Open Access Article
Open Access ArticlePotential application of metallacarboranes as an internal reference: an electrochemical comparative study to ferrocene†
Jewel Ann Maria
Xavier
 a,
Clara
Viñas
a,
Clara
Viñas
 a,
Encarnación
Lorenzo
a,
Encarnación
Lorenzo
 bcd,
Tania
García-Mendiola
bcd,
Tania
García-Mendiola
 *bc and
Francesc
Teixidor
*bc and
Francesc
Teixidor
 *a
*a
aInstitut de Ciencia de Materials de Barcelona (ICMAB-CSIC), Campus de la UAB, Bellaterra, Spain. E-mail: teixidor@icmab.es
bDepartamento Quimica Analítica y Analisis Instrumental, Universidad Autónoma de Madrid, Spain
cInstitute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, Ciudad Universitaria de Cantoblanco, 28049, Madrid, Spain
dIMDEA-Nanociencia, Ciudad Universitaria de Cantoblanco, 28049, Madrid, Spain
First published on 4th March 2022
Abstract
Ferrocene and its derivatives have been extensively used as an internal reference in electrochemical processes. Yet, they possess limitations such as solvent restrictions that require chemical modifications. In this regard, we have studied the use of metallacarboranes [3,3′-M(1,2-C2B9H11)2]− (M = Co, Fe) as general internal reference systems and have proven their suitability by thoroughly investigating their electrochemical properties in both aqueous and organic electrolytes without any derivatization.
To evaluate any electrochemical process, it is always necessary that it be compared with another electrochemical process that serves as a reference. Typically, we use reference electrodes such as the standard hydrogen electrode (SHE), normal hydrogen electrode (NHE), reversible hydrogen electrode (RHE), the various forms of Ag/AgCl electrodes, or saturated calomel electrode (SCE), among others. In all of these, water is a key component. What happens when we want to compare that electrochemical process of interest which takes place in water to the same one in an organic solvent or to a very similar one having compounds insoluble in water but soluble in an organic solvent? What if we simply want to compare a series of electrochemical processes in water and in organic solvents?1 Can we use the electrodes that work in aqueous solutions for non-aqueous systems as well?2 Yes, but after the initial few experiments the reference potentials are altered and unmeasurable junction potentials develop.3 Hence, using the same reference electrodes to measure aqueous or non-aqueous redox systems is not the most appropriate in principle as the potentials measured are not directly comparable. One way to avoid this problem is to use as internal reference a highly reversible electrochemical couple that is soluble in organic solvents. This led to the extensive use of ferrocene,4 or cobaltocene5 or decamethylferrocene,6 as the internal reference redox systems (Table S3, ESI†). While ferrocene is insoluble in water, derivatives of ferrocene such as ferrocene carboxylic acid (FCA),7 ferrocenemethanol (FcMe)8 and ferrocene acetic acid (FAA)4a which go through one-electron oxidation to the ferricinium state, similar to ferrocene, are soluble in water. These are generally referred to as internal standards for a quasi-reference electrode (QRE).9 Thus, with QRE, it became possible to compare the same electroactive couple in both water and non-aqueous media, but this is not highly accurate because the functional modifications on the parent electroactive species does indeed affect the redox potential of the electrochemical couple.10 Up until now, we have discussed about the electrochemical couples that were originally opted for organic solvents which had to be chemically modified to be adapted to aqueous solvents. However, there is a highly reversible redox active electrolyte of great significance in aqueous media, namely the ferricyanide to ferrocyanide couple.11 It is noteworthy that all the internal standards described are complexes in which one of their electroactive pairs fulfills the 18e− rule and are octahedrally coordinated to avoid, as much as possible, a preceding and following interaction of the metal with the solvent so that it cannot significantly alter the formal potential (E0′) value. Indeed, all these participate in outer sphere electron transfer mechanisms. As a rule, all the possible internal standards for aqueous media are either anions or alcohols and those for non-aqueous media are neutral. Therefore, there is no internal standard currently available that is suitable, without any modification, for both water and organic solvents.
Metallacarboranes form an interesting class of polyhedral carborane clusters, akin to ferrocene, having an empirical formula of [M(C2B9H11)2]−, (M = Co, Fe), where the metal occupies a shared vertex merging the two icosahedra units producing a θ shape molecular anion.12 Interestingly, these compounds can be soluble in both aqueous and non-aqueous systems without any structural modifications depending on the cation, unlike ferrocene. Structurally, due to the 3-dimensional nature of the cluster, a ‘canopy effect’ is produced which better shields the redox active metal center from the external solvent environment in comparison to ferrocene which offers only a 2-dimensional rigidity to the metal center (Scheme 1). Notably, M′[3,3′-M(1,2-C2B9H11)2] (M = Co, Fe), when M′ = Na, K is water soluble and when M′ = Cs is soluble in electrochemically common non-aqueous solvents.13 When M′ = H, it is soluble in both the mediums but as it is a strong acid, it can lead to possible interactions with the solvent. Therefore, for this study, we have focused on the salts of alkaline ions. Herein, we discuss the potential application of [M(C2B9H11)2]− as an internal reference by conducting a comparative study with the currently accepted and widely employed internal reference ferrocene and its analogues.
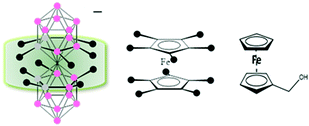 | ||
| Scheme 1 Schematic representation of the ‘canopy effect’ for [3,3′-M(1,2-C2B9H11)2]− (M = Co, Fe) in comparison to the planar ferrocene (Fc) and ferrocene methanol (FcMe). | ||
These carborane clusters are gaining popularity among a broad scientific community owing to their attractive properties such as non-localized negative charge and 3D-aromaticity,14 ability to form hydrogen, Cc–H⋯O and dihydrogen, Cc–H⋯B–H, bonds,15 water solubility13 and easy derivatization similar to organic compounds.16 In this study, we have focused on the electrochemical properties of [3,3′-Co(1,2-C2B9H11)2]− (denoted as [o-COSAN]−) and [3,3′-Fe(1,2-C2B9H11)2]− (denoted as [o-FESAN]−) exploiting the reversible redox active pairs, Co3+/2+ and Fe3+/2+ and comparing its aqueous and non-aqueous behavior in dry acetonitrile and water as solvents with ferrocene (Fc) and ferrocene methanol (FcMe) in the same solvents. The study was undertaken using cyclic voltammetry (CV) with a three-electrode system of glassy carbon (working), Pt wire (auxiliary) and Ag/AgCl or saturated calomel electrode (SCE) (reference).
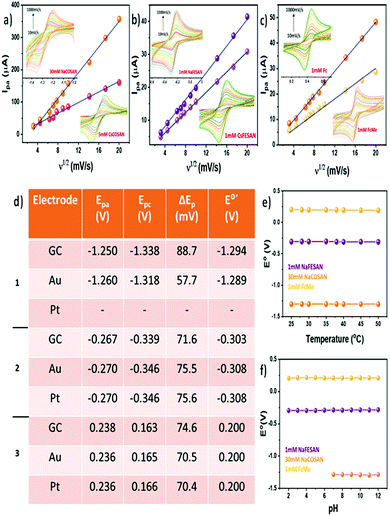
Electrolytes play a significant role in determining the charge-transfer kinetics in any electrochemical process. Hence, it is important to investigate the effect of different electrolytes on a redox system. Consequently, a series of CV measurements were carried out using different electrolytes such as KNO3, NaCl, phosphate buffer, among others, with Na[o-COSAN] and Na[o-FESAN] in comparison to FcMe (Fig. S1, ESI†). The experiments showed that the metallacarboranes behaved reversibly with negligible variations in the formal potential (E0′) (Table S1, ESI†) in different electrolytes and among them, KNO3 was chosen as the electrolyte for the subsequent experiments. A high concentration of the electrolyte is required to ensure efficient charge transfer by increasing the conductivity of the solution as well as limiting the migration of the analyte.17 CV measurements were performed by sequentially increasing the concentration of the electrolyte to understand the impact of varying electrolyte concentrations. Expectedly, at lower concentrations of the electrolyte the charge transfer was limited and hence the peak potential separation (ΔEp) was large in all the compounds investigated including FcMe (Fig. S2, ESI†). Even in non-aqueous solvent, where tetrabutylammonium perchlorate (TBAP) was used as the electrolyte, a similar tendency was observed (Fig. S3, ESI†). The optimum concentration of the electrolyte for the study was chosen as 0.3 M. Another important parameter which greatly affects the electrochemical behaviour of a redox system is the scan rate (ν). Scan rate dictates the rate of change of potential and a higher scan rate would imply a faster decrease in the diffusion layer, thereby producing higher peak currents.18Fig. 1a–c shows the linear dependency of the anodic peak current (ipa) with the square root of the scan rate (ν1/2) in accordance to the Randles–Sevcik equation. Fig. 1a and b show the variation of the anodic peak current for [o-COSAN]− and [o-FESAN]−, respectively (Fig. S4a–c, ESI†). A similar trend was also observed for the experiments performed in organic electrolyte using Cs[o-COSAN] and Cs[o-FESAN] in comparison to Fc (Fig. S4d–f, ESI†). Therefore, it can be inferred that the redox process of [o-COSAN]− and [o-FESAN]− is a diffusion-controlled reversible process. The formal potential (E0′) and the peak potential separation (ΔEp) were also constant with small variations in different scan rates. These results are encouraging and asserts the capability of [3,3′-M(1,2-C2B9H11)2] (M = Co, Fe) as an internal reference.
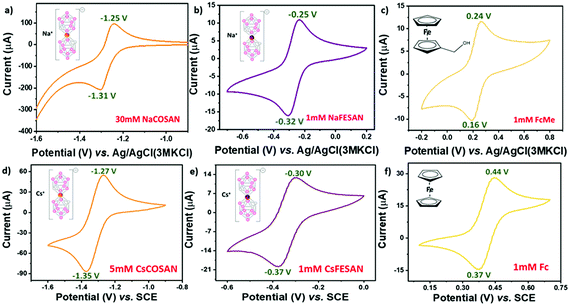
A major factor to contemplate while considering any new redox system as a reference for electrochemical studies is to understand its behaviour in a solvent system. Previously, we have shown that due to the ability of the metallacarboranes to form strong hydrogen and di-hydrogen bonds, [o-COSAN]− has a remarkable ability to self-assemble in the form of vesicles and micelles in an aqueous environment.19 Hence, to substantiate that the self-assembling ability of [o-COSAN]− and [o-FESAN]− has negligible influence on the E0′ of the redox couple, CV measurements were performed in water solutions with different concentration of the Na salts of the metallacarboranes (Fig. S5, ESI†). The concentration was varied from 5–50 mM for Na[o-COSAN] and 1–65 mM for Na[o-FESAN]. To notice, while the peak potential separation (ΔEp) remained in the range for one-electron transfer process for Na[o-COSAN] in all concentrations, for Na[o-FESAN] at higher concentrations (from 50 mM), the ΔEp was larger (Table S2, ESI†). This can be ascribed to the high barrier rendered to the electron transfer kinetics due to the formation of vesicles in Na[o-FESAN] whereas in the case of Na[o-COSAN], the vesicle formation did not show any profound effect on the electrochemical response. Nevertheless, both the metallacarboranes showed highly reversible electrochemical behavior for a wide range of concentration with a constant formal potential of −1.282 V and −0.283 V for Na[o-COSAN] and Na[o-FESAN], respectively.
The pH of an electrolytic solution greatly influences the Nernstian equilibrium of an electrochemical process. Depending on the pH, the redox activity of the electroactive species may vary and in case of metals an acidic pH can even lead to corrosion.20 Hence, it becomes important to ascertain the electrochemical response of the internal references in varying pH conditions. Subsequently, pH dependent CV experiments were performed using 0.1 M Britton–Robinson (BR) buffer as the electrolyte (Fig. S6, ESI†). Upon varying the pH from 2–12, the formal potential (E0′) and the peak potential separation (ΔEp) were fairly constant for Na[o-FESAN] at −0.291 V and 74 mV, respectively, and comparable to the tendency of FcMe (Fig. 1f, ESI†). However, for Na[o-COSAN] as the potential window was very close to the water reduction peak, at acidic pH it was difficult to observe an electrochemical response. Conversely, at basic pH, the E0′ was constant at −1.285 V (Fig. 1f) while some fluctuations were observed in the ΔEp (Fig. S7, ESI†). In general, most of the electrochemical experiments are carried out at ambient temperature. But while considering the ability of a compound to be used as an internal reference it is relevant to subject it to harsh conditions and understand the behaviour. Therefore, CV measurements in temperature ranges from 25–50 °C were carried out (Fig. S8, ESI†). From the experiments, it was observed that the temperature did not influence the formal potential (E0′) and the peak potential separation (ΔEp) in Na[o-COSAN] and Na[o-FESAN] and that the compounds were thermally stable throughout the measurement (Fig. 1e and Fig. S9, ESI†). Different working electrode materials can influence the electrochemical response due to adsorption, difference in electron transfer kinetics as well as occurrence of electrode specific chemical reactions. Fig. 1d describes the various peak potentials along with formal and peak separation potentials for different electrodes such as glassy carbon, Au and Pt. Changing the electrode material had negligible influence on the potentials for Na[o-COSAN] as well as Na[o-FESAN] and hence, suggests that these metallacarboranes interact minutely with the electrode materials (Fig. S10, ESI†). Equivalent studies were also performed in non-aqueous solvents and similar results were obtained (Fig. S11, ESI†). Interestingly, the Cs salts of the metallacarboranes are partially soluble in water, particularly Cs[o-FESAN]. The comparison of the CVs of Na[o-FESAN] and Cs[o-FESAN] in aqueous solvent shows similar peak potentials with slight reduction in peak currents (Fig. S12, ESI†). Hence, Cs[o-FESAN] can ideally be used as an internal reference in both solvent systems, but preferably in organic solvents.
In this article, we have demonstrated the redox reversible behavior of [3,3′-M(1,2-C2B9H11)2] (M = Co, Fe) in different electrochemical environments and conditions with regard to Fc and FcMe. Fig. 2 shows the electrochemical response for the compounds investigated with the E0′ as −1.28 V, −1.27 V, −0.28 V and −0.29 V (vs. Ag/AgCl) as well as ΔEp as 60 mV, 80 mV, 70 mV and 70 mV for Na[o-COSAN], Cs[o-COSAN], Na[o-FESAN] and Cs[o-FESAN], respectively. The main characteristics required for an internal reference is to have constant peak potentials and redox behaviour with high thermal and chemical stability. The various CV experiments performed have shown that the metallacarboranes, particularly [o-FESAN]−, has a steady redox response with remarkable thermal and chemical stability and a E0′ very near to NHE, closer than Fc. All these results are suggestive of the potential use of [3,3′-M(1,2-C2B9H11)2]− (M = Co, Fe) as an internal reference in both aqueous and non-aqueous solvents, without any structural or functional modifications. Precisely, the same internal reference for both organic and aqueous electrolytes. As a proof of concept we have tested these electrodes as internal references in a THF:water mixture and have proven a superior performance than the conventional Ag/AgCl electrode that experienced a drift.
This work was funded by the Spanish Ministry of Science, through the “Severo Ochoa” Programme for Centres of Excellence in R&D (CEX2019-000917-S) as well as the Ministerio de Economia y Competitividad (PID2019-106832RB-I00), (PID2020-116728RB-I00, CTQ2015-71955-RED ELECTROBIONET) and Generalitat de Catalunya (2017SGR1720) are appreciated. J. A. M. Xavier acknowledges DOC-FAM programme under the Marie Sklodowska-Curie grant agreement no. 754397 and is enrolled in the PhD programme in UAB. Support from the Community of Madrid (TRANSNANOAVANSENS S2018/NMT-4349), is acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. Zanello, Inorganic Electrochemistry, The Royal Society of Chemistry, 2003 Search PubMed.
- A. A. J. Torriero, S. W. Feldberg, J. Zhang, A. N. Simonov and A. M. Bond, J. Solid State Electrochem., 2013, 17, 3021–3026 CrossRef CAS.
- M. P. S. Mousavi, S. A. Saba, E. L. Anderson, M. A. Hillmyer and P. Bühlmann, Anal. Chem., 2016, 88, 8706–8713 CrossRef CAS PubMed.
- (a) R. R. Gagne, C. A. Koval and G. C. Lisensky, Inorg. Chem., 1980, 19, 2854–2855 CrossRef CAS; (b) G. Gritzner and J. Kuta, Pure Appl. Chem., 1984, 56, 461–466 CrossRef.
- M. J. A. Shiddiky, A. A. J. Torriero, C. Zhao, I. Burgar, G. Kennedy and A. M. Bond, J. Am. Chem. Soc., 2009, 131, 7976–7989 CrossRef CAS PubMed.
- (a) M. Matsumoto and T. W. Swaddle, Inorg. Chem., 2004, 43, 2724–2735 CrossRef CAS PubMed; (b) I. Noviandri, K. N. Brown, D. S. Fleming, P. Gulyás, P. A. Lay, A. Masters and L. Phillips, J. Phys. Chem. B, 1999, 103, 6713–6722 CrossRef CAS.
- S. McCormack, N. R. Russell and J. F. Cassidy, Electrochim. Acta, 1992, 37, 1939–1944 CrossRef CAS.
- H. M. A. Amin, Y. Uchida, E. Kätelhön and R. G. Compton, J. Electroanal. Chem., 2021, 880, 114891 CrossRef CAS.
- K. Z. Brainina, A. V. Tarasov and M. B. Vidrevich, Chemosensors, 2020, 8, 15 CrossRef CAS.
- (a) A. A. J. Torriero, Med. Analyt. Chem. Int. J., 2019, 2, 2–4 Search PubMed; (b) A. A. J. Torriero, Electrochim. Acta, 2014, 137, 235–244 CrossRef CAS; (c) A. Paul, R. Borrelli, H. Bouyanfif, S. Gottis and F. Sauvage, ACS Omega, 2019, 4, 14780–14789 CrossRef CAS PubMed.
- (a) S. D. Collyer, F. Davis, A. Lucke, C. J. M. Stirling and S. P. J. Higson, J. Electroanal. Chem., 2003, 549, 119–127 CrossRef CAS; (b) P. Fischer, P. Mazúr and J. Krakowiak, Molecules, 2022, 27, 560 CrossRef CAS PubMed.
- (a) R. A. Wiesboeck and M. F. Hawthorne, J. Am. Chem. Soc., 1964, 86, 1642 CrossRef CAS; (b) R. N. Grimes, Carboranes, Elsevier Inc., New York, 3rd edn, 2016 Search PubMed.
- M. Tarrés, C. Viñas, P. González-Cardoso, M. M. Hänninen, R. Sillanpää, V. Dordovic, M. Uchman, F. Teixidor and P. Matejcek, Chem. – Eur. J., 2014, 20, 6786 CrossRef PubMed.
- (a) C. Masalles, J. Llop, C. Viñas and F. Teixidor, Adv. Mater., 2002, 14, 826–829 CrossRef CAS; (b) J. Poater, M. Solà, C. Viñas and F. Teixidor, J. Am. Chem. Soc., 2020, 142, 9396 CrossRef CAS PubMed.
- (a) D. Brusselle, P. Bauduin, L. Girard, A. Zaulet, C. Viñas, F. Teixidor, I. Ly and O. Diat, Angew. Chem., Int. Ed., 2013, 52, 12114–12118 CrossRef CAS PubMed; (b) C. E. Housecroft, J. Organomet. Chem., 2015, 798, 218 CrossRef CAS; (c) M. J. Hardie and C. L. Raston, Chem. Commun., 2001, 905 RSC.
- D. Olid, R. Núñez, C. Viñas and F. Teixidor, Chem. Soc. Rev., 2013, 42, 3318–3336 RSC.
- (a) H. P. Bennetto, Annu. Rep. Prog. Chem., Sect. A: Gen., Phys. Inorg. Chem., 1973, 70, 223–248 CAS; (b) N. Matubayasi, Surface tension and related thermodynamic quantities of aqueous electrolyte solutions, Taylor & Francis, Boca Raton, 2014 Search PubMed; (c) S. Srinivasan, Electrode/Electrolyte Interfaces Structure and Kinetics of Charge Transfer, Springer US, Boston, MA, 2006, pp. 27–92.
- A. J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications/Allen, Wiley, New York, 1980 Search PubMed.
- (a) P. Bauduin, S. Prevost, P. Farràs, F. Teixidor, O. Diat and T. Zemb, Angew. Chem., Int. Ed., 2011, 50, 5298–5300 CrossRef CAS PubMed; (b) M. Uchman, V. Ďorďovič, Z. Tošner and P. Matějíček, Angew. Chem., Int. Ed., 2015, 54, 14113 CrossRef CAS PubMed; (c) D. C. Malaspina, C. Viñas, F. Teixidor and J. Faraudo, Angew. Chem., Int. Ed., 2020, 59, 3088 CrossRef CAS PubMed.
- N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart and J. L. Dempsey, J. Chem. Educ., 2018, 95, 197–206 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2cc00424k |
| This journal is © The Royal Society of Chemistry 2022 |