanol treatment.2 The washing could also increase the surface area and roughness of the aerogel by removing other impurities.44 For Fe3+ and Pt4+, the performances decreased after washing, which might be because the regeneration cannot remove all of the pollutants, leading to fewer binding sites available for the next cycle.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Peydayesh, S. Bolisetty, T. Mohammadi and R. Mezzenga, Langmuir, 2019, 35(11), 4161–4170 CrossRef CAS PubMed.
- M. Peydayesh, M. K. Suter, S. Bolisetty, S. Boulos, S. Handschin, L. Nyström and R. Mezzenga, Adv. Mater., 2020, 32(12), 1907932 CrossRef CAS PubMed.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke and H. J. Schellnhuber, Nature, 2009, 461(7263), 472–475 CrossRef PubMed.
- A. A. Olajire, Energy, 2010, 35(6), 2610–2628 CrossRef CAS.
- R. Ghahremani, B. Baheri, M. Peydayesh, S. Asarehpour and T. Mohammadi, Res. Chem. Intermed., 2015, 41(12), 9845–9862 CrossRef CAS.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Chem. Soc. Rev., 2019, 48(2), 463–487 RSC.
- S. Yu, H. Pang, S. Huang, H. Tang, S. Wang, M. Qiu, Z. Chen, H. Yang, G. Song and D. Fu, Sci. Total Environ., 2021, 800, 149662 CrossRef CAS PubMed.
- B.-M. Jun, Y. A. Al-Hamadani, A. Son, C. M. Park, M. Jang, A. Jang, N. C. Kim and Y. Yoon, Sep. Purif. Technol., 2020, 247, 116947 CrossRef CAS.
- L. Rani, J. Kaushal, A. L. Srivastav and P. Mahajan, Environ. Sci. Pollut. Res., 2020, 1–26 Search PubMed.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48(10), 2783–2828 RSC.
- M. F. Ghazvini, M. Vahedi, S. N. Nobar and F. Sabouri, J. Environ. Chem. Eng., 2020, 9(1), 104790 CrossRef.
- R. M. Rego, G. Kuriya, M. D. Kurkuri and M. Kigga, J. Hazard. Mater., 2021, 403, 123605 CrossRef CAS PubMed.
- Q. Chen, Q. He, M. Lv, Y. Xu, H. Yang, X. Liu and F. Wei, Appl. Surf. Sci., 2015, 327, 77–85 CrossRef CAS.
- Y. Cao, H. Zhang, F. Song, T. Huang, J. Ji, Q. Zhong, W. Chu and Q. Xu, Materials, 2018, 11(4), 589 CrossRef PubMed.
- Y. Cao and R. Mezzenga, Adv. Colloid Interface Sci., 2019, 269, 334–356 CrossRef CAS PubMed.
- M. Usuelli, T. Germerdonk, Y. Cao, M. Peydayesh, M. Bagnani, S. Handschin, G. Nyström and R. Mezzenga, Nanoscale, 2021, 13(29), 12534–12545 RSC.
- T. Jin, M. Peydayesh, H. Joerss, J. Zhou, S. Bolisetty and R. Mezzenga, Environ. Sci. Water Res. Technol., 2021, 7(10), 1873–1884 RSC.
- M. Peydayesh, T. Suta, M. Usuelli, S. Handschin, G. Canelli, M. Bagnani and R. Mezzenga, Environ. Sci. Technol., 2021, 55(13), 8848–8858 CrossRef CAS PubMed.
- G. Kontopidis, C. Holt and L. Sawyer, J. Dairy Sci., 2004, 87(4), 785–796 CrossRef CAS PubMed.
- Z. Chavoshpour-Natanzi and M. Sahihi, Food Hydrocolloids, 2019, 96, 493–502 CrossRef CAS.
- C. L. Luu, T. T. Van Nguyen, T. Nguyen and T. C. Hoang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6(2), 025004 Search PubMed.
- Y. Wang, H. Chen, X. Hu and H. Yu, Analyst, 2016, 141(15), 4647–4653 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130(42), 13850–13851 CrossRef PubMed.
- O. G. Nik, X. Y. Chen and S. Kaliaguine, J. Membr. Sci., 2012, 413, 48–61 CrossRef.
- Nonappa and E. Kolehmainen, Soft Matter, 2016, 12(28), 6015–6026 RSC.
- G. M. Reddy, G. M. Peters, B. P. Tatman, T. S. Rajan, S. M. Kock, J. Zhang, B. G. Frenguelli, J. T. Davis, A. Marsh and S. P. Brown, Mater. Adv., 2020, 1(7), 2236–2247 RSC.
- A. Wickramasinghe, Y. Xiao, N. Kobayashi, S. Wang, K. P. Scherpelz, T. Yamazaki, S. C. Meredith and Y. Ishii, J. Am. Chem. Soc., 2021, 143(30), 11462–11472 CrossRef CAS PubMed.
- N. Zhang, R. Jin, G. Mao, J. Tan, L. Chen, C. Li and J. Wang, Inorg. Chim. Acta, 2021, 120674 Search PubMed.
- X.-Y. Xu, C. Chu, H. Fu, X.-D. Du, P. Wang, W. Zheng and C.-C. Wang, Chem. Eng. J., 2018, 350, 436–444 CrossRef CAS.
- K. Chakarova, I. Strauss, M. Mihaylov, N. Drenchev and K. Hadjiivanov, Microporous Mesoporous Mater., 2019, 281, 110–122 CrossRef CAS.
- J. Yang, Y. Dai, X. Zhu, Z. Wang, Y. Li, Q. Zhuang, J. Shi and J. Gu, J. Mater. Chem. A, 2015, 3(14), 7445–7452 RSC.
- J. Ioannou, A. Donald and R. Tromp, Food Hydrocolloids, 2015, 46, 216–225 CrossRef CAS.
- M. Peydayesh, T. Greber, I. Haechler, A. Armanious, X. Jia, M. Usuelli, M. Bagnani and R. Mezzenga, Adv. Sustainable Syst., 2021, 2100309 Search PubMed.
- R. Kardani, M. Asghari, N. F. Hamedani and M. Afsari, J. Ind. Eng. Chem., 2020, 83, 100–110 CrossRef CAS.
- Y. Jiang, C. Liu, J. Caro and A. Huang, Microporous Mesoporous Mater., 2019, 274, 203–211 CrossRef CAS.
- D. Li, H. Furukawa, H. Deng, C. Liu, O. M. Yaghi and D. S. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(1), 191–196 CrossRef CAS PubMed.
- D. Li, E. M. Jones, M. R. Sawaya, H. Furukawa, F. Luo, M. Ivanova, S. A. Sievers, W. Wang, O. M. Yaghi and C. Liu, J. Am. Chem. Soc., 2014, 136(52), 18044–18051 CrossRef CAS PubMed.
- M. Peydayesh and R. Mezzenga, Nat. Commun., 2021, 12(1), 1–17 CrossRef PubMed.
- F. Zhao, C. Su, W. Yang, Y. Han, X. Luo, C. Li, W. Tang, T. Yue and Z. Li, Appl. Surf. Sci., 2020, 527, 146862 CrossRef CAS.
- Y. Zhang, X. Xu, C. Yue, L. Song, Y. Lv, F. Liu and A. Li, Chem. Eng. J., 2021, 404, 126546 CrossRef CAS.
- J. Guo, X. Fan, J. Wang, S. Yu, M. Laipan, X. Ren, C. Zhang, L. Zhang and Y. Li, Chem. Eng. J., 2021, 130588 CrossRef CAS.
- Q. Zhu, Y. Chu, Z. Wang, N. Chen, L. Lin, F. Liu and Q. Pan, J. Mater. Chem. A, 2013, 1(17), 5386–5393 RSC.
- N. Chen and Q. Pan, ACS Nano, 2013, 7(8), 6875–6883 CrossRef CAS PubMed.
- W.-h. Li, Q.-y. Yue, Z.-h. Ma, B.-y. Gao, Y.-j. Li and H.-x. Zhao, Water Sci. Technol., 2013, 67(2), 284–292 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods. See DOI: 10.1039/d2cc00695b |
| This journal is © The Royal Society of Chemistry 2022 |