Themed issue on drinking water oxidation and disinfection processes
Tom
Bond
*a,
Wenhai
Chu
b,
Urs
von Gunten
cd and
Maria José
Farré
e
aDepartment of Civil and Environmental Engineering, University of Surrey, Guildford, GU2 7XH, UK. E-mail: t.bond@surrey.ac.uk
bState Key Laboratory of Pollution Control and Resources Reuse, College of Environmental Science and Engineering, Tongji University, China
cEawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland
dSchool of Architecture, Civil and Environmental Engineering (ENAC), École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
eICRA, Catalan Institute for Water Research, Emili Grahit 101-17003, Girona, Spain
Historical overview
In the mid-1800s John Snow established that cholera was a waterborne disease. Snow was a medical doctor in London, UK, who curbed a cholera outbreak by suggesting the handle of a pump providing public drinking water was removed. His discovery, though slow to gain acceptance, eventually led to the spread of centralised drinking water treatment and distribution through industrialised countries in the early 20th century.Centralised drinking water treatment was based upon sand filtration and chlorination.1,2 By controlling the spread of waterborne diseases such as cholera, typhoid and dysentery, their application became a public health triumph. For example, from 1900 to 1947 the lifespan of the average American increased from 47 to 63. It has been estimated that ∼50% of the increase in lifespan among city dwellers was due to the introduction of drinking water treatment.1,3
The pre-eminence of chlorination was largely unchallenged until the 1970s, when Johannes Rook, a Dutch chemist, reported that four chlorinated and/or brominated trihalomethanes were generated during drinking water treatment from reactions between chlorine and natural organic matter.4
One member of this group is chloroform (also known as trichloromethane), which has become the most studied disinfection byproduct. Coincidentally, this chemical links the careers of Rook and Snow. The latter was also a pioneer of anaesthesia and personally administered chloroform to Queen Victoria for pain relief during the birth of her eighth and ninth children. Meanwhile, soon after Rook's breakthrough, chloroform was listed as an animal carcinogen5 and an epidemiological study correlated cancer mortality with drinking water obtained from the Mississippi River.6 Taken together, these studies sent shockwaves amongst the scientific community.
Regulatory limits for trihalomethanes were imposed in many countries by the end of the 1970s. Subsequently the use of alternative drinking water disinfectants – in particular, ozone, monochloramine, chlorine dioxide or physical treatment such as ultraviolet (UV) light (Table 1) – has increased, often due to concerns about the health risks posed by chlorination disinfection byproducts and regulatory limits for trihalomethanes. For example, between 1998 and 2007 the proportion of US drinking water utilities using monochloramine increased from 11 to 30%, with equivalent increases for ozone, chlorine dioxide and UV light from 2 to 9%, 4 to 8% and 0 to 2%, respectively.7 Meanwhile, in Italy, the prevalence of chlorine dioxide has increased to the extent where it is used as a final disinfectant for 31% of public drinking water supplies.8 Parts of Europe, notably the Netherlands and areas of Germany, Switzerland and Austria, took more drastic action and phased out the routine use of chlorination as a drinking water disinfectant, a change also driven by consumer dislike of the taste and odour associated with a chlorine residual.9 Instead, drinking water in these regions is distributed without a residual disinfectant. Despite the described increases in alternative disinfectants, chlorine remains the commonest disinfectant used worldwide to this day.
| Free chlorine (HOCl and −OCl) | Chloramines (mainly NH2Cl) | Chlorine dioxide (ClO2) | Ozone (O3) | UV light | |
|---|---|---|---|---|---|
| Stable residual? | Yes | Yes | Yes | No | No |
| Efficacy for: | |||||
| Bacteria | Excellent | Good | Excellent | Excellent | Good |
| Protozoa | Fair to poor | Poor | Good | Good | Excellent |
| Viruses | Excellent | Fair | Excellent | Excellent | Good |
| Efficacy for iron(II) and manganese(II) | Excellent (iron), limited (manganese) | Limited | Excellent | Excellent | None |
| Key chemical transformation products | Chlorinated, brominated, iodinated byproducts, e.g. trihalomethanes, haloacetic acids, haloacetonitriles, MX | Less chlorinated and brominated byproducts than chlorine; more NDMA, cyanogens and iodinated byproducts | Limited halogenated byproducts. Decomposes to chlorite and chlorate | Bromate. Low molecular weight acids, carbonyls and aldehydes | Medium pressure lamps without filters enhance halo-nitromethanes in downstream chlorine/chloramine disinfection |
Over 700 disinfection byproducts are now known from reactions involving chlorine and alternative disinfectants.10 Multiple epidemiological studies since the 1970s have reaffirmed the link between cancer and long-term exposure to chlorinated drinking water, the bladder being the organ most frequently affected. For example, it was recently estimated that 4.9% (∼6500 in total) of cases of bladder cancer in 28 European countries can be attributed to chlorinated drinking water.11 However, over time, the evidence for trihalomethanes explaining this association has weakened, since they are no longer regarded as animal carcinogens under typical drinking water conditions.12 Instead, the causal agents for this association are unknown and trihalomethanes can be viewed as a surrogate parameter representing all chlorination disinfection byproducts, both in drinking water regulations and as an exposure metric in epidemiological studies.
Oxidation and disinfection processes in drinking water treatment
One definition of drinking water disinfection is the removal or inactivation of pathogenic microorganisms to a level where they are not considered harmful to human health. This definition emphasises that, despite the plethora of chemical pollutants known to occur in drinking water at trace concentrations, ensuring its excellent microbiological quality remains the primary objective of treatment. This is not to say that controlling chemical pollutants is unnecessary or unimportant, more that this should not be achieved by compromising the microbiological quality of drinking water.13 By the above definition, widespread treatment processes such as sand filtration, and coagulation-flocculation-sedimentation, can be viewed as disinfection steps, since they remove pathogenic microorganisms. Indeed, the World Health Organization (WHO) advocates a multi-barrier approach to securing the microbial safety of drinking water.14 Nonetheless, the term disinfection process is normally reserved for the final step of drinking water treatment, i.e. treatment with chlorine or an alternative disinfectant (Table 1), which serves as the final barrier before treated water enters the distribution system. The performance of these processes is normally defined by the product of disinfectant concentration and time (C × T or CT) in specially designed contact tanks. The hydraulics of these tanks are a key factor regarding disinfection efficiency.7 The CT concept determines the amount of inactivation credit for a particular chemical disinfectant and is applied in a conservative way to provide a safety factor.Chemical disinfectants can inactivate pathogens by oxidising cell walls and reacting with the intercellular nucleic acids (DNA and RNA) which store genetic information. Thus, they are also strong oxidants (Table 1) and can be applied for oxidation rather than disinfection. For instance, chlorine dioxide and ozone can be used for the oxidation of reduced inorganic compounds such as manganese(II) and iron(II) (Table 1) often found in groundwater into particulate forms readily removed by downstream filtration processes. As this indicates, oxidation processes are normally applied earlier in the water treatment works than disinfection processes.
Chlorine is now much less widely used for oxidation, due to concerns about the higher levels of disinfection byproducts produced from its reactions with natural organic matter present earlier in the treatment works. Under water treatment conditions chlorine exists in the form of hypochlorous acid (HOCl) and hypochlorite (−OCl), collectively known as free chlorine (Table 1). Hypochlorous acid is the stronger oxidant (Table 1) and a better disinfectant than hypochlorite; their relative abundance is pH dependent (pKa = 7.5).
When free chlorine mixes with ammonia, chloramines (sometimes called combined chlorine) rapidly form. These reactions can be utilised to generate chloramines in a controlled fashion during water treatment, with monochloramine the main product (Table 1). Chloramines are less active oxidants and disinfectants than free chlorine and form lower levels of chlorinated and brominated byproducts (Table 1). They are highly stable and are often used to maintain a disinfectant residual in longer distribution systems. However, chloramines can enhance the formation of nitrogenous byproducts such as N-nitrosodimethylamine (NDMA) relative to chlorine. NDMA is a carcinogenic byproduct with a WHO guideline value of 100 ng L−1.14
In the UK, China and the USA ozone is primarily applied for the oxidation of anthropogenic trace pollutants (or micropollutants), notably pesticides and antibiotics, particularly when combined with downstream granular activated carbon adsorption. Ozone is also a highly effective disinfectant (Table 1) and has been widely used for this purpose in Europe since the beginning of the 20th century.15 When ozone is applied to source waters containing significant ambient concentrations of bromide (Br−), the formation of bromate, a possibly carcinogenic inorganic disinfection byproduct regulated in the US, China and the EU/UK, can be a concern.
Advanced oxidation processes (AOPs) are emerging technologies mainly used in situations where micropollutants recalcitrant to ozone and/or activated carbon are present. A variety of configurations exist, often involving combined processes such as O3/H2O2, UV/H2O2, UV/O3 and most recently UV/chlorine.16,17 All are characterised by in situ formation of the hydroxyl radical (˙OH), a very strong oxidant with low selectivity.
UV light by itself is effective for the inactivation of a wide range of microorganisms, importantly including the protozoan parasite Cryptosporidium, which is chlorine resistant. UV light has historically been mainly used for the disinfection of small groundwater systems; its use is increasing in other large-scale applications, for example, New York City's main drinking water sources are disinfected using UV light.18 UV light alone does not form halogenated byproducts, but it initiates photochemical reactions which may produce non-halogenated byproducts when applied in high doses beyond those typically used for disinfection purposes.
Chlorine dioxide (ClO2) is an excellent oxidant and disinfectant, which forms limited organic halogenated byproducts (Table 1), because it partially decomposes into chlorine.19 Its application for water treatment is relatively common in Italy, and would perhaps be more typical elsewhere if it did not decompose to chlorite (ClO2−) and chlorate (ClO3−), which are both subject to regulatory limits or guidelines due to concerns about their toxicity.13
While chemicals used for oxidation and/or disinfection can fully mineralise natural organic matter and micropollutants into carbon dioxide and water, at the economically feasible doses typically applied during water treatment, the level of complete mineralisation is modest. Rather, the parent compound (or precursor) is transformed, via various mechanistic pathways, into oxidation or disinfection byproducts.17 Thus, the drinking water disinfectants/oxidants in Table 1, and other emerging technologies not shown, are all perhaps best thought of as chemical transformation processes.
Future trends and research needs
Each discussed process has its own characteristic set of chemical transformation products (Table 1). Their identity is a function not only of the chemical transformation process, and water quality parameters (e.g. pH, temperature), but of the chemical functionality of the parent compound(s) present in a given water source. As analytical chemical instrumentation becomes ever more sensitive and sophisticated, so the number of recorded chemical transformation products increases. There is a danger that their number becomes so overwhelmingly large that it is difficult to know which should be prioritised for in-depth toxicological studies (e.g. animal testing) or considered for water quality regulations. It is suggested that researchers focus on transformation products belonging to groups known as potent toxicants, while multidisciplinary computational predictions, which integrate information about reaction kinetics, transformation product identity and toxicological consequences may allow reduction of the experimental load.17Drinking water supplies are increasingly likely to be treated by alternative disinfectants such as chlorine dioxide, ozone and UV light in the future. A related strategy may be to apply ozone or UV light for primary disinfection followed by a small amount of chlorine/chloramines/chlorine dioxide to maintain a stable residual for the distribution system. Introducing additional oxidation processes or switching from chlorine to an alternative oxidant/disinfectant can have unintended and surprising consequences on water quality. For example, in terms of byproduct formation, switching from chlorine to chloramines has the potential to enhance formation of NDMA, cyanogens and iodinated byproducts (Table 1). Moreover, it can solubilise the surface of lead pipes, which does not happen in the presence of a chlorine residual, due to the latter's stronger oxidising capacity.21 Therefore, changes of this type need a cautious and balanced investigation on a site by site basis.
Ozone and UV light leave no stable residual for distribution systems (Table 1), which is often seen as a disadvantage. However, experience from parts of Europe shows that high-quality drinking water can be produced in these circumstances, but this requires a very rigorous approach concentrating on water resources protection, natural attenuation, multibarrier water treatment, and careful maintenance of distribution and household plumbing systems.9 Extensive replacement of drinking water distribution systems is very expensive, with the cost typically passed onto tap water consumers, so will only happen with widespread political and public support and appropriate pricing models.
Changes of this type would gain traction if there was clearer scientific evidence that switching disinfectants was beneficial from a public health perspective (e.g. if it was associated with reduced incidence of bladder cancer) across a range of source water types. There is a need for more epidemiological studies with this focus, and for equivalent toxicological data, i.e. those linked to key public health or environmental health outcomes. A likely consequence of increased public trust in the quality of tap water is reduced consumption of bottled water. Since plastic is now recognised amongst the most pervasive and persistent pollutants on Earth,22 and as bottled water has a much higher carbon footprint than tap water, this change would bring significant environmental benefits.
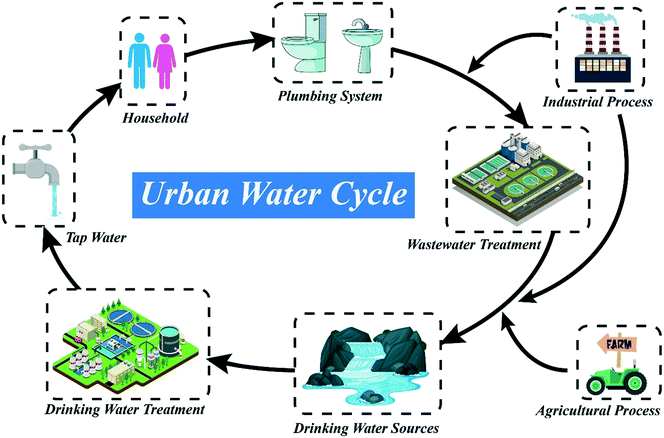
As the impacts of climate change and population growth bite, so the availability of pristine water resources will decrease. Drinking water providers are increasingly relying on freshwater sources impacted by wastewater effluent (Fig. 1) or algae, or turning to desalination of seawater. The characteristics of these water types, and the precursors they contain, can be quite different to those of pristine source waters – and so can the transformation products they generate. Wastewater effluent, in particular, is an important source of drinking water micropollutants (e.g. pharmaceuticals, endocrine disruptors). A common research theme is therefore to assess whether transformation products arising from micropollutants are actually more toxic or hazardous than the parent compound. It is arguable that micropollutants found in wastewater should be targeted during municipal wastewater (sewage) treatment, rather than during drinking water treatment, and more research in this area may be productive.
As a closing thought from the guest editors of this themed issue, it is hoped that gathering a series of related articles will prove valuable for the reader, as it will allow him or her to see a cross-section of the latest multidisciplinary research in the area, many aspects of which are touched upon in this editorial.
References
- D. Sedlak, Water 4.0: The Past, Present, and Future of the World's Most Vital Resource, Yale University Press, 2015 Search PubMed.
- M. J. McGuire, Eight revolutions in the history of US drinking water disinfection, J. - Am. Water Works Assoc., 2006, 123–149 CrossRef CAS.
- D. Cutler and G. Miller, The role of public health improvements in health advances: The twentieth-century United States, Demography, 2005, 42, 1–22 CrossRef PubMed.
- J. J. Rook, Formation of haloforms during chlorination of natural waters, Water Treat. Exam., 1974, 23, 234–243 Search PubMed.
- NCI, National Cancer Institute. Report on Carcinogenesis Bioassay of Chloroform. NTIS PB-264018, Bethesda, MD., 1976 Search PubMed.
- T. Page, R. H. Harris and S. S. Epstein, Drinking water and cancer mortality in Louisiana, Science, 1976, 193, 55–57 CrossRef CAS PubMed.
- AWWA, Water Quality and Treatment: A Handbook on Drinking Water, American Water Works Association, American Society of Civil Engineers, McGraw-Hill, 6th edn, 2011 Search PubMed.
- C. Collivignarelli and S. Sorlini, Trihalomethane, chlorite and bromate formation in drinking water oxidation of Italian surface waters, J. Water Supply: Res. Technol.--AQUA, 2004, 53, 159–168 CrossRef CAS.
- F. Rosario-Ortiz, J. Rose, V. Speight, U. von Gunten and J. Schnoor, How do you like your tap water?, Science, 2016, 351, 912–914 CrossRef CAS PubMed.
- S. D. Richardson and M. J. Plewa, To regulate or not to regulate? What to do with more toxic disinfection byproducts?, J. Environ. Chem. Eng., 2020, 8, 103939 CrossRef CAS.
- I. Evlampidou, L. Font-Ribera, D. Rojas-Rueda, E. Gracia-Lavedan, N. Costet, N. Pearce, P. Vineis, J. J. K. Jaakkola, F. Delloye, K. C. Makris, E. G. Stephanou, S. Kargaki, F. Kozisek, T. Sigsgaard, B. Hansen, J. Schullehner, R. Nahkur, C. Galey, C. Zwiener, M. Vargha, E. Righi, G. Aggazzotti, G. Kalnina, R. Grazuleviciene, K. Polanska, D. Gubkova, K. Bitenc, E. H. Goslan, M. Kogevinas and C. M. Villanueva, Trihalomethanes in drinking water and bladder cancer burden in the European Union, Environ. Health Perspect., 2020, 128, 017001–14 CrossRef PubMed.
- S. E. Hrudey and J. Fawell, 40 years on: what do we know about drinking water disinfection by-products (DBPs) and human health?, Water Sci. Technol.: Water Supply, 2015, 15, 667–674 CAS.
- WHO, Chlorine Dioxide, Chlorite and Chlorate in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality, Geneva, Switzerland, 2016 Search PubMed.
- WHO, Guidelines for drinking-water quality: fourth edition incorporating the first addendum, Geneva, Switzerland, 2017 Search PubMed.
- C. von Sonntag and U. von Gunten, Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications, IWA Publishing, London, UK, 2012 Search PubMed.
- Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications, ed. M. I. Stefan, IWA Publishing, London, UK, 2017 Search PubMed.
- U. von Gunten, Oxidation Processes in Water Treatment: Are We on Track?, Environ. Sci. Technol., 2018, 52, 5062–5075 CrossRef CAS PubMed.
- Scientific American, Turning on the Zap: New York City Readies World's Largest UV Drinking-Water Disinfection Plant, 2012, Available at: https://www.scientificamerican.com/article/nyc-uv-drinking-water-disinfection/ (accessed 10 August 2020) Search PubMed.
- V. Rougé, S. Allard, J. P. Croué and U. Von Gunten, In Situ Formation of Free Chlorine during ClO2 Treatment: Implications on the Formation of Disinfection Byproducts, Environ. Sci. Technol., 2018, 52, 13421–13429 CrossRef PubMed.
- Metcalf & Eddy, Inc., G. Tchobanoglous, F. L. Burton, R. Tsuchihashi and H. D. Stensel, Wastewater Engineering: Treatment and Resource Recovery, McGraw-Hill Education, 5th edn, 2014 Search PubMed.
- D. L. Sedlak and U. von Gunten, The Chlorine Dilemma, Science, 2011, 331, 42–44 CrossRef CAS PubMed.
- C. Ostle, R. C. Thompson, D. Broughton, L. Gregory, M. Wootton and D. G. Johns, The rise in ocean plastics evidenced from a 60-year time series, Nat. Commun., 2019, 10, 1622 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2020 |