Open Access Article
Open Access ArticleNatural products' role against COVID-19
Ananda da Silva
Antonio
 a,
Larissa Silveira Moreira
Wiedemann
a,
Larissa Silveira Moreira
Wiedemann
 a and
Valdir Florêncio
Veiga-Junior
a and
Valdir Florêncio
Veiga-Junior
 *ab
*ab
aChemistry Department, Institute of Exact Sciences, Federal University of Amazonas, Avenida Rodrigo Octávio, 6200, Coroado, CEP: 69.077-000, Manaus, AM, Brazil. E-mail: valdir.veiga@gmail.com
bChemical Engineering Section, Military Institute of Engineering, Praça General Tibúrcio, 80, Praia Vermelha, Urca, CEP: 22.290-270, Rio de Janeiro, RJ, Brazil
First published on 19th June 2020
Abstract
COVID-19 is a viral disease caused by a new severe acute respiratory syndrome (SARS-CoV-2), which has quickly resulted in a pandemic. As a great threat to global public health, the development of a treatment has become vital, and a rush to find a cure has mobilized researchers from all areas across the world. Synthetic drugs, such as hydroxychloroquine, have gained attention. However, the efficacy of repositioned drugs is still under evaluation, and besides, some severe side effects are a cause for concern. This emphasizes the urgency for treatment options, which can be both safe and effective. With this in mind, natural products could be an important resource in the development of COVID-19 treatment, as they have already contributed in the past to treatments against other viruses, such as HIV, MERS-CoV, and influenza. Natural products are described long term as bioactive substances and some phytochemical classes such as flavonoids, alkaloids, and peptides are known antiviral bioproducts, and have been virtually tested with success against COVID-19. However, important issues still need to be addressed as to their bioavailability and true efficacy in vivo. This review intends to systematically evaluate the natural metabolites that could potentially be used against this new disease looking at their natural sources, mechanism of action and previous pharmacological usages. The aim is to provide a starting point for this research area in order to speed up the establishment of anti-SARS-CoV-2 bioproducts.
Introduction
Viral infections are currently considered a persistent public health issue. Viruses can be defined as submicroscopic non-living agents responsible for several human illnesses, mainly composed of RNA or DNA strains. The most famous examples of viral diseases are human immunodeficiency virus (HIV), herpes simplex virus, hepatitis, influenza and dengue virus.1–3 The major concern regarding this type of infection is related to the intrinsic characteristics of viruses, such as their replication mechanism effects and mutation capacities. The propagation of viral infections in a living organism relies on the inoculation of the virus in a healthy cell. After inoculation, the virus replicates itself using the host cell's mechanisms. Thus, it can profoundly modify organism functioning and metabolism, so that vital functions can break down. In addition, viruses can undergo mutation as a response to biotic and abiotic characteristics regarding the host.Antiviral treatment development becomes tricky, as it depends on the knowledge of its pathobiology. The inoculation of viruses in the host cells makes it difficult to find drugs that do not cause strong side effects.1,4,5 As we are aware of its molecular structure, inoculation, and replication mechanism, it is possible to develop drugs that can specifically combat the virus with severe adverse effects to the host. The capability of the virus to develop drug resistance is another challenge to be faced, as it increases the outspreading as a pandemic disease.1,2,6
COVID-19 is a viral disease caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2). It is a human strain of the Coronaviridae family, also known as coronaviruses, a large group of single-stranded enveloped RNA viruses which uses both mammals and reptiles as their host, including, bats, and snakes.4,5 The SARS-CoV-2 was first detected in Wuhan, China in December 2019 and declared a pandemic on March 13, 2020. Fifteen days (March 28) after its classification as a pandemic, COVID-19 had infected 571![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 689 people across the World with 26
689 people across the World with 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 confirmed deaths.7 As COVID-19 mortality rate remains unclear due to difficulties in tracking cases and different containment protocols used by each country, its unprecedented rapid spread throughout the world has created urgency for a treatment to be developed. For instance, in 2020 May 23, nearly three months after COVID-19 classification as pandemic, the global number of infected people was 5
493 confirmed deaths.7 As COVID-19 mortality rate remains unclear due to difficulties in tracking cases and different containment protocols used by each country, its unprecedented rapid spread throughout the world has created urgency for a treatment to be developed. For instance, in 2020 May 23, nearly three months after COVID-19 classification as pandemic, the global number of infected people was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103
103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006 with 333
006 with 333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401 fatal cases.7
401 fatal cases.7
Although coronaviruses have been known to humankind since the 1960s, most of their strains which were contagious to humans causes clinical conditions usually related to the common cold. Since the first discovery, three more lethal forms have arisen, the SARS-CoV-1 on 2003, the MERS-CoV on 2012 and the SARS-CoV-2 in 2019. Although it is the third pandemic occurrence of a virus from this family, there is still no vaccine for any type of coronavirus infection. Since SARS-CoV-1 outbreak, technologies have progressed in that the viral genome is able to be sequenced within days, and thus its pathobiology can be deduced.4,5 Virus pathobiology becomes important as drugs and vaccines development aim to interrupt the viral inoculation and replication mechanisms or create antibodies.4,8
Although it is possible to stablish virus genome within days, drugs and vaccines development is not as simple. Both cases require several safety and efficacy analysis which can make all process last for at least a year.9 A strategy that is being used in COVID-19 pandemic is the repurposing of commercial drugs, an approach which has been controversial. Since there is no prospect for the rapid development of vaccines, palliative treatments to combat this pandemic scenario are our best options.4
Through human history and evolution, natural products play an essential role in the development and treatment of several diseases. Essential oils and extracts derived from plants and animals are considered a remarkable source of bioactive metabolites.1,10–13 Bioactivities of natural products have being widely applied in pharmaceutical industry and ethnobotany, such as inflammation, cancer, oxidative process and viral infections. Several antiviral bioproducts have already been described by the activity against Dengue virus, Coronavirus, Enterovirus, Hepatitis B, Influenza virus and HIV.1–3 Thus, bioproducts could be friends in the fight against SARS-CoV-2, through enabling the development of specific chemotherapies to COVID-19. In this paper, we provide insights on the potential of bioproducts in face of this new threat.
SARS-CoV-2 inoculation and replication
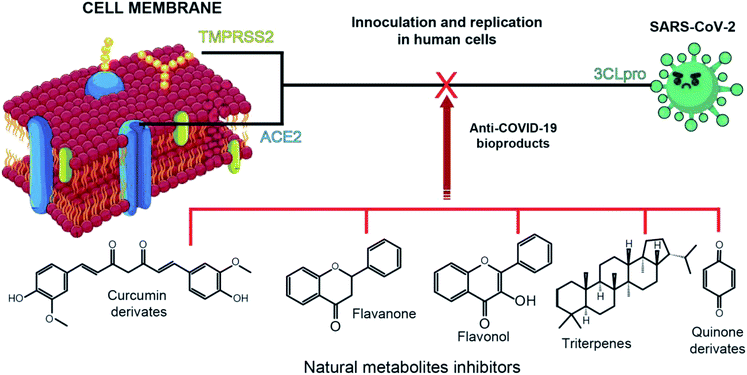
The SARS-CoV-2 genomic sequence enabled the identification of the main proteins and enzymes involved in its inoculation and replication processes. Fortunately, its genomic sequence closely resembles SARS-CoV-1 (79.5%) and one strain of coronaviruses which in only contagious in bats (96%).8 The genomic data also demonstrates that SARS-CoV-2 inoculation and viral replication in humans host occur mainly through three major proteins and enzymes. These proteins are the papain-like protease (ACE2), spike protein (TMPRSS2), and the 3 chymotrypsin-like protease (3CLpro) (Fig. 1).4,5,14Among those three structures, the ACE2 and TMPRSS2 are part of the host cell. The angiotensin-converting enzyme type 2 (ACE2) is an analogue of the angiotensin converting enzyme type I (ACE) and part of the renin–angiotensin system responsible for the blood pressure regulation. Most of anti-COVID-19 development has being focused on the ACE2 inhibition,4,15,16 although acting on such a central pressure control system is probably not the best approach. The spike protein with which SARS-CoV-2 interacts (TMPRSS2) is the transmembrane protease serine type 2. It was estimated that after SARS-CoV-2 binding with ACE2, host TMPRSS2 promotes virus fusion with the infected cell and its activation by cleavage of virus protein.17 At last, the 3CLpro is responsible for the proteolytic cleavage of virus polypeptide in 11 non-structural proteins responsible for its replication.18 Thus, 3CLpro occurrence only within SARS-CoV-2 and not in the host cell dragged attention as a possible inhibition site for COVID-19 treatment development.
This similarity of SARS-CoV-1 and SARS-CoV-2 can serve as a short-cut in specific treatment development for COVID-19, as well as a “universal” treatment for coronaviruses infections, as a great deal of research has already been conducted based on those coronaviruses strains.8,19
Anti-coronaviruses metabolites from natural sources
The development of vaccines against coronaviruses faces several challenges inherent to its establishment as safe and efficient, production and distribution. Therefore, supportive treatments are more achievable in a short amount of time in pandemic control. Treatment is primarily based on the control of symptoms and the inhibition of viral replication. Furthermore, prevention through social measures can limit the transmission.20An issue in the development of any antiviral treatment, including synthetic drugs, is the selectivity towards the virus and not the host metabolism, in order words, producing an efficient treatment with low toxicity. In this regard, the pharmaceutical industry often turns to natural products with antiviral activity.1
Several natural sources from flora and fauna have been tested in terms of their anti-SARS-CoV-1 activity and used as a scaffold in drug development since its outbreak in 2003 (Table 1). These natural metabolites include flavones, flavonols, fatty acids, tannins, terpenes, and alkaloids. The diversity of these chemical classes is related to the different mechanism used by each phytochemical class capable to inhibit coronaviruses. However, the chemical structures of these natural metabolites presents common features that corroborate the conclusions of Jo et al. (2019),5 who, through in silico analysis, showed that SARS-CoV-1 inhibition requires chemical structures containing a hydrophobic aromatic ring, hydroxyl groups and carbohydrates moieties. Although, not all of the anti-SARS-CoV-1 compounds (Table 1) present the aromatic ring, their molecular structures have lipophilic and hydrophilic regions and the ability to form multiple hydrogen bonds through hydroxyl groups.
| Molecular structure | Compound/extract type | Natural source | IC50 | Method to assess in vitro activity | Ref. |
|---|---|---|---|---|---|
| a Cell survival (%). b In vivo (human) test. c In vivo (mice) test; n.i = not informed. | |||||
|
Isotheaflavin-3-gallate | Black tea | 7 μm | Fluorogenic substrate peptide assay | 21 |
|
Tannic acid | Black tea | 3 μm | Fluorogenic substrate peptide assay | 21 |
|
Leukamenin (1) | Lactuca sativa | n.i | n.i | 12 |
| Glaucocalyxin (2) | |||||
| Pseurata (3) | |||||
|
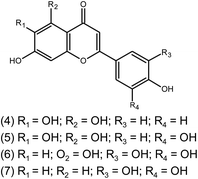
Scutellarein (4) | Scutellaria | 10 μM | Fluorogenic substrate peptide assay | 12 |
| Quercetagetin (5) | |||||
| Myricetin (6) | |||||
| Robinetin (7) | |||||
|
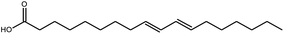
Isolinoleic acid | Mucuna pruriens | 50 μM | n.i | 12 |
|
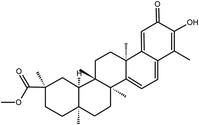
Pristimerin | Celastrus orbiculatus | 5.5 mM | n.i | 12 |
|
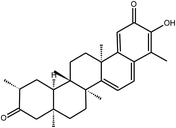
Tingenone | Celastrus orbiculatus | 9.9 mM | n.i | 12 |
|
Iguesterin | Celastrus orbiculatus | 2.6 mM | n.i | 12 |
|
Ethanol extract/Lycorine | Lycoris radiata | 886.6 μg mL−1/15.7 mM | MTS assay | 22 |
|
Ethanolic extract/Friedelanol | Euphorbia neriifolia | 132.4a | MRC-5 system | 23 |
|
Ethanolic extract/Friedelin | Euphorbia neriifolia | 109.0a | MRC-5 system | 23 |
|
Ethanolic extract/Epitaraxerol | Euphorbia neriifolia | 111.0a | MRC-5 system | 23 |
|
Glycyrrhizin | 300 mg L−1b | n.i | 24 | |
|
Mycalamide A | Mycale sp. | 0.2 μg kg−1c | n.i | 11 |
|
Tetrandrine | Stephania tetrandra | 295.6 nM | MRC-5 system | 25 |
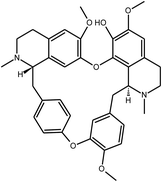
|
Fangchinoline | Stephania tetrandra | 919.2 nM | MRC-5 system | 25 |
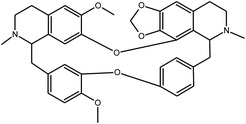
|
Cepharanthine | Stephania tetrandra | 729.7 nM | MRC-5 system | 25 |
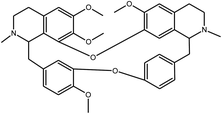
|
Tryptanthrin | Strobilanthes cusia | 1.52 μM | MTT assay | 26 |
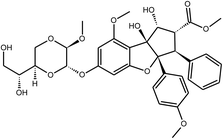
|
Silvestrol | Aglaia foveolata | 3 nM | MRC-5 system | 27 and 28 |
| — | Ethanol extract | Artemisia annua | 1053.0 μg mL−1 | MTS assay | 29 |
| — | Chloroform extract | Pyrrosia lingua | 2378.0 μg mL−1 | MTS assay | 29 |
| — | Ethanol extract | Lindera aggregate | 1374.0 μg mL−1 | MTS assay | 29 |
Several of the anti-SARS-CoV-1 natural metabolites (Table 1) also have other bioactive properties against other virus types or diseases. For instance, the marine sponge mycalamide A and its analogue known as mycalamide B, both present bioactivity against the Herpes virus.11 Meanwhile, the flavonol myricetin has antiviral activity against leukemia, HIV and the influenza virus.30 Additionally, lycorine is known for its broad pharmaceutical applications as an antioxidant, antibacterial, antitumoral, anti-inflammatory and anticancer.22
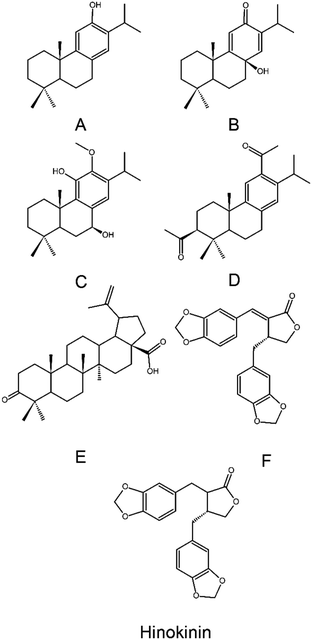
Natural products have huge potential in coronavirus treatment as can be observed in the history of SARS-CoV-1 combat. Wen et al. (2007)31 demonstrated that the natural metabolites, ferruginol (A), 8β-hydroxyabiet-9(11),13-dien-12-one (B), 7β-hydroxydeoxycryptojaponol (C), 3β,12-diacetoxyabiet-6,8,11,13-tetraene (D), betunolic acid (E), and savinin (F) had IC50 values ranging between 0.63 (betunolic acid) to 1.57 μM (3β,12-diacetoxyabiet-6,8,11,13-tetraene). Despite these values are higher than the synthetic drug, they represent a remarkable bioactivity within natural products. As bioproducts containing those metabolites were not specifically design to be anti-coronaviruses, but are commonly exploited and consumed by humans, their low IC50 values prove that SARS-CoV-1 treatment can be assessed in a sustainable way. In addition, the evaluation of their molecular structure and inhibitory mechanism can provide new and innovative scaffolds for drug development. These metabolites include four abietane-type diterpenes (A–D), one triterpene (E) and one lignan (F) (Fig. 2).31
Abietane-type diterpenes are metabolites that can be found in conifer angiosperm families, such as Araucariaceae, Cupressaceae, Phyllocladaceae, Asteraceae, and Lamiaceae. With more than 200 compounds, this class of metabolites presents bioactivities including antiulcer, antitumor, anti-inflammatory, anti-diabetic, antimicrobial, antileishmanial, antimalarial, and cardioprotection. It is worth mentioning that compounds E and F are also both found in Cupressaceae species.31 Lignoid classes have recently been reviewed regarding their antiviral activity. Along with their huge diversity of bioactive compounds, the dibenzylbutyrolactones in particular present antiviral activity. Examples of this structural class are savinin (compound F) and hinokinin (Fig. 2), which are active against SARS-CoV-1 and HIV, respectively.32
Repurposed drugs against COVID-19
In early March 2020, the antimalarial hydroxychloroquine (HCQ) and chloroquine (CQ) displayed in vitro and in vivo activity against SARS-CoV-2 in small geospecific test groups.33 Hydroxychloroquine and chloroquine are synthetic derivates of quinine, an alkaloid extract from the bark of Remija and Cinchona (Rubiaceae) species, widely used as antimalarial drug. All the three chemical species are toxic to humans, causing a decrease of cardiac functions inducing arrhythmias or hypotension effects in single and continuous intake.34 In an adult man, poisoning by a single ingestion of quinine can occur in a range from 10 to 15 g, whilst with chloroquine and hydroxychloroquine this ranges from 2 to 5 g,34 emphasizing the danger of these compounds in preventive treatment with no proper evaluation of effective dose.During nearly three months, several claims regarding the use of hydroxychloroquine and chloroquine were spread through public based on preliminary data of this drug repurposing as “the cure” for COVID-19.33,35 Those pretentions news induced several people to self-medication with no medical attention of cardiac functions.35 Although global population is anxious to scientific community provide a reliable treatment, the usage of HCQ and CQ in COVID-19 combat is still in preliminary test fase.36 Such assertive treatment request several safety and randomized clinical test with unbiased data obtained in trials within different ethnicities, which is being a hard task to accomplish in face of the supposed underreporting of COVID-19 in several countries.
Another drug drawing attention in COVID-19 treatment is the remdesivir, which act as a RNA polymerase inhibitor.37 The inhibition of RNA-dependent RNA polymerase is a strategy in virus treatment to develop high specific antivirus, reducing the damage to the host cells. RNA polymerase of viruses is responsible for its replication mechanism as its promotes a −1 ribosomal frameshifting that is fundamental to synthesize their structure protein.38 Remdesivir have successfully inhibit SARS-CoV-1 and MERS-CoV in primary human airway epithelial cell cultures, with EC50 of approximately 0.07 μM and 50% cytotoxic less than 10 μM.11
Whilst the attention hydroxychloroquine dragged in early pandemic scenario, other drugs also presented similar results to HCQ and CQ in silico efficacy in inhibit SARS-CoV-2. Among those drugs it can be emphasized the Nelfinavir, which presented a binding energy to SARS-CoV-2 close to the values described for chloroquine, −8.4 and −8.0 kcal mol−1, respectively.39–41 Despite repurposing drugs are an approach which aid to reduce the time necessary to control a pandemic scenario,41 reckless usage of preliminary data can become a public health issue.
Possible anti-COVID-19 natural products
Although the development of bioactive natural products against a specific disease, such as COVID-19, is faster than vaccine development, it is still an arduous task due to the diversity of natural metabolites, their chemical complexity and extraction. Virtual screening for bioactive compounds is a useful tool in natural products research in order to shortening the time spend in phytochemical screening of several natural products extracts. This approach is known as in silico analysis by molecular docking.39,42,43Nearly 45 days after COVID-19 become pandemic, natural metabolites of different chemical classes presented promising data on virtual molecular docking (Table 2). Despite the distinct molecular structure, several chemical classes, such as flavanones, flavonols, alkaloids, fatty acids, quinones, terpenes and steroids presented similar binding energy or docking score to repurposed drugs (e.g.: remdesivir and chloroquine) with proteins involved in COVID-19 replication (Table 2), including ACE2, 3CLpro and TMPRSS2. Most of docking evaluation were focused in inhibitors of ACE2 (Table 2) as a possible consequence of the first finding regarding COVID-19 replication and the implication of this enzyme in the formation of the risk group.44,45
| Natural metabolite | Binding energy (kcal mol−1) | Ref. | Natural metabolite | Binding energy (kcal mol−1) | Ref. |
|---|---|---|---|---|---|
| a Repurposed drug used as reference. | |||||
| Binding with 3CLpro | Binding with ACE2 | ||||
| Taiwanhomoflavone A | −9.60 | 39 | Taiwanhomoflavone A | −7.60 | 39 |
| Epicatechin-(4β,8)-epicatechin-(4β,6)-catechin | −10.60 | 39 | Epicatechin-(4β,8)-epicatechin-(4β,6)-catechin | −8.20 | 39 |
| Epicatechin-(4′,8)-epigallocatechin | −10 | 39 | Epicatechin-4-epigallocatechin | −7.20 | 39 |
| Quercetin 3-glucosyl-(1,4)-rhamnoside | −9.90 | 39 | Quercetin 3-glucosyl-(1,4)-rhamnoside | −6.50 | 39 |
| Lactucopicrin 15-oxalate | −8.20 | 39 | Lactucopicrin 15-oxalate | −8.30 | 39 |
| Lactucopicrin | −7.80 | 39 | Lactucopicrin | −8.30 | 39 |
| Vitetrifolin D | −7.60 | 39 | Vitetrifolin D | −7.30 | 39 |
| Myricitrin | −8.90 | 39 | Myricitrin | −7.10 | 39 |
| Apigenin | −7.80 | 39 | Apigenin | −7.10 | 39 |
| Kaempferol | −7.80 | 39 | Kaempferol | −7.20 | 39 |
| (−)-Asperlicin C | −9.70 | 39 | (−)-Asperlicin C | −9.50 | 39 |
| Cassameridin | −9.30 | 39 | Cassameridin | −8.10 | 39 |
| Oriciacridone F | −9.10 | 39 | Oriciacridone F | −6.70 | 39 |
| Remdesivira | −8.20 | 39 | Remdesivira | −7.80 | 39 |
| Afzelin | −8.80 | 39 | Afzelin | −7.10 | 39 |
| Isoquercitrin | −8.20 | 39 | Isoquercitrin | −7.80 | 39 |
| Amentoflavone | −9.28 | 70 | Silybin | −10.572 | 40 |
| Glabrolide | −9.16 | 70 | Tetrahydrocurcumin | −8.009 | 40 |
| Zeylanone | −9.12 | 70 | Corydine | −6.041 | 40 |
| 5,7,3′,4′-tetrahydroxy-2'-(3,3-dimethylallyl) isoflavone | −29.57 | 71 | Aloin | −8.383 | 40 |
| Mirycitrin | −22.13 | 71 | Isoaloresin | −7.835 | 40 |
| Methyl rosmarinate | −20.62 | 71 | Quercetin | −8.664 | 40 |
| Amaranthin | −18.14 | 71 | Withaferin A | −9.631 | 40 |
| Betulinic acid | −4.23 | 42 | Hinokinin | −7.11 | 40 |
| Cryptotanshinone | −6.23 | 42 | Philligenin | −7.807 | 40 |
| N-cis-feruloyltyramine | −6.25 | 42 | Chloroquinea | −8.019 | 40 |
| Sugiol | −6.04 | 42 | Narigin | −6.85 | 15 |
| Nelfinavira | −10.72 | 52 | Naringenin | −6.05 | 15 |
| Lupinavir | −9.41 | 52 | Hesperidin | −4.21 | 15 |
| Kaempferol | −8.58 | 52 | Hesperetin | −6.09 | 15 |
| Quercetin | −8.47 | 52 | Neohesperidin | −3.78 | 15 |
| Apigenine-7-glucoside | −7.83 | 52 | Nobiletin | −5.42 | 15 |
| Zingerol | −5.40 | 52 | Hupehemonside | −7.1 | 55 |
| Gingerol | −5.38 | 52 | Pseudojervine | −6.8 | 55 |
| (E)-β-Farnesene | −27.56 | 67 | Imperialine-3-β-D-glucoside | −7.1 | 55 |
| α-Copaene | −20.08 | 67 | Verdine | −6.6 | 55 |
| Epicatechin-gallate | −6.67 | 52 | Zhebeininoside | −6.8 | 55 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Binding with HSPA5 (Heat Shock Protein A5) | Binding with NSP1 (non-structural protein of SARS-CoV-2) | ||||
| Diadiazin | −8.6 | 43 | Esculin | −6.88 | 72 |
| Genestein | −7.5 | 43 | Lactose | −11.66 | 72 |
| Formotein | −7.5 | 43 | aRemdesivir | −5.80 | 72 |
| Biochanin A | −6.9 | 43 | Gingerenone | −4.39 | 72 |
| Palmitic acid | −5.5 | 43 | Shogaol | −2.64 | 72 |
| Chlorogenic acid | −6.5 | 43 | |||
| Caffeic acid | −6.2 | 43 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Binding with TMPRSS2 | |||||
| Silybin | −11.928 | 40 | Isoaloresin | −9.759 | 40 |
| Tetrahydrocurcumin | −8.793 | 40 | Withaferin A | −11.242 | 40 |
| Corydine | −7.91 | 40 | Hinokinin | −7.67 | 40 |
| Aloin | −9.185 | 40 | Philligenin | −9.503 | 40 |
| Baicalin | −8.46 | 21 | Excavatolide M | −14.38 | 62 |
| Geniposide | −14.69 | 62 | Schisphenin A | −14.27 | 62 |
| Dictyosphaeric acid A | −14.02 | 62 | Citocoline | −13.96 | 62 |
| Durumolide K | −13.92 | 62 | 5-Methoxyhydnocarpin | −13.92 | 62 |
| Microcarpin | −13.31 | 62 | Curtisian L | −13.38 | 62 |
| Isogemichalcone B | −13.07 | 62 | (−)-Epicatechin 3-O-(3′-O-methyl) gallate | −13.10 | 62 |
ACE2 inhibitors
Since ACE2 has been indicated as the major receptor of SARS-CoV-2 viruses in humans, attention has been given to understanding its regulation as a form to treat this virus.As a part of the renin-angiotensin system, the main function of ACE2 is to convert angiotensin II, a strong vasoconstrictor, to angiotensin (structural forms I, III, IV, V, VI and VII), a vasodilator that contributes in the maintenance and reduction of the blood pressure by counter-regulating ACE. Despite it being an analogue of ACE, their similarity is only approximately 42%.46 An issue regarding ACE2 and coronaviruses infections is that most of the chronic treatment of hypertension and diabetes involves the use of ACE inhibitors (ACEIn).47 These substances are also known to cause the expression of ACE2 to be upregulated, putting the patient in the risk group of COVID-19.4,5 In fact, most of the COVID-19 confirmed patients that presented severe or fatal forms of the infection had comorbidities, hypertension or diabetes in particular.4,8 Meanwhile, commonly used ACEIn present in hypertensive medicines, such as perindopril, enalapril, and losartan, do not cause any inhibition of ACE2.46,47 ACE2 upregulation by ACEIn is currently attributed to its partial capacity to cleave angiotensin I. As angiotensin I concentration increases due to the ACE inhibition, ACE2 mRNA is enhanced to compensate.48
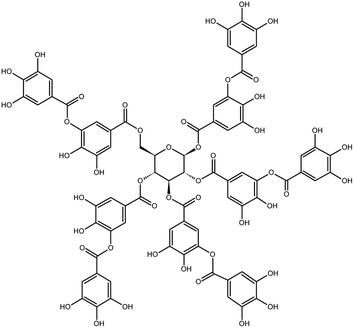
Several natural products present ACE inhibition activity and are extensively used in ethnobotanics, and in some cases are deeply rooted in the human diet.47,49 The application of bioproducts, like ACE inhibitors, is widespread, primarily because the synthetic substances, such as enalapril, were developed using a natural metabolite as a scaffold. This demonstrates their reliability as a new medicine sources; they present less side effects than synthetic drugs; and, in some cases, even natural extracts can present lower IC50 values.49
There are at least 300 plants that have ACE inhibitors activity, including some well-know medicinal and food species, such as cinnamon (Cinnamomum zeylanicum Blume or Cinnamomum verum J. Presl.), pepper (Capsicum spp.), olive (Olea europaea L.), hawthorn (Crataegus pinnatifida Bunge), black nightshade (Solanum nigrum L.), passion fruit (Passiflora edulis Sims) and grape (Vitis vinifera L).47,50,51 ACE inhibitors from natural products belong to several phytochemical classes, including flavonoids, xanthones, alkaloids, peptides, terpenes, and tannins. Some compounds have presented both AceIn and ACE2 inhibitor activity, for example, phenolic compounds like myricetin and quercetin glycosylated derivates.12,50–52 The ability to inhibit both, ACE and ACE2, is caused by their closely related active sites, which are distinct mainly in terms of the smaller intramolecular size of ACE2 sites.48,49
Briefly, ACE2In natural metabolites are of the same chemical classes of ACEIn and can be readily obtained in medium polarity extracts of angiosperms species such as roots and barks.15,39,40,50
ACE and ACE2 inhibitors present amphiphilic molecular structures usually with an aromatic moiety, in order to enable their interaction with the protein, a similar pattern to the observed with SARS-CoV-1 inhibitors.21,22,24,39 Pharmacophere analysis of suggested natural metabolites capable to inhibit SARS-CoV-2 through ACE2 inhibitions usually have structural features in accordance with Lipinski's rule with molecular weight lower than 500 Da, less than 5 hydrogen bond donors and a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P under 5.51,53,54 The number of hydrogen bond acceptors were more variable among suggested phytochemicals against COVID-19, ranging until 20.51,53 For instance, ACEIn peptides have as first residue an aromatic amino acid, and the third is a hydrophobic one.49 The sequence trending necessary for peptides to be ACEIn was extensively exploited by Daskaya-Dikmen et al. (2017).49
P under 5.51,53,54 The number of hydrogen bond acceptors were more variable among suggested phytochemicals against COVID-19, ranging until 20.51,53 For instance, ACEIn peptides have as first residue an aromatic amino acid, and the third is a hydrophobic one.49 The sequence trending necessary for peptides to be ACEIn was extensively exploited by Daskaya-Dikmen et al. (2017).49
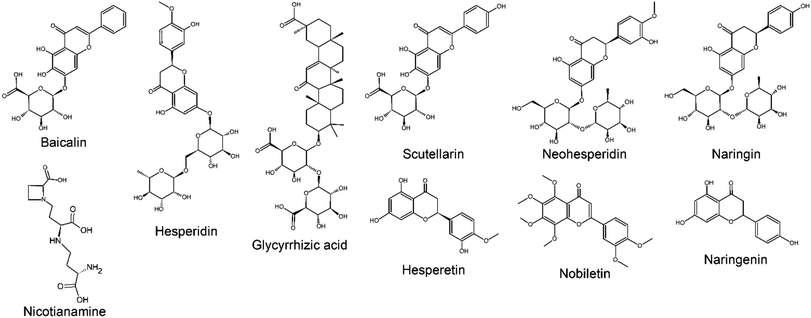
The first in silico screening for anti-SARS-CoV-2 natural metabolites was within traditional chinese herbs, such as species of the Citrus genus.15 The virtual molecular docking of Chinese herb metabolites with the ACE2 against SARS-CoV-2 suggested 11 natural products capable of inhibiting it.15 The natural metabolites suggested as possible bioactive substances against within Chinese medicinal botanic species includes baicalin (baicalein-7-O-glucuronide), scutellarin (scutellarein-7-glucuronide), hesperetin, nicotianamine, glycyrrhizin, naringin, naringenin, hesperidin, neohesperidin, and nobiletin (Fig. 3).15,21,55 Similar to ACEin natural ACE2 inhibitors screened to combat SARS-CoV-2 are classified as alkaloids, flavonols, flavanones, terpenes, limonoids, lignans, terpenoids, tannins, phenolic acids and fatty acids.15,21,51,53,55 Disregarding the distinct software's and molecular docking models, the class with major representatives and better affinity results are within flavonoids (Table 2).
Although the first in silico study of anti-COVID-19 natural products emphasizes flavanones with lower distribution on flora, such as naringin and naringenin,15 recent finds indicates glycosylated derivates of quercetin to present promising inhibition activity with lower binding energy than −8.3 kcal mol−1.51,53 These flavonoids, described by Joshi et al. (2020)51 includes quercetin-3-glucuronide-7-glucoside and quercetin 3-vicianoside, which can be found in Indian long pepper (Piper longum L.), turmeric (Curcuma longa L.) and wormwood (Artemisia absinthium L.)51 Considering inhibition of COVID-19 through ACE2 the flavolignan silybin have presented the lowest binding affinity until end of 2020 May (Table 2). Silybin is one of the major metabolites obtained from milk thistle seeds (Silybum marianum), a plant with traditional usage in chemopreventive, anti-inflammatory agent and in the treatment of digestive disorders.56
Two important points regarding the anti-SARS-2 activity of flavonoids must be highlighted: (1) glycosylated forms are more active than their respective aglycon; and (2) extracts and fractions are significantly more effective than the isolated compounds.30
Although most of promising natural metabolites against SARS-CoV-2 binding to ACE2 are within flavonoids, cases within other classes, such as limonoids (tetranortriterpenoids) and alkaloids deserts attention. Alisha and Tripti (2020)53 demonstrated that the limonoid 6-α-acetoxygedunin has an even lower binding affinity to ACE2 than any flavonoid. Limonoids are major compounds produced by Meliaceae family, mainly within the genus Carapa, such as C. guianensis, an Amazonian species known as andiroba, widely consumed as an anti-inflammatory.57 The presence of anti-COVID-19 within well-known species is promising on the development of chemotherapies as their exploitation is already stablished.
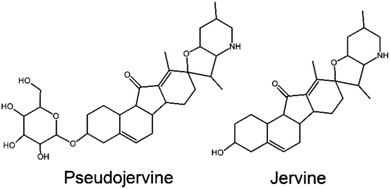
Molecular docking in the chromatographic fingerprint of a Chinese medicinal plant applied in the treatment of COVID-19 has also characterized extracts and alkaloids of the Veratrum nigrum L. as ACE2 inhibitors of SARS-CoV-2.55 Most of the suggested alkaloids are typical of the Liliaceae genera, such as hupehemonside, pseudojervine, and imperialine. However, Veratrum species are known to be toxic when consumed after decoction due the occurrence of a specific group of alkaloids, which are either major or minor compounds in their chemical profile, though, the incidents of human intoxication is rare and usually accidental.58 One of the alkaloids reported through molecular docking as anti-SARS-CoV-2 is an analogue of jervine (Fig. 4), a toxic alkaloid.55,58 Thus, despite the presence of several possible anti-COVID-19 alkaloids, special care needs to be take when considering the use of Veratrum species.
TMPRSS2 inhibitors
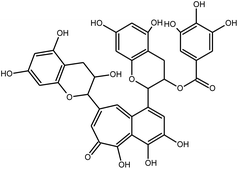
TMPRSS2 inhibitions search is the lowest within the major replication proteins (Table 2) despite molecular docking also indicate it as a strategy in COVID-19 treatment.14 TMPRSS2 is already known for its involvement in the inoculation and replication of influenza virus, cancer, and the SARS-CoV-1.59 TMPRSS2 natural inhibitors includes flavonoids, terpenes and peptides. For instance, the flavonoids baicalein and baicalin (Fig. 3), which have already been reported as a down-regulators of the TMPRSS-2 expression,60 were also indicated on in silico studies against COVID-19.21,55 It is worth mentioning that baicalein was proposed by molecular docking studies to also be an ACE2 inhibitor.15,55 Indeed, it is ideal to the candidate metabolite be applied to interact with different binding sites of the virus, in order to increase its possible bioactivity in vivo.39Baicalin and baicalein are good examples of the potential of virtual molecular screening in the search for anti-COVID-19 natural metabolites. After its promising data within molecular docking,15 enriched fractions with both compounds were tested in vitro presenting antiviral efficacy similar to those obtained by repurposed drug. In vitro tests were performed by fluorescence resonance energy transfer protease assay and with Vero E6 cells contaminated with COVID-19. Within both assay, baicalein had the most promising results with IC50, EC50 and selectivity index (SI) to the SARS-CoV-2 3CLpro of 0.94 μM, 1.69 μM and 118, respectively, while baicalin presented the values 6.41 μM, 10.27 μM and 19, respectively. For instance, chloroquine EC50 was 1.13 μM, with a SI of 88.61 This data61 confirms that in silico experiments, such as those obtained for baicalein,21,55 can give promising insights on possible anti-COVID-19 natural metabolites. The major natural source of baicalein are Scutellaria and Oroxylum genera, mainly in the roots of S. baicalensis and the seeds of Oroxylum indicum.62 Both flavonoids have therapeutic properties as neuroprotective, antioxidant, anti-inflammatory, renal protector and anticancer.63 In addition, these flavonoids also presented remarkable activity as inhibitors of viruses, such as the Zika virus.59
In addition to already know human TMPRSS2 inhibitors, Rahman et al. (2020)62 demonstrated by in silico studies that iridoids, diterpenes and lignans are promising anti-SARS-CoV-2 through TMPRSS2 interaction. The inhibition of TMPRSS2 requires similar structural features as those previously described for ACE2 inhibitors, presence of hydroxy moieties for hydrogen binding and presence of aromatic rings.
Rahma and coworkers62 suggested 12 natural metabolites with binding energy with TPMRSS2 ranging from −11.06 to −14.69 kcal mol−1 (Table 2). The natural metabolite with greater inhibition potential was the geniposide, an iridoid found in Gardenia genus (Rubiaceae) and endemic in Central America and China.62 Such values are higher than any molecular docking focused on ACE2 inhibition. Natural source of the 12 metabolites suggested as anti-COVID-19 by Rahma and coworkers62 included marine soft corals (Formosan gorgonian and Alcyoniidae family), free-floating algae from Sargassum genus, mushrooms of Paxillus genus and several species of angiosperms, such as magnolia-vine (Shisandra sphenanthera) green tea (Camellia sinensis) and the branched asphodel (Asphodelus ramosus).
3CLpro inhibitors
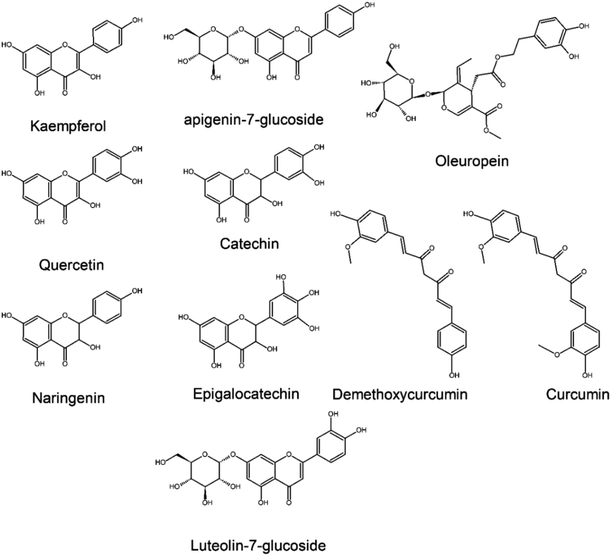
The inhibition of 3CLpro, the main protein of SARS-CoV-2, has received more attention within researchers as it could prevent virus inoculation on the host.16,63,64 Although the 3CLpro is a enzyme specific to the virus, the one within SARS-CoV-2 has a large structural similarity with the one present in SARS-CoV-1 (96.08%).63 Gurung et al. (2020)63in silico analysis demonstrated that the terpenoids bonducellpin D and caesalmin B and the flavonoid 5,7-dimethoxyflavanone-4′-O-β-d-glucopyranoside have binding affinity with 3CLpro of SARS-CoV-1, SARS-CoV-2 and MERS-CoV ranging from −8 to −11 kcal mol−1, an outstanding value compared to repurposed drugs (Table 2). Despite the terpenoids and flavonoids described by Gurung and coworkers are common of Chinese herbs (Caesalpinia minax) and an European mistletoe (Viscum album), the simultaneous inhibition of different strains of coronaviruses is promising to develop a chemotherapy against the virus family.Khaerunnisa et al. (2020)52 evaluated, by molecular docking, natural metabolites capable of inhibiting the 3Cpro of SARS-CoV-2, emphasizing the prominent results of kaempferol, quercetin, luteolin-7-glucoside, demethoxycurcumin, naringenin, apigenin-7-glucoside, oleuropein, catechin, curcumin, and epigallocatechin (Fig. 5).51,65,66 The anti-SARS-CoV-2 activity of these flavonoids is advantageous as they can be easily found and are well-distributed in angiosperm botanical families, including Lauraceae, Lamiaceae, Apiaceae, and Leguminosae.30,64
In addition to the promising results observed to flavonoids, volatile terpenoids are also specialized metabolites that present some very interesting preliminary results that indicate a possible use of these substances. In this case, the already existing supply-chains from industries that produce essential oils increase the sustainability of this kind of prospection. The mono and sesquiterpenes geraniol, linalool, (E)-β-farnesene and (E)-nerolidol presented in silico inhibition of 3CLpro with binding energy of −24.71, −24.05, −27.56 and −26.44 kcal mol−1, respectively.67 These compounds can be found in several plants species with ancient and very well-known uses as foods, medicinal and aromatics, such as lemon balm (Melissa officinalis), lemongrass (Cymbopogon citratus), lavender (Lavandula angustifolia), geranium (Pelargonium graveolens), basil (Ocimum basilicum), mandarin (Citrus reshni), cinnamon (Cinnamomum zeylanicum), chamomile (Matricaria recutita), ginger (Zingiber officinale) and copaiba (Copaifera sp.).67
As TMPRSS2 inhibitors, molecular docking for 3CLpro inhibitors also suggest algae as a potential source of anti-COVID-19 metabolites. Gentile et al. (2020)64 molecular docking evaluation of a marine drug metabolite database evidence that algae polyphenols, known as phlorotannins, and quercetin derivates can be applied in chemotherapy development against SARS-CoV-2. These compounds had already been isolated from Sargassum genus species.64
RNA polymerase inhibitors
A new course in anti-SARS-CoV-2 chemotherapy development is through an extremely specific mechanisms to inhibit viruses replication, the RNA polymerase inhibitors. Metabolites with this property are expected to be less toxic then ACE2 or TMPRSS2 inhibitors, which binds to the host cell. Although RNA polymerase inhibitors are less toxic, their exploitation applied to coronaviruses treatment is still rare. Bibliography survey only suggests two substances which inhibits RNA translation of coronaviruses, the remdesivir and a synthetic 1,4-diazepane derivate (IC50 of 0.45 μM).68 Molecular docking also suggested four commercial drugs which efficacy still need to be proven.37 Despite its potential in virus treatment, natural inhibitors of SARS-CoV-2 RNA polymerase screened so far in essential oils had docking values lower than the commercial ref. 67. As natural inhibitors of Dengue and Chikungunya viruses RNA polymerase had already been described within natural extracts,69 it is expected that there are also natural metabolites able to inhibit SARS-CoV-2 RNA translation still undiscover.Conclusions
In the face of this great global challenge, we are striving for a COVID-19 treatment that can be quickly produced and easily distributed. Natural products could provide an answer to this dilemma, as they often have low toxicity and are used in the pharmaceutical industry for their bioactivity, including antiviral. The similarity between SARS-CoV-1 and COVID-19 also brings light to the development of new drugs or even vaccine. Great hope has emerged from the possible anti-SARS-CoV-2 activity of flavonols, flavanones, and flavanols and the fact that these metabolites have a large occurrence within angiosperm plants. As most of the present research is theoretical or does not present analytical validation, a long path is still ahead in terms of biological analysis and optimized extraction and production. The systematic evaluation presented here intend to reinforce this research effort.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq) and FAPERJ.References
- M. Denaro, A. Smeriglio, D. Barreca, C. De Francesco, C. Occhiuto, G. Milano and D. Trombetta, Phytother. Res., 2019, 1–27, DOI:10.1002/ptr.6575.
- L. T. Lin, W. C. Hsu and C. C. Lin, J. Tradit. Complement. Med., 2014, 4, 24–35 CrossRef PubMed.
- W. Hussain, K. S. Haleem, I. Khan, I. Tauseef, S. Qayyum, B. Ahmed and M. N. Riaz, Future Virol., 2017, 12, 299–308 CrossRef CAS.
- H. Zhang, J. M. Penninger, Y. Li, N. Zhong and A. S. Slutsky, Intensive Care Med., 2020, 46, 586–590 CrossRef CAS PubMed.
- S. Jo, S. Kim, D. H. Shin and M. S. Kim, J. Enzyme Inhib. Med. Chem., 2020, 35, 145–151 CrossRef CAS PubMed.
- K. Dhama, K. Sharun, R. Tiwari, M. Dadar, Y. S. Malik, K. P. Singh and W. Chaicumpa, Hum. Vaccines Immunother., 2020 DOI:10.1080/21645515.2020.1735227.
- World Heath Organization, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/, accessed May 2020.
- L. Wang, Y. Wang, D. Ye and Q. Liu, Int. J. Antimicrob. Agents, 2020, 105948, DOI:10.1016/j.ijantimicag.2020.105948.
- S. Bambini and R. Rappuoli, Drug Discov. Today, 2009, 14, 252–260 CrossRef CAS PubMed.
- S. Y. Lee and S. J. Hur, Food Chem., 2017, 228, 506–517 CrossRef CAS PubMed.
- M. Donia and M. T. Hamann, Lancet Infect. Dis., 2003, 3, 338–348 CrossRef CAS PubMed.
- V. Kumar, Y. S. Jung and P. H. Liang, Expert Opin. Ther. Pat., 2013, 23, 1337–1348 CrossRef CAS PubMed.
- A. Ahmad, M. U. Rehman and K. M. Alkharfy, Eur. Rev. Med. Pharmacol. Sci., 2020, 24(7), 4030–4034 CAS.
- M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T. S. Schiergens, G. Herrler, N.-H. Wu, A. Nitsche, M. A. Müller, C. Drosten and S. Pöhlmann, Cell, 2020, 181, 1–10 CrossRef PubMed.
- F. Meneguzzo, R. Ciriminna, F. Zabini and M. Pagliaro, Processes, 2020, 8, 549 CrossRef CAS.
- Q. Cui, C. Huang, X. Ji, W. Zhang, F. Zhang and L. Wang, Preprint, 2020 DOI:10.20944/preprints202002.0047.v1.
- L. W. Shen, H. J. Mao, Y. L. Wu, Y. Tanaka and W. Zhang, Biochimie, 2017, 142, 1–10 CrossRef CAS PubMed.
- M. T. ul Qamar, S. M. Alqahtani, M. A. Alamri and L. Chen, J. Pharm. Anal., 2020 DOI:10.1016/j.jpha.2020.03.009.
- M. L. Agostini, E. L. Andres, A. C. Sims, R. L. Graham, T. P. Sheahan, X. Lu, E. C. Smith, J. B. Case, J. Y. Feng, R. Jordan, A. S. Ray, T. Cihlar, D. Siegel, R. L. Mackman, M. O. Clarke, R. S. Baric and M. R. Denison, mBio, 2018, 9, 1–15 CrossRef PubMed.
- Y. Yang, F. Peng, R. Wang, K. Guan, T. Jiang, G. Xu, J. Sun and C. Chang, J. Autoimmun., 2020, 109, 102434 CrossRef CAS PubMed.
- C. N. Chen, C. P. C. Lin, K. K. Huang, W. C. Chen, H. P. Hsieh, P. H. Liang and J. T. A. Hsu, J. Evidence-Based Complementary Altern. Med., 2005, 2, 209–215 CrossRef PubMed.
- M. khalifa, E. Attia, J. Fahim and M. Kamel, J. Adv. Biomed. Pharm. Sci., 2018, 1, 41–49 CrossRef.
- F. R. Chang, C. T. Yen, M. Ei-Shazly, W. H. Lin, M. H. Yen, K. H. Lin and Y. C. Wu, Nat. Prod. Commun., 2012, 7, 1415–1417 CrossRef CAS PubMed.
- J. Cinatl, B. Morgenstern, G. Bauer, P. Chandra, H. Rabenau and H. W. Doerr, Lancet, 2003, 361, 2045–2046 CrossRef CAS.
- D. E. Kim, J. S. Min, M. S. Jang, J. Y. Lee, Y. S. Shin, C. M. Park, J. H. Song, H. R. Kim, S. Kim, Y. H. Jin and S. Kwon, Biomolecules, 2019, 9, 696 CrossRef CAS PubMed.
- Y. C. Tsai, C. L. Lee, H. R. Yen, Y. S. Chang, Y. P. Lin, S. H. Huang and C. W. Lin, Biomolecules, 2020, 10, 366 CrossRef CAS PubMed.
- C. Müller, F. W. Schulte, K. Lange-Grünweller, W. Obermann, R. Madhugiri, S. Pleschka, J. Ziebuhr, R. K. Hartmann and A. Grünweller, Antiviral Res., 2018, 150, 123–129 CrossRef PubMed.
- M. T. Islam, C. Sarkar, D. M. El-Kersh, S. Jamaddar, S. J. Uddin, J. A. Shilpi and M. S. Mubarak, Phytother. Res., 2020 DOI:10.1002/ptr.6700.
- S. Y. Li, C. Chen, H. Q. Zhang, H. Y. Guo, H. Wang, L. Wang, X. Zhang, S. N. Hua, J. Yu, P. G. Xiao, R. S. Li and X. Tan, Antiviral Res., 2005, 67, 18–23 CrossRef CAS PubMed.
- H. Zakaryan, E. Arabyan, A. Oo and K. Zandi, Arch. Virol., 2017, 162, 2539–2551 CrossRef CAS PubMed.
- C. C. Wen, Y. H. Kuo, J. T. Jan, P. H. Liang, S. Y. Wang, H. G. Liu, C. K. Lee, S. T. Chang, C. J. Kuo, S. S. Lee, C. C. Hou, P. W. Hsiao, S. C. Chien, L. F. Shyur and N. S. Yang, J. Med. Chem., 2007, 50, 4087–4095 CrossRef CAS PubMed.
- Q. Cui, R. Du, M. Liu and L. Rong, Molecules, 2020, 25, 183 CrossRef CAS PubMed.
- P. Gautret, J.-C. Lagier, P. Parola, V. T. Hoang, L. Meddeb, M. Mailhe, B. Doudier, J. Courjon, V. Giordanengo, V. E. Vieira, T. Dupont, S. Honoré, P. Colson, E. Chabrière, B. La Scola, J.-M. Rolain, P. Brouqui and D. Raoult, Int. J. Antimicrob. Agents, 2020 DOI:10.1016/j.ijantimicag.2020.105949.
- R. Thanacoody, J. Med., 2016, 44, 197–198 Search PubMed.
- S. Tasnim, M. M. Hossain and H. Mazunder, Journal of Preventive Medicine & Public Health, 2020, 53, 171–174 Search PubMed.
- C. Funck-Brentano and J. Salem, Lancet, 2020 DOI:10.1016/S0140-6736(20)31174-0.
- A. A. Elfiky, Life Sci., 2020, 253, 117592 CrossRef CAS PubMed.
- S. J. Park, Y. G. Kim and H. J. Park, J. Am. Chem. Soc., 2011, 133, 10094–10100 CrossRef CAS PubMed.
- R. Joshi, S. Jagdale, S. Bansode, S. S. Shankar, M. Tellis, V. K. Pandya, A. Giri and M. Kulkarni, J. Biomol. Struct. Dyn., 2020 DOI:10.1080/07391102.2020.1760137.
- M. Pandit and N. Latha, Preprints, 2020 DOI:10.21203/rs.3.rs-22687/v1.
- V. Chandel, S. Raj, B. Rathi and D. Kuma, Chem. Biol. Lett., 2020, 7, 166 CAS.
- D. hai Zhang, K. lun Wu, X. Zhang, S. qiong Deng and B. Peng, J. Integr. Med., 2020, 18, 152–158 CrossRef PubMed.
- A. Elfiky, J. Biomol. Struct. Dyn., 2020 DOI:10.1080/07391102.2020.1761881.
- Y. Yang, M. S. Islam, J. Wang, Y. Li and X. Chen, Int. J. Biol. Sci., 2020, 16, 1708–1717 CrossRef CAS PubMed.
- L. Fang, G. Karakiulakis and M. Roth, Lancet Respir. Med., 2020, 2600, 30116 Search PubMed.
- M. L. Huang, X. Li, Y. Meng, B. Xiao, Q. Ma, S. S. Ying, P. S. Wu and Z. S. Zhang, Clin. Exp. Pharmacol. Physiol., 2010, 37, e1–e6 CrossRef CAS PubMed.
- J. M. Barbosa-Filho, V. K. M. Martins, L. A. Rabelo, M. D. Moura, M. S. Silva, E. V. L. Cunha, M. F. V. Souza, R. N. Almeida and I. A. Medeiros, Rev. Bras. Farmacogn., 2006, 16, 421–446 CrossRef.
- G. I. Rice, D. A. Thomas, P. J. Grant, A. J. Turner and N. M. Hooper, Biochem. J., 2004, 383, 45–51 CrossRef CAS PubMed.
- C. Daskaya-Dikmen, A. Yucetepe, F. Karbancioglu-Guler, H. Daskaya and B. Ozcelik, Nutrients, 2017, 9, 1–19 CrossRef PubMed.
- G. S. Patten, M. Y. Abeywardena and L. E. Bennett, Crit. Rev. Food Sci. Nutr., 2016, 56, 181–214 CrossRef CAS PubMed.
- T. Joshi, P. Sharma, S. Mathpal, H. Pundir, V. Bhatt and S. Chandra, Eur. Rev. Med. Pharmacol. Sci., 2020, 24, 4529 CAS.
- S. Khaerunnisa, H. Kurniawan, R. Awaluddin and S. Suhartati, Prepr, 2020 DOI:10.20944/preprints202003.0226.v1.
- K. Alisha and S. Tripti, ChemRxiv, 2020 DOI:10.26434/chemrxiv.12320273.v1.
- L. Guan, H. Yang, Y. Cai, L. Sun, P. Di, W. Li, G. Li and U. Tang, MedChemComm, 2019, 10, 148 RSC.
- J. Cheng, Y. Tang, B. Bao and P. Zhang, ChemRxiv, 2020 DOI:10.26434/chemrxiv.11955273.v2.
- R. Gazak, D. Wakterová and V. Kren, Curr. Med. Chem., 2007, 14, 315 CrossRef CAS PubMed.
- K. Ninomiya, S. Miyazawa, K. Ozeki, N. Matsuo, O. Muraoka, T. Kikushi, T. Yamada, R. Tanaka and T. Morikawa, Int. J. Mol. Sci., 2016, 17, 591 CrossRef PubMed.
- T. Minatani, H. Ohta, E. Sakai, T. Tanaka, K. Goto, D. Watanabe and H. Miyaguchi, Forensic Toxicol., 2018, 36, 200–210 CrossRef CAS.
- A. Oo, B. T. Teoh, S. S. Sam, S. A. Bakar and K. Zandi, Arch. Virol., 2019, 164, 585–593 CrossRef CAS PubMed.
- D. Xu, Q. Chen, Y. Liu and X. Wen, Oncotarget, 2017, 8, 105561–105573 CrossRef PubMed.
- H. Su, S. Yao, W. Zhao, M. Li, J. Liu and W. Shang, bioRxiv, 2020 DOI:10.1101/2020.04.13.038687.
- N. Rahman, Z. Basharat, M. Yousuf, G. Castaldo, L. Rastrelli and H. Khan, Molecules, 2020, 25, 2271 CrossRef CAS PubMed.
- A. B. Gurung, M. A. Ali, J. Lee, M. A. Farah and K. M. Al-Anazi, Life Sci., 2020, 255, 117831 CrossRef CAS PubMed.
- D. Gentile, V. Patamia, A. Scala, M. T. Sciortino, A. Piperno and A. Rescifina, Mar. Drugs, 2020, 18, 225 CrossRef CAS PubMed.
- J. S. Rane, A. Chattterjee and A. Kumar, ChemRxiv, 2020 DOI:10.26434/chemrxiv.12094203.v1.
- A. I. Owis, M. S. El-Hawary, D. El Amir, O. M. Aly, U. R. Abdelmonhsen and M. S. Kamel, RSC Adv., 2020, 10, 16570 RSC.
- J. K. R. da Silva, P. L. B. Figueiredo, K. G. Byler and W. N. Setzer, Int. J. Mol. Sci., 2020, 21, 3426 CrossRef CAS PubMed.
- T. Hermann, in RNA Therapeutics, Topics in Medicinal Chemistry, ed. A. Garner, Springer International Publishing, 2017, vol. 27 Search PubMed.
- A. F. C. D. S. Oliveira, R. R. Teixeira, A. S. Oliveira, A. P. M. Souza, M. L. Silva and S. O. Paula, Molecules, 2017, 22, 505 CrossRef PubMed.
- O. O. Olubiyi, M. Olagunju, M. Keutmann and B. Strodel, Preprint, 2020 DOI:10.20944/preprints202004.0161.v2.
- M. T. ul Qamar, S. M. Alqahtani, M. A. Alamri and L.-L. Chen, J. Pharm. Anal., 2020 DOI:10.1016/j.jpha.2020.03.009.
- A. Sharma, V. Tiwari and R. Sowdhamini, Preprint, 2020 DOI:10.26434/chemrxiv.12091356.v1.
| This journal is © The Royal Society of Chemistry 2020 |