Rh(I)-Catalyzed intramolecular [3 + 2] cycloaddition reactions of yne-vinylidenecyclopropanes†
Kang-Hua
Rui
a and
Min
Shi
 *abc
*abc
aKey Laboratory for Advanced Materials and Institute of Fine Chemicals, School of Chemistry & Molecular Engineering, East China University of Science and Technology, Meilong Road No. 130, Shanghai, 200237, China
bState Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071, P. R. China
cState Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, University of Chinese Academy of Science, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: mshi@mail.sioc.ac.cn
First published on 3rd April 2019
Abstract
A cationic Rh(I) complex-catalyzed intramolecular [3 + 2] cycloaddition reaction of newly developed yne-vinylidenecyclopropanes (VDCPs) has been established, affording an efficient synthesis of fused [6.5]-bicyclic structures in moderate to good yields under mild conditions. Moreover, the reaction exhibits a broad substrate scope along with good functional group tolerance.
Introduction
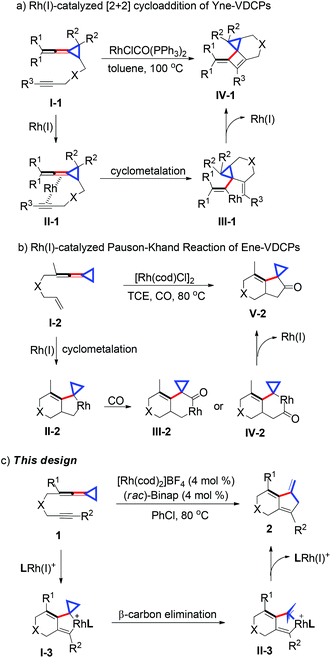
Bicyclic ring structures widely exist in natural products and are essential skeletons ubiquitously contained in some biologically important compounds possessing various biological activities.1 Therefore, developing new methods to synthesize bicyclic scaffolds has been intensively pursued by the synthetic community. During the past few decades, rhodium-catalyzed C–C bond activation reactions and cycloadditions have emerged as one of the most powerful and straightforward methods in terms of good atom economy and mild reaction conditions for the construction of complex polycyclic frameworks.2 Methylenecyclopropanes (MCPs),3,4 vinylcyclopropanes (VCPs)5,6 and vinylidenecyclopropanes (VDCPs)7,8 as highly strained but readily accessible and adequately reactive molecules have been frequently used in the rapid construction of polycyclic skeletons since they are fascinating building blocks for organic synthesis. Recently, many interesting transformations have been explored using novel functionalized alkylidenecyclopropanes as substrates upon transition metal catalysis. These reactions can provide diversified polycyclic products with high chemo- and regioselectivities under mild conditions. Our research group has long been interested in discovering transition metal-catalyzed intramolecular cycloadditions of functionalized VDCPs for the construction of polycyclic frameworks. Previously, we reported an intramolecular [2 + 2] cycloaddition of yne-VDCPs I-1 for the production of cyclopropane containing polycyclic compounds (Scheme 1a).9 In this cycloaddition reaction, the coordination of the rhodium(I) complex to the internal double bond of the allene unit and the alkyne moiety formed intermediate II-1, which subsequently underwent cyclometalation/reductive elimination to afford the corresponding polycyclic products IV-1. In 2012, a highly efficient Rh(I)-catalyzed Pauson–Khand-type [2 + 2 + 1] cycloaddition of ene-VDCPs I-2 with CO was achieved by our group (Scheme 1b).10 The reaction proceeded through a cyclometalation of I-2 with a Rh(I) catalyst to give rhodacyclic intermediate II-2, which underwent the insertion of CO to provide two regioisomers, III-2 and IV-2. These reactive species underwent reductive elimination to afford the corresponding cyclopropane containing polycyclic adducts V-2 in good yields. In this context, we report a cationic Rh(I) complex-catalyzed intramolecular [3 + 2] cycloaddition of newly developed yne-VDCPs 1 to afford a variety of bicyclic derivatives 2 with good functional group tolerance under mild conditions. On the basis of previous work, we envisaged that the cyclometalation of the Rh catalyst with the alkyne group and the double bond adjacent to the VDCP moiety could initially give intermediate I-3, which would directly undergo a β-carbon elimination to form the corresponding π-allyl Rh(III) intermediate II-3. The subsequent reductive elimination of intermediate II-3 could give the desired bicyclic product 2 (Scheme 1c). In this intramolecular [3 + 2] cycloaddition reaction, the cyclopropane could act as a three-carbon synthon to give the corresponding ring-opened products rather than the cyclopropane ring retaining polycyclic products. It should be mentioned here that in 2014 we disclosed a gold(I)-catalyzed cycloisomerization of yne-VDCPs for the synthesis of bicyclic derivatives.8j However, these reactions underwent 5-exo-dig and 6-exo-dig cyclizations at the same time, giving bicyclic derivatives as well as VDCP-rearranged products as product mixtures in most cases since gold(I) catalysts are the most powerful soft Lewis acids for electrophilic activation of C–C triple bonds, rendering difficulties in the control of the regioselectivity. In addition, the gold-catalyzed reaction was limited to the use of terminal alkynes as substrates. On the other hand, rhodium(I) catalysts prefer to undergo oxidative addition in the reactions using ene-VDCPs or yne-VDCPs as substrates. Therefore, it is possible to overcome the shortage in the control of regioselectivity in the gold(I) catalyzed transformation and enable extension of the substrate scope and to afford bicyclic products exclusively if using a rhodium(I) complex as the catalyst.Results and discussion
Our initial examination started with the tosylamide tethered yne-VDCP substrate 1a. Thus, a solution of 1a in 1,2-dichloroethane (DCE) was heated at 80 °C in the presence of 4 mol% of [Rh(cod)2]BF4 and 4 mol% of (rac)-Binap. To our delight, the desired bicyclic product 2a was exclusively produced in 45% yield (Table 1, entry 1). With this encouraging result, we then focused our efforts on seeking out the optimal reaction conditions. Screening of solvents indicated that PhCl was the best choice, providing 2a in 83% yield (Table 1, entries 1–4). A subsequent survey of other phosphine ligands revealed that using (rac)-Binap as the ligand gave 2a in the highest yield (Table 1, entries 5–7). In addition, using NaBArF as a coordinating anion afforded 2a in 72% yield under otherwise identical conditions and raising the reaction temperature to 90 °C did not give a better result (Table 1, entries 8–9).| Entrya | Catalyst | Ligand | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), catalyst (4 mol%), ligand (4 mol%), and solvent (2 mL) were used; 6–10 h. b Isolated yield. c The reaction was conducted at 90 °C. | ||||
| 1 | [Rh(cod)2]BF4 | (rac)-Binap | DCE | 45 |
| 2 | [Rh(cod)2]BF4 | (rac)-Binap | Dioxane | 62 |
| 3 | [Rh(cod)2]BF4 | (rac)-Binap | CH3CN | 21 |
| 4 | [Rh(cod) 2 ]BF 4 | (rac)-Binap | PhCl | 83 |
| 5 | [Rh(cod)2]BF4 | t BuXPhos | PhCl | 48 |
| 6 | [Rh(cod)2]BF4 | dppe | PhCl | 68 |
| 7 | [Rh(cod)2]BF4 | dppf | PhCl | 61 |
| 8 | [Rh(cod)2]BArF | (rac)-Binap | PhCl | 72 |
| 9c | [Rh(cod)2]BF4 | (rac)-Binap | PhCl | 78 |
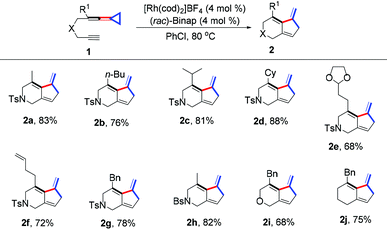
With the optimal reaction conditions in hand, we next examined the scope of the substrates and the results are shown in Table 2. For yne-VDCPs 1b–1e, in which R1 could be various primary or secondary alkyl groups, the desired products 2b–2e were given in good yields ranging from 68–88%. The R1 group could also be a 1-butenyl or benzyl group, giving the corresponding products 2f and 2g in 72% and 78% yields, respectively. Moreover, with the substrates 1h–1j, in which the yne and VDCP moieties are connected by BsN, oxygen atom and carbon anchor, the corresponding products 2h–2j were obtained in moderate to good yields as well.
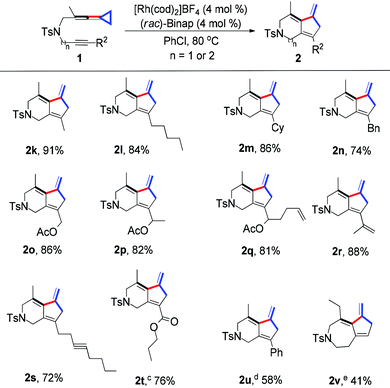
Next, various substituents at the alkynyl moiety were explored (Table 3). When R2 was a methyl, pentyl or cyclohexyl group, the desired products 2k–2m were obtained in good to excellent yields. R2 could also be a benzyl group, giving the corresponding product 2n in 74% yield. For substrates 1o–1q, in which the alkyne moiety contained a primary or secondary acetoxyalkyl group, the desired products 2o–2q were produced in good yields ranging from 81–86%. In order to further explore the substrate scope of this protocol, substrates 1r and 1s, in which their alkynyl moieties have an olefinic group and an internal alkyne unit, were synthesized and the expected products 2r and 2s were produced in 88% and 72% yields respectively under the standard conditions. Previously, we also reported a thermal induced intramolecular [2 + 2] cycloaddition of alkynone-vinylidenecyclopropanes under heating conditions.11a Substrate 1t bearing an α,β-unsaturated alkynyl ester was also tolerated, affording the desired product 2t in 76% yield. Notably, in this case, substrate 1t could be partially converted into a thermal-induced intramolecular [2 + 2] cycloaddition byproduct 3t in 20% yield.11 Moreover, using 1u as the substrate, in which R2 = Ph, the desired product 2u was obtained in 58% yield along with its thermal-induced [2 + 2] cycloadduct 3u as a product mixture. Extending the carbon chain with a (CH2)2 tether, the desired product 2v could be exclusively obtained in 41% yield if using 1,2-dichloroethane (DCE) as the solvent. All these results indicated a broad substrate scope for this Rh(I)-catalyzed synthetic protocol.
| a Reaction conditions: yne-VDCP 1 (0.10 mmol), [Rh(cod)2]BF4 (4.0 mol%), (rac)-Binap (4.0 mol%), and PhCl (2.0 mL) were added and the reaction mixture was stirred for 6–8 h. b Isolated yield. c Byproduct 3t was obtained in 20% yield (see Scheme 2). d Byproduct 3u was obtained in 29% yield. e DCE was employed as the solvent in place of PhCl. |
|---|
|
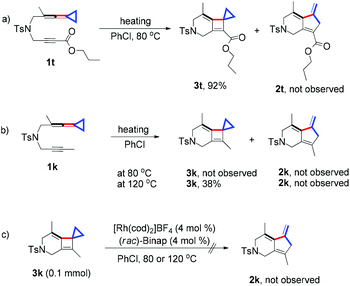
Control experiments were conducted to probe the mechanism of this cycloaddition reaction. In the case of 1t, upon heating 1t at 80 °C in PhCl for 12 h in the absence of the cationic Rh(I) catalyst, product 3t could be obtained in 92% yield without formation of product 2t (Scheme 2a). For substrate 1k, although none of the [2 + 2] cycloadduct 3k could be detected at 80 °C, 38% yield of 3k could be afforded upon heating at 120 °C in PhCl for 48 h also without formation of 2k (Scheme 2b). Furthermore, treating 3k under the standard conditions did not give the corresponding [3 + 2] cycloadduct 2k even if carrying out the reaction at 120 °C (Scheme 2c). This result suggested that the cyclobutene moiety could not undergo oxidative addition with the cationic Rh(I) complex, although the oxidative addition to cyclobutenones could take place smoothly.12 Therefore, the initial oxidative addition of yne-VDCP 1 to give intermediate I-3 is essential in this Rh(I)-catalyzed transformation (Scheme 1c).
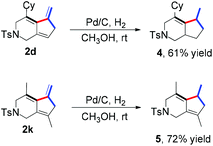
Further transformations of the present product 2 were also examined briefly. The hydrogenation of 2d and 2k in methanol under H2 atmosphere in the presence of Pd/C could give the corresponding products 4 and 5 in 61% and 72% yields, respectively (Scheme 3).
Conclusions
In conclusion, we have developed a novel Rh(I)-catalyzed intramolecular [3 + 2] cycloaddition of yne-VDCP substrates, giving the corresponding fused [6.5]-bicyclic products in good yields under mild conditions. The substrate scope is broad along with good functional group tolerance allowing the easy generation of various carbo- and heterobicyclic compounds under mild conditions. This Rh(I)-catalyzed synthetic strategy significantly extended the substrate scope for the construction of bicyclic derivatives as compared with those of gold(I)-catalyzed cycloisomerizations.8j Additional efforts on the investigation of the mechanistic details of this intramolecular [3 + 2] cycloaddition reaction and application of this protocol to the synthesis of biologically active substances are currently ongoing in our laboratory.Experimental section
General experimental methods
Melting points were determined on a digital melting point apparatus and temperatures were uncorrected. NMR spectra were recorded with a Bruker spectrometer at 400 MHz (1H NMR), and 100 MHz (13C NMR) in CDCl3, respectively. Chemical shifts were reported in ppm downfield from internal TMS. Organic solvents used were dried by standard methods when necessary. Commercially available reagents were used without further purification. All reactions were monitored by TLC with Huanghai GF254 silica gel coated plates. Flash column chromatography was carried out using 300–400 mesh silica gel at increased pressure. Infrared spectra were recorded on a PerkinElmer PE-983 spectrometer with absorption in cm−1. Mass spectra were recorded by ESI and HRMS was measured on an HP-5989 instrument.General procedure for the synthesis of 2
A 10 mL dried tube was charged with yne-VDCP 1 (0.1 mmol, 1.0 equiv.), [Rh(cod)2]BF4 (4.0 mol%) and (rac)-Binap (4.0 mol%). The reaction tube was evacuated and backfilled with argon (repeated three times). Then, PhCl (2.0 mL) was added into the tube. The reaction mixture was stirred at 80 °C for 6–8 h. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography (SiO2) to give the corresponding product 2.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful for the financial support from the National Basic Research Program of China [(973)-2015CB856603], the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000 and sioczz201808), the National Natural Science Foundation of China (20472096, 21372241, 21572052, 20672127, 21421091, 21372250, 21121062, 21302203, 21772037, 21772226, 20732008, 21772037, 21772226 and 21861132014), and the Fundamental Research Funds for the Central Universities 222201717003.Notes and references
- (a) Y. Kavanagh, M. O'Brien and P. Evans, Tetrahedron, 2009, 65, 8259 CrossRef CAS; (b) K. Takeda and M. Toyota, Tetrahedron, 2011, 67, 9909 CrossRef CAS; (c) A.-C. Guevel and D. J. Hart, J. Org. Chem., 1996, 61, 473 CrossRef CAS PubMed; (d) Y.-L. Kuo, M. Dhanasekaran and C.-K. Sha, J. Org. Chem., 2009, 74, 2033 CrossRef CAS PubMed; (e) K. Takahashi, K. Takeda and T. Honda, Tetrahedron Lett., 2010, 51, 3542 CrossRef CAS; (f) J. S. Beckett, J. D. Beckett and J. E. Hofferberth, Org. Lett., 2010, 12, 1408 CrossRef CAS PubMed.
- For selected reviews, see: (a) J. A. Varela and C. Saa, Chem. Rev., 2003, 103, 3787 CrossRef CAS PubMed; (b) K. Tanaka, Synlett, 2007, 1977 CrossRef CAS; (c) T. Shibata and K. Tsuchikama, Org. Biomol. Chem., 2008, 6, 1317 RSC; (d) S. Perreault and T. Rovis, Chem. Soc. Rev., 2009, 38, 3149 RSC; (e) M. Murakami and T. Matsuda, Chem. Commun., 2011, 47, 1100 RSC; (f) C. Aissa, Synthesis, 2011, 3389 CrossRef; (g) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410 CrossRef CAS PubMed; (h) P.-H. Chen, B. A. Billett, T. Tsukamoto and G. Dong, ACS Catal., 2017, 7, 1340 CrossRef CAS PubMed; (i) G. Chen, X. Jiang, C. Fu and S. Ma, Chem. Lett., 2010, 39, 78 CrossRef CAS; (j) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed.
- Recent reviews of MCPs, see: (a) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (b) I. Nakamura and Y. Yamamoto, Adv. Synth. Catal., 2002, 344, 111 CrossRef CAS; (c) A. Brandi, S. Cicchi, F. M. Cordero and A. Goti, Chem. Rev., 2003, 103, 1213 CrossRef CAS PubMed; (d) L.-X. Shao and M. Shi, Curr. Org. Chem., 2007, 11, 1135 CrossRef CAS; (e) H. Pellissier, Tetrahedron, 2010, 66, 8341 CrossRef CAS; (f) G. Audran and H. Pellissier, Adv. Synth. Catal., 2010, 352, 575 CrossRef CAS; (g) A. Masarwa and I. Marek, Chem. – Eur. J., 2010, 16, 9712 CrossRef CAS PubMed; (h) L. Yu and R. Guo, Org. Prep. Proced. Int., 2011, 43, 209 CrossRef CAS; (i) M. Shi, J.-M. Lu, Y. Wei and L.-X. Shao, Acc. Chem. Res., 2012, 45, 641 CrossRef CAS PubMed; (j) A. Brandi, S. Cicchi, F. M. Cordero and A. Goti, Chem. Rev., 2014, 114, 7317 CrossRef CAS PubMed; (k) H. Pellissier, Tetrahedron, 2014, 70, 4991 CrossRef CAS; (l) L. Yu, M. Liu, F. Chen and Q. Xu, Org. Biomol. Chem., 2015, 13, 8379 RSC.
- For some more recent papers related to MCPs, please see: (a) A. Fürstner and C. Aïssa, J. Am. Chem. Soc., 2006, 128, 6306 CrossRef PubMed; (b) M. Shi, L.-P. Liu and J. Tang, J. Am. Chem. Soc., 2006, 128, 7430 CrossRef CAS PubMed; (c) C. Aïssa and A. Fürstner, J. Am. Chem. Soc., 2007, 129, 14836 CrossRef PubMed; (d) S. Simaan and I. Marek, J. Am. Chem. Soc., 2010, 132, 4066 CrossRef CAS PubMed; (e) K. Chen, M. Jiang, Z. Zhang, Y. Wei and M. Shi, Eur. J. Org. Chem., 2011, 7189 CrossRef CAS; (f) S. Cui, Y. Zhang and Q. Wu, Chem. Sci., 2013, 4, 3421 RSC; (g) J. C. Timmerman, B. D. Robertson and R. A. Widenhoefer, Angew. Chem., Int. Ed., 2015, 54, 2251 CrossRef CAS PubMed; (h) Z.-Z. Zhu, K. Chen, L.-Z. Yu, X.-Y. Tang and M. Shi, Org. Lett., 2015, 17, 5994 CrossRef CAS PubMed; (i) B. Yao, Y. Li, Z. Liang and Y. Zhang, Org. Lett., 2011, 13, 640 CrossRef CAS PubMed; (j) K. Chen, Z.-Z. Zhu, Y.-S. Zhang, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 6645 CrossRef CAS PubMed; (k) K. Chen, Z.-Z. Zhu, J.-X. Liu, X.-Y. Tang, Y. Wei and M. Shi, Chem. Commun., 2016, 52, 350 RSC; (l) M. Araya, M. Gulías, I. Fernández, G. Bhargava, L. Castedo, J. L. Mascareñas and F. López, Chem. – Eur. J., 2014, 20, 10255 CrossRef CAS PubMed; (m) D.-H. Zhang, X.-Y. Tang, Y. Wei and M. Shi, Chem. – Eur. J., 2013, 19, 13668 CrossRef CAS PubMed; (n) D.-H. Zhang, Y. Wei and M. Shi, Chem. – Eur. J., 2012, 18, 7026 CrossRef CAS PubMed; (o) B. Cao, Y. Wei and M. Shi, Chem. Commun., 2018, 54, 14085 RSC.
- Recent reviews of VCPs, see: (a) Y. Wang and Z.-X. Yu, Acc. Chem. Res., 2015, 48, 2288 CrossRef CAS PubMed; (b) Z.-X. Yu, Y. Wang and Y. Wang, Chem. – Asian J., 2010, 5, 1072 CrossRef CAS PubMed; (c) D. K. Brownsey, E. Gorobets and D. J. Derksen, Org. Biomol. Chem., 2018, 16, 3506 RSC; (d) M. Meazza, H. Guo and R. Rios, Org. Biomol. Chem., 2017, 15, 2479 RSC; (e) V. Ganesh and S. Chandrasekaran, Synthesis, 2016, 48, 4347 CrossRef CAS; (f) J. E. Baldwin, Chem. Rev., 2003, 103, 1197 CrossRef CAS PubMed; (g) T. Sato, K. Izawa, J. L. Aceña, H. Liu and V. A. Soloshonok, Eur. J. Org. Chem., 2016, 2757 CrossRef CAS.
- For some more recent papers related to VCPs, please see: (a) P. A. Wender, C. O. Husfeld, E. Langkopf and J. A. Love, J. Am. Chem. Soc., 1998, 120, 1940 CrossRef CAS; (b) P. A. Wender, A. J. Dyckman, C. O. Husfeld, D. Kadereit, J. A. Love and H. Rieck, J. Am. Chem. Soc., 1999, 121, 10442 CrossRef CAS; (c) P. A. Wender and T. J. Williams, Angew. Chem., Int. Ed., 2002, 41, 4550 CrossRef CAS; (d) P. A. Wender, A. B. Lesser and L. E. Sirois, Angew. Chem., Int. Ed., 2012, 51, 2736 CrossRef CAS PubMed; (e) P. A. Wender, L. O. Haustedt, J. Lim, J. A. Love, T. J. Williams and J. Y. Yoon, J. Am. Chem. Soc., 2006, 128, 6302 CrossRef CAS PubMed; (f) R. Shintani, H. Nakatsu, K. Takatsu and T. Hayashi, Chem. – Eur. J., 2009, 15, 8692 CrossRef CAS PubMed; (g) F. Inagaki, K. Sugikubo, Y. Miyashita and C. Mukai, Angew. Chem., Int. Ed., 2010, 49, 2206 CrossRef CAS PubMed; (h) K. Sugikubo, F. Omachi, Y. Miyanaga, F. Inagaki, C. Matsumoto and C. Mukai, Angew. Chem., Int. Ed., 2013, 52, 11369 CrossRef CAS PubMed; (i) C. H. Liu and Z. X. Yu, Angew. Chem., Int. Ed., 2017, 56, 8667 CrossRef CAS PubMed; (j) X. Hong, M. C. Stevens, P. Liu, P. A. Wender and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 17273 CrossRef CAS PubMed; (k) P. Liu, L. E. Sirois, P. H. Y. Cheong, Z. X. Yu, I. V. Hartung, H. Rieck, P. A. Wender and K. N. Houk, J. Am. Chem. Soc., 2010, 132, 10127 CrossRef CAS PubMed; (l) P. A. Wender, D. N. Fournogerakis, M. S. Jeffreys, R. V. Quiroz, F. Inagaki and M. Pfaffenbach, Nat. Chem., 2014, 6, 448 CrossRef CAS PubMed; (m) L. Jiao, C. X. Yuan and Z. X. Yu, J. Am. Chem. Soc., 2008, 130, 4421 CrossRef CAS PubMed; (n) L. Jiao, M. Lin and Z. X. Yu, J. Am. Chem. Soc., 2011, 133, 447 CrossRef CAS PubMed; (o) D.-H. Liu and Z.-X. Yu, Synlett, 2018, 29, 764 CrossRef.
- Recent reviews of VDCPs, see: (a) M. Shi, L.-X. Shao, J.-M. Lu, Y. Wei, K. Mizuno and H. Maeda, Chem. Rev., 2010, 110, 5883 CrossRef CAS PubMed; (b) B.-L. Lu, L.-Z. Dai and M. Shi, Chem. Soc. Rev., 2012, 41, 3318 RSC; (c) D.-H. Zhang, X.-Y. Tang and M. Shi, Acc. Chem. Res., 2014, 47, 913 CrossRef CAS PubMed; (d) S. Yang and M. Shi, Acc. Chem. Res., 2018, 51, 1667 CrossRef CAS PubMed.
- For some more recent papers related to VDCPs, please see: (a) S. Yang, K.-H. Rui, X.-Y. Tang, Q. Xu and M. Shi, J. Am. Chem. Soc., 2017, 139, 5957 CrossRef CAS PubMed; (b) S. Yang, Q.-Z. Li, C. Xu, Q. Xu and M. Shi, Chem. Sci., 2018, 9, 5074 RSC; (c) S. Yang, Q. Xu and M. Shi, Chem. – Eur. J., 2016, 22, 10387 CrossRef CAS PubMed; (d) S. Yang, W. Yuan, Q. Xu and M. Shi, Chem. – Eur. J., 2015, 21, 15964 CrossRef CAS PubMed; (e) D.-Y. Li, Y. Wei, I. Marek, X.-Y. Tang and M. Shi, Chem. Sci., 2015, 6, 5519 RSC; (f) A. V. Stepakov, A. G. Larina, V. M. Boitsov, V. V. Gurzhiy, A. P. Molchanov and R. R. Kostikov, Tetrahedron Lett., 2014, 55, 2022 CrossRef CAS; (g) M.-Z. Miao, J. Cao, J.-J. Zhang, X. Huang and L.-L. Wu, J. Org. Chem., 2013, 78, 2687 CrossRef CAS PubMed; (h) N. U. Zhanpeisov, K. Mizuno, M. Anpo and J. Leszczynski, Int. J. Quantum Chem., 2004, 96, 343 CrossRef CAS; (i) H. Maeda, T. Hirai, A. Sugimoto and K. Mizuno, J. Org. Chem., 2003, 68, 7700 CrossRef CAS PubMed; (j) W. Yuan, X. Tang, Y. Wei and M. Shi, Chem. – Eur. J., 2014, 20, 3198 CrossRef CAS PubMed.
- B.-L. Lu and M. Shi, Angew. Chem., Int. Ed., 2011, 50, 12027 CrossRef CAS PubMed.
- W. Yuan, X. Dong, M. Shi, P. McDowell and G.-G. Li, Org. Lett., 2012, 14, 5582 CrossRef CAS PubMed.
- For selected examples related to thermal induced cyclizations, see: (a) S. Yang, Q. Xu and M. Shi, Tetrahedron, 2016, 72, 584 CrossRef CAS; (b) I. Nakamura, T. Nemoto, Y. Yamamoto and A. de Meijere, Angew. Chem., Int. Ed., 2006, 45, 5176 CrossRef CAS PubMed; (c) I. Nakamura, R. Nagata, T. Nemoto, M. Terada, Y. Yamamoto, T. Späth and A. de Meijere, Eur. J. Org. Chem., 2007, 4479 CrossRef CAS; (d) X.-Y. Tang and M. Shi, J. Org. Chem., 2009, 74, 5983 CrossRef CAS.
- (a) M. A. Huffman, L. S. Liebeskind and W. T. Pennington, Organometallics, 1990, 9, 2194 CrossRef CAS; (b) M. A. Huffman, L. S. Liebeskind and W. T. Pennington, Organometallics, 1992, 11, 255 CrossRef CAS; (c) T. Xu, H. M. Ko, N. A. Savage and G. Dong, J. Am. Chem. Soc., 2012, 134, 20005 CrossRef CAS PubMed; (d) M. A. Huffman and L. S. Liebeskind, J. Am. Chem. Soc., 1991, 113, 2771 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of new compounds. See DOI: 10.1039/c9qo00343f |
| This journal is © the Partner Organisations 2019 |

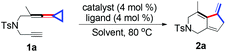
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)