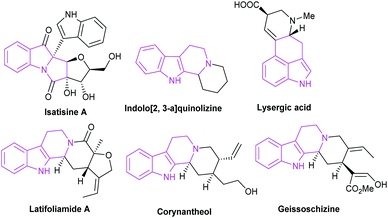
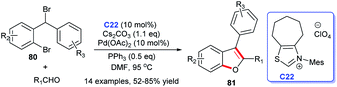Open Access Article
Open Access ArticleRecent advances of N-heterocyclic carbenes in the applications of constructing carbo- and heterocyclic frameworks with potential biological activity
Mei-Mei Li a,
Xiaozhen Chenb,
Yun Deng*a and
Jun Lu*ac
a,
Xiaozhen Chenb,
Yun Deng*a and
Jun Lu*ac
aState Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. E-mail: ljaaa111@163.com; dengyun@cdutcm.edu.cn
bChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
cInstitute of Integrated Bioinformedicine & Translational Science, Hong Kong Baptist University Shenzhen Research Institute and Continuing Education, Shenzhen 518000, China
First published on 26th November 2021
Abstract
In recent years, N-heterocyclic carbenes (NHCs) have established themselves as a masterful and promising type of organocatalyst for the speedy construction of medicinally and biologically significant molecules from common and accessible small molecules. In particular, various cyclic scaffolds, including carbocycles and heterocycles, have been synthesized using NHCs via cycloaddition reaction. An exhaustive review focused on the chemistry of NHCs in these cyclic molecules has yet to be reported. In this contribution, a general picture of the utilization of NHCs in constructing twelve kinds of bioactive cyclic skeletons is firstly presented. We provide a systematic and comprehensive overview from the perspective of cycloaddition reactions; moreover, the limitations, challenges, and future prospects were discussed.
1 Introduction
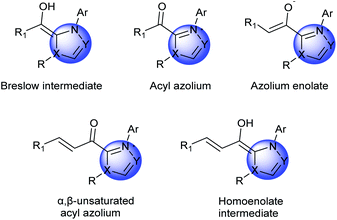
Cyclic organic frameworks, especially heterocyclic frameworks, are widely distributed motifs in numerous biologically active molecules, including natural products and pharmaceutical molecules, which occupy a crucial position in organic and medicinal chemistry.1 The interest toward the discovery of novel and elegant approaches for the construction of these complex carbocycles and heterocycles has been enduring, due to their potential in the production of pharmaceutically active compounds.2 Organocatalysts are tremendously attractive for assembling these bioactive cyclic compounds. So far, a variety of organocatalysts with excellent reaction performances have been discovered.3NHC catalysis4 is deemed to be a significant organic synthesis strategy, which shows the unique property of the polarity inversion for many carbonyl compounds, then produces nucleophilic acyl anion intermediates, thereby reacting with a variety of electrophilic substrates. Apart from the applications of NHCs in umpolung strategies, NHCs simultaneously function as catalysts in non-umpolung transformation.5 These reactions mainly involve several types of important intermediates: Breslow intermediates, homoenolate intermediates, α,β-unsaturated acyl azoliums, saturated acyl azoliums, azolium enolates and azolium dienolates (Fig. 1).
In view of the unique catalytic properties, various types of reactions have been realized via NHC catalysis, with C–C bond and C-heteroatom formation reactions being ubiquitous, including cross-benzoin, homoenolate, especially cycloaddition/annulation reactions.6 Certainly, the flourishing development of carbene catalysis has opened the door to a new era in the construction of bioactive cyclic scaffolds.7 The cycloaddition reaction has long been recognized as the most effective method for synthesizing cyclic scaffolds, leading to four-to eight-membered cyclic structure compounds being constructed. NHC-catalyzed cycloaddition/annulation reactions provide a facile and elegant access to a library of ring skeletons, such as indoles, pyrazoles, pyrroles, and fused ring compounds with important physiological activities, and important intermediates in the pharmaceutical synthesis process, or key pharmacophores as well as structural modules of drugs.8
We herein summarize and analyze literatures during the last ten years related to the synthesis of common biologically active cyclic organic skeletons, such as indoles, pyrazoles, pyrroles, quinolines, lactams and lactones catalyzed by NHCs, providing a comprehensive overview of the applications of NHCs to fabricate bioactive carbocycles and heterocycles, then discussing the substantial limitations, technical challenges and future prospects for the applications of NHCs in this field (Fig. 2).
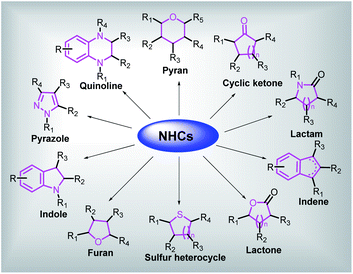 | ||
| Fig. 2 A general picture of utilization of NHCs in constructing several kinds of bioactive cyclic skeletons. | ||
2 Applications of NHCs in the construction of biologically active cyclic skeletons
2.1 Applications in the synthesis of indole derivatives
Indole skeleton compounds exist widely in various medicinal plants, most of which have been found possessing crucial biological activities and being broadly used in medicine and pesticides.9 As a pharmaceutical key skeleton with extensive bio-activities, indole derivatives are attractive synthetic targets for the drug discovery.10Owing to their complex and diverse structures, the synthesis of indole alkaloids is a hot research target in the field of organic chemistry.11 The construction of indole skeletons was an unprecedented challenge over a long period of time. In recent years, the emergence of NHCs has provided a powerful strategy for the synthesis of these relatively complex cyclic compounds, especially those containing chiral centers.
In the past decade, reports on the synthesis of indole derivatives catalyzed by NHCs sprang up rapidly. In this section, we summarize the synthesis protocols of indole derivative skeletons mainly include spiroindole derivatives and other indole derivatives catalyzed by chiral NHCs.
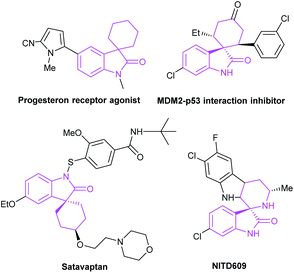
The spirocyclic indoxyl scaffold has been found as the core structure of many bioactive molecules and pharmaceuticals (Fig. 3), and often found applications for the treatment of human cancers and neurodegenerative diseases.12 In view of the biological importance, there's an urgent demand for the preparation of this type of framework. In recent years, there appears many researches on the efficient construction of NHC-catalyzed indole derivatives. In this review, we classify and explain the construction of spiroindole skeleton from the perspective of cycloaddition reaction.
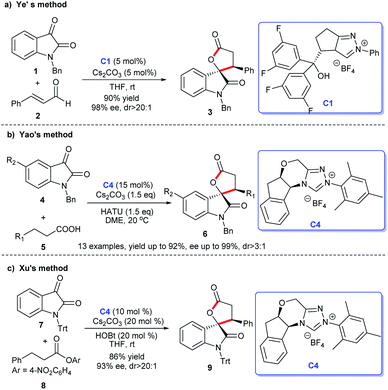
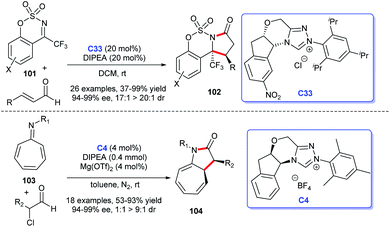
2.1.1.1 [3 + 2] Annulation to construct five-membered spirooxindole compounds. Regarding the construction of chiral five-membered spiroxindole compounds, the [3 + 2] cycloaddition reaction demonstrates a pivotal position. For the preparation of spiroxindole skeleton compounds, isatin is employed as a very common raw material. Ye et al. reported NHC-catalyzed [3 + 2] annulation of enals 2 and isatins 1, resulting in the corresponding spirocyclic oxindolo-ε-butyrolactones 3 in good yield with acceptable diastereo- and enantioselectivities. Authors employed the chiral NHC bearing a proximal hydroxy group derived from L-pyroglutamic acid as a catalyst, the reaction taking place under mild conditions with a low catalysis loading (Scheme 1a).13
Moreover, an [3 + 2] annulation for the synthesis of spirocyclic oxindolo-γ-butyrolactones 6 was demonstrated using substituted propionic acid 5 and isatin 4 as raw materials via the catalysis of chiral NHC derived from triazolium salt C4. This reaction was provided with good to high yields with excellent enantioselectivities (Scheme 1b). A variety of isatins with different substituents and substituted propionic acid were well tolerated under the optimized reaction conditions.14
Subsequently, the use of β-functionalization of saturated carboxylic esters afforded the spiroxindole lactones 9. In this [3 + 2] reaction, isatin 7 was still used as the basic material and a HOBt-assisted NHC-catalysis strategy was developed (Scheme 1c). The authors proved that the addition of HOBt achieved the enhancement of diastereoselectivity and enantioselectivities.15 These reactions provided strategies for the preparations of the spiroindole lactone ring of the [3 + 2] cycloaddition reaction with isatin as the substrate.
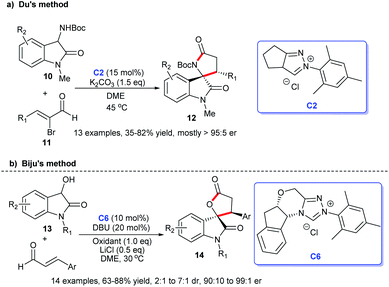
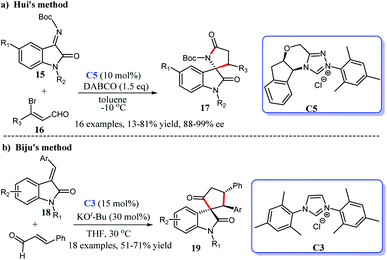
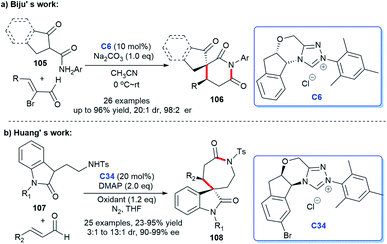
In addition, the [3 + 2] cycloaddition reactions between C-3 saturated heteroatom substituted isatin and different types of reactive aldehydes were developed separately. In 2015, Du and co-workers demonstrated that NHC can catalyze the [3 + 2] annulation of α-bromoenals 11 and 3-aminooxindoles 10 leading to the synthesis of spiroxindole γ-butyro-lactams 12 (Scheme 2a). They conducted a series of experiments furnishing good substrate tolerance and high enantioselectivities. The work offered an approach for rapid access to the 3,2′-spiropyrrolidine oxindole skeleton.16 2017, Indian researcher Biju disclosed the enantioselective synthesis of spiro γ-butyrolactones 14 [3 + 2] annulation of enals with 3-hydroxy oxindoles 13 under NHC-catalysis C6 in the presence of oxidant and LiCl (Scheme 2b). The reaction afforded good yields and excellent er values, among which NHC-bound homoenolate was the key intermediate in the formation of spiro compounds.17
After the carbonyl group at the C-3 position is modified as a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond or a C
C double bond or a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond, the isatin still shows excellent reactivity. In 2016, the interception of isatin N-boc-ketimines 15 and 3-bromoenals 16 resulted in the enantioselective synthesis of spiro[indoline-3,2′-pyrroles] 17 with one quaternary chiral center via a chiral NHC-catalysis C5. A screening of various solvents, catalysis and bases achieved a mild to good yield and excellent enantioselectivities (>95
N double bond, the isatin still shows excellent reactivity. In 2016, the interception of isatin N-boc-ketimines 15 and 3-bromoenals 16 resulted in the enantioselective synthesis of spiro[indoline-3,2′-pyrroles] 17 with one quaternary chiral center via a chiral NHC-catalysis C5. A screening of various solvents, catalysis and bases achieved a mild to good yield and excellent enantioselectivities (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er) (Scheme 3a).18 The same year, Biju's group published the diastereoselective synthesis of cyclopentanone-fused spiro-oxindoles by the [3 + 2] reaction of isatilidene derivatives 18 and α,β-unsaturated aldehydes. In the presence of NHC generated from the imidazolium salt C3 and base, the desired cyclo-pentanone-fused spiroxindoles 19 bearing an all-carbon quaternary were obtained in moderate to good yields (51–71%) (Scheme 3b).19
5 er) (Scheme 3a).18 The same year, Biju's group published the diastereoselective synthesis of cyclopentanone-fused spiro-oxindoles by the [3 + 2] reaction of isatilidene derivatives 18 and α,β-unsaturated aldehydes. In the presence of NHC generated from the imidazolium salt C3 and base, the desired cyclo-pentanone-fused spiroxindoles 19 bearing an all-carbon quaternary were obtained in moderate to good yields (51–71%) (Scheme 3b).19
2.1.1.2 [4 + 2] and [3 + 3] Annulations to construct six-membered spiroxindole compounds. Spiro six-membered oxindoles bearing a quaternary stereo-center at the C3 position are significant moieties appearing in various biological natural products and synthetic drug molecules (Fig. 4),20 and thus can serve as a precursor to synthesize derivatives with a wide spectrum of biological activities. Enormous endeavours have been contributed for their synthesis and synthetic protocols to spiro six-membered oxindoles have been well disclosed.
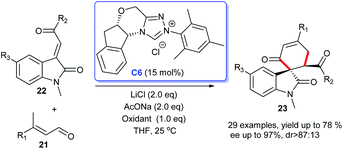
In 2016, an enantioselective version of the oxidative NHC-catalyzed spirocarbocyclic oxindoles synthesis was developed by Liu's group (Scheme 4). The [4 + 2] cycloaddition of 3-alkylenyloxindoles 22 with enals 21 resulted in the desired six-membered spiroxindoles 23. The use of NHC generated from salt C6 in cooperation with AcONa and LiCl was found to be optimal for the reaction. A variety of substitution patterns on the benzene ring of 3-alkylenyloxindoles as well as substitution on the enals were tolerated well under the optimized reaction condition to achieve the desired products in good yields and high enantioselectivities.21 The reaction could be carried out smoothly at room temperature, which made the process highly practical.
The enantioselective synthesis of spiroindole containing a six-membered lactam ring was efficiently achieved through oxidative carbine catalysis using NHC generated from chiral triazolium salt C7 and oxidant with LiCl as a cooperative Lewis acid (Scheme 5). In this process, treating the 2-aminoacrylates with oxindole-derived enals gave the corresponding products 26 in excellent yields (up to 98%).22 Nonetheless, the drawbacks of this approach still existed, such as the optical purity hasn't been investigated and the reaction temperature could be further optimized.
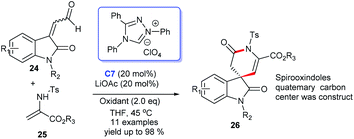
In 2019, Enders and colleagues designed an interesting formal [3 + 3] cycloaddition of isatin-derived enals 24 and cyclic N-sulfonyl ketimines 25. In this reaction, the NHC catalyst and DQ have proven to be indispensable for superior yields and ee values. Finally, the [3 + 3] annulation afforded highly enantioselective six-membered lactam rings fused at the C3 position of spiroxindoles 26 (Scheme 6).23
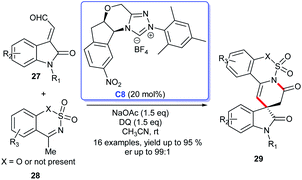
Subsequently, the enantioselective [3 + 3] cycloaddition of isatin-derived enals 27 with ethynyl-ethylene carbonates 28 through a cooperative NHC C8/Cu catalytic process was reported by Gong's group.24 With these feasible approaches in hand, the six-membered lactones and lactams 29 fused at the C3 position of spiroindoles could be easily obtained.
2.1.1.3 [3 + 4] Annulation to construct seven-membered rings fused at C3 position of spiroxindole compounds. In view of their attractive biological activities and extensive applications in human diseases, the diversified spiroxindoles incited synthetic chemists' tremendous concern. Quantities of protocols have been opened up for the preparation of these compounds with four-, five-, six-, or seven-membered rings fused at the C3 position. Among them, the catalytic asymmetric synthesis of seven-membered spiroxindoles was less reported relatively, mainly owing to the difficulty in forming the unstable large rings with spiro-quaternary stereocenters.25
NHC-catalyzed [3 + 4] cycloaddition reactions via a homoenolate intermediate have proven to be a prominent approach to seven-membered heterocycles.
In 2018, Ye and co-workers designed an [3 + 4] annulation of aurone-derived azadienes 31 and isatin-derived enals 30 under NHC-catalysis C10 leading to the practical and efficient synthesis of spirocyclic oxindole-benzofuroazepinones 32 (Scheme 7a). Moreover, the cycloadducts were afforded in good yields with excellent enantioselectivities and gram-scale preparation was achieved. Numerous researches showed these oxindole-benzofuroazepinones have highly potential bioactivities, the exploitation of this preparation method provided the basis for the exploration of the biological activity of which.26
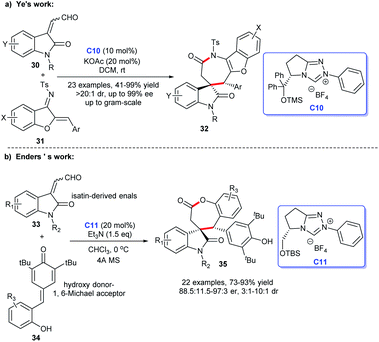 | ||
| Scheme 7 The enantioselective synthesis of spirocyclic oxindole-ε-lactones and lactams from isatin-derived enals. | ||
In the same year, Enders et al. discovered a NHC C11-catalyzed [3 + 4] cycloaddition of isatin-derived enals 33 and p-quinone methides 34 resulting in a good-yield and high-stereoselectivity synthesis of spirocyclic oxindole-ε-lactones 35 (Scheme 7b).25
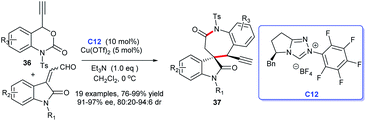
Subsequently, on the basis of previous reports, an additional approach to spirocyclic oxindole seven-membered-ε-lactams was described by Gong group in 2019.24 In this study, ethynylethylene carbonates 36 with enals derived from isatin were employed as suitable substrates and triazole salt C12 and Cu(OTf)2 as co-catalyst giving the corresponding products 37 in good to excellent yields with high enantioselectivities (Scheme 8).
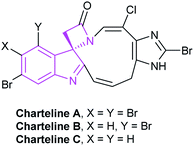
2.1.1.4 [2 + 2] Annulation to construct four-membered spirooxindole compounds. Compared with other spiroindole rings, four-membered spiroxindole compounds are rarely reported relatively. With the combination of two outstanding heterocycles of indole and β-lactam, spirocyclic indolo-β-lactam is one of the leading structures of some bioactive natural products, such as chartelines extracted from the marine bryozoan Chartella papyracea (Fig. 5). In view of the unique structure and potential bioactivities, the synthesis of spirocyclic indolo-β-lactams is an important task for organic synthesis.27
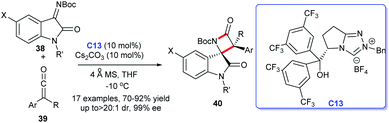
In 2014, the first efficient and atom-economic NHC-catalyzed enantioselective synthesis for spirocyclic oxindolo-β-lactams was disclosed by Ye's group. The reaction was achieved with isatin-derived ketimines and ketenes as substrates, the bifunctional NHC C13 with a free hydroxyl group as catalysis (Scheme 9).28 A variety of substitution pattern on the benzene ring of isatin-derived ketimines 38 as well as substitutions on ketene 39 were tolerated well under the optimized reaction conditions. This process provided new channel for the preparation of 3-position quaternary lactam ring of spiroindoles 40 which was difficult to synthesize.
Owing to their complicated structures and diverse biological activities, indole-fused heterocyclic compounds, which is the combination of indole structure with other heterocyclic scaffolds containing bioactive centers have drawn considerable attention of organic chemists.
In 2013 and 2015, Lu and co-workers completed NHC-catalyzed cycloaddition leading to the preparation of functionalized 3,4-dihydropyrano[3,2-b] indol-2-ones 43 (ref. 30) and indolo[2,3-a] quinolizidines 44,31 respectively. In the presence of NHC C2 generated from the triazolium salt and base, the desired products were obtained in moderate to good yields (Scheme 10). With this elegant approach in hand, indole-fused lactones and lactams analogs were easily prepared.
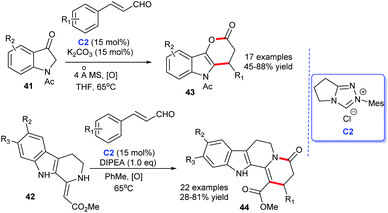 | ||
| Scheme 10 The enantioselective synthesis of indole-fused six-membered heterocycle rings from cinnamaldehyde derivatives. | ||
In 2017, Ye's group disclosed an [3 + 3] annulation accessing to indolo[2,3-b]dihydrothiopyranones from bromoenals and indoline-2-thiones 47 catalyzed by NHC C15.32 Soon after, on the basis of the abovementioned study, the same group reported their achievements in NHC-catalyzed [3 + 3] cycloaddition, they accomplished the synthesis of dihydro-pyridinone-fused indoles 46 via conducting the reactions with bromoenals and indolin-2-imines 45 as raw materials for cycloaddition reactions (Scheme 11).33 In the process, they developed a unified strategy for the synthesis of indole-fused six-membered heterocycles catalyzed by NHC C14. This reaction obtained satisfactory substrate suitabilities and good yields. However, the stereoselectivity of the reaction was not achieved.
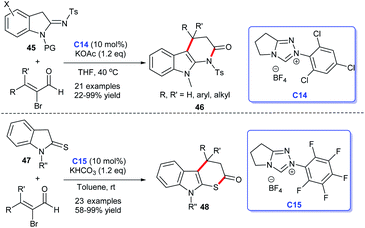 | ||
| Scheme 11 The enantioselective synthesis of indole-fused six-membered heterocycle rings from α-bromocinnamaldehyde derivatives. | ||
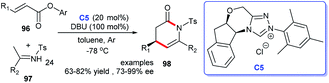
Construction of the indole-fused heterocyclic scaffolds using NHC-activated 2-bromoenals as substrate was continually studied. In 2019, the efficiently asymmetric [3 + 4] cycloaddition of 3-formylindol-2-methylmalonates 49 with 2-bromoenals to functionalized azepino[1,2-a]indoles 50 promoted by NHC catalyst C5 was reported. The authors pointed out that the 3-formyl group in indoles served as an essential mediated group in the generation of cycloaddition products under moderate conditions (Scheme 12).34 This reaction took place under mild conditions with low catalyst loading. Moreover, the reaction exhibited good substrate tolerance and excellent yields with good to excellent stereoselectivities.
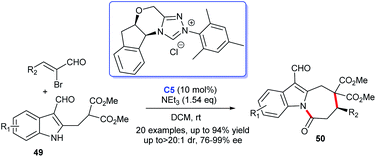 | ||
| Scheme 12 The enantioselective synthesis of indole-fused seven-membered lactams from α-bromocinnamaldehyde derivatives. | ||
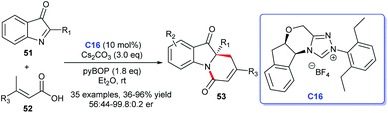
Besides, by taking advantage of chiral NHC catalysis cooperated with pyBOP strategy, Du et al. reported the [4 + 2] annulation of α,β-unsaturated carboxylic acids 52 bearing γ-H and 2-aryl-3H-indol-3-ones 51 to afford C2-quaternary indolin-3-ones 53 (Scheme 13).35 The cycloadducts were obtained in good yields with good to excellent enantioselectivities. A wide variety of substituted 2-aryl-3H-indol-3-ones and α,β-unsaturated carboxylic acids were well tolerated under the optimized reaction conditions. These synthetic methods about indole-fused scaffolds provide effective pathways for drug discovery.
2.2 Applications in the synthesis of pyrazole derivatives
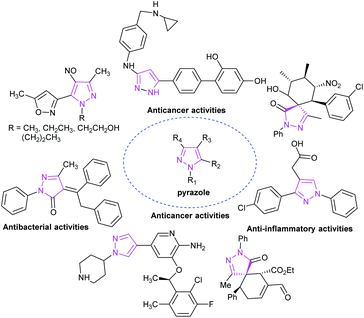
As a representative of a five-membered heterocyclic ring, the pyrazole skeleton contains two nitrogen atoms and is the core structural unit of many synthetic drugs and natural compounds. Pyrazole derivatives and related analogues display a broad scope of biological and pharmaceutical activities,36 including antitumor, antibacterial, antifungal and other biological activities (Fig. 7).37During to the characteristics of high efficiency, low toxicity, and the ability to modify multiple substituents, pyrazole skeleton has become the focus of scientific researchers around the world. The synthesis of pyrazole derivatives is also a hot topic in organic synthetic chemistry. The pyrazole derivative 1-phenyl-3-methylpyrazolone was first artificially synthesized by L. Knott from ethyl acetoacetate and phenyl-hydrazine in 1883. Since then, the door to the synthesis of pyrazole derivatives has been opened, and a large number of pyrazole derivatives have been synthesized.
In recent years, the synthesis of heterocyclic compounds catalyzed by NHCs has drawn considerable attention, and reports on the synthesis of pyrazole derivatives catalyzed by NHCs are abundant.
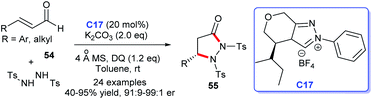
In 2016, Yang and coworkers developed a feasible approach to access to pyrazolidinone products 55 via NHC C17-catalyzed [3 + 2] cycloaddition of enals and protected hydrazine with suitable electronic properties, disclosing an enantioselective amination of enals' β-carbon (Scheme 14). In this reaction, α,β-unsaturated acyl azolium intermediate derived from NHC was the core intermediate to form the new C–N bond asymmetrically.38
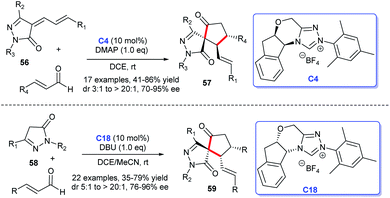
In the same year, Enders group reported that they obtained the five-membered spiropyrazolones 57 from the [3 + 2] cycloaddition of unsaturated pyrazolones 56 with enals under the catalysis of chiral NHC C4. The reaction showed good yields and excellent stereoselectivities.39 Soon afterwards, as their continuous work in this direction, the research group published another work about NHC-catalyzed the three-component and one-pot synthesis of spirocyclic pyrazolones 59 (Scheme 15). They employed two molecules of the enal and pyrazolone 58 as substrates to obtain the products via a [3 + 2] cycloaddition in excellent stereoselectivities and good yields.40 These reactions exhibited good substrate and functional group tolerance.
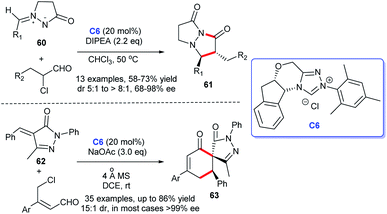
In 2018, Zhong and coworkers disclosed a highly asymmetric [3 + 2] annulation of α-chloroaldehydes with 3-oxopyrazolidin-1-ium-2-ides 60 catalyzed by chiral NHC C6, leading to the synthesis of a series of bicyclic pyrazolidinones 61.41 Subsequently, Zhong's group kept on reporting their research on the synthesis of pyrazole derivatives under the same catalyst chiral NHC. They framed spirocyclohexane pyrazolone skeletons 63 through [4 + 2] annulation of α-arylidene pyrazolinones 62 and γ-chloroenals without the participation of oxidant. The reaction presented great advantages in substrate tolerance and stereoselectivity. This protocol furnishes new thinking for the establishment of spirocyclohexane pyrazolone skeletons (Scheme 16).42
2.3 Applications in the synthesis of quinoline derivatives
Compounds with quinoline as the core structure are abundant in natural products. Quinoline is one of the leading structures processing strong biological activities and many potential medicinal values. They are widely used in new materials, pharmaceuticals, agrochemicals, etc.The quinoline-based compounds are the focus of drug discovery due to their extensive physiological activities. As early as 1988, Evans assigned quinoline as a “privileged scaffold” in medicinal chemistry.43 The prominent antimalarial drug quinine and compounds with antiinflammatory and antitumor activities are based on the quinoline skeleton (Fig. 8).
For acquiring quinoline derivatives, concise and efficient chemical synthesis is the principal channel. In recent years, because of their simple preparations, stable structures, and low toxicity, NHCs have become very effective organic catalysts for synthesizing heterocyclic skeletons. Therefore, NHCs are also diffusely employed in the construction of quinoline skeletons.
In 2018, Biju and coworkers developed a method for the synthesis of 4-difluoromethylquinolines 65 through intramolecular cyclization of aldimines 64 under the catalysis of NHC C2. The generation of NHC from precursor bicyclic triazolium salt in the presence of DBU as the base was the key step for the conversion. The substrate scope of the reaction was examined and a tentative mechanism in Scheme 17 for this NHC-catalyzed 4-difluoromethyl-quinolines synthesis was described as: aldimine 64 can be converted into tetrahedral intermediate A by combining with the NHC, then undergoes a rapid proton transfer to create aza-Breslow intermediate B, which could form an NHC-bound intermediate C by intercepting the electron-poor olefin intramolecularly. When a base excess is present, elimination of the carbonate occurs, resulting in intermediate D, which can undergo a 1,3-H shift and eventually lead to quinoline 65.44 In this study, it was proposed that addition of NHC catalyst C2 to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of 64 resulting in formation of the Breslow intermediate, conjugate addition and tautomerization under alkaline conditions then afforded the cycloaddition products 65.
N of 64 resulting in formation of the Breslow intermediate, conjugate addition and tautomerization under alkaline conditions then afforded the cycloaddition products 65.
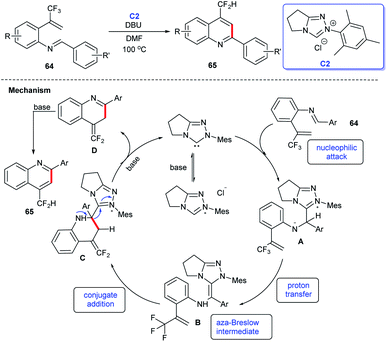 | ||
| Scheme 17 The synthesis of 4-difluoromethylquinolines catalyzed by NHC and the tentative mechanism of the reaction. | ||
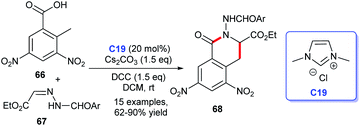
Later, Yao et al. demonstrated the [4 + 2] annulation of 2-methyl-3,5-dinitrobenzoic acid 66 and acylhydrazone 67 leading to the synthesis of isoquinolinones 68 by using a cooperative NHC C19 and DCC catalytic strategy (Scheme 18). The reaction showed good yields.45
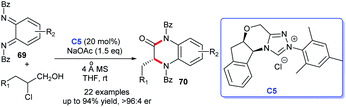
In 2019, the asymmetric synthesis of dihydroquinoxaline derivatives was described by Chi's group. In this process, triazolium C5 has proven to be effective for the production of highly stereoselective dihydroquinoxaline derivatives 70 in the presence of NaOAc. The aminated chloroaldehydes and cyclo-hexadiene-1,2-diimines were employed as suitable substrates and afforded the corresponding functional dihydroquinoxaline derivatives in excellent yields with brilliant enantioselectivities (Scheme 19).46 In view of the previous synthetic routes, Chi's method showed advantages with a gentle reaction condition and widespread substrate applicability, and products with high purity were achieved.
2.4 Applications in the synthesis of pyran derivatives
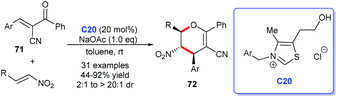
The pyran skeleton and its analogues are detected in numerous natural products or synthetic compounds, many of which possess significant biological activities. For instance, the most common pyran structure–tetrahydropyrans, pyrones and benzopyrones are privileged structures existing in numerous natural products and bioactive molecules (Fig. 9).47 These scaffolds showed promising applications in the field of medicinal chemistry. Therefore, economical and efficient synthetic methods for these molecules are highly desirable. The bloom of NHC catalysts provides excellent routes for their acquisition.48Ye's group demonstrated the synthesis of multiple substituted dihydropyrans 72 by [4 + 2] annulation of oxodienes 71 and nitroalkenes in the presence of NHC C20. The cycloaddition products were given in good yields with nice diastereoselectivities. Furthermore, they declared that the NHC catalysis behaved good applicability to β-substituted nitroalkenes, which was not feasible in the presence of DABCO or phosphine (Scheme 20).49
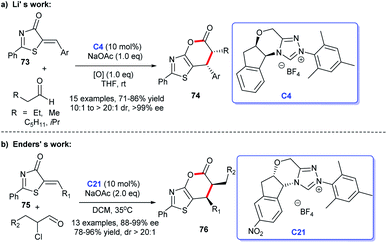
Subsequently, Li et al. provided a method to prepare functionalized thiazolopyrones. They received the target [4 + 2] cycloadducts via the reaction between aliphatic aldehydes and thiazolones 73 in the presence of NHC C21 generated from the triazolium C4 and NaOAc, the desired products 74 were obtained in moderate to good yields50 (Scheme 21a). An additional approach to functionalized thiazolopyrones 76 was reported by Enders group in 2017 (Scheme 21b). In this study, α-chloroaldehydes and 5-alkenylthiazolones 75 were exploited to engage in the asymmetric [4 + 2] heterocycloaddition.51
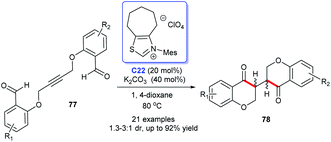
Following these pioneering reports, Zhou and co-workers constructed the bisbenzopyrone 78 scaffold through the intramolecular hydroacylation–Stetter reaction by the catalysis of NHC C22 in 2018. A sequence of bisbenzopyrones was acquired with good to excellent yields (Scheme 22).52
2.5 Applications in the synthesis of furan derivatives
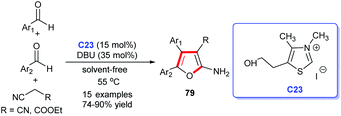
Furan is a pharmacophore structure discovered in numerous natural products and thus can act as a precursor to synthesize derivatives with a wide spectrum of biological activities.53 In addition, furan derivatives are widely employed in many other areas, including agriculture, materials and other fields. In view of the application importance, several methodologies were developed for the synthesis of furan derivatives, NHC-catalyzed synthesis method, of course, included.In 2011, Yao and colleagues disclosed a solvent-free cycloaddition of malononitrile (or ethylcyano-acetate) with two molecular aromatic aldehydes catalyzed by NHC generated from the thiazolium salt C23 and DBU (Scheme 23). Finally, the substituted furans 79 were obtained efficiently, meanwhile, the reactions showed good substrate and functional group tolerance.54
On the basis of the previous study, the same group completed the construction of furans skeleton via the [3 + 2] annulation of aryl aldehydes with a series of substituted 2′-bromodiphenyl bromomethanes 80 by using a cooperative NHC-palladium C22 complex and PPh3 catalytic strategy (Scheme 24).
This protocol exhibited the characteristics of simple operation, good substrate applicability, and good catalyst tolerance, offering a new novel strategy for obtaining benzo[b]furans.55
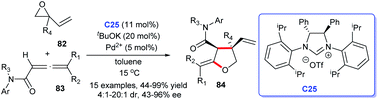
The enantioselective synthesis of polyfunctional chiral tetrahydrofurans was achieved through carbene–palladium complex catalysis using NHC generated from chiral imidazolium salt and tBuOK (Scheme 25) by Hou's group. The process starting from vinyl epoxides 82 and α-allenic amides 83 proceeded favourably in toluene. The asymmetric [3 + 2] annulation for accessing polyfunctional chiral tetrahydrofurans 84 acquired good yields with high diastereo- and enantioselectivities by NHC–Pd catalysis C25. They clued that chiral NHC serving as a particular ligand in this reaction.56
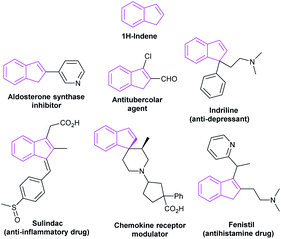
2.6 Applications in the synthesis of indenes
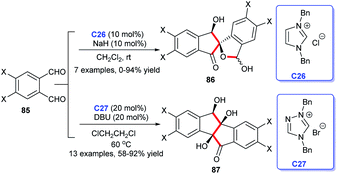
The indene skeleton was significant moieties found in many natural compounds possessing interesting bioactivities and pharmaceutical activities (Fig. 10). Moreover, indene derivatives were widely used in materials science, including new fluorescent materials and catalytic materials. In recent years, many attempts have been made to build this special skeleton by organic chemists. Herein, we summarize the NHC-catalyzed synthesis of indene derivatives.In 2011, Cheng et al. described that two molecules of phthalaldehydes 85 dimerized in the presence of NHC C26 or C27, leading to an asymmetry synthesis of indenones.57 The adoption of different salts, bases and solvents leading to different condensation products 86 and 87 respectively. In this work, the NHC-catalysis successively mediated intermolecular benzoin condensation, intramolecular aldol reaction and benzoin condensation to obtain corresponding indenones. This work has reflected the diversity of NHC as organocatalysis (Scheme 26).
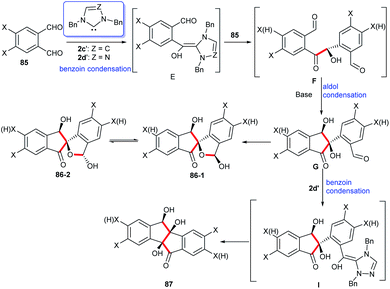
To further explain the effect of different reaction conditions on the products, the mechanism of intermolecular condensation was proposed in Scheme 27. In the reaction between imidazole or triazole carbene and phthalaldehydes 85, they form a benzoin condensation via a Breslow intermediate E which then gives rise to R-hydroxylketones F. There has been a possibility that the intramolecular aldol condensation of F may yield cis-dihydroxylindenone G due to the presence of hydrogen bonds between the two cis-substituted hydroxyl groups. As a result of the intramolecular addition of 2-hydroxyl to aldehyde moiety G, hemiacetals of spiro-indenones 86 exist in solution as a mixture of epimer 86-1 and epimer 86-2. Accompanying with an intramolecular condensation of benzoin between two carbonyl groups the hydroxylindenones G are converted into the fully cis-trihydroxylindeno[2,1-a]inden-5-ones 87.
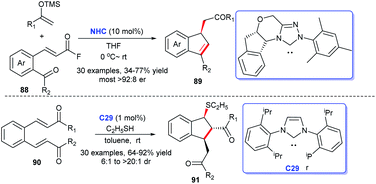
In 2017, Lupton and coworkers disclosed an approach to obtain functionalized indenes 89 exploiting α,β-unsaturated acyl fluorides 88 and TMS enol ethers as raw materials with NHC catalyzing (Scheme 28). The reaction realized the efficient synthesis of highly stereoselective functionalized indenes. Thirty-one examples were tested via generality investigation. The results exhibited a broad substrate scope and excellent functional group tolerance.58 In 2019, He's group acquired a series of sulfenylated indanes 91 via sulfa-Michael–Michael cascade reaction of thiols with o-formyl chalcones 90 mediated by NHC C29.59 Only 1% of NHC sufficed to promote the reaction, which made the process highly practical.
2.7 Applications in the synthesis of lactams
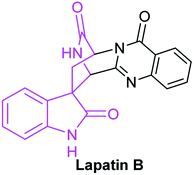
Great quantities of compounds containing lactam structure have outstanding biological activities. For instance, lactam skeletons are the essential pharmacophores of penicillin antibiotics. Thalidomide containing spiro-glutarimide skeleton is a widely exploited drug for cancer treatment.60 Lapatin B bearing a δ-lactam moiety and processing antibacterial activity, was isolated from Trichoderma penicillium (Fig. 11).In addition, lactam skeletons are often used as intermediates to synthesize complex organic compounds. Therefore, their special properties and important applications have attracted chemists' widespread attention. With the widespread application of NHC catalysts, reports regarding to synthesis of lactam compounds catalyzed by NHC are numerous.61
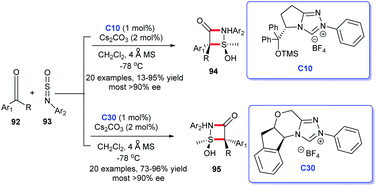
Only 1% of triazolium salt C10 or C30 and 2% of Cs2CO3 sufficed to promote the reaction. Surprisingly, under the same other conditions, two different types of NHC precursors triazolium salts C10 and C30 led to the completely distinct four-membered cycloaddition products, which reflects the “smart” of NHC catalysts. However, the harsh reaction condition (−78 °C) thereby prevented its large-scale synthetic applications. In addition, Xia et al. offered a powerful strategy for the synthesis of β-lactams. They utilized NHC–Pd complex to catalyze the [2 + 2] cycloaddition of imine's allyl derivatives and benzyl chlorides in the presence of CO.63
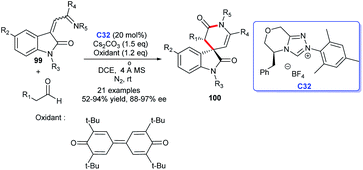
A [2 + 4] cycloaddition promoted by oxidative carbine catalysis using NHC generated from chiral triazolium salt C32 and 3,3′,5,5′-tetra-tert-butyldi-phenoquinone as oxidant was disclosed by Ren's group in 2019. Employing isatin derivatives 99 and aldehydes as substrates in the process could result in the enantioselective synthesis of oxindole δ-lactams (Scheme 31).66 The reaction took place under mild conditions with low catalyst loading. In view of the previous synthetic approaches, the reaction temperature was optimized, conducing to the large-scale preparation of products.
2.8 Applications in the synthesis of lactones
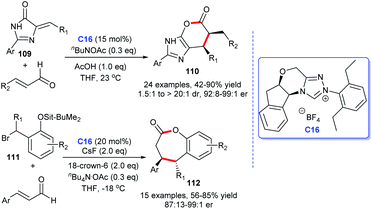
The lactone ring structure is extensively distributed in a large number of synthetic and natural organic compounds, many of which exhibit significant biological activities.70 For instance, vernolepin is used as an antitumor drug, butyrolactone is used as a neurotransmitter, natural product, and ieodomycins B shows antimicrobial activity, and so on (Fig. 12). These excellent activities of lactone ring compounds stimulated the great interest of synthetic chemists. Several routes have been developed to construct the lactone ring scaffold, among which, the NHCs were also found to be efficient catalysts for the lactone rings.71Scheidt and co-workers developed an [4 + 2] annulation accessing to imidazole fused six-membered lactones 110 with imidazolidinones 109 and α,β-unsaturated aldehydes as starting materials in 2013.72 The reaction was promoted by using a cooperative NHC and AcOH catalytic strategy, with the corresponding products afforded in moderate to good yields with up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. On the basis of 110, the same group focused on the core structure lactone, and subsequently, developed a protocol to synthesis 2-benzoxopinones 112, via the NHC-catalyzed [4 + 3] cycloaddition by treating α,β-unsaturated aldehydes with o-quinone methides for the formation of 2-benzoxopinones. NHC catalyzes the formation of cycloaddition products by forming homoenolate intermediates with aldehydes (Scheme 34).73
1 er. On the basis of 110, the same group focused on the core structure lactone, and subsequently, developed a protocol to synthesis 2-benzoxopinones 112, via the NHC-catalyzed [4 + 3] cycloaddition by treating α,β-unsaturated aldehydes with o-quinone methides for the formation of 2-benzoxopinones. NHC catalyzes the formation of cycloaddition products by forming homoenolate intermediates with aldehydes (Scheme 34).73
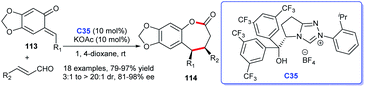
An additional approach to benzo-ε-lactones was described by Ye and co-workers. In the study, o-quinone methides 113 and enals were adopted as substrates and NHC derived from triazolium salt C35 was proven to be effective for the generation of desired seven-membered lactone rings 114 (Scheme 35).74 The reaction exhibited mild reaction conditions, high yields, and good stereoselectivities. The construction of five-membered lactone rings through NHC–Lewis acid cooperative catalysis was reported by She et al. during the same period. They synthesized a series of butenolides by [3 + 2] cycloaddition of alkynyl aldehydes and β, γ-unsaturated α-ketoesters.75
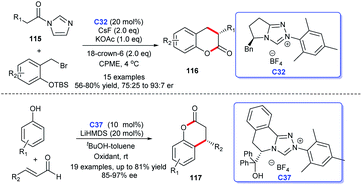
Coumarin is a typical aromatic lactone, and coumarin scaffolds were discovered in lots of natural products, including bioactive compounds. Thus, coumarins received great attention of chemists. In recent years, reports on the synthesis of coumarin skeleton compounds catalyzed by NHCs have gradually increased (Scheme 36). In 2015, Scheidt's group opened up a new synthetic strategy of dihydrocoumarins 116 under the catalysis of NHCs. The study is a continuation of “a dual activation” strategy based on their previous report.76 In 2017, You and co-workers demonstrated that NHC could catalyze the [3 + 3] cycloaddition of enals with phenols resulting in the synthesis of 4-aryl-3,4-dihydrocoumarins 117 in good yields with good to excellent enantioselectivities. Meanwhile, the reaction also showed good substrate applicability.77 In addition, Enders's group exploited enals to react with malonates bearing an ortho-hydroxy phenyl with the NHC-catalysis, leading to the efficient synthesis of coumarin scaffolds 117 called cyclopenta-[c]-fused chromenones.78
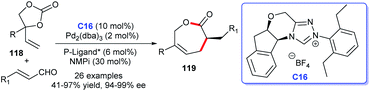
The efficient asymmetric acquisition of ε-caprolactones was developed by Glorius and co-workers in 2018. The corresponding cycloaddition products 119 were achieved in good yields with excellent enantioselectivities through cooperative catalysis of NHC–Pd and P-ligand*. Vinylethylene carbonates 118 and enals were employed as substrates for the [5 + 2] cycloaddition reaction (Scheme 37). Twenty-six examples were investigated demonstrating good substrate tolerance and wide functional group applicability for the reaction.79
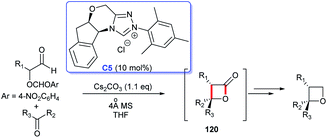
In 2015, NHC-catalyzed [2 + 2] cycloaddition to construct four-membered lactone ring was developed by Smith's group (Scheme 38). The reaction starting from perfluoroketones and α-aroyloxyaldehydes proceeded smoothly in THF, catalyzed by NHC generated from triazolium salt C5, affording the cycloadducts four-membered lactones 120, as a precursor for the synthesis of chiral multisubstituted four-membered cyclic ethers in good yields, with excellent diastereo- and enantioselectivities.80 This reaction took place under mild conditions with low catalyst loading, providing an excellent strategy for the construction of the quaternary lactone rings.
2.9 Applications in the synthesis of cyclic ketones
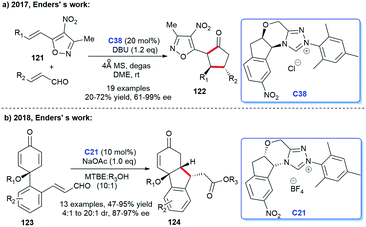
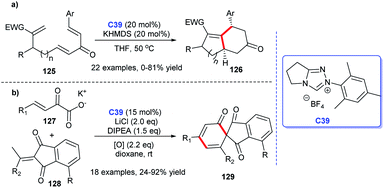
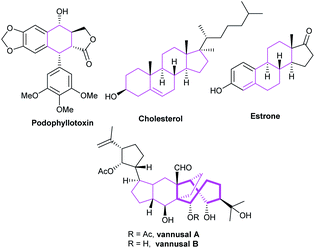
The NHC catalysis was also found to be an efficacious strategy for the preparation of cyclic ketones.81 In 2017, Enders's group demonstrated the asymmetric synthesis of a variety of 3,5-diaryl cyclohexenones 122 with the assistance of chiral NHC C38. They achieved the [4 + 2] cycloaddition products through the Breslow intermediate from the reaction of NHC with enals (Scheme 39a).82 Subsequently, the group reported the NHC-catalyzed [3 + 2] annulation reaction for obtaining cyclopentanone derivatives.83 In 2018, with the catalysis of chiral NHC C21, the same group synthesized cyclohexenone derivatives-tricyclic fluorene skeletons, through an intramolecular homoenolate Michael addition esterification reaction (Scheme 39b).84 These reactions exhibited wide substrates applicability, medium to good yields, and excellent region- and stereo selectivities.In 2019, Lupton and co-workers reported their new strategy for synthesizing cyclohexanones with the catalysis of NHC. They exploited acrylates 125 undergoing intermolecular [5 + 1] cycloaddition to achieve corresponding functionalized cyclopentanones 126 in moderate to good yields (Scheme 40a).85 Therefore, the stereoselectivity of the reaction needs to be further explored. In the same year, a synthesis strategy regarding to spirocyclohexanones catalyzed by NHC was reported by Huang et al. They employed 2-ethylidene 1,3-indandiones and potassium 2-oxo-3-enoates as substrates to conduct the [3 + 3] annulation reaction. The enantioselective synthesis of spirocyclohexanones 129 was achieved through oxidative carbene catalysis using NHC generated from triazolium salt C39 and oxidant with LiCl as a cooperative Lewis acid (Scheme 40b).86 With these protocols in hand, spiro- and fused cyclohexanones were easily afforded.
2.10 Applications in the construction of several complex carbocyclic skeletons
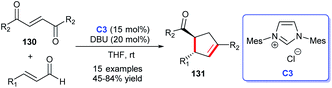
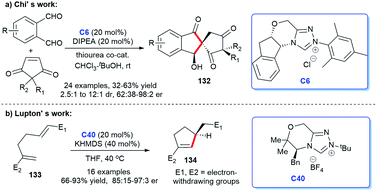
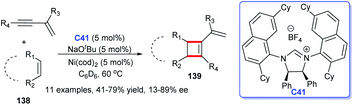
In addition to heterocyclic skeletons, carbocyclic skeletons are significant moieties discovered in natural products and biologically active molecules (Fig. 13).87 Therefore, the construction of carbocyclic skeletons has achieved considerable attention.88 For NHCs, there have been increasing applications in the synthesis of almost all types of cyclic compounds, and they are also widely used in the synthesis of carbocyclic skeletons.Following these pioneering reports, in 2018, an all-carbon spirocycle scaffold 132 was constructed by one-step asymmetric [4 + 1] annulation through catalysis of NHC C6 (Scheme 42a). In this study, they employed thiourea, which proved essential for the reaction, as a co-catalyst to unseal the reaction. The research opened the door to NHC-catalyzed synthesis of all-carbon spirocyclic skeletons for the first time.90 In the same year, Lupton and co-workers synthesized a series of multi-substituted cyclopentenes 134 through intramolecular cycloaddition reaction under the catalysis of NHC C40. Additionally, the highly stereoselective reaction was accomplished through the β-azolium ylide, which was reported lesser than Breslow intermediate (Scheme 42b).91
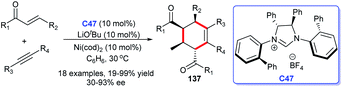
In 2015, a novel approach to chiral multi-substituted cyclohexenes was disclosed by Ogoshi and co-workers. In this study, a [2 + 2 + 2] cycloaddition of alkynes and two molecules of enones took place by using a cooperative NHC C47 and Ni(cod)2 catalytic strategy. The multi-substituted cyclohexenes bearing four contiguous chiral centers 137 were obtained in moderate to good yields and ee values (Scheme 44).87
2.11 Applications in the synthesis of sulfur heterocycles
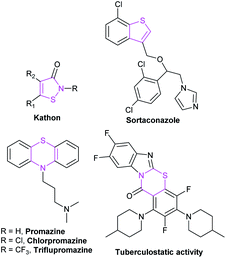
Organic sulfur-containing heterocyclic compounds exist widely in nature. They are the core fragments of many drugs and natural alkaloids, which show good biological activities in many aspects. Moreover, sulfur-containing heterocyclic compounds are broadly used in medicines, pesticides, dyes and other fields, such as the antipsychotic drug promazine, the antibacterial agent Kathon, sulfur dyes and so on (Fig. 14). Therefore, the research on sulfur-containing heterocyclic compounds has become an important part of organic chemistry. In recent years, as more and more excellent catalytic properties of NHCs have been discovered, synthetic methods of sulfur-containing heterocycles catalyzed by NHCs have also been developed.Owing to its remarkable biological activities and challenging structure, the synthesis of thiazinone skeleton is a research hotspot in the field of NHC catalysis.
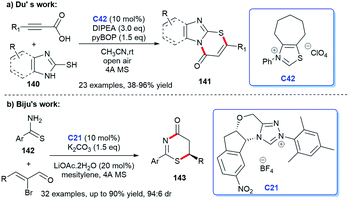
In 2018, imidazo fused thiazinones 141 was synthesized by Du et al. In the presence of NHC C42 generated from the thiazolium, alkynyl acids were converted into alkynyl acylazolium intermediates, then reacted with mercaptoimidazoles 140 in situ to form C–S bond, leading to the corresponding [3 + 3] cycloadducts (Scheme 46a).94 In 2019, Biju and co-workers reported their [3 + 3] cycloaddition between enals and thioamides 142 catalyzed by chiral NHC C21, resulting in a variety of functionalized thiazinones 143 with high stereoselectivities (Scheme 46b).95 Thirty-two reactions were carried out to prove the good functional group applicability.
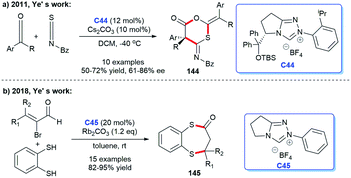
Ye's team has also made great progress in the establishment of NHC-catalyzed sulfur-containing heterocyclic skeletons. In 2011, Ye's group reported that they completed the asymmetric synthesis of 1,3-oxathian-6-ones 144 catalyzed by chiral NHC via [2 + 2 + 2] cycloadditions of isothiocyanates and two molecules of ketones.96 In this study, NHC generated from C44 was proven to be the effective catalyst (Scheme 47a). In 2018, the same group developed an NHC C45-catalyzed [3 + 4] annulation reaction, affording benzo[1,5]di-thiepin-2-ones 145 with 1,2-benzenedithiol and bromoenals as starting materials (Scheme 47b).97 This reaction provided ideas for the establishment of seven-membered sulfur-containing heterocycles.
2.12 Applications in the synthesis of other heterocyclic compounds
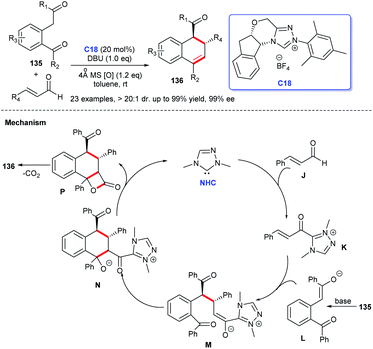
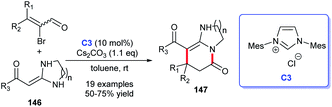
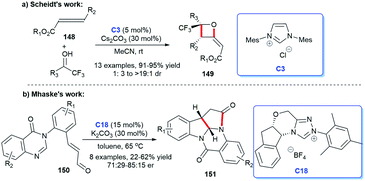
In 2011, Yao and co-workers disclosed the construction of imidazo-fused heterocyclic skeleton, which was catalyzed by NHC C3 and a privileged motif in diverse bioactive molecules.98 They employed bromoenals and HKAs as substrates, under the catalysis of NHC, to form C–C and C–N bond, leading to the acquisition of imidazo(pyrido)[1,2-a]pyridine and tetrahydro-benzo[b]imidazo[1,2,3ij][1,8] naphthyridine 147 (Scheme 48).99In 2018, Scheidt et al. developed an asymmetric NHC-catalyzed [2 + 2] cycloaddition strategy for multi-substituted oxetanes 149 (Scheme 49a).100 In 2019, Jin et al. obtained challenging chromeno[4,3-b]pyrrole derivatives containing four chiral centers with the catalysis of chiral NHC.101 In the same year, Mhaske and coworkers prepared deoxy-cruciferane alkaloid scaffold 151, which was a class of biologically active skeletons, via intramolecular annulation reaction catalyzed by NHC C18 (Scheme 49b).102
Axially chiral biaryl skeletons have emerged as indispensable structures in organic chemistry.103 They are found in a lot of natural products, drugs, catalysts, and some important chiral ligands (Fig. 15).104 As a class of smart catalysts, chiral NHC catalyzed annulation reactions provide an efficient way for the construction of axially chiral biaryl scaffolds.105
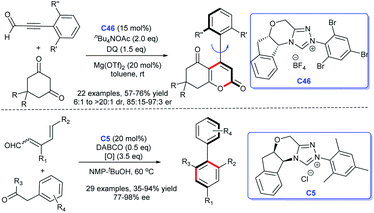
In 2018, Wang's group built axially chiral molecule scaffolds with the catalysis of NHC.106 In 2019, Zhu et al. developed a chiral NHC C46-catalyzed asymmetric [4 + 2] cycloaddition, resulting in a series of atroposelective arene.107 In the same year, Shi et al. completed the efficient synthesis of biaryl atropisomers under the cooperative catalysis of NHC C5 and Pd (Scheme 50).108 These approaches open a new avenue for the diverse synthesis of axially chiral molecule scaffolds.
3 Conclusions and outlook
In summary, we have reviewed the general protocols for synthesizing carbocycles and heterocycles containing indoles, pyrazoles, pyrroles, quinolines, lactams, lactones and other cyclic compounds assisted by NHCs from the perspective of cycloaddition reactions, wishing to advertise the readers of achievements and challenges that lie ahead in the field of NHC-mediated the construction of bioactive skeletons. In these studies, NHCs catalyze simple molecules to build complex heterocyclic skeletons, which is determined by the attractive features of NHCs. The current applications of smart NHCs in the catalytic synthesis of the organic framework have already unleashed a significant impact on the field of organic chemistry and medicinal chemistry. In general, high-efficient atomic utilization, mild reaction conditions, good functional group compatibility, and excellent stereoselectivity enable this NHC catalysis strategy to be applied in more aspects. Based on these outstanding advances, new challenges are likely to be conquered in the future.From the perspective of this review, we proposed several challenging aspects, and the suggested directions for the future investigation in this area mainly include the following:
(i) The synthetic approaches of NHC catalysts are relatively miscellaneous, meanwhile, the low yield is the direction, which is urgently needed to be settled in the future.
(ii) The development of a completely innovative NHC catalyst may bring a more concise and efficient approach for the synthesis of these complex chiral cyclic skeletons;
(iii) The mechanism of NHC catalysis is expected to be further clarified, then promoting the production of chiral bioactive molecules on an industrial scale, making greater contributions to the efficient synthesis of bioactive natural products and drugs;
(iv) In addition to the applications highlighted in this review, the applications of NHCs in other fields will also be increasingly developed.
(v) Based on these previous studies, more simple and outstanding organic catalysts will be disclosed for assembling biologically active organic cyclic frameworks;
NHCs provide opportunities to create complex biologically active scaffolds from simple molecules. It is reasonable to believe that the chemistry of NHCs will continue to flourish and will lead to amazing results.
Conflicts of interest
The authors confirm that this article content has no conflicts of interest.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (81803374), Shenzhen Science and Technology Innovation Fund (JCYJ20180302174121208), China Postdoctoral Science Foundation (2020M673565XB), and Xinglin Scholar Research Promotion Project of CDUTCM (BSH2019027 and BJRC2020002).References
- Q. Ren, M. Li, Y. Deng, A. Lu and J. Lu, Pharmacol. Res., 2021, 165, 105377 CrossRef CAS PubMed; Z.-H. Chen, W.-S. Li, Z.-Y. Zhang, H. Luo, J.-R. Wang, H.-Y. Zhang, Z.-R. Zeng, B. Chen, X.-W. Li and Y.-W. Guo, Org. Lett, 2021, 23, 5621 CrossRef PubMed; X.-L. Hu, Q.-W. He, H. Long, L.-X. Zhang, R. Wang, B.-L. Wang, J.-H. Feng, Q. Wang, J.-Q. Hou, X.-Q. Zhang, W.-C. Ye and H. Wang, J. Nat. Prod., 2021, 84, 1954 CrossRef PubMed.
- W.-J. Wu, M.-M. Li, B. Liu and Y. Wu, Eur. J. Org. Chem., 2019, 3169 CrossRef CAS; M.-M. Li, Y. Wu and B. Liu, Org. Lett., 2019, 21, 575 CrossRef PubMed.
- Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Acc. Chem. Res., 2015, 48, 740 CrossRef CAS PubMed.
- M. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 486 CrossRef PubMed.
- P.-C. Chiang and J. W. Bode, N-Heterocyclic Carbenes, 2010, p. 399 Search PubMed.
- Q. Zhao, G. Meng, S. P. Nolan and M. Szostak, Chem. Rev., 2020, 120, 1981 CrossRef CAS PubMed.
- M. Balci, Synthesis, 2018, 50, 1373 CrossRef CAS; J. Mahatthananchai and J. W. Bode, Acc. Chem. Res., 2014, 47, 696 CrossRef PubMed.
- A. Thakur and J. Louie, Acc. Chem. Res., 2015, 48, 2354 CrossRef CAS PubMed.
- H.-E. A. Saad, S. H. El-Sharkawy and W. T. Shier, Planta Med., 1995, 61, 313 CrossRef CAS PubMed; C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef PubMed.
- G.-Y. Li, B.-G. Li, T. Yang, J.-F. Yan, G.-Y. Liu and G.-L. Zhang, J. Nat. Prod., 2006, 69, 1374 CrossRef CAS PubMed.
- D. F. Taber and P. K. Tirunahari, Tetrahedron, 2011, 67, 7195 CrossRef CAS PubMed.
- S. Haddad, S. Boudriga, T. N. Akhaja, J. P. Raval, F. Porzio, A. Soldera, M. Askri, M. Knorr, Y. Rousselin and M. M. Kubicki, New J. Chem., 2014, 39, 520 RSC; S. M. Rajesh, S. Perumal, J. Menéndez, P. Yogeeswari and D. Sriram, MedChemComm, 2011, 2, 626 RSC; D. Kathirvelan, J. Haribabu, B. S. R. Reddy, C. Balachandran and V. Duraipandiyan, Bioorg. Med. Chem. Lett., 2015, 25, 389 CrossRef CAS PubMed.
- L. H. Sun, L. T. Shen and S. Ye, Chem. Commun., 2011, 47, 10136 RSC.
- Y. Xie, C. Yu, T. Li, S. Tu and C. Yao, Chem.–Eur. J., 2015, 21, 5355 CrossRef CAS PubMed.
- J. Xu, S. Yuan, M. Miao and Z. Chen, J. Org. Chem., 2016, 81, 11454 CrossRef CAS PubMed.
- D. Jiang, S. Dong, W. Tang, T. Lu and D. Du, J. Org. Chem., 2015, 80, 11593 CrossRef CAS PubMed.
- S. Mukherjee, S. Joseph, A. Bhunia, R. G. Gonnade, S. R. Yetra and A. T. Biju, Org. Biomol. Chem., 2017, 15, 2013 RSC.
- C. Wang, S. Zhu, G. Wang, Z. Li and X.-P. Hui, Eur. J. Org. Chem., 2016, 2016, 5653 CrossRef CAS.
- A. B. A. Patra, S. R. Yetra, R. G. Gonnade and A. T. Biju, Org. Chem. Front., 2015, 2, 1584 RSC.
- Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432 CrossRef CAS PubMed; M. Collins, V. Hudak and R. Bender, Bioorg. Med. Chem. Lett., 2004, 14, 2185 CrossRef PubMed; H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36 CrossRef PubMed.
- H. Yao, Y. Zhou, X. Chen, P. Zhang, J. Xu and H. Liu, J. Org. Chem., 2016, 81, 8888 CrossRef CAS PubMed; L. Shen, W. Jia and Y. Song, Chin. J. Chem., 2014, 32, 814 CrossRef.
- L. L. Zhao, X. S. Li, L. L. Cao, R. Zhang, X. Q. Shi and J. Qi, Chem. Commun., 2017, 53, 5985 RSC.
- D. Enders, Q. Liu, X. Chen, S. Li and K. Rissanen, Adv. Synth. Catal., 2019, 361, 1991 CrossRef.
- Z. J. Zhang, L. Zhang, R. L. Geng, J. Song, X. H. Chen and L. Z. Gong, Angew. Chem., Int. Ed., 2019, 58, 12190 CrossRef CAS PubMed.
- Q. Liu, S. Li, X. Y. Chen, K. Rissanen and D. Enders, Org. Lett., 2018, 20, 3622 CrossRef CAS PubMed.
- Z. H. Gao, K. Q. Chen, Y. Zhang, L. M. Kong, Y. Li and S. Ye, J. Org. Chem., 2018, 83, 15225 CrossRef CAS PubMed.
- L. Chevolot, A. M. Chevolot, M. Gajhede, C. Larsen, U. Anthoni and C. Christophersen, J. Am. Chem. Soc., 1985, 107, 4542 CrossRef CAS; U. Anthoni, K. Bock, L. Chevolot, C. Larsen, P. H. Nielsen and C. Christophersen, J. Org. Chem., 1987, 52, 5638 CrossRef.
- H. M. Zhang, Z. H. Gao and S. Ye, Org. Lett., 2014, 16, 3079 CrossRef CAS PubMed.
- W. Zhang, J. Bah, A. Wohlfarth and J. Franzén, Chem.–Eur. J., 2011, 17, 13814 CrossRef CAS PubMed; W. Gu, Y. Zhang, X. J. Hao, F. M. Yang, Q. Y. Sun, S. L. Morris-Natschke, K. H. Lee, Y. H. Wang and C. L. Long, J. Nat. Prod., 2014, 77, 2590 CrossRef PubMed.
- Y. Lu, W. Tang, Y. Zhang, D. Du and T. Lu, Adv. Synth. Catal., 2013, 355, 321 CrossRef CAS.
- S. Hu, B. Wang, Y. Zhang, W. Tang, M. Fang, T. Lu and D. Du, Org. Biomol. Chem., 2015, 13, 4661 RSC.
- L. Yi, K.-Q. Chen, Z.-Q. Liang, D.-Q. Sun and S. Ye, Adv. Synth. Catal., 2017, 359, 44 CrossRef CAS.
- L. Yi, Y. Zhang, Z. F. Zhang, D. Sun and S. Ye, Org. Lett., 2017, 19, 2286 CrossRef CAS PubMed.
- S.-Y. Zhu, Y. Zhang, X.-F. Chen, J. Huang and S.-H. Shi, Chem. Commun., 2019, 55, 4363 RSC.
- S. Fang, S. Jin, R. Ma, T. Lu and D. Du, Org. Lett., 2019, 21, 5211 CrossRef CAS PubMed.
- X. Liang, J. Zang, M. Zhu, Q. Gao, B. Wang, W. Xu and Y. Zhang, ACS Med. Chem. Lett., 2016, 7, 950 CrossRef CAS PubMed.
- Y. Zhang, S. Wu, S. Wang, K. Fang, G. Dong, N. Liu, Z. Miao, J. Yao, J. Li, W. Zhang, C. Sheng and W. Wang, Eur. J. Org. Chem., 2015, 2015, 2030 CrossRef CAS; H. S. Ibrahim, S. M. Abou-Seri, M. Tanc and M. M. Elaasser, Eur. J. Med. Chem., 2015, 103, 583 CrossRef PubMed.
- X. Wu, B. Liu, Y. Zhang, M. Jeret, H. Wang, P. Zheng, S. Yang, B. A. Song and Y. R. Chi, Angew. Chem., Int. Ed., 2016, 55, 12280 CrossRef CAS PubMed.
- D. Enders, S. Li, L. Wang, P. Chauhan, A. Peuronen and K. Rissanen, Synthesis, 2016, 49, 1808 CrossRef.
- L. Wang, S. Li, P. Chauhan, D. Hack, A. R. Philipps, R. Puttreddy, K. Rissanen, G. Raabe and D. Enders, Chem.–Eur. J., 2016, 22, 5123 CrossRef CAS PubMed.
- L. Yang, Y. Lv, F. Wang and G. Zhong, Org. Biomol. Chem., 2018, 16, 4433 RSC.
- C. Zhao, K. Shi, G. He, Q. Gu, Z. Ru, L. Yang and G. Zhong, Org. Lett., 2019, 21, 7943 CrossRef CAS PubMed.
- K. Takahashi, K. Cho, A. Iwai, T. Ito and N. Iwasawa, Chem.–Eur. J., 2019, 25, 13504 CrossRef CAS PubMed.
- A. Patra, F. Gelat, X. Pannecoucke, T. Poisson, T. Besset and A. T. Biju, Org. Lett., 2018, 20, 1086 CrossRef CAS PubMed.
- Y. Hu, D. Pan, L. Cong, Y. Yao, C. Yu, T. Li and C. Yao, ChemistrySelect, 2018, 3, 1708 CrossRef CAS.
- R. Huang, X. Chen, C. Mou, G. Luo, Y. Li, X. Li, W. Xue, Z. Jin and Y. R. Chi, Org. Lett., 2019, 21, 4340 CrossRef CAS PubMed.
- X. Han, G. R. Peh and P. E. Floreancig, ChemInform, 2013, 2013, 1193 Search PubMed; D. Liu, X. M. Li, L. Meng, C. S. Li, S. S. Gao, Z. Shang, P. Proksch, C. G. Huang and B. G. Wang, J. Nat. Prod., 2011, 74, 1787 CrossRef CAS PubMed.
- Z. Y. Wang, Q. Liu, K. K. Wang, M. Liu, Y. Han, A. Sun and X. Ma, Asian J. Org. Chem., 2021, 10, 766 CrossRef CAS.
- X. Y. Chen, L. H. Sun and S. Ye, Chem.–Eur. J., 2013, 19, 4441 CrossRef CAS PubMed.
- L. Lin, Y. Yang, M. Wang, L. Lai, Y. Guo and R. Wang, Chem. Commun., 2015, 51, 8134 RSC.
- D. Enders, S. Li, X.-Y. Chen, H. Sheng, C. von Essen and K. Rissanen, Synthesis, 2017, 50, 1047 CrossRef.
- M. Zhao, J. L. Liu, H. F. Liu, J. Chen and L. Zhou, Org. Lett., 2018, 20, 2676 CrossRef CAS PubMed.
- D. S. Mortensen, A. L. Rodriguez, K. E. Carlson, J. Sun, B. S. Katzenellenbogen and J. A. Katzenellenbogen, J. Med. Chem., 2001, 44, 3838 CrossRef CAS PubMed.
- C. Yu, J. Lu, T. Li, D. Wang, B. Qin, H. Zhang and C. Yao, Synlett, 2011, 2011, 2420 CrossRef.
- Y. Jia, T. Li, C. Yu, B. Jiang and C. Yao, Org. Biomol. Chem., 2016, 14, 1982 RSC.
- W. Y. Wang, J. Y. Wu, Q. R. Liu, X. Y. Liu, C. H. Ding and X. L. Hou, Org. Lett., 2018, 20, 4773 CrossRef CAS PubMed.
- Y. Cheng, J.-H. Peng, Y.-J. Li, X.-Y. Shi and M.-S. Tang, J. Org. Chem., 2011, 76, 1844 CrossRef CAS PubMed.
- C. Zhang and D. W. Lupton, Org. Lett., 2017, 19, 4456 CrossRef CAS PubMed.
- Z.-N. Feng, J.-Y. Luo, Y. Zhang, G.-F. Du and L. He, Org. Biomol. Chem., 2019, 17, 4700 RSC.
- S. Mondal, A. Ghosh, S. Mukherjee and A. T. Biju, Org. Lett., 2018, 20, 4499 CrossRef CAS PubMed.
- C. He, Y. Zhou, Z. Li, J. Xu and X. Chen, Org. Chem. Front., 2021, 8, 1569 RSC.
- T. Y. Jian, L. He, C. Tang and S. Ye, Angew. Chem., Int. Ed., 2011, 50, 9104 CrossRef CAS PubMed.
- P. Xie, B. Qian, H. Huang and C. Xia, Tetrahedron Lett., 2012, 53, 1613 CrossRef CAS.
- Y. Xiao, J. Wang, W. Xia, S. Shu, S. Jiao, Y. Zhou and H. Liu, Org. Lett., 2015, 17, 3850 CrossRef CAS PubMed.
- W. Xia, H. Yao, D. Liu, L. Zhao, Y. Zhou and H. Liu, Adv. Synth. Catal., 2016, 358, 1864 CrossRef CAS.
- C. He, Z. Li, J. Xu and H. Ren, J. Org. Chem., 2019, 84, 12177 CrossRef CAS PubMed.
- Z. Z. Zhang, Y. Zhang, H. X. Duan, Z. F. Deng and Y. Q. Wang, Chem. Commun., 2020, 56, 1553 RSC.
- C. He, Z. Li, H. Zhou and J. Xu, Org. Lett., 2019, 21, 8022 CrossRef CAS PubMed.
- D. Liu, Z. Hu, Y. Zhang, M. Gong, Z. Fu and W. Huang, Chem.–Eur. J., 2019, 25, 11223 CAS.
- L. Liu, D. Guo and J. Wang, Org. Lett., 2020, 22, 7025 CrossRef CAS PubMed.
- Y.-T. Wu, R. Zhang, X.-Y. Duan, H.-F. Yu, B.-Y. Sun and J. Qi, Chem. Commun., 2020, 56, 9854 RSC.
- E. O. McCusker and K. A. Scheidt, Angew. Chem., Int. Ed., 2013, 52, 13616 CrossRef PubMed.
- J. Izquierdo, A. Orue and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634 CrossRef CAS PubMed.
- H. Lv, W. Q. Jia, L. H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607 CrossRef CAS PubMed.
- J. Qi, X. Xie, R. Han, D. Ma, J. Yang and X. She, Chem.–Eur. J., 2013, 19, 4146 CrossRef CAS PubMed.
- A. Lee and K. A. Scheidt, Chem. Commun., 2015, 51, 3407 RSC.
- G. T. Li, Z. K. Li, Q. Gu and S. L. You, Org. Lett., 2017, 19, 1318 CrossRef CAS PubMed.
- Q. Liu, X. Y. Chen, R. Puttreddy, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2018, 57, 17100 CrossRef CAS PubMed.
- S. Singha, T. Patra, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2018, 140, 3551 CrossRef CAS PubMed.
- A. T. Davies, A. M. Slawin and A. D. Smith, Chem.–Eur. J., 2015, 21, 18944 CrossRef CAS PubMed.
- S. Shee, S. Mukherjee, R. G. Gonnade and A. T. Biju, Org. Lett., 2020, 22, 5407 CrossRef CAS PubMed.
- X. Y. Chen, Q. Liu, P. Chauhan, S. Li, A. Peuronen, K. Rissanen, E. Jafari and D. Enders, Angew. Chem., Int. Ed., 2017, 56, 6241 CrossRef CAS PubMed.
- X. Y. Chen, S. Li, H. Sheng, Q. Liu, E. Jafari, C. von Essen, K. Rissanen and D. Enders, Chem.–Eur. J., 2017, 23, 13042 CrossRef CAS PubMed.
- T. Shu, S. Li, X. Y. Chen, Q. Liu, C. von Essen, K. Rissanen and D. Enders, Chem. Commun., 2018, 54, 7661 RSC.
- X. B. Nguyen, Y. Nakano, N. M. Duggan, L. Scott, M. Breugst and D. W. Lupton, Angew. Chem., Int. Ed., 2019, 58, 11483 CrossRef CAS PubMed.
- Y. Gao, D. Liu, Z. Fu and W. Huang, Org. Lett., 2019, 21, 926 CrossRef CAS PubMed.
- R. Kumar, H. Tokura, A. Nishimura, T. Mori, Y. Hoshimoto, M. Ohashi and S. Ogoshi, Org. Lett., 2015, 17, 6018 CrossRef CAS PubMed.
- K. C. Nicolaou, A. Ortiz and H. Zhang, Angew. Chem., Int. Ed., 2010, 40, 5648 Search PubMed; K. C. Nicolaou, A. Ortiz, H. Zhang, P. Dagneau, A. Lanver, M. P. Jennings, S. Arseniyadis, R. Faraoni and D. E. Lizos, J. Am. Chem. Soc., 2010, 132, 7138 CrossRef CAS PubMed.
- R. R. Paul, K. C. S. Lakshmi, E. Suresh and V. Nair, Tetrahedron Lett., 2013, 54, 2046 CrossRef CAS.
- S. Zhuo, T. Zhu, L. Zhou, C. Mou, H. Chai, Y. Lu, L. Pan, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2019, 58, 1784 CrossRef CAS PubMed.
- L. Scott, Y. Nakano, C. Zhang and D. W. Lupton, Angew. Chem., Int. Ed., 2018, 57, 10299 CrossRef CAS PubMed.
- S. Perveen, Z. Zhao, G. Zhang, J. Liu, M. Anwar and X. Fang, Org. Lett., 2017, 19, 2470 CrossRef CAS PubMed.
- S. Ogoshi, R. Kumar, E. Tamai, A. Ohnishi, A. Nishimura, Y. Hoshimoto and M. Ohashi, Synthesis, 2016, 48, 2789 CrossRef.
- K. Sun, S. Jin, J. Zhu, X. Zhang, M. Gao, W. Zhang, T. Lu and D. Du, Adv. Synth. Catal., 2018, 360, 4515 CrossRef CAS.
- A. Ghosh, S. Barik and A. T. Biju, Org. Lett., 2019, 21, 8598 CrossRef CAS PubMed.
- X. N. Wang, L. T. Shen and S. Ye, Org. Lett., 2011, 13, 6382 CrossRef CAS PubMed.
- F. Xia, X. Y. Chen and S. Ye, J. Org. Chem., 2018, 83, 15178 CrossRef CAS PubMed.
- A. Scribner, R. Dennis, J. Hong, S. Lee and T. Biftu, Eur. J. Med. Chem., 2007, 42, 1334 CrossRef CAS PubMed; C. Hamdouchi, J. Ezquerra, J. A. Vega, J. J. Vaquero, J. Alvarez-Builla and B. A. Heinz, Bioorg. Med. Chem. Lett., 1999, 9, 1391 CrossRef PubMed; T. H. Al-Tel and R. A. Al-Qawasmeh, Eur. J. Med. Chem., 2010, 45, 5848 CrossRef PubMed.
- C. Yao, W. Jiao, Z. Xiao, Y. Xie, T. Li, X. Wang, R. Liu and C. Yu, RSC Adv., 2013, 3, 10801 RSC.
- S. S. Lopez, A. A. Jaworski and K. A. Scheidt, J. Org. Chem., 2018, 83, 14637 CrossRef CAS PubMed.
- T. Li, J. Wang, J. Xu, J. Jin, Y. R. Chi and Z. Jin, Org. Lett., 2020, 22, 326 CrossRef CAS PubMed.
- M. M. Ahire, M. D. Pol, D. S. Kavale, R. G. Gonnade and S. B. Mhaske, Org. Biomol. Chem., 2019, 17, 7135 RSC.
- S. R. Laplante, P. J. Edwards, L. D. Fader, A. Jakalian and O. Hucke, Chemmedchem, 2015, 6, 505 CrossRef PubMed.
- Y. Lu, S. Wu, Y. Yue, S. He, J. Li, J. Tang, W. Wang and H. B. Zhou, ACS Med. Chem. Lett., 2016, 7, 1185 CrossRef CAS PubMed; R. Noyori and H. Takaya, ChemInform, 1990, 23, 345 Search PubMed.
- T. Li, C. Mou, P. Qi, X. Peng, S. Jiang, G. Hao, W. Xue, S. Yang, L. Hao, Y. R. Chi and Z. Jin, Angew. Chem., Int. Ed., 2021, 60, 9362 CrossRef CAS PubMed.
- C. Zhao, D. Guo, K. Munkerup, K. W. Huang, F. Li and J. Wang, Nat. Commun., 2018, 9, 611 CrossRef PubMed.
- K. Xu, W. Li, S. Zhu and T. Zhu, Angew. Chem., Int. Ed., 2019, 58, 17625 CrossRef CAS PubMed.
- J. Diesel, D. Grosheva, S. Kodama and N. Cramer, Angew. Chem., Int. Ed., 2019, 58, 11044 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |