Open Access Article
Open Access ArticlePlanetary metrics for the absolute environmental sustainability assessment of chemicals†
Victor
Tulus
 ,
Javier
Pérez-Ramírez
,
Javier
Pérez-Ramírez
 * and
Gonzalo
Guillén-Gosálbez
* and
Gonzalo
Guillén-Gosálbez
 *
*
Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland. E-mail: jpr@chem.ethz.ch; gonzalo.guillen.gosalbez@chem.ethz.ch
First published on 27th October 2021
Abstract
Environmental assessments in green chemistry often rely on simplified metrics that enable comparisons between alternative routes but fail to shed light on whether they are truly sustainable in absolute terms, viz. relative to the Earth's ecological capacity. To expand our currently limited knowledge of the extent to which chemicals are environmentally sustainable, here we analyse 492 chemical products through the lens of seven planetary boundaries representing critical biophysical limits that should never be exceeded. We found that most of them transgress these environmental guardrails, mainly the ones strongly connected to greenhouse gas emissions (i.e., climate change, ocean acidification and biosphere integrity). However, their levels of transgression fail to correlate with their carbon footprints, currently the focus of most studies, implying that chemicals entailing higher greenhouse gas emissions are not necessarily less environmentally sustainable in absolute terms. Our work points towards the need to embrace absolute sustainability criteria in current environmental assessments, which will require agreeing on how to allocate shares of the planet's ecological capacity among anthropogenic activities, including chemicals’ production. This work's absolute environmental sustainability assessment (AESA) method, which could complement standard life cycle assessment approaches, might help experimental researchers working in green chemistry develop truly ‘green’ products. The AESA method should be taken as a starting point to devise holistic approaches for quantifying the absolute environmental impact of chemicals to guide research and policymaking more sensibly.
Introduction
The chemical industry faces the challenge of transitioning towards more sustainable production patterns while keeping its competitive edge. Emerging trends towards this overarching goal include shifting to renewable carbon (e.g., via carbon capture and utilisation [CCU]1 or biomass use2), adopting circular economy principles for emissions and waste reduction (e.g., recycling of plastics waste3 and CO2 utilisation), and electrifying process units, among others.4 Realising this sustainability transition will require suitable key performance indicators to effectively screen low-carbon technologies and develop optimal pathways.For many years, experimental work in green chemistry was guided by general principles and associated metrics, like the E-factor, atom economy or other mass-based metrics.5 However, these single-factor metrics started showing practical limitations evaluating the greenness of a chemical due to the continuous advances in the field and the inherently complex nature of the chemical routes.6 Accordingly, the current trend is to replace single-factor metrics by multi-factor indicators to provide evidence of a significant advance in green chemistry, which should be applied in any experimental work.7 Among the indicators available, those based on life cycle assessment (LCA) have been recommended and are gaining broad interest in the community.7 However, which specific LCA indicators should be employed in green chemistry and how to interpret them are still open questions.
LCA is a tool that quantifies the environmental impacts and resources used throughout a product's life cycle, from raw materials acquisition through production, storage, transportation, and final use and disposal. In the past decades, strong methodological developments in LCA have led to a range of life cycle impact assessment (LCIA) methods.8 These LCIA methods provide multi-factor metrics that quantify the damage on three main areas of protection—i.e., human health, ecosystem quality, and resources—from the life cycle inventory of feedstocks, energy use, emissions, and waste. Examples of such methods include the Eco-indicator 99,9 CML 2001,10 IMPACT 2002+,11 TRACI 2.1,12 EPS2015,13 and ReCiPe 2016,14 currently implemented in software packages like GaBi,15 SimaPro,16 openLCA,17 Umberto18 and CMLCA.19
Interpreting multi-factor LCIA results is often challenging, especially when burden-shifting occurs, i.e., some impacts improve at the expense of worsening others.20 Multi-criteria decision-support tools were used to simplify the analysis by aggregating indicators based on weights, derived via systematic approaches21 or expert elicitation.9 However, such weights are often subjective and can be controversial, being implausible that any consensus will ever be reached on their values.
In recent years, LCIA methods and their aggregated variants have become widely used in industrial ecology and process systems engineering, making LCA the predominant approach to quantify the environmental burdens of products and services,8 including chemicals and fuels.4,22–24 In green chemistry applications, LCIA methods can be used to screen alternative synthesis routes for chemicals displaying the same (or comparable) functionalities and identify critical hotspots in the chemicals’ supply chain.
However, standard LCIA methods, besides being hard to interpret, cannot quantify the chemicals’ absolute environmental sustainability level accurately, i.e., the extent to which chemicals could contribute to safely operating our planet, considering its finite carrying capacity. This limitation stems from the lack of environmental thresholds establishing maximum allowable impact limits within which a system could be deemed sustainable.25 For example, we may know that chemical A's global warming potential (GWP, also referred to as carbon footprint) is larger than chemical B's. Nevertheless, this information is insufficient to establish whether they are sustainable in absolute terms due to the absence of environmental thresholds (i.e., are A's and B's carbon footprint scores admissible?).26
The need to define environmental thresholds has recently emerged in the LCA literature, leading to the concept of absolute environmental sustainability assessments (AESA), which quantify impacts relative to some global limits. For example, thresholds were defined for terrestrial acidification based on the Earth's carrying capacity to interpret the impact results in this category.27 More recently, AESA methods based on the Planetary Boundaries (PBs) concept started to emerge.
The PBs framework—initially introduced by Rockström et al.28 and later updated with slight modifications by Steffen and colleagues29—identifies a set of biophysical limits within which humanity should operate our planet safely. The nine interlinked Earth-system processes, all essential for maintaining the Earth's resilience, include (1) climate change, (2) stratospheric ozone depletion, (3) ocean acidification, (4) biogeochemical flows, (5) land-system change, (6) freshwater use, (7) change in biosphere integrity, (8) atmospheric aerosol loading, and (9) introduction of novel entities. For most of the Earth-system processes, one or more control variables were identified, and quantitative thresholds (global boundaries) were defined that delimit a safe operating space (SOS) for humanity, which should never be exceeded to preserve the planet's stability.
The PBs concept, in its current state, only focuses on the well-being of the planet Earth and multiple challenges towards the road of true absolute sustainability—i.e., from the environmental, economic and social perspectives combined—remain open.30 These challenges will be addressed by the newly formed Earth Commission (EC), which is planning to expand the PBs framework by exploring a safe and just corridor for humanity.31 The EC will focus on defining more relevant control variables and more accurate boundaries’ ranges at global and regional scales.31 Additional research efforts aim to quantify the interactions between the PBs to understand complex feedback loops potentially amplifying anthropogenic impacts on the Earth system.32
Even though the work related to the PBs concept is still in its infancy, the benefits of AESA methods33,34 for LCA developed within this framework are unfolding new avenues for evaluating the environmental pillar of sustainability in industrial systems. As a proof-of-concept, Ryberg et al. applied a novel AESA method to assess the EU laundry washing sector.35 Ryberg and colleagues also assessed the absolute environmental sustainability level of a Danish utility company.36 Furthermore, capitalising on existing AESA methods, González-Garay et al. quantified the absolute environmental sustainability level of several CCU routes for green methanol production.37 In more recent work, Galán-Martín et al. extended this analysis to the main building blocks in the petrochemical industry, proposing a new method to quantify the impact on biosphere integrity.34 D'Angelo et al. evaluated low-carbon ammonia synthesis routes through the lens of seven PBs,38 while Samaroo et al. investigated how to achieve absolute environmental sustainability across integrated industrial networks for ammonia production considering a set of PBs-based metrics.39 Li et al. explored a possible route for China's carbon neutrality for the combined production of electricity, methanol and ammonia, focusing on the climate change PB.40 Valente et al. analysed whether multiple green hydrogen production routes could become environmentally sustainable substitutes for heavy road transportation fuels considering the PBs.41 Moreover, Wheeler et al.42 and Ehrenstein et al.43 embedded the PBs concept into optimisation models to design sustainable bioethanol and hydrogen supply chains, respectively.
AESA methods could provide a sound multi-factor evaluation framework to assess emerging and existing chemical routes. Moreover, they could generate valuable insight into the environmental sustainability footprints of current chemicals, uncovering their major global impacts. Certainly, there seems to be a general agreement that shifting to renewable carbon should be a priority in the chemical sector. However, to our knowledge, no previous study quantified the percentage reduction in carbon footprint needed in chemicals to operate within the safe operating space. Likewise, whether chemicals could potentially pose a threat to other critical Earth-system processes remains unclear. Hence, shedding light on whether (and by how much) current chemicals exceed their share of the Earth's ecological capacity could help to guide research more sensibly and develop more effective regulations.
Here we apply a multi-factor AESA method based on the PBs concept for assessing chemical technologies, and use it to evaluate 492 chemicals, providing clear guidelines for interpreting the multi-dimensional results. We find that most of these chemicals heavily transgress the climate change, ocean acidification and change in biosphere integrity PBs, while some also exceed the biogeochemical flow (nitrogen) PB. We also show how the carbon footprint, widespread in single-factor environmental assessments, fails to correlate with the absolute environmental sustainability level of chemicals. Overall, our results highlight the benefits of applying multi-factor AESA methods for assessing chemicals and guiding research and regulations more sensibly.
Methods
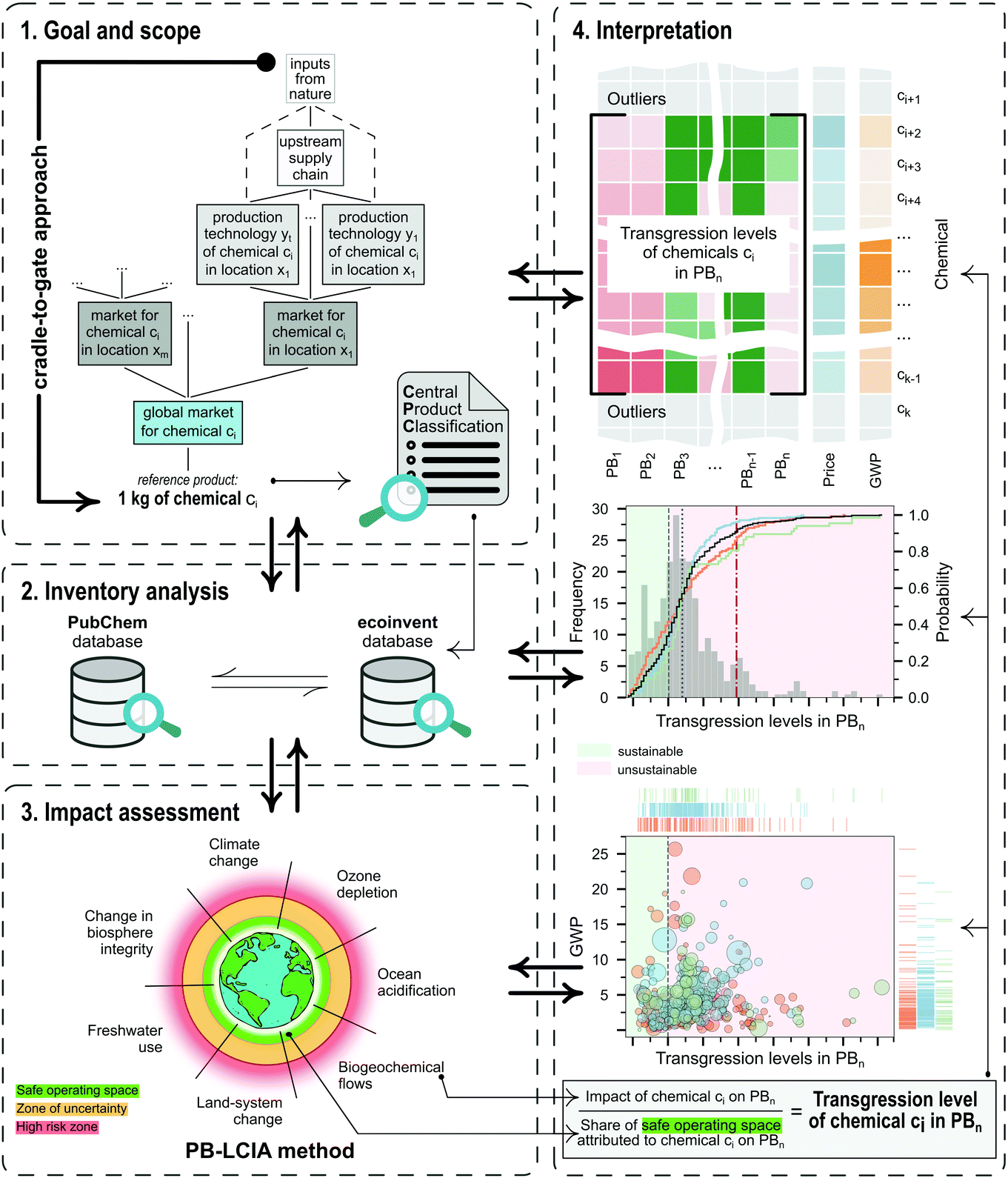
To illustrate the potential of applying AESA methods to evaluate chemicals, we carried out a systematic absolute environmental sustainability assessment of 492 chemicals following the four LCA phases described in the ISO 14040 and 14044 standards44,45 (Fig. 1), as detailed below.Phase 1: Goal and scope definition
The main goal of this study is to quantify the absolute environmental sustainability level of a set of chemicals currently produced at an industrial scale. The analysis quantifies the impact of each chemical relative to its share of the safe operating space, which defines a maximum threshold above which the chemical should be deemed environmentally unsustainable.As shown in Fig. 1, the functional unit (FU) is set to one kg of the reference product (chemical) of a specific global market. Usually, a global market combines multiple regional markets, each encompassing several plants operating within a geographical region and implementing one or multiple standard technologies. The analysis adopts a cradle-to-gate scope covering all the upstream inputs from the technosphere and nature, contributing to the plants as part of their supply chains. The main motivation for this choice is that we prefer to avoid making assumptions concerning the use phase of the chemicals (not reported in the database), which would add significant uncertainties to the analysis.
Focusing on global markets enables the evaluation of the average burden of a chemical manufactured by the predominant technologies rather than by marginal processes. Moreover, reducing the granularity of the data avoids assessing duplicated chemicals produced at regional scales, which could shift the global distribution of impact values, as discussed later in the article. Chemicals lacking a global production market were aggregated into one global market using mass allocation, mimicking the procedure implemented in the ecoinvent database.
It should be noted that each chemical in the dataset has its own FU (i.e., a relative reference unit to which the input and output data are normalised). This FU should not be used to carry out direct comparisons between chemicals because they show different functionalities. However, it is possible to compare each chemical with an ideal environmentally sustainable counterpart, which would display an impact equal to the share of the global ecological budget assigned to it. Accordingly, we determine each chemical's transgression level (i.e., the ratio of impact score to the share of ecological budget), as explained in Phase 4: Interpretation, enabling the comparison of each chemical against its ‘ideal’ absolute sustainable analogue.
The dataset of chemicals focuses on divisions 33–36 of the United Nations Central Product Classification (CPC) v2.1.46 Divisions 33 to 36 – as part of section 3 of CPC, ‘Other transportable goods, except metal products, machinery and equipment’ – encompasses the following categories: ‘Coke oven products; refined petroleum products; nuclear fuel’ (33), ‘basic chemicals’ (34), ‘other chemical products; man-made fibres’ (35), ‘rubber and plastics products’ (36). Finally, chemicals in these four categories were reorganised for convenience into three groups, i.e., organic, inorganic, and other (non-classifiable) chemicals. Further details are elaborated in section 1 of the ESI.†
Phase 2: Inventory analysis
Phase 3: Impact assessment
The life-cycle impact assessment method connected to the PBs framework (PB-LCIA) translates flows of inventory entries to impacts expressed in the units of the control variables (CV) of the Earth-system processes. We quantify the impact in seven out of nine Earth-system processes (also referred to as PBs), discarding the ‘atmospheric aerosol loading’ and the ‘introduction of novel entities’ PBs, since the former has no defined global boundary, and the latter lacks a control variable yet.The PB-LCIA method used in this study relies on the characterisation factors (CFs) of Ryberg et al.33 (CFs for six PBs) and those proposed by Galán-Martín et al.34 (CFs for biosphere integrity PB), as follows:
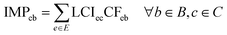 | (1) |
Phase 4: Interpretation
Eqn (1) provides the impact of a chemical in the CVs of the PBs. Similarly, a standard multi-factor LCIA method would provide the impact in a set of categories without further processing the results. However, impact values as such cannot shed light on whether the chemical is environmentally sustainable. To address this fundamental question, here we go beyond standard multi-factor LCIA methods to compute a transgression level (TL) metric that relates the impact of the chemical with the share of the safe operating space (SOS) allocated to it (Fig. 1 and eqn (2)). The PBs define an SOS for humanity, which should not be exceeded by all the anthropogenic activities jointly. Hence, to quantify the transgression level, we first allocate a share of this global ecological budget to the chemical and then evaluate whether its impact exceeds this quota.In mathematical terms, the transgression level of chemical c in the CV of PB b, denoted by TLbc, is computed as follows:
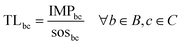 | (2) |
Note that our method provides a range of LCA metrics in the form of transgression levels. However, these are easier to interpret than standard LCA indicators, as transgression levels above one indicate that the chemical is unequivocally environmentally unsustainable. Hence, a new experimental route should be deemed appealing if it outperforms the business-as-usual alternative and at the same time shows transgression levels below one in all the PBs. Certainly, being better than the current technology is not enough if the new route exceeds the PBs. If trade-offs arise between categories, priority should be given to the climate change and biosphere integrity PBs, currently regarded as core boundaries through which the others operate.29
Equal per capita (EPC) is based on the equality principle stating that every person has the same moral right to access the Earth's ecological budget (the SOS). Translating one's personal share of the SOS to a specific activity, i.e., the production of a chemical, requires an additional upscaling method.50 Here, we used the upscaling method based on the economic value of the chemical, as in Ryberg et al.35 and other works,51,52 assuming that it may be used as a proxy for human wellbeing,53i.e., higher economic value promotes further wellbeing and should result in larger shares of the SOS. Hence, in the EPC approach, the share of the SOS of a chemical c (sosEPCbc) is determined as follows:
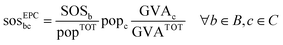 | (3) |
By contrast, the grandfathering (GF) approach is based on the sovereignty principle. According to this principle, the share allocated to each anthropogenic activity should be proportional to its current contribution to the global pressure exerted on the environment (eqn (4)). The downside of this approach is that it allocates shares of the SOS only among already existing activities without applying any moral criteria for differentiation.
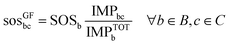 | (4) |
Eqn (3) requires the GVA of chemical c—defined as gross output (revenues) less intermediate consumption and net indirect taxes—, a piece of information often unavailable in the public domain. To overcome this limitation, we compute an upper bound on the GVA of chemicals, accounting only for the revenues generated from their global sales at basic prices (GVAc < pricecDEMc). Here, the idea is to follow a conservative approach to overestimate the share assigned to the chemical and underestimate the transgression levels. Following this approach and combining eqn (2) and (3), a lower bound on the transgression level of the chemical from the EPC perspective (TLEPCbc) can be computed as:
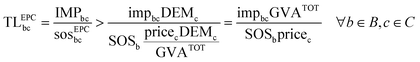 | (5) |
According to eqn (5), the TL of a chemical could be reduced by decreasing its impact and/or increasing its price. Impacts could be decreased (to some extent) through appropriate strategies implemented across the chemical supply chain. In contrast, global markets in a free economy dictate the prices, so they cannot be controlled at will by the producer, at least for general chemicals.
Similarly, combining eqn (2) and (4), the transgression level of the chemical from the GF perspective (TLGFb) is computed as follows:
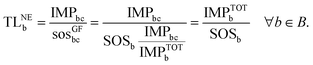 | (6) |
Note that the GF approach leads to the same transgression levels for all the chemicals. Hence, it fails to discriminate among them and, in general, among any anthropogenic activity. All data related to the calculation of TLs is available in section 4 of the ESI.†
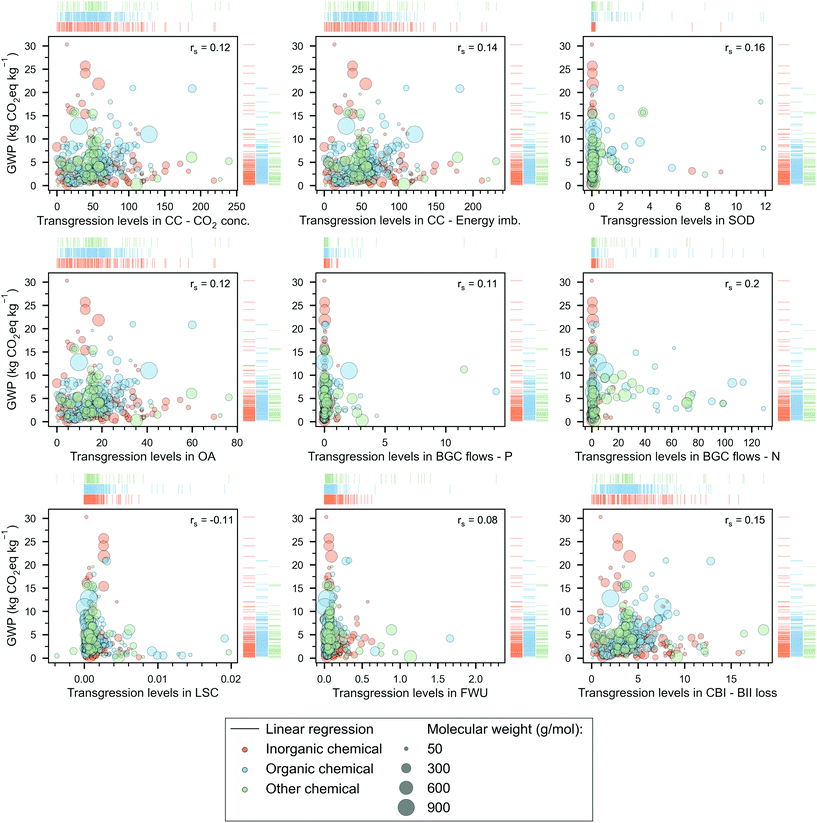 | ||
| Fig. 3 Global warming potential (GWP) of chemicals versus their transgression levels (TLEPC) in the PBs. The chemicals, represented by bubbles – whose size denotes the molecular weight –, are grouped into three categories, ‘inorganic’ (orange), ‘organic’ (blue) and ‘other’ (green). We indicate the Spearman's rank correlation coefficient (rs) between the TLs (in a specific PB) and GWP scores on the top right of the scatter plot. Rug plots on the sides of each scatter plot show the individual distribution of the chemicals according to their category. The notation for the PBs is as follows: climate change (CC) with control variables of CO2 concentration (CO2 conc.) and energy imbalance (Energy imb.), stratospheric ozone depletion (SOD), ocean acidification (OA), biogeochemical (BGC) flows with control variables of phosphorus (P) and nitrogen (N), land-system change (LSC), freshwater use (FWU), change in biosphere integrity (CBI) with control variable of loss of biodiversity intactness index (BII loss). Note that 21 chemicals with GWP scores ranging from 48 to 3907 kg CO2eq per kg of chemical are omitted here (for visualisation purposes) but are shown in Table S-4 of the ESI.† | ||
Results and discussion
Quantifying the absolute environmental sustainability level of chemicals
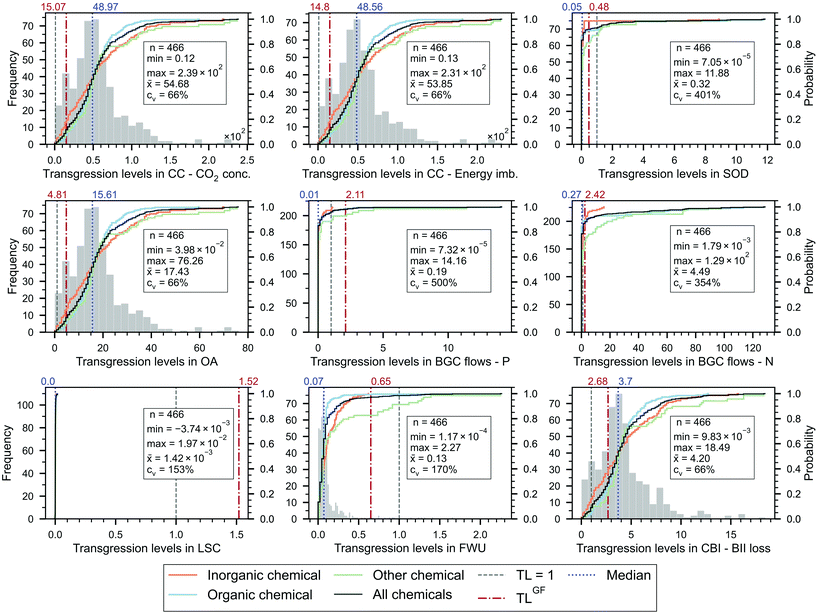
We start by analysing the TL of the chemicals (Fig. 2), finding that the overwhelming majority of them (99.4%) transgress at least one PB, while none of them transgresses all the PBs simultaneously. Hence, only three of the 466 chemicals evaluated (0.6%) respect their ecological budget and, thus, should be deemed absolute sustainable (according to the EPC sharing principle applied), i.e., hexamethyldisilazane (HMDS), selenium and trimesoyl chloride. These chemicals show relatively low impact scores and, at the same time, high unitary prices (154, 76 and 1421 USD2018 per kg, respectively) that grant them a large ecological budget.Most of the chemicals heavily transgress the GHG-related PBs, i.e., climate change (CC) – CO2 concentration and energy imbalance (99.4% of the chemicals with TL > 1), ocean acidification (OA) (98.5%) and change in biosphere integrity (CBI) (92.3%). These results are consistent with the fact that the chemical industry is a hard-to-abate sector that consumes large amounts of fossil resources (1.12 Gt) and generates about 2.0 Gt CO2eq cradle-to-gate emissions.1
In contrast, chemicals display weaker links with other Earth-system processes. Notably, only 0%, 1.5%, 7.1%, and 4.9% of the chemicals transgress the land-system change (LSC), freshwater use (FWU), stratospheric ozone depletion (SOD) and biogeochemical (BGC) flow of phosphorous (P), respectively—all of them more strongly connected to the agricultural sector. 126 chemicals (27%) exceed the nitrogen (N) flow quota, some of them to a large extent, e.g., TL = 128.9 for monoethanolamine. From the chemicals transgressing the BGC flow of N, only 14 are fertilisers or pesticides, ten of which fixate N in their chemical structure. Similarly, other 38 chemicals that exceed the N flow budget also fixate N in their chemical structure, while the rest consume N upstream in their supply chain.
All the chemicals operate within the SOS defined for LSC (maximum TL of 0.02), and only seven slightly transgress the FWU PB. Uranium hexafluoride (UF6), used for uranium enrichment, transgresses the latter category the most (TL = 2.3). Four enriched uranium elements with different concentrations, used in the fuel for light-water nuclear reactors, also exceed the FWU PB (by 1.2–1.4 times). These molecules, together with UF6, consume large amounts of freshwater, yet their high prices (841 USD2018 per kg U) offset their high impacts, resulting in moderate TLs.
Nine chemicals show negative TLs in the LSC PB, i.e., potassium chloride, potassium sulfate, potassium hydroxide, lithium carbonate, vinyl chloride, toluene diisocyanate, propylene, ethylene, and butadiene. These negative scores, very low in absolute value (−1 × 10−7 to −1 × 10−3), could be explained by some small LCI entries of ‘land transformation to the forest’ (higher in absolute value than the positive entries labelled as ‘land transformation from the forest’), which are associated with the disposal of waste materials in landfills.
The TL scores of the chemicals are highly scattered, with the minimum and maximum TLs differing in as much as five orders of magnitude in the SOD, BGC flows and FWU PBs, and three orders in the others. This is also observed in the coefficients of variation (cv), particularly high in the SOD, BGC flows, FWU and LSC PBs (at most 500% for BGC flow of P). The cv of the latter PB is additionally affected by the near-zero mean and the negative values already discussed (recall that cv is given by the ratio of the standard deviation to the mean).
Comparing the EPC and GF sharing approaches, we find that in the GHG-related PBs, the former often leads to higher TLs (89.9%, 90.3%, 89.9% and 73.2% probability of TLEPC > TLGF in CC – CO2 conc., CC – Energy imb., OA and CBI PBs, respectively). In contrast, the opposite occurs in the SOD, BGC flows, LSC and FWU PBs—8.2%, 1.9% (P flow), 13.7% (N flow), 0% and 3% probability of TLEPC > TLGF, respectively. Note that TLEPC > TLGF implies that the share of the SOS allocated to the chemical based on economic considerations is lower than the share resulting from the grandfathering approach, i.e., sosEPC < sosGF. However, qualitatively, both approaches tend to lead to the same outcome (i.e., when one share is transgressed according to a principle, it is also transgressed with the other and vice versa). This holds for all the PBs except for the BGC flows of P and N and LSC PBs, which are transgressed only by some chemicals (or none in the case of LSC PB) with the EPC principle based on economic criteria and by all with the GF approach.
Is GWP a good proxy of absolute environmental sustainability level?
We next study the relationship between the TL and the GWP, finding that larger GWP values do not necessarily lead to larger TLs—Spearman's rank correlation coefficients (rS) in the range (−0.11, 0.16), see Fig. 3. Said differently, a molecule A with a larger carbon footprint than that of B is not necessarily less sustainable in absolute environmental terms than the latter. This poor correlation is explained by two main observations: (1) not all the PBs impacts correlate with GWP (Fig. S-1 in the ESI†), and (2) the price of the product, which dictates its ecological budget (i.e., more expensive chemicals are allowed to pollute more), does not correlate either with the carbon footprint (Fig. S-2 in the ESI†). Notably, from eqn (5), TLEPC is directly proportional to the impacts in the PBs and inversely proportional to the unitary price of the chemical. Hence, at least in the GHG-related PBs, the TL should correlate with the GWP because the carbon footprint drives the impact in these PBs. However, this does not occur because the unitary price fails to correlate with the GWP. Furthermore, the other TLs also fail to correlate with the GWP because neither the impact on their PBs nor the price correlates with the carbon footprint.Note that there are 21 chemicals with high GWP scores, e.g., lithium (48.1 kg CO2eq per kg), previously mentioned, UF6 (146.7 kg CO2eq per kg), and uranium enriched at 4.2% (3907.2 kg CO2eq per kg), which are not outliers but were omitted in Fig. 3 to ease the visual analysis of the data (complete list of these chemicals is provided in Table S-4 of the ESI†).
Absolute environmental sustainability level of selected relevant chemicals
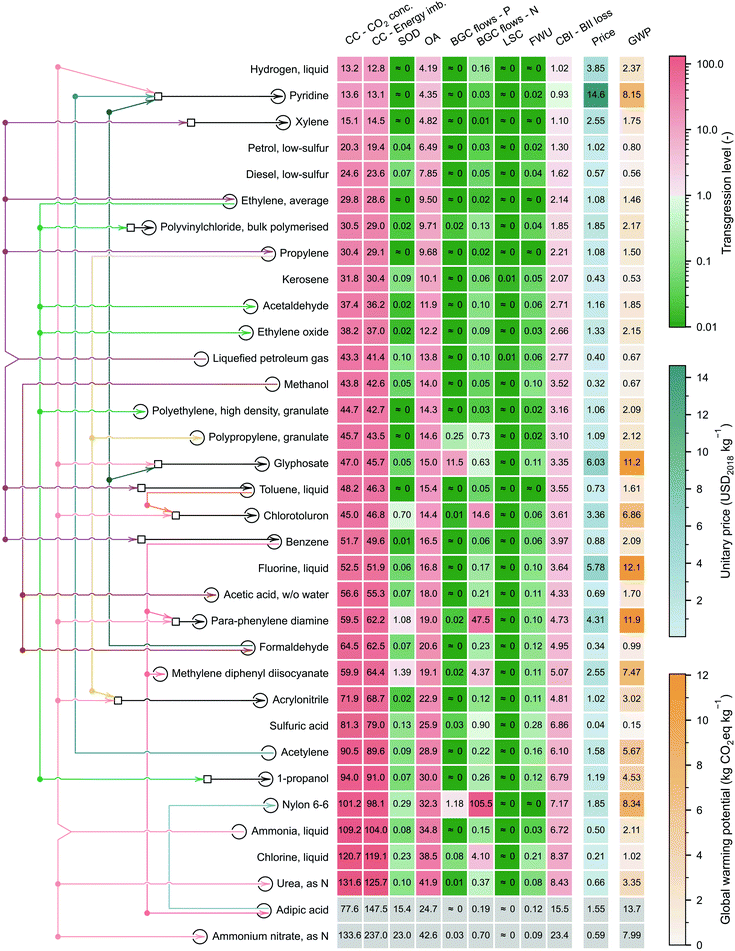
We next select 34 chemicals for a more in-depth discussion based on their relevance, focusing on analysing their TLEPC and GWP scores and prices (Fig. 4). These chemicals sit at different levels in the synthesis tree of the chemical industry. In Fig. 4, the network of connections qualitatively represents the direct or indirect uses of chemicals in producing other chemicals. For instance, petrol, diesel, kerosene, naphtha, and liquefied petroleum gas (LPG) are produced from crude oil in a petroleum refinery. The latter two, naphtha and LPG, serve as raw materials in the steam cracking process to produce ethylene and propylene. Additionally, through catalytic reforming, naphtha and LPG can yield benzene, toluene, and xylene. Moreover, these platform chemicals are used as precursors of other chemicals. For example, ethylene is used to produce ethylene oxide and acetaldehyde via direct oxidation and can also be polymerised to obtain polyethylene. Furthermore, ethylene is indirectly involved in the production of polyvinylchloride and 1-propanol. Finally, Fig. 4 also shows other chemicals widely used in the chemical sector, e.g., hydrogen or sulfuric acid.The chemicals shown in Fig. 4 are ordered by their TLs in CC – energy imbalance PB, regarded as the most stringent CV of the core PBs,29 the other two being the CVs of CO2 concentration (CC PB) and loss of biodiversity intactness index (CBI PB).54 Consistent with our previous results and focusing on the most heavily transgressed PBs (i.e., the GHG-related ones: CC, OA and CBI), we find again that the TLs of these selected chemicals do not correlate with the GWP. For example, sulfuric acid shows the lowest GWP (0.15 kg CO2eq per kg) but ranks 26th out of 34 chemicals in terms of TL in the CC PB. Similarly, fluorine shows the largest GWP (12.1 kg CO2eq per kg) but displays only the 20th largest TL in the CC PB. This poor correlation further reinforces the observation that the carbon footprint should not be taken as a proxy of absolute environmental sustainability level.
The mismatch between the TLs in the GHG-related PBs and the carbon footprint is due to the poor correlation between the carbon emissions and the prices, which affect the quotas (lower prices lead to tighter ecological budgets). Hence, while a pattern seems to emerge from the analysis of the GWP, the same is not true for the TLs. Notably, chemicals sitting downstream in the synthesis tree require more production steps and, therefore, tend to lead to larger scores in GWP. However, they also tend to have larger prices, which results in larger quotas and, therefore, lower TLs. Whether one factor (larger impacts due to more complex production routes) offsets the other (higher prices and quotas) depends on the specific case. For example, the GWP of 1 kg of pyridine is 3.9 times that of 1 kg of ammonia. Still, its price is 29.2 times higher, which results in a smaller TL of pyridine in the GHG-related PBs relative to its precursor, ammonia. Conversely, the GWP of 1 kg of ammonium nitrate is 3.8 times greater than that of ammonia, but its price is only 18% higher, resulting in a higher TL with respect to ammonia. Furthermore, comparing chlorine with hydrogen, both involved in the production of many chemicals (either directly in their synthesis or elsewhere in their supply chains), we find that hydrogen shows a much lower TL in the GHG-related PBs (at most 13.2 vs. 120.7 for chlorine), despite having a much higher carbon footprint (2.37 vs. 1.02 kg CO2eq per kg). These disparities are due to the high hydrogen price (3.85 vs. 0.21 USD2018 per kg for chlorine), which grants hydrogen a larger ecological budget, leading to a 10-fold difference in TLs.
Polymerisation (e.g., propylene to polypropylene), oxidation (e.g., methanol to formaldehyde) or ammoxidation (e.g., propylene to acrylonitrile) tend to increase the TLs because the price increment is insufficient to counteract the impact increase. Notably, focusing on the CC – energy imbalance PB, when propylene (TL = 29.1, GWP of 1.50 kg CO2eq per kg and price of 1.08 USD2018 per kg) is oxidised in the presence of ammonia to acrylonitrile via the Sohio process, the GWP increases 2-fold, while the price remains almost the same, resulting in a much higher TL (i.e., 68.7).
The TLs in the other PBs, i.e., the ones not directly linked to GHG emissions, are always relatively low, excluding some high TLs in the BGC – N flow (e.g., nylon 6–6, para-phenylenediamine, or chlorotoluron), and to a lesser extent, in the BGC – P flow (e.g., nylon 6–6 or glyphosate). We clarify that due to the inverse modelling and the cradle-to-gate scope of the analysis, our calculations underestimate the impact in the BGC – N flow PB (see section 7 of the ESI†), so the TLs could be higher in this category. We finally note that a cradle-to-grave scope covering the use phase of the chemicals could lead, in some cases, to significantly different results. For example, fuels show relatively low carbon footprints on a cradle-to-gate basis, compared to some platform chemicals (e.g., 0.56 kg CO2eq per kg of diesel vs. 0.67 kg CO2eq per kg of methanol). However, expanding the scope to the ‘grave’ would increase the overall GWP score (and, consequently, the TL values in the GHG-related PBs) by roughly 3.1 kg CO2eq per kg of fuel.
Conclusions and outlook
Here we discussed the use of absolute environmental sustainability methods for assessing chemical technologies, quantifying the impact of 492 chemicals through the lens of seven PBs, all essential to maintaining the stability of the Earth system. We found that an overwhelming majority of them (99.4%) are environmentally unsustainable due to the transgression of at least one PB, exceeding in some cases the allowable budget by more than 200 times. The transgression levels are exceptionally high in the carbon-related PBs (i.e., climate change, ocean acidification, and change in biosphere integrity). In contrast, they are almost negligible or very moderate in the remaining ones.Our results also show that GWP is not a good proxy of absolute environmental sustainability performance in chemicals, as it fails to correlate with their transgression level. This is because the environmental sustainability level of chemicals can depend on their prices (higher prices, more ecological budget), which do not correlate with the GWP scores. Notably, chemicals sitting downstream in the chemical industry tend to show larger carbon footprints but not necessarily larger transgression levels. Furthermore, there are other critical ecological impacts on the PBs that do not correlate at all with carbon emissions.
The outcome of this study has broad implications for the chemical industry. First, the need to embrace absolute environmental sustainability criteria in assessing chemicals is highlighted. This paradigm shift will require agreeing on how to assign shares of the SOS to them, which will allow setting priorities to make the chemical sector more sustainable. Second, it follows that the carbon footprint (or any other single-factor metric) should not be the only metric of concern, although fossil chemicals show the highest transgression levels in the carbon-related PBs. Notably, a fair and insightful assessment of chemicals and their labelling as ‘green’ would require transgression metrics that consider both a range of impact levels and their associated thresholds.
The AESA method presented herein should complement the studies of experimental researchers working in the area of green chemistry, who already use mass-based metrics and LCA. This method benefits from the integration of life-cycle principles and goes beyond standard LCIA metrics by explicitly comparing the impact scores against Earth-sytem's thresholds. Ideally, sustainability assessments should also cover the economic and social dimensions, linking to the sustainable development goals (SDGs). This will require further progress in the PBs framework to define other control variables, which would allow to explore a safe and just corridor for humanity. Further research should also focus on regionalised assessments and social indicators tailored to chemicals production.
Overall, this work aims to raise awareness of the need to go beyond standard multi-factor LCAs in green chemistry studies to embrace absolute sustainability criteria based on the Earth's ecological capacity. This holistic approach will allow identifying pathways that outperform the current technologies and do so in a way that is entirely consistent with the Earth's biophysical limits. Assessments similar to the one presented herein will help guide research more sensibly and allocate resources more judiciously to produce more sustainable chemicals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication was created as part of NCCR Catalysis, a National Centre of Competence in Research funded by the Swiss National Science Foundation.References
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- H. Chang, I. Bajaj, G. W. Huber, C. T. Maravelias and J. A. Dumesic, Green Chem., 2020, 22, 5285–5295 RSC.
- M. Rabnawaz, I. Wyman, R. Auras and S. Cheng, Green Chem., 2017, 19, 4737–4753 RSC.
- I. Ioannou, S. C. D'Angelo, Á. Galán-Martín, C. Pozo, J. Pérez-Ramírez and G. Guillén-Gosálbez, React. Chem. Eng., 2021, 6, 1179–1194 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- M. G. T. C. Ribeiro and A. A. S. C. Machado, Green Chem. Lett. Rev., 2013, 6, 1–18 CrossRef CAS.
- P. Jessop, Green Chem., 2020, 22, 13–15 RSC.
- G. Finnveden, M. Z. Hauschild, T. Ekvall, J. B. Guinée, R. Heijungs, S. Hellweg, A. Koehler, D. Pennington and S. Suh, J. Environ. Manage., 2009, 91, 1–21 CrossRef PubMed.
- M. Goedkoop and R. Spriensma, The Eco-indicator 99: A damage oriented method for Life Cycle Impact Assessment, 2000 Search PubMed.
- Handbook on Life Cycle Assessment: Operational Guide to the ISO Standards, ed. J. B. Guinée, H. de Bruijn, R. van Duin, M. A. J. Huijbregts, M. Gorree, R. Heijungs, G. Huppes, R. Kleijn, A. de Koning, L. van Oers, A. Wegener Sleeswijk, S. Suh and H. A. Udo de Haes, Springer Netherlands, Dordrecht, 2002, vol. 7 Search PubMed.
- O. Jolliet, M. Margni, R. Charles, S. Humbert, J. Payet, G. Rebitzer and R. Rosenbaum, Int. J. Life Cycle Assess., 2003, 8, 324 CrossRef.
- J. C. Bare, Tool for the Reduction and Assessment of Chemical and other Environmental Impacts (TRACI): Version 2.1 User's Manual, 2003 Search PubMed.
- B. Steen, The EPS 2015d impact assessment method – an overview, 2015 Search PubMed.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. D. M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- Sphera Solutions GmbH, GaBi, https://gabi.sphera.com/software/, (accessed 14 June 2021).
- PRé Sustainability B.V., SimaPro, https://simapro.com/, (accessed 14 June 2021).
- GreenDelta, openLCA, https://www.openlca.org/, (accessed 14 June 2021).
- ifu Hamburg GmbH, Umberto, https://www.ifu.com/en/umberto/, (accessed 14 June 2021).
- R. Heijungs, CMLCA, http://www.cmlca.eu/, (accessed 14 June 2021).
- I. M. Algunaibet and G. Guillén-Gosálbez, J. Cleaner Prod., 2019, 229, 886–901 CrossRef.
- T. L. Saaty, Eur. J. Oper. Res., 1990, 48, 9–26 CrossRef.
- A. Nikolopoulou and M. G. Ierapetritou, Comput. Chem. Eng., 2012, 44, 94–103 CrossRef CAS.
- C. Jiménez-González and M. R. Overcash, Green Chem., 2014, 16, 3392–3400 RSC.
- J. Kleinekorte, L. Fleitmann, M. Bachmann, A. Kätelhön, A. Barbosa-Póvoa, N. Von Der Assen and A. Bardow, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 203–233 CrossRef PubMed.
- A. Bjørn and M. Z. Hauschild, Int. J. Life Cycle Assess., 2015, 20, 1005–1018 CrossRef.
- M. Z. Hauschild, in Procedia CIRP, Elsevier B.V., 2015, vol. 29, pp. 1–7 Search PubMed.
- A. Bjørn, M. Margni, P. O. Roy, C. Bulle and M. Z. Hauschild, Ecol. Indic., 2016, 63, 1–13 CrossRef.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. I. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. De Wit, T. Hughes, S. Van Der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. A. Foley, Nature, 2009, 461, 472–475 CrossRef PubMed.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers, S. Sorlin and S. Sörlin, Science, 2015, 347, 1–10 CrossRef PubMed.
- M. Z. Hauschild, S. Kara and I. Røpke, CIRP Ann., 2020, 69, 533–553 CrossRef.
- J. Rockström, J. Gupta, T. M. Lenton, D. Qin, S. J. Lade, J. F. Abrams, L. Jacobson, J. C. Rocha, C. Zimm, X. Bai, G. Bala, S. Bringezu, W. Broadgate, S. E. Bunn, F. DeClerck, K. L. Ebi, P. Gong, C. Gordon, N. Kanie, D. M. Liverman, N. Nakicenovic, D. Obura, V. Ramanathan, P. H. Verburg, D. P. van Vuuren and R. Winkelmann, Earth's Future, 2021, 9, 31 CrossRef.
- S. J. Lade, W. Steffen, W. de Vries, S. R. Carpenter, J. F. Donges, D. Gerten, H. Hoff, T. Newbold, K. Richardson and J. Rockström, Nat. Sustain., 2020, 3, 119–128 CrossRef.
- M. W. Ryberg, M. Owsianiak, K. Richardson and M. Z. Hauschild, Ecol. Indic., 2018, 88, 250–262 CrossRef.
- Á. Galán-Martín, V. Tulus, I. Díaz, C. Pozo, J. Pérez-Ramírez and G. Guillén-Gosálbez, One Earth, 2021, 4, 565–583 CrossRef.
- M. W. Ryberg, M. Owsianiak, J. Clavreul, C. Mueller, S. Sim, H. King and M. Z. Hauschild, Sci. Total Environ., 2018, 634, 1406–1416 CrossRef CAS PubMed.
- M. W. Ryberg, T. K. Bjerre, P. H. Nielsen and M. Hauschild, J. Ind. Ecol., 2021, 25, 765–777 CrossRef.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2019, 12, 3425–3436 RSC.
- S. C. D'Angelo, S. Cobo, V. Tulus, A. Nabera, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2021, 9, 9740–9749 CrossRef.
- N. Samaroo, N. Koylass, M. Guo and K. Ward, Green Chem., 2020, 22, 6547–6559 RSC.
- Y. Li, S. Lan, M. W. Ryberg, J. Pérez-Ramírez and X. Wang, iScience, 2021, 24, 102513 CrossRef CAS PubMed.
- A. Valente, V. Tulus, Á. Galán-Martín, M. A. J. Huijbregts and G. Guillén-Gosálbez, Sustainable Energy Fuels, 2021, 5, 4637–4649 RSC.
- J. Wheeler, Á. Galán-Martín, F. D. Mele and G. Guillén–Gosálbez, AIChE J., 2021, 67, e17131 CrossRef CAS.
- M. Ehrenstein, Á. Galán-Martín, V. Tulus and G. Guillén-Gosálbez, Appl. Energy, 2020, 276, 115486 CrossRef CAS.
- ISO 14040:2006(E), Environmental management—Life cycle assessment—Principles and framework, Geneva, 2016 Search PubMed.
- ISO 14044:2006(E), Environmental management—Life cycle assessment—Requirements and guidelines, Geneva, 2016 Search PubMed.
- United Nations, Central Product Classification, Ver.2.1, 2015 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B. A. Shoemaker, P. A. Thiessen, B. Yu, L. Zaslavsky, J. Zhang and E. E. Bolton, Nucleic Acids Res., 2021, 49, D1388–D1395 CrossRef CAS PubMed.
- P. L. Lucas, H. C. Wilting, A. F. Hof and D. P. van Vuuren, Glob. Environ. Chang, 2020, 60, 102017 CrossRef.
- A. W. Hjalsted, A. Laurent, M. M. Andersen, K. H. Olsen, M. W. Ryberg and M. Hauschild, J. Ind. Ecol., 2021, 25, 6–19 CrossRef.
- A. Bjørn, C. Chandrakumar, A.-M. Boulay, G. Doka, K. Fang, N. Gondran, M. Z. Hauschild, A. Kerkhof, H. King, M. Margni, S. McLaren, C. Mueller, M. Owsianiak, G. Peters, S. Roos, S. Sala, G. Sandin, S. Sim, M. Vargas-Gonzalez and M. W. Ryberg, Environ. Res. Lett., 2020, 15, 083001 CrossRef.
- M. W. Ryberg, M. M. Andersen, M. Owsianiak and M. Z. Hauschild, J. Cleaner Prod., 2020, 276, 123287 CrossRef.
- R. Dworkin, Philos. Public Aff., 1981, 10, 185–246 Search PubMed.
- H. O. Pörtner, R. J. Scholes, J. Agard, E. Archer, A. Arneth, X. Bai, D. Barnes, M. Burrows, L. Chan, W. L. Cheung, S. Diamond, C. Donatti, C. Duarte, N. Eisenhauer, W. Foden, M. A. Gasalla, C. Handa, T. Hickler, O. Hoegh-Guldberg, K. Ichii, U. Jacob, G. Insarov, W. Kiessling, P. Leadley, R. Leemans, L. Levin, M. Lim, S. Maharaj, S. Managi, P. A. Marquet, P. McElwee, G. Midgley, T. Oberdorff, D. Obura, E. Osman, R. Pandit, U. Pascual, A. P. F. Pires, A. Popp, V. Reyes-García, M. Sankaran, J. Settele, Y. J. Shin, D. W. Sintayehu, P. Smith, N. Steiner, B. Strassburg, R. Sukumar, C. Trisos, A. L. Val, J. Wu, E. Aldrian, C. Parmesan, R. Pichs-Madruga, D. C. Roberts, A. D. Rogers, S. Díaz, M. Fischer, S. Hashimoto, S. Lavorel, N. Wu and H. T. Ngo, Scientific outcome of the IPBES-IPCC co-sponsored workshop on biodiversity and climate change, IPBES and IPCC, Bonn, Germany, 2021 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02623b |
| This journal is © The Royal Society of Chemistry 2021 |

![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif)