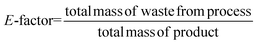Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Complementary green analytical procedure index (ComplexGAPI) and software†
Justyna
Płotka-Wasylka
 *ab and
Wojciech
Wojnowski
*ab and
Wojciech
Wojnowski
 a
a
aDepartment of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, PL-80-233 Gdańsk, Poland
bBioTechMed Center, Gdańsk University of Technology, 80-233 Gdańsk, Poland. E-mail: juswasyl@pg.edu.pl; plotkajustyna@gmail.com
First published on 18th October 2021
Abstract
It is not easy to find appropriate tools for the evaluation of the “green” nature of analytical methodologies which involve the use of compounds, materials, or chemicals manufactured prior to the analytical step. Here, we propose a new metric for the evaluation of analytical procedures based on the GAC attributes. The proposed solution expands on the well-known green analytical procedure index by adding additional fields pertaining to the processes performed prior to the analytical procedure itself. Each field of the hexagon that was added to the GAPI pictogram corresponds to a different aspect of the described process and is coloured green if certain requirements are met. To showcase the utility of the proposed metric, it was used to evaluate analytical protocols for the determination of pesticides in urine samples. We believe that, following GAPI's success, ComplexGAPI will also gain attention and eventually trust and acceptance from the chemical community. To facilitate the use of this tool, we have created freeware software for generating the ComplexGAPI pictograms.
Introduction
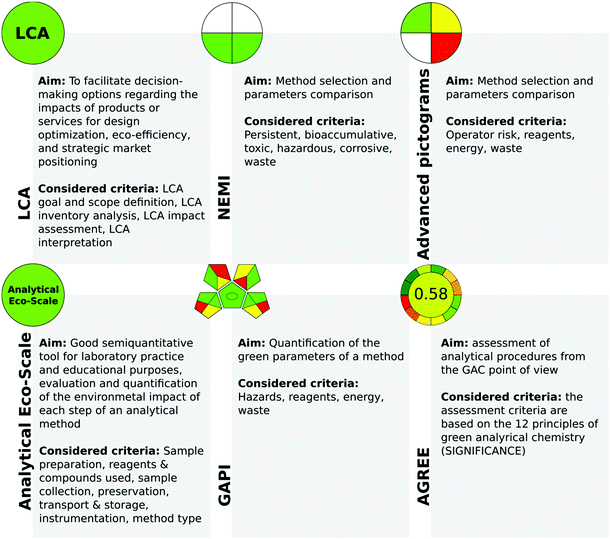
One of the prominent approaches in chemistry is the philosophy of green chemistry which aims to conduct processes in accordance with the principles of sustainable development. Nowadays, green chemistry principles are widely applied in numerous areas, from government policies, through industrial management, to educational practice and technology development. In the circular economy concept, it is important to balance economic growth, resource sustainability, and environmental protection. Thus it can be claimed that green and sustainable chemistry is a path towards changing the attitudes and paradigms in chemical manufacturing and production.1 Moreover, the general concept of green chemistry permeates its areas, leading to the revaluation of approaches and giving rise to new ways of thinking. In the particular case of analytical chemistry, the term green analytical chemistry (GAC) is commonly used.Finding the right way to assess the green character of an analytical procedure is challenging since many different parameters must be taken into consideration.2 It is generally accepted that hard data on actual environmental impacts are needed to claim that a process or product is sustainable. Here, the developed tools for assessing the greenness of the given analytical procedures come to the rescue (Fig. 1). These tools are juxtaposed and described in detail in several published reviews.3–5 However, in this area, some questions can be raised as follows: How many parameters are evaluated by these tools? Are these metrics easy to use? How effective are these methods for assessing the green nature of an analytical procedure? The answers to these questions are important, as there are still many examples for analytical protocols reported in the literature that are claimed to be green and eco-friendly by their authors with little tangible evidence, e.g., in the form of a greenness metric score. Furthermore, such new protocols should be compared with the previously developed methodologies (Tobiszewski, Anal. Chem.).6
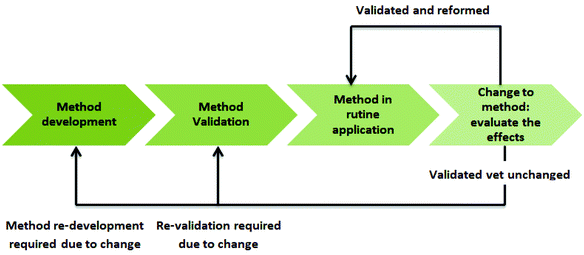
Analytical protocols are used to gather data in numerous application areas, which are then used as the basis for making decisions, and so their validity is of high importance.7 Thus, these data must be characterized by consistent quality. Such quality can be ensured by using a tool called life cycle assessment (LCA) which allows assessing the potential impacts of products, processes, or services through production, usage, and disposal.8 The life cycle concept is adaptable to analytical methodologies if an analytical protocol as a process and the output of this process in the form of reportable results are considered.9 However, it is not often applied in the area of analytical chemistry. In fact, it needs standardized guidelines to ensure the high quality of its application. Moreover, the impact assessment methods need to be extended by further human and ecosystem health indicators.10
The LCA of an analytical protocol includes quality-by-design (QbD) approaches in every step of the development of a new procedure, its validation and operational applications.4 Moreover, LCA includes additional elements, such as the identification of an analytical target profile (ATP) – a set of criteria that define what will be measured (e.g., analyte content and impurity content) and the performance criteria to be achieved by the measurement (e.g., validation parameters), but without specifying the method.9 With these features in mind, the LCA of an analytical procedure can be broken down into three stages: method design, method qualification, and continued method verification (Fig. 1).
One of the earliest tools for the assessment of the greenness of analytical procedures is the National Environmental Methods Index (NEMI, Fig. 2).11 Although NEMI as a greenness assessment tool has its advantages (e.g., it is easy to read by potential users), it also has some drawbacks. The NEMI symbol presents each threat as being below or above a certain value, and therefore it cannot be considered quantitative. Furthermore, this tool does not take into consideration such issues as energy, chemical and reagent consumption, and the amount of waste generated. In addition, searching for each chemical used in the procedure in official lists (EPA TRI list, Resource Conservation and Recovery Act list, etc.) is time-consuming. Therefore, it has been modified by de la Guardia et al.12 who proposed the use of a colour scale to improve clarity (Fig. 2).
 | ||
| Fig. 2 Characterization of the most popular metrics for the evaluation of the green character of analytical procedures. | ||
Another very popular and often used metric is the analytical Eco-scale proposed by Gałuszka et al.13 In this tool, the penalty points are considered and subtracted from a base of 100. The higher the score, the more sustainable the analytical procedure is. The analytical Eco-Scale is characterized by many advantages such as simplicity of use and semi-quantitative calculation of the amounts of chemicals and wastes, information about the environmental impacts of analytical approaches is provided quantitatively, and different aspects of environmental impacts are evaluated. Its drawbacks however include the lack of additional quantifiers capable of discriminating between the micro- and macro-scale of method applications. In addition, the result is not informative in the case of a negative environmental impact, and as such does not facilitate the improvement of the method during the design stage in this aspect.
Recently, two metrics, the Analytical GREEnness calculator (AGREE) and the Red-Green-Blue model, have been introduced.14,15 AGREE is a comprehensive, flexible, and straightforward evaluation approach that produces an easily interpretable and informative result. In AGREE, the considered criteria are taken from the 12 principles of GAC and are transformed into a unified 0–1 scale. One of the advantages of this metric is the availability of freeware software which makes its applications more straightforward.
The RGB model uses three colours to represent the main attributes of the assessed method.15 These attributes cover analytical performance (red), compliance with the principles of green chemistry (green), and practical effectiveness (blue). The final colour assigned to the evaluated methodology is a result of the additive synthesis of the primary colours, the intensities of which are expressed by the Colour Score parameter on the scale of 0–100%, distinguishing three separate ranges. These ranges allow the simplification of the application of the RGB model for the assessment of analytical procedures and distinguish the limited number of resultant/final colours of a method. In addition, the quantitative parameter, called “method brilliance”, integrating all primary colours, is provided. The evaluation using the RGB model is performed using Excel worksheets.
The RGB model inspired a new perspective on the implementation of sustainable development principles in analytical chemistry, leading to the formulation of the so-called 12 principles of White Analytical Chemistry (WAC).16 This concept incorporates the main assumptions of GAC, while also addressing the additional expectations. WAC aims to maintain the integrity of the various parameters without directly prioritizing any of the attributes assessed. As the aspiration for sustainable development is striving for a “white” method, the authors of WAC propose the application of the term “white” as a synonym for a well-balanced analytical procedure used in a given application.
In 2018, the green analytical procedure index (GAPI) tool was reported17 and has since been used by many scientists to evaluate the green nature of the developed procedures, making it relatively successful and already established at the time of writing. The GAPI metric uses a pictogram to classify the greenness of each step of an analytical methodology, applying a colour scale, with two or three levels of evaluation for each stage. In GAPI, reagents, procedures, and instrumentation are evaluated. Thus, many factors are considered, including chemical health and environmental hazard, waste amount and type, and energy requirements. Furthermore, GAPI presents information on the entire analytical protocol. What is very important is that the compact pictogram of GAPI allows for an at-a-glance comparison of several methods and easy selection of the greenest method for a particular study. It could be stated that GAPI evidently indicates the weakest points in analytical procedures.
Considering the above-mentioned tools it could be concluded that they are sufficient and provide reliable and factual results. They do, however, have certain shortcomings when viewed through the lens of the spirit of the original stipulations of green analytical chemistry. GAC is a multi-step approach, and one of its axioms is that the new analytical procedure will meet the desired requirements from the sustainability point of view (Fig. 3).
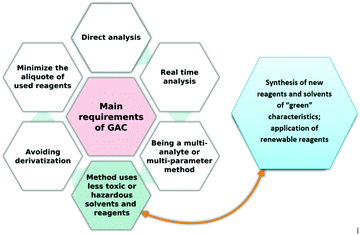
When re-visiting these original stipulations we can point to an issue with the current assessment tools. Nowadays, many new solvents, sorbents, reagents, columns, etc. are produced in order to enhance not only the efficiency but also the green character of a developed procedure and this part, i.e., production/synthesis of new, specific reagents, solvents or other materials prior to sample preparation and final analysis should also be evaluated. While some available metrics for measuring the aspects of a chemical process relating to the principles of green chemistry could be used to assess this stage of method development (e.g., e-factor, step economy, atom economy, etc.),18 their application would not be convenient or time-saving. Therefore, we propose a complex green analytical procedure index (ComplexGAPI), an easy tool that complements the existing GAPI metric. One hexagonal field was added to the original GAPI graph and it reflects the processes performed prior to the sample preparation step and final analysis. We believe that by following the path of GAPI success, ComplexGAPI will also gain attention, trust and acceptance from the chemical community. To facilitate the use of the tool, we have created freeware software for generating ComplexGAPI pictograms.
Complementary green analytical procedure index
The premise behind the development of the tool was to allow it to assess as much information as possible about a given analytical methodology, including processes performed prior to analysis, thus providing a more comprehensive evaluation of the procedure's ‘greenness’. We believe that the tool proposed here meets these criteria. The complex green analytical procedure index (ComplexGAPI) is a tool that covers all aspects of an analytical procedure, from sample collection, its transport, preservation, and storage to sample preparation and final analysis, but also these aspects and processes which are performed prior to the general analytical methodology. This modification of the original GAPI tool was motivated by questions from many chemists who applied GAPI in their laboratory practice but were finding it difficult to evaluate such processes as the synthesis of new ionic liquids (ILs), deep eutectic substances (DESs), nanoparticles (NPs), etc., and other materials used in the separation step, e.g., phases for columns.ComplexGAPI was created based on the same principles which guided the development of GAPI: the analytical eco-scale13 and the eco-scale.19 In addition, some requirements taken from the CHEM2120 tool were also taken into consideration in ComplexGAPI development. This makes the new metric easy to use for those who are already familiar with these tools and have used them to assess the green nature of the analytical procedures. They will in fact find the assessment process much more straightforward and less time-consuming thanks to the availability of the software for ComplexGAPI. The ComplexGAPI metric expands the pictogram created for GAPI by adding an additional hexagonal field at its bottom. This field corresponds to the ‘green’ character of pre-analysis processes. It covers such aspects as yield and conditions, reagents and solvents, instrumentation, work up and purification of the end products (Fig. 4). As in GAPI, the modified tool utilizes a colour scale, with two or three levels of evaluation for each stage. The created pictogram can be used to evaluate and quantify – from green to yellow to red – the low, medium and high environmental impacts associated with each stage of the pre-analysis process and the analytical methodology. Each field reflects a different feature of the described processes and analytical protocol and is filled green if certain requirements are met. The complex green analytical procedure index parameters are described in Table 1.
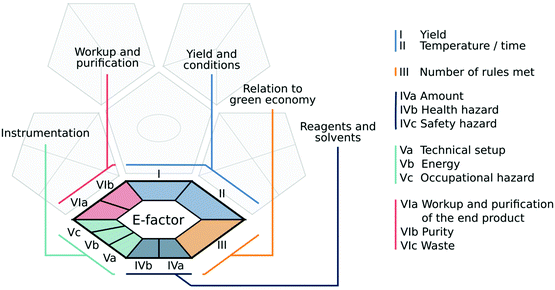 | ||
| Fig. 4 The ComplexGAPI pictogram, with the original GAPI pictogram greyed out in the background, and particular fields of the added hexagonal glyph grouped and colour-coded for clarity. | ||
The design of ComplexGAPI
A basic requirement for the creation of ComplexGAPI, as a modification of GAPI, is legibility, simplicity and user-friendliness. At the same time, it is required to cover the whole range of parameters that characterize the analytical protocol as well as pre-analysis processes (reagents, conditions, and techniques). As the GAPI tool has been described in detail,17 we will only cover the parameters covered by the additional hexagonal glyph describing the aspects related to the processes taking place prior to the analytical protocol.The conditions of the process performers are also evaluated by ComplexGAPI. Here, temperature and time are taken into consideration jointly, as these factors are closely related. A perfect situation would be if the reaction takes place quickly and at room temperature, however, the use of a higher temperature is often needed to perform synthesis during a satisfactory period of time.19 Cooling is even more troublesome, as often only fixed temperatures (for instance 0 °C for an ice bath, or −5 °C for an acetone/ice bath) are available. In addition, avoiding moisture is sometimes recommended to obtain reproducible results,19 and this is not an easy task. In fact, such a step requires the use of inert gases, Schlenk lines, gloveboxes, etc., which affects the economy of the whole procedure, but also its overall duration. Thus, temperature and time are considered jointly with colours green, yellow and red corresponding to particular threshold values.
| Requirement | Green chemistry component | Points | Result in the economy of synthesis/reaction/process |
|---|---|---|---|
| Design | Application of experimental design to reduce or eliminate the use or generation of hazardous substances | 1 | It aids in finding methods and techniques to speed up chemical reactions using small amounts of reagents to produce equivalent results at the same price point |
| Assessment of the chemical product's life cycle, including its design, manufacture, use, and ultimately disposal | 1 | It might also lead to a reduction in the number of synthetic steps and result in increased production and plant capacity while reducing energy and water consumption | |
| Use | Use of raw materials, elimination of wastes and avoiding the use of toxic and/or hazardous reagents and solvents | 1 | It aids in finding methods and techniques to speed up chemical reactions using small amounts of reagents to produce equivalent results at the same price point |
| Prevention of pollution by waste minimization and avoidance of toxic and hazardous substances | 1 | Using fewer chemicals for product manufacturing results in reduced waste, which in turn reduces the cost of disposing and treatment of chemical wastes | |
| Effort | Effort to minimize the expenditure of energy and chemicals | 1 | Using fewer chemicals for product manufacturing results in reduced waste, which in turn reduces the cost of disposing and treatment of chemical waste |
| Effort to use harmless reactants, alternative solvents, and new pathways of synthesis | 1 |
Considering the generated waste, it was decided to use the E-factor parameter which takes into account not only waste by-products and leftover reactants, but also spent catalysts and catalyst supports, solvent losses, and anything else that can be regarded as a waste.27 Thus, it can be said that the E-factors are derived from the amount of solvents, reagents, and consumables used per unit mass of product made, and the appropriate equation for its calculation can be used (eqn (1)). Sometimes it is easier to calculate the E-factor from a different viewpoint, since accounting for the losses and exact waste streams is difficult. In such a case, eqn (2) should be used.
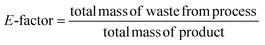 | (1) |
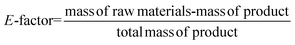 | (2) |
Waste prevention can be achieved if most of the solvents and the reagents are recyclable (e.g., catalysts, acids or bases that are bound to a solid phase can be filtered off, regenerated, and reused in a subsequent run). In such a way, these compounds are not included as by-products. This is also the case with water which is a significant by-product of many chemical syntheses and other processes and is generally harmless, so its mass can be omitted from the total mass of waste in the calculation. However, in the case when the water is severely contaminated and difficult to reclaim in a form pure enough to apply or discharge to a publicly owned wastewater treatment facility, its mass must be taken into consideration for E-factor calculation.27 The higher the E-factor of a chemical process, the greater is the waste generated, the greater its negative environmental impact, and the less sustainable it is. This is why this factor was included in ComplexGAPI to show the green character of the overall process. In this case, only the value of the E-factor is included in the graph to facilitate the comparisons of different methodologies used at the same chemical scale.
Software
The proposed tool is accompanied by a simple piece of software that facilitates the use of ComplexGAPI for assessing the greenness of analytical procedures. It was developed in Python using the default28 Tkinter library. The graphical user interface of the software is shown in Fig. S2.† Its use, outlined in the ESI,† is straightforward. The user chooses parameters corresponding to both the pre-analysis processes and the sample preparation and analysis stages from drop-down menus, and the corresponding ComplexGAPI pictogram is generated live for immediate reference. When ready, the pictogram can be saved either as a raster image (.png) or vector graphic (.svg).The software is available under the open-source MIT license and can be downloaded from mostwiedzy.pl/complexgapi. The code is made available in an open repository.
Case study
To showcase the utility and convenience of ComplexGAPI, the greenness of three reported analytical methodologies for the determination of pesticides in urine samples was assessed and juxtaposed and evaluated using the developed tool.The procedures are as follows: Procedure 1 (in situ-IL-DLLME-HPLC): magnetic nanoparticle-assisted in situ ionic liquid dispersive liquid–liquid microextraction (in situ-IL-DLLME) coupled to high performance liquid chromatography (HPLC);29 Procedure 2 (SFO-DLLME-GC-MS): dispersive liquid–liquid microextraction (DLLME) based on solidification (SFO) of deep eutectic solvent (DES) droplets combined with gas chromatography-mass spectrometry (GC-MS);30 and Procedure 3 (SB-μ-SPE-GC-MS): membrane-protected stir-bar supported micro-solid-phase extraction (SB-μ-SPE) coupled to GC-MS.31
These procedures differ in many aspects, starting from the processes performed prior to the analysis, through the sample preparation step, ending at the final determination. In the first procedure, Fe3O4 magnetic nanoparticles and the ionic liquid ([N4,4,4,4][N(CN)2]) were synthesized and characterized before the analytical procedure. The DLLME extraction technique was used to isolate the analytes, while HPLC was applied for the final determination.
In the second procedure, DES (menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenylacetic acid) was synthesized and applied as an extractant. The extraction solvent was forced to pass through a glass filter under an N2 stream and it was dispersed as fine droplets in the sample solution. Due to the low density of the synthesized extractant, it was collected on top of the sample solution without centrifugation.
phenylacetic acid) was synthesized and applied as an extractant. The extraction solvent was forced to pass through a glass filter under an N2 stream and it was dispersed as fine droplets in the sample solution. Due to the low density of the synthesized extractant, it was collected on top of the sample solution without centrifugation.
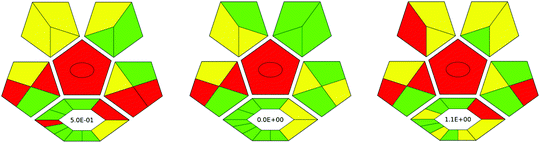
In the third procedure, the layered double hydroxide/graphene (LDH-G) hybrid was synthesized by co-precipitation and used as a sorbent in SB-μ-SPE extraction. Furthermore, GC-MS was applied for the final determination of the analytes in urine samples. The result of the evaluation of these procedures for pesticide determination in urine ComplexGAPI is shown in Fig. 5.
By juxtaposing the results of the assessment of the selected procedures for the pesticide determination in urine samples, it is evident where these procedures differ and which aspects should be focused on to avoid certain issues. It should be noted that all methods require the transport of samples and their storage.
The procedure based on DLLME, which in turn is based on the solidification of DES droplets and GC-MS (Procedure 2) seems to be greener than the other two methodologies. This is mainly because the processes related to the synthesis of DES as well as the micro-extraction procedure are based on non-hazardous reagents. In fact, DES synthesis is a very simple process. In this case, 4.68 g of menthol was mixed with 1.36 g of phenylacetic acid in a glass tube and the mixture was heated for 1 h at 60 °C in a water bath. The synthesis occurs in 100% yield and no wastes are generated during this part (E-factor = 0). No further steps are required. The procedure requires small amounts of reagents for the analytical separation, and thus, a few millilitres of wastes are generated. The critical point of Procedure I is the amount of waste generated that is not recycled. In fact, the synthesis of nanoparticles consists of several steps and requires a large aliquot of reagents. The resulting solution requires heating at 180 °C for 20 h. The black magnetite microspheres were thoroughly washed with ethanol and deionized water several times and then dried under vacuum at 50 °C for 24 h. Considering the analytical procedure, the protocol should be refined with respect to waste production and its regeneration. Procedure III fails in many aspects, including reagent consumption, waste generation, and conditions used in the synthesis processes. The synthesis part involves numerous steps, a large volume of reagents as well as their aliquots, and application of high temperature for long periods of time. The procedure does not support green economy and it is characterized by a higher E-factor. This is why, in comparison to all the evaluated procedures in terms of the green character, the last one is the lowest-scoring and future modifications are recommended.
Conclusions
As the interest in green analytical chemistry increases, fresh perspectives on metrics that allow the evaluation of the analytical procedures are required. This is why the GAPI tool was proposed in 2018. Although it is widely applied by the researchers, its limitation is that it does not allow the evaluation of the processes which occur before analytical methodology, meaning synthesis and other reactions, preparation of stationary phases, etc. In order to meet the needs of the users of the GAPI tool which allows the assessment of the green nature of the analytical procedure, an improved tool, the complementary green analytical procedure index (ComplexGAPI), has been developed, which not only allows the assessment of the analytical procedure in terms of its environmental friendliness but also those processes which precede the analytical procedure itself. An additional hexagonal field was added to the GAPI pictogram to reflect the greenness aspect of the following parameters: yield and conditions, reagents and solvents, instrumentation and workup, and purification. These elements can be used to evaluate the syntheses/manufacturing of organic compounds or solvents, nanomaterials, or stationary phases. The advantage of the ComplexGAPI tool is the availability of software that will facilitate the use of such a solution. The use of the ComplexGAPI to assess the green nature of the entire protocol enables finding at a first glance where the considered procedures differ and to which parts attention should be paid to avoid certain issues. We recommend following the principles of green chemistry in every aspect of laboratory work.Author contributions
J. P.-W. is the originator of the ComplexGAPI idea and the visual image, while W. W. has developed the software that facilitates the use of ComplexGAPI for assessing the greenness of analytical procedures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. P.-W. would like to express her thanks to the Gdańsk University of Technology for support within the Iconic Scholars program (32/WCh-BR/2021 (RI-23.2021)). J. P.-W would also like to thank Reaxys base (Elsevier) which was helpful during the optimization of the parameters used.Notes and references
- M. Poliakoff, P. Licence and M. W. George, Curr. Opin. Green Sustainable Chem., 2018, 13, 146, DOI:10.1016/j.cogsc.2018.04.011.
- C. Turner, Pure Appl. Chem., 2013, 85, 2217, DOI:10.1351/pac-con-13-02-05.
- M. Espino, F. J. V. Gomez, J. Boiteux, M. de los Ángeles Fernández and M. F. Silva, Comprehesive Foodomics, 2021, vol. 2, p. 825. DOI:10.1016/B978-0-08-100596-5.22822-9.
- M. Gamal, I. A. Naguib, D. S. Panda and F. F. Abdallah, Anal. Methods, 2021, 13, 369, 10.1039/D0AY02169E.
- A. Gutiérrez-Serpa, R. González-Martín, M. Sajid and V. Pino, Talanta, 2021, 225, 122053, DOI:10.1016/j.talanta.2020.122053.
- M. Tobiszewski, Anal. Methods, 2016, 8, 2993, 10.1039/C6AY00478D.
- M. K. Parr and A. H. Schmidt, J. Pharm. Biomed. Anal., 2017, 17, 31221, DOI:10.1016/j.jpba.2017.06.020.
- J. Pryshlakivsky and C. Searcy, J. Cleaner Prod., 2021, 309, 127344, DOI:10.1016/j.jclepro.2021.127344.
- O. G. Bhusnure, Life Cycle Assessment (Lca) Approach To Analytical Method Development: A Review, World J. Pharm. Pharm. Sci., 2015, 4, 933 Search PubMed.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123, 10.1039/C4GC01153H.
- L. H. Keith, L. U. Gron and J. L. Young, Chem. Rev., 2007, 107, 2695, DOI:10.1021/cr068359e.
- M. de la Guardia and A. Sergio, Green Analytical Chemistry: Theory and Practice, Elsevier, Amsterdam, 2011 Search PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61, DOI:10.1016/j.trac.2012.03.013.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076, DOI:10.1021/acs.analchem.0c01887.
- P. M. Nowak and P. Kościelniak, Anal. Chem., 2019, 91, 10343, DOI:10.1021/acs.analchem.9b01872.
- P. M. Nowak, R. Wietecha-Posłuszny and J. Pawliszyn, TrAC, Trends Anal. Chem., 2021, 138, 116223, DOI:10.1016/j.trac.2021.116223.
- J. Płotka-Wasylka, Talanta, 2018, 181, 204, DOI:10.1016/j.talanta.2018.01.013.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32, DOI:10.1021/acssuschemeng.7b03505.
- K. van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3, DOI:10.1186/1860-5397-2-3.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2017, 17, 3111, 10.1039/C5GC00340G.
- D. Hariyati Adam, Elvina, M. N. Sari Hasibuan, R. Syahputra, L. Habibah Pasaribu and Suriyani, Int. J. Sci. Technol. Res., 2020, 9, 471 Search PubMed.
- N. O. Bedenik and N. Zidak, Eurasian J. Business Manage., 2019, 7(2), 49, DOI:10.15604/ejbm.2019.07.02.005.
- S. K. Sharma, A. Chaudhary and R. V. Singh, Gray chemistry verses green chemistry: Challenges and opportunities, Rasayan J. Chem., 2008, 1, 68 CAS.
- B. A. de Marco, B. S. Rechelo, E. G. Tótoli, A. C. Kogawa and H. R. N. Salgado, Saudi Pharm. J., 2019, 27, 1, DOI:10.1016/j.jsps.2018.07.011.
- R. Cucciniello and D. Cespi, Recycling within the chemical industry: The circular economy era, Recycling, 2018, vol. 3, pp. 6–9 Search PubMed.
- R. Shanghi, The Need For Green Chemistry: Environment Friendly Alternatives, Narosa Publishing House, New Delhi, 2003 Search PubMed.
- R. A. Sheldon, Green Chem., 2017, 19, 18, 10.1039/C6GC02157C.
- Python Software Foundation, 2001.
- X. Li, D. Zeng, Y. Liao, M. Tsunoda, Y. Zhang, X. Xie, R. Wang, L. Li, W. Hu, S. Deng and Y. Song, Microchem. J., 2020, 159, 105350, DOI:10.1016/j.microc.2020.105350.
- A. Jouyban, M. A. Farajzadeh and M. R. Afshar Mogaddam, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1124, 114, DOI:10.1016/j.jchromb.2019.06.004.
- M. Sajid, C. Basheer, M. Daud and A. Alsharaa, J. Chromatogr., A, 2017, 1489, 1, DOI:10.1016/j.chroma.2017.01.089.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02318g |
| This journal is © The Royal Society of Chemistry 2021 |