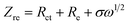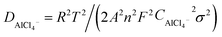High-performance aluminum-ion batteries based on AlCl3/caprolactam electrolytes†
Cheng
Xu
,
Wenyang
Zhang
,
Pan
Li
,
Shimeng
Zhao
,
Yiqun
Du
,
Huixin
Jin
,
Youjian
Zhang
,
Zihan
Wang
and
Jianxin
Zhang
 *
*
School of Materials Science and Engineering, Shandong University, Jinan, 250061, China. E-mail: jianxin@sdu.edu.cn
First published on 18th November 2019
Abstract
Aluminum-ion batteries (AIBs) have become a promising energy storage system due to their excellent cycling performance and safety properties. However, many problems persist in the electrolyte system as well as high cost and harsh working environments. In this study, we prepared two novel electrolytes, namely, AlCl3/caprolactam (CPL) and AlCl3/urea/CPL for use in AIBs. The AlCl3/CPL electrolyte exhibited a specific capacity of 136 mA h g−1 at a current density of 5 A g−1. The performance of AIBs was improved when an appropriate amount of urea was added. The AlCl3/urea/CPL electrolyte achieved a high initial specific capacity of 151 mA h g−1 at 5 A g−1 and reached 132 mA h g−1 with a high coulombic efficiency of ∼98% after 3000 cycles. The CPL electrolyte improved the cutoff voltage and initial discharge voltage of AIBs, and the capacity of AIBs was significantly improved when compared with that of the reported electrolyte. This work provides innovative insights into AIBs, and CPL will be a promising electrolyte for AIBs.
Nowadays, either limited resources or environmental destruction can seriously restrict societal development. Therefore, it has become imperative to explore renewable energies such as solar, wind, ocean, and bioenergy.1 There will be a huge demand for electrochemical energy storage devices with superior capacity, lower cost, and higher security.2–5 The lithium-ion battery was once one of the most popular energy storage systems; however, it is difficult to meet the current demand due to the deficiencies of lithium and expensive production costs.6 Aluminum-ion batteries (AIBs) have many advantages of low cost, abundant Al sources, low flammability, and high theoretical specific capacity. Its theoretical specific capacity is up to 2980 mA h g−1 due to aluminum tri-electron participating in the electrode reaction.7 AIBs have been one of the most potential electrochemical energy storage devices to replace lithium-ion batteries.
Dai's group developed rechargeable AIBs with high-rate capability,8 with aluminum metal as the anode and a 3D graphitic foam as the cathode. Since then, the development of cathode materials for AIBs has attracted the attention of many researchers; consequently, carbon materials,9–12 oxides,13,14 sulfides,15,16 selenides,17 sulfur,18 iodine,19 and tellurium20 have been developed. Graphite/graphene may be one of the most promising cathode materials,21–24 and the battery has a better electrochemical performance when the active material and carbon material are combined.25–27 Electrolyte systems for AIBs include [EMIm]Cl,8 acetamide,28 urea,29,30 molten salts,31 LiCl/KCl,32 and Et3NHCl.33–35 However, the problems of high cost, low capacity, and harsh working environment have not been effectively resolved, which have hindered the applications of AIBs. These electrolytes allow the reversible deposition of aluminum on the anode, and the interaction/deintercalation of AlCl4− on the cathode.36
Herein, we prepared two new low-cost electrolytes, namely, AlCl3/caprolactam (CPL) and AlCl3/urea/CPL for AIBs. The AlCl3/CPL electrolyte improved the cutoff voltage of AIBs, and the battery exhibited a specific capacity of 136 mA h g−1 at a current density of 5 A g−1. Moreover, the performance of the AlCl3/CPL electrolytes was improved when an appropriate amount of urea was added, and a battery with AlCl3/urea/CPL as the electrolyte achieved a high initial specific capacity of 151 mA h g−1 at 5 A g−1.
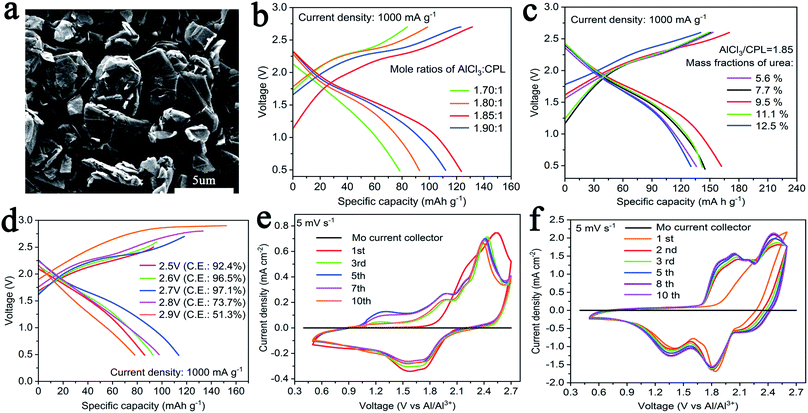
CPL and AlCl3 were mixed in a weighing bottle and evenly stirred in an Ar-filled glove box to yield the electrolyte. In order to reveal the performance of the electrolyte, we chose commercial graphite (C; particle size: ∼2 μm; purchased directly from the factory) as the cathode material. The surface morphology of C showed the characterization of a 2-D layered structure like that of traditional graphite (Fig. 1a). Here, C and natural graphite were characterized by powder X-ray diffraction (XRD), as shown in Fig. S1.† Both C and natural graphite have sharp peaks at 26.55°, corresponding to (002), and natural graphite has better crystallinity.
Fig. 1b shows the charge–discharge curves of AIBs with various molar ratios of AlCl3/CPL as the electrolyte at a current density of 1 A g−1: coin cells with a molar ratio of AlCl3/CPL electrolyte of 1.85 yield a better discharge capacity. As shown in Fig. 1c, the performance of AIBs was considerably improved when an appropriate amount of urea was added, and AIBs have an optimum performance when the mass ratio of urea is 9.5%. Next, AlCl3/CPL (1.85) and AlCl3/urea/CPL (9.5%) were used as the electrolyte for testing the electrochemical performance of AIBs. Fig. 1d shows the charge–discharge curves of AIBs with AlCl3/CPL as the electrolyte, revealing that the appropriate cutoff voltage was 2.7 V. As shown in Fig. S2a,† the cutoff voltage of AIBs with AlCl3/urea/CPL as the electrolyte may be set at 2.7 V, but the coulombic efficiency is low; therefore, the cutoff voltage was set at 2.6 V (Fig. S2b†). Fig. 1e and f shows the CV curves of AIBs at a scan rate of 5 mV s−1; evidently, the current density of the AlCl3/urea/CPL electrolyte is higher than that of the AlCl3/CPL electrolyte. The initial CV curve is inconsistent with the other cycles, and this was probably due to the side reactions occurring during charging. It is clear that there are two oxidation peaks at 1.79–2.23 and 2.26–2.6 V; the corresponding reduction peaks are at 1.18–1.48 and 1.61–1.96 V (Fig. 1f), which were different from those for the CV curves (oxidation peak: 2.13–2.54 and 2.28–2.65 V; reduction peak: 1.25–1.90 V) of the AlCl3/CPL electrolyte (Fig. 1e). Mo foil (black line) has no oxidation peaks between 0.5 and 2.7 V, revealing the suitability of Mo as a current collector for AIBs.
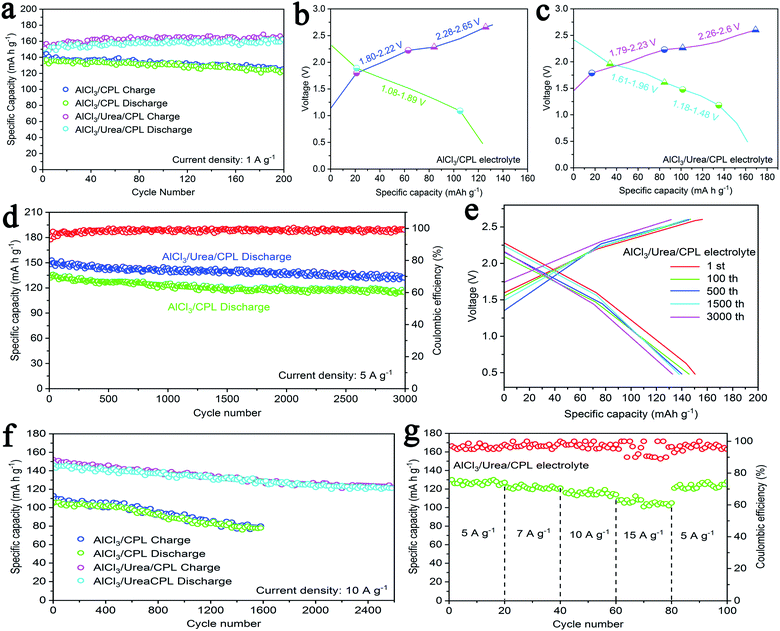
The cycling performance of the AlCl3/urea/CPL electrolyte at a current density of 1 A g−1 exhibited a high specific capacity of 161 mA h g−1, which was preferable to that of the AlCl3/CPL electrolyte (138 mA h g−1, Fig. 2a). The charge–discharge curves of AIBs at 1 A g−1 are shown in Fig. 2b and c; the charge–discharge voltage plateaus were fairly consistent with the oxidation/reduction peaks (Fig. 1e and f). AIBs with AlCl3/CPL or AlCl3/urea/CPL as the electrolyte have a high initial discharge voltage of ∼2.4 V and cutoff voltage of 2.7 and 2.6 V, respectively; this high voltage could increase the capacity of AIBs. Static cycling for the AlCl3/urea/CPL electrolyte at 5 A g−1 denoted a high initial specific capacity of 151 mA h g−1, delivering 132 mA h g−1 with a high coulombic efficiency of ∼98% after 3000 cycles (Fig. 2d). The charge–discharge curves of the AlCl3/urea/CPL electrolyte at the 1st, 100th, 500th, 1500th, and 3000th cycles are almost identical with capacity decay of only 0.004% per cycle (Fig. 2e). The AlCl3/CPL electrolyte exhibited an initial capacity of 136 mA h g−1 and reached 115 mA h g−1 after 3000 cycles. At a super-high current density of 10 A g−1, the AlCl3/urea/CPL electrolyte exhibited a specific capacity with little decay of about 121 mA h g−1 after 2600 cycles (Fig. 2f). For comparison, the capacity of AlCl3/CPL was also tested, showing a discharge capacity of 78 mA h g−1 after 1600 cycles. Therefore, AIBs displayed more stable cycling performance at an ultrahigh current density when urea was added. As shown in Fig. 2g, the rate performances of the AlCl3/urea/CPL electrolyte at current densities of 5, 7, 10, and 15 A g−1 were measured to evaluate the kinetics, and the battery could exhibit a specific capacity of 105 mA h g−1 at 15 A g−1. As shown in Fig. S3,† the cycling performance of a coin cell without the active materials exhibited a negligible specific capacity of 3 mA h g−1, revealing that Mo or Fe had no contribution toward the capacity of AIBs. Fig. S4a and b† show a comparison of the surface morphologies of the initial aluminum and aluminum anode after 1000 cycles of charging–discharging. It may be concluded that there was no aluminum dendrite formation during the battery reaction. As shown in Fig. S5a,† the discharging capacities of batteries with different masses of graphite indicated that the entire amount of graphite participated in the electrochemical reaction.8 AIBs took only 1 min to be fully discharged at 10 A g−1 (Fig. S5b†), revealing that chloroaluminate anions have the ability of rapid diffusion and intercalation when a graphite layer is used.
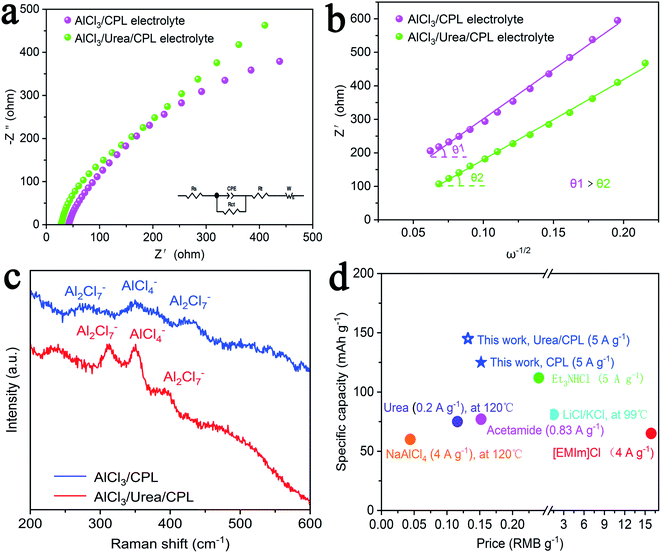
Fig. 3a and b shows a comparison of the Nyquist plots of AlCl3/CPL and AlCl3/urea/CPL electrolytes; these plots include two parts: a semicircle in the high-frequency region and a sloping line in the low-frequency region. The ion diffusion coefficient in the electrodes could be calculated from the plots in the low-frequency region according to the following equations:
where D is the ion diffusion coefficient, R is the gas constant, T is the absolute temperature, A is the surface area, n is the number of electrons, F is the Faraday's constant, C is the concentration, and σ is the Warburg factor. A smaller semicircle (Fig. 3a, according to the curvature of the arc) for AlCl3/urea/CPL reveals that it has the smallest charge transfer resistance. The relationship between the impedance and phase angle is shown in Fig. 3b, and the tilt angle relation (θ1 > θ2) demonstrates that the AlCl3/urea/CPL electrolyte yields a smaller Warburg factor, which corresponds to a larger AlCl4− ion diffusion coefficient when using graphite cathodes. The impedance relationship corresponded to the intensities of AlCl4− and Al2Cl7− in the electrolyte (Fig. 3c), and the intensity for AlCl3/urea/CPL was higher than that for the AlCl3/CPL electrolyte. The conductivity of the electrolyte significantly increased after the addition of urea (Table S1†), and it reached the maximum value at the ratio of 9.5%. It is possible to conclude that the higher concentrations of AlCl4− and Al2Cl7− may be the reason for the better performance of the AlCl3/urea/CPL electrolyte. Moreover, both AlCl3/CPL and AlCl3/urea/CPL electrolytes have a high performance–price ratio (Fig. 3d). Table S2† lists the electrochemical performance of the reported electrolyte, and the performances of AlCl3/CPL and AlCl3/urea/CPL electrolytes were considerably improved.
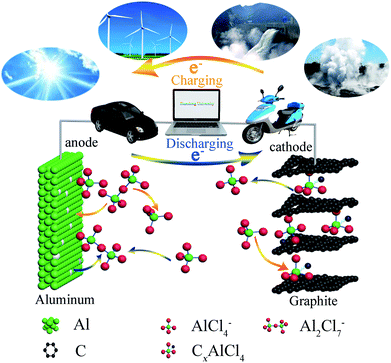
The energy storage mechanism and chloroaluminate anions' intercalation/deintercalation of AIBs could be tentatively proposed (Fig. 4).37–39 We proposed that the mechanism of aluminum deposition and ion intercalation for AIBs during charging and discharging could be expressed as follows:
AlCl3/CPL electrolyte:
| Anode: 4Al2Cl7− + 3e− ↔ Al + 7AlCl4− | (1) |
| Cathode: xC + AlCl4− ↔ CxAlCl4 + e− | (2) |
AlCl3/urea/CPL electrolyte:48
| Anode: 4Al2Cl7− + 3e− ↔ Al + 7AlCl4− | (3) |
| 2[AlCl2·(urea)2]+ + 3e− ↔ Al + AlCl4− + 4(urea) | (4) |
| Cathode: xC + AlCl4− ↔ CxAlCl4 + e− |
When AlCl3/CPL is used as the electrolyte of the AIB, AlCl4− intercalates into C layers and combines with carbon atoms to form CxAlCl4 on the cathode during charging (eqn (2)); Al2Cl7− decomposes into Al and AlCl4− on the anode and aluminum deposits on the aluminum foil (eqn (1)). During discharging, the reverse reaction of the above occurs. When urea was added to the AlCl3/CPL electrolyte, there was a reversible reaction at the anode, which [AlCl2·(urea)2]+ would break down into Al, AlCl4−, and urea (eqn (4)).
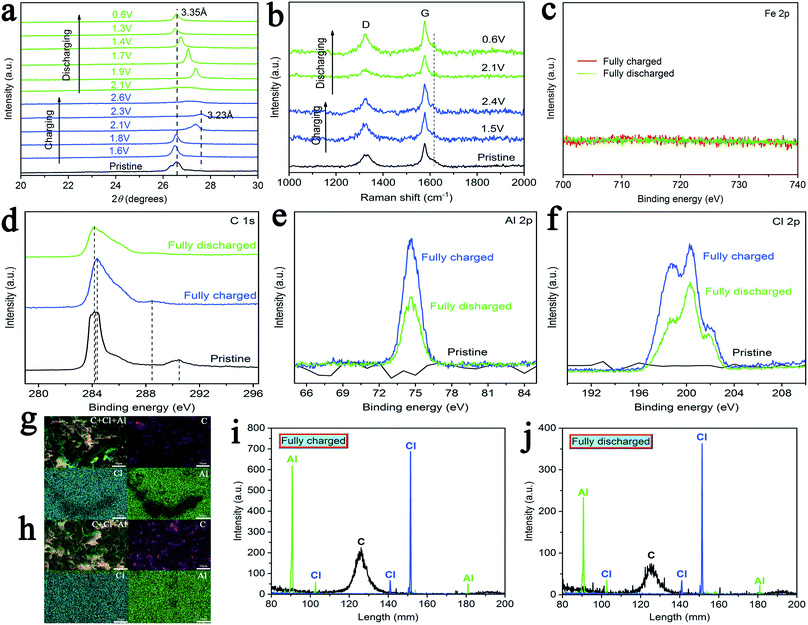
In order to further explain the mechanism of chloroaluminate anions' intercalation/deintercalation during charging–discharging, cathodes (AIBs with AlCl3/urea/CPL as the electrolyte) with different cutoff voltages were characterized by X-ray diffraction (XRD). Fig. 5a shows the XRD patterns of cathodes, and the peak at 26.5° (d ≈ 3.35 Å) of pristine cathode shifts to the peak at ∼27.58° (d ≈ 3.23 Å) when the cutoff voltage changes from 1.6 to 2.3 V. The peak was recovered when the battery was fully discharged, and the reversible change in peaks indicated AlCl4− intercalation/deintercalation when using the C cathode. Subsequently, Raman spectra were obtained to investigate the structure of C cathodes (AlCl3/urea/CPL) for different cutoff voltages. The D-band (∼1331 cm−1) is related to the presence of structural defects and disorders in the carbon material (Fig. 5b).40–42 The G-band is involved in the E2g vibration mode of the sp2-bonded carbon atoms.43–47 Evidently, the G-band split a new peak at 1617 cm−1, and this peak weakens with discharging. The reversible splitting of the G-band illustrated AlCl4− intercalation/deintercalation during charging–discharging.
As shown in Fig. 5c–f and S8,† X-ray photoelectron spectra (XPS) was utilized to prove the chemical nature of the intercalated species in C cathodes (AlCl3/CPL). As shown in Fig. 5c, no peak was found in the XPS of Fe 2p, which proved that stainless steel was not involved in the battery reaction. XPS spectrum shows two C 1s peaks at about 284.2 and 290.4 eV for the pristine cathode (Fig. 5d). When fully charged, it gradually developed two new peaks at 284.3 and 288.4 eV. As shown in Fig. 5e and f, the intensities of Al 2p and Cl 2p (fully discharged) were reduced when compared with those of the cathodes (fully charged). This confirmed the electrochemical oxidation of graphite by the intercalation of AlCl4− anions (eqn (2)). The peak (fully discharged) without complete recovery was probably caused by the residual electrolyte in the C cathodes or an irreversible reaction.
Lastly, energy-dispersive spectrometry (EDS) and electron probe microanalyzer (EPMA) were used for the qualitative analyses of the elements in the fully charged–discharged cathodes (AlCl3/urea/CPL). As shown in Fig. 5g and h, the EDS elemental mapping images of C, Al, and Cl revealed that the elements were evenly distributed. Fig. 5i and j shows the EPMA spectrum of the fully charged–discharged C cathode; the intensities of Al and Cl decreased with the discharging of AIBs.
Conclusions
In summary, we assembled two novel AIBs with low-cost AlCl3/CPL and AlCl3/urea/CPL ionic liquids as the electrolyte; commercial graphite was used as the cathode. AIBs with AlCl3/CPL as the electrolyte delivered a high discharge capacity of 138 mA h g−1 at a current density of 1 A g−1 and exhibited 115 mA h g−1 at a current density of 5 A g−1 after 3000 cycles. Moreover, we found that the performance of AIBs could be improved when an appropriate amount of urea was added to AlCl3/CPL. AIBs with AlCl3/urea/CPL as the electrolyte achieved high specific capacity of 161 mA h g−1 at 1 A g−1, reaching 132 mA h g−1 with a high coulombic efficiency of ∼98% at 5 A g−1 after 3000 cycles. The energy storage mechanism and the intercalation/de-intercalation of AlCl4− were confirmed by a series of material characterization analyses. This work proves that the addition of urea can significantly improve the electrochemical performance of AlCl3/CPL. We believe that the addition of other potential electrolytes could also improve the performance, and the new electrolyte obtained in this work can be extended to other cathode materials used for AIBs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- M. Z. Jacobson, Energy Environ. Sci., 2019, 2, 148 RSC.
- Z. G. Yang, J. L. Zhang, M. C. W. K. Meyer, X. C. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577 CrossRef CAS PubMed.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911 CrossRef CAS PubMed.
- W. Manalastas Jr, S. Kumar, V. Verma, L. Zhang, D. Yuan and M. Srinivasan, ChemSusChem, 2019, 12, 379 CrossRef PubMed.
- M. Kaltenbrunner, M. S. White, E. D. Głowacki, T. Sekitani, T. Someya, N. S. Sariciftci and S. Bauer, Nat. Commun., 2012, 3, 1 Search PubMed.
- G. A. Elia, K. Marquardt, K. Hoeppner, S. Fantini, R. Lin, E. Knipping, W. Peters, J. F. Drillet, S. Passerini and R. Hahn, Adv. Mater., 2016, 28, 7564 CrossRef CAS PubMed.
- Q. F. Li and N. J. Bjerrum, J. Power Sources, 2002, 110, 1 CrossRef CAS.
- M. C. Lin, M. Gong, B. G. Lu, Y. P. Wu, D. Y. Wang, M. Y. Guan, M. Angell, C. X. Chen, J. Yang, B. J. Hwang and H. J. Dai, Nature, 2015, 520, 325 CrossRef PubMed.
- C. Xu, S. Zhao, Y. Du, W. Zhang, P. Li, H. Jin, Y. Zhang, Z. Wang and J. Zhang, ChemElectroChem, 2019, 6, 3350 CrossRef CAS.
- Y. Hu, S. Debnath, H. Hu, B. Luo, X. Zhu, S. Wang, M. Hankel, D. J. Searles and L. Wang, J. Mater. Chem. A, 2019, 7, 15123 RSC.
- J. Smajic, A. Alazmi, N. Batra, T. Palanisamy, D. H. Anjum and P. M. F. J. Costa, Small, 2018, 14, 1803584 CrossRef PubMed.
- Z. Zhou, N. Li, Y. Yang, H. Chen, S. Jiao, W.-L. Song and D. Fang, Adv. Energy Mater., 2018, 8, 1801439 CrossRef.
- H. Lu, Y. Wan, T. Wang, R. Jin, P. Ding, R. Wang, Y. Wang, C. Teng, L. Li, X. Wang, D. Zhou and G. Xue, J. Mater. Chem. A, 2019, 7, 7213 RSC.
- X. Zhang, G. Zhang, S. Wang, S. Li and S. Jiao, J. Mater. Chem. A, 2018, 6, 3084 RSC.
- S. Wang, Z. Yu, J. Tu, J. Wang, D. Tian, Y. Liu and S. Jiao, Adv. Energy Mater., 2016, 6, 1600137 CrossRef.
- S. Wang, S. Jiao, J. Wang, H.-S. Chen, D. Tian, H. Lei and D.-N. Fang, ACS Nano, 2017, 11, 469 CrossRef CAS PubMed.
- W. Xing, D. F. Du, T. H. Cai, X. C. Li, J. Zhou, Y. M. Chai, Q. Z. Xue and Z. F. Yan, J. Power Sources, 2018, 401, 6 CrossRef CAS.
- X. W. Yu, M. J. Boyer, G. S. Hwang and A. Manthiram, Chem, 2018, 4, 586 CAS.
- H. J. Tian, S. L. Zhang, Z. Meng, W. He and W. Q. Han, ACS Energy Lett., 2017, 2, 1170 CrossRef CAS.
- X. Zhang, S. Jiao, J. Tu, W.-L. Song, X. Xiao, S. Li, M. Wang, H. Lei, D. Tian, H. Chen and D. Fang, Energy Environ. Sci., 2019, 12, 1918 RSC.
- C. Liu, Z. Liu, Q. Li, H. Niu, C. Wang, Z. Wang and B. Gao, J. Power Sources, 2019, 438, 226950 CrossRef CAS.
- X. Dong, H. Xu, H. Chen, L. Wang, J. Wang, W. Fang, C. Chen, M. Salman, Z. Xu and C. Gao, Carbon, 2019, 148, 134 CrossRef CAS.
- Y. Hu, S. Debnath, H. Hu, B. Luo, X. Zhu, S. Wang, M. Hankel, D. J. Searles and L. Wang, J. Mater. Chem. A, 2019, 7, 15123 RSC.
- H. Huang, F. Zhou, X. Shi, J. Qin, Z. Zhang, X. Bao and Z.-S. Wu, Energy Storage Materials, 2019, 23, 664 CrossRef.
- C. Li, S. Dong, P. Wang, C. Wang and L. Yin, Adv. Energy Mater., 2019, 9, 1902352 CrossRef CAS.
- D. Kim, D.-J. Yoo, M. T. Otley, A. Prokofjevs, C. Pezzato, M. Owczarek, S. J. Lee, J. W. Choi and J. F. Stoddart, Nat. Energy, 2019, 4, 51 CrossRef CAS.
- Z. Zhao, Z. Hu, R. Jiao, Z. Tang, P. Dong, Y. Li, S. Li and H. Li, Energy Storage Materials, 2019, 22, 228 CrossRef.
- N. Canever, N. Bertrand and T. Nann, Chem. Commun., 2018, 54, 11725 RSC.
- H. D. Jiao, C. Wang, J. G. Tu, D. H. Tian and S. Q. Jiao, Chem. Commun., 2017, 53, 2331 RSC.
- M. Angella, C. J. Pan, Y. M. Rong, C. Z. Yuana, M. C. Lin, B. J. Hwang and H. J. Dai, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 834 CrossRef PubMed.
- Y. Song, S. Q. Jiao, J. G. Tu, J. X. Wang, Y. J. Liu, H. D. Jiao, X. H. Mao, Z. C. Guo and D. J. Fray, J. Mater. Chem. A, 2017, 5, 1282 RSC.
- J. Wang, X. Zhang, W. Q. Chu, S. Q. Liu and H. J. Yu, Chem. Commun., 2019, 55, 2138 RSC.
- H. Y. Xu, T. W. Bai, H. Chen, F. Guo, J. B. Xi, T. Q. Huang, S. Y. Cai, X. Y. Chu, J. Ling, W. W. Gao, Z. Xu and C. Gao, Energy Storage Materials, 2019, 17, 38 CrossRef.
- Z. Yu, S. Jiao, S. Li, X. Chen, W.-L. Song, T. Teng, J. Tu, H.-S. Chen, G. Zhang and D.-N. Fang, Adv. Funct. Mater., 2019, 29, 1806799 CrossRef.
- H. Yang, H. Li, J. Li, Z. Sun, K. He, H.-M. Cheng and F. Li, Angew. Chem., Int. Ed., 2019, 58, 11978 CrossRef CAS PubMed.
- Y. Wu, M. Gong, M.-C. Lin, C. Yuan, M. Angell, L. Huang, D.-Y. Wang, X. Zhang, J. Yang, B.-J. Hwang and H. Dai, Adv. Mater., 2016, 28, 9218 CrossRef CAS PubMed.
- A. S. Childress, P. Parajuli, J. Y. Zhu, R. Podila and A. M. Rao, Nano Energy, 2017, 39, 69 CrossRef CAS.
- P. Bhauriyal, A. Mahata and B. Pathak, Phys. Chem. Chem. Phys., 2017, 19, 7980 RSC.
- L. Y. Zhang, L. Chen, H. Luo, X. F. Zhou and Z. P. Liu, Adv. Energy Mater., 2017, 7, 1700034 CrossRef.
- G. S. Zakharova, C. Jahne, A. Popa, C. Taschner, T. Gemming, A. Leonhardt, B. Buchner and R. Klingeler, J. Phys. Chem. C, 2012, 116, 8714 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- Y. Wang, F. Su, C. D. Wood, J. Y. Lee and X. S. Zhao, Ind. Eng. Chem. Res., 2008, 47, 2294 CrossRef CAS.
- Z. F. Li, C. Bommier, Z. S. Chong, Z. L. Jian, T. W. Surta, X. F. Wang, Z. Y. Xing, J. C. Neuefeind, W. F. Stickle, M. Dolgos, P. A. Greaney and X. L. Ji, Adv. Energy Mater., 2017, 7, 1602894 CrossRef.
- M. Balabajew, H. Reinhardt, N. Bock, M. Duchardt, S. Kachel, N. Hampp and B. Roling, Electrochim. Acta, 2016, 211, 679 CrossRef CAS.
- R. J. Nemanich, S. A. Solin and D. Gúerard, Phys. Rev. B: Solid State, 1977, 16, 2965 CrossRef CAS.
- C. X. Li, S. H. Dong, R. Tang, X. L. Ge, Z. W. Zhang, C. X. Wang, Y. P. Lu and L. W. Yin, Energy Environ. Sci., 2018, 11, 3201 RSC.
- X. D. Huang, Y. Liu, H. W. Zhang, J. Zhang, O. Noonan and C. Z. Yu, J. Mater. Chem. A, 2017, 5, 19416 RSC.
- J. Li, J. Tu, H. Jiao, C. Wang and S. Jiao, J. Electrochem. Soc., 2017, 164, A3093 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9se00941h |
| This journal is © The Royal Society of Chemistry 2020 |