Pd-Catalyzed coupling reaction of cyclobutanols with propargylic carbonates†
Penglin
Wu
a,
Minqiang
Jia
*a and
Shengming
Ma
 *ab
*ab
aResearch Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, P. R. China
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn
First published on 1st April 2019
Abstract
An efficient approach of Pd-catalyzed ring opening coupling reaction of cyclobutanols with propargylic carbonates was realized to provide a series of multisubstituted δ-allenyl ketones. The reaction had a wide substrate scope with tolerance to different functional groups.
Introduction
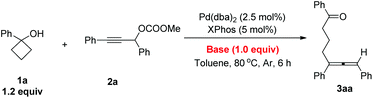
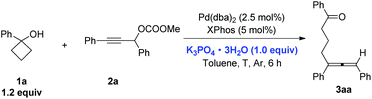
An allene unit is an important type of molecular structure and found widespread in numerous natural products and bioactive molecules.1 Thus, the synthesis of differently substituted allenes has attracted a lot of attention.2 The coupling reaction of propargylic carbonates with different metal regents was an efficient method for the construction of multi-substituted allenes.3 Recently, we found that cyclopropanols may be used as an efficient coupling reagent with propargylic carbonates for the synthesis of different substituted allenes. The reaction has a wide substrate scope under mild reaction conditions.4 To expand the scope of such a reaction, we envisioned that cyclobutanols which are easily available from cyclobutanones5 could also be used in this type of coupling reaction via Pd catalysts as pioneered by Uemura.6,7 In this paper, we report a general method for the synthesis of different substituted δ-allenyl ketones from propargylic carbonates.Firstly, standard reaction conditions with cyclopropanols were used for the coupling reaction of cyclobutanol 1a with propargylic carbonate 2a. Unfortunately, the reaction mixture was stirred at 50 °C for 21 h and then 80 °C for 7 h to afford the desired allene product 3aa in only 28% NMR yield with 46% of carbonate 2a being recovered (Scheme 1). These results revealed that the reactivity of cyclobutanol was much lower than the cyclopropanol, which was possibly due to their different ring strains (the ring strains of cyclopropane and cyclobutane are 29.0 and 26.3 kcal mol−1, respectively8).
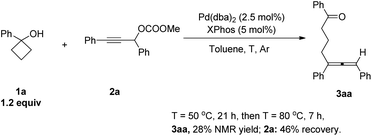 | ||
| Scheme 1 Reaction of cyclobutanol 1a with propargylic carbonate 2a under the standard conditions of cyclopropanol. | ||
Results and discussion
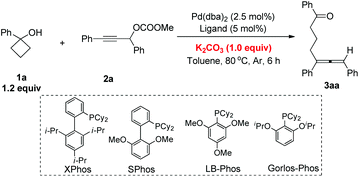
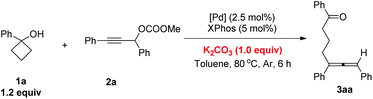
Based on these results, we decided to further screen the reaction conditions with cyclobutanol 1a and propargylic carbonate 2a as the model substrates. Firstly, different carbonate salts were added in toluene at 80 °C with Pd(dba)2 and XPhos as the catalysts. K2CO3 and Cs2CO3 provided the highest yields (Table 1, entries 1–4). K3PO4·3H2O had almost the same effect (Table 1, entry 5). Other bases such as t-BuOK, NaHCO3, CsOPiv, DIPEA, and Et3N were also tested demonstrating inferior results (Table 1, entries 6–10). Finally, we also found that the very freshly prepared carbonate 2a can be used directly to give the corresponding allene 3aa in 80% NMR yield without the addition of any base (Table 1, entry 11). However, 1.0 equiv. of base was required for the (slightly) aged 2a.| Entry | Base | NMR yield of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Recovery of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.24 mmol), 2a (0.20 mmol), Pd(dba)2 (2.5 mol%), XPhos (5.0 mol%), and base (1.0 equiv.) in toluene (1.0 mL) at 80 °C. b NMR yield with CH2Br2 as the internal standard. c The carbonate 2a was used immediately after chromatography on silica gel; reaction time: 3 h. | |||
| 1 | Li2CO3 | 59 | 3 |
| 2 | Na2CO3 | 54 | 10 |
| 3 | K2CO3 | 80 | 0 |
| 4 | Cs2CO3 | 80 | 0 |
| 5 | K3PO4·3H2O | 79 | 0 |
| 6 | t-BuOK | Complicated | — |
| 7 | NaHCO3 | 55 | 12 |
| 8 | CsOPiv | 0 | 96 |
| 9 | DIPEA | 51 | 12 |
| 10 | Et3N | 61 | 3 |
| 11c | — | 80 | 0 |
Then, a range of different phosphine ligands were screened. The use of PPh3 and dppp led to complicated results (Table 2, entries 1 and 2). When other phosphine ligands such as PCy3, dppf, dppm, LB-Phos and Gorlos-Phos were used, the starting material 2a was recovered in 36–75% with no product 3aa being observed (Table 2, entries 3–7). Fortunately, SPhos and rac-BINAP resulted in a moderate yield of product 3aa with 5–10% yield of 2a remaining (Table 2, entries 8 and 9). Finally, it was observed that XPhos was still the best ligand, which provided the allene product in 80% yield and no 2a was recovered (Table 2, entry 10).
| Entry | Ligand | NMR yield of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Recovery of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.24 mmol), 2a (0.20 mmol), Pd(dba)2 (2.5 mol%), ligand (5.0 mol%), and K2CO3 (1.0 equiv.) in toluene (1.0 mL) at 80 °C. b NMR yield with CH2Br2 as the internal standard. | |||
| 1 | PPh3 | Complicated | — |
| 2 | dppp | Complicated | — |
| 3 | PCy3 | 0 | 75 |
| 4 | dppf | 0 | 74 |
| 5 | dppm | 0 | 36 |
| 6 | LB-Phos | 0 | 63 |
| 7 | Gorlos-Phos | 0 | 39 |
| 8 | SPhos | 56 | 10 |
| 9 | rac-BINAP | 63 | 5 |
| 10 | XPhos | 80 | 0 |
Subsequently, with XPhos as the optimal ligand, Pd(II) salts were tested. Different Pd(II) sources such as Pd(OAc)2, PdCl2 and Pd(TFA)2 gave 9–28% yields of product 3aa with 31–81% recovery of the starting material 2a (Table 3, entries 2–4).
| Entry | [Pd] | NMR yield of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Recovery of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.24 mmol), 2a (0.20 mmol), [Pd] (2.5 mol%), XPhos (5.0 mol%), and K2CO3 (1.0 equiv.) in toluene (1.0 mL) at 80 °C. b NMR yield with CH2Br2 as the internal standard. | |||
| 1 | Pd(dba) 2 | 80 | 0 |
| 2 | Pd(OAc)2 | 28 | 31 |
| 3 | PdCl2 | 13 | 73 |
| 4 | Pd(TFA)2 | 9 | 81 |
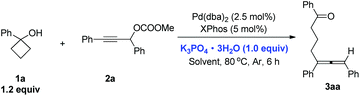
Considering the convenience, K3PO4·3H2O was used as the base for further study. Solvents were further screened. THF, dioxane, CH3CN and DCM all gave the desired product 3aa albeit with lower yields (49–69%, Table 4, entries 2–5). When DMF was used, the reaction was complicated and 14% of 3aa was observed (Table 4, entry 6).
| Entry | Solvent | NMR yield of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Recovery of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.24 mmol), 2a (0.20 mmol), Pd(dba)2 (2.5 mol%), XPhos (5.0 mol%), and K2CO3 (1.0 equiv.) in solvent (1.0 mL) at 80 °C. b NMR yield with CH2Br2 as the internal standard. | |||
| 1 | Toluene | 79 | 0 |
| 2 | THF | 69 | 0 |
| 3 | Dioxane | 61 | 0 |
| 4 | CH3CN | 56 | 0 |
| 5 | DCM | 49 | 0 |
| 6 | DMF | 14 | — |
Finally, lowering the temperature to 70 °C or 60 °C can both provide 81% yield of 3aa (Table 5, entries 2 and 3). However, the reaction at 60 °C on 1 mmol scale led to the minor recovery of the propargylic carbonate 2a. Decreasing the temperature further to 50 °C resulted in a lower yield of 3aa with 53% recovery of the starting material 2a (Table 5, entry 4).
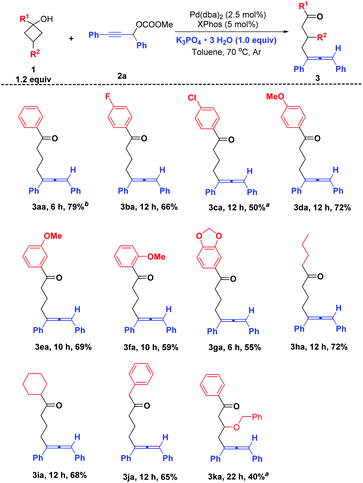
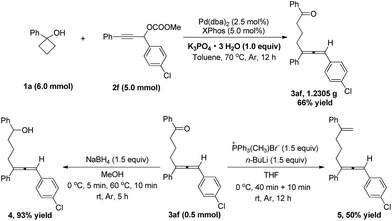
With the optimized reaction conditions above (Table 5, entry 2), the substrate scope of various cyclobutanols 1 with the propargylic carbonates 2a was investigated. All the reactions were carried out on 1 mmol scale and the results are summarized in Scheme 2. When R1 is an aryl group, besides phenyl, the substituent group on the phenyl group can be either an electron-withdrawing group (F, Cl) or an electron-donating group (OMe), providing the allene products in 50–79% yields (Scheme 2, 3aa–3ga). In addition, the substituents on the phenyl rings at the ortho, meta, and para positions may all be tolerated (Scheme 2, 3da–3fa). And the piperonyl group can be tolerated well providing 55% yield of 3ga. Except for the aryl group, R1 can be different alkyl groups such as n-butyl, cyclohexyl and benzyl to provide the desired allene products in 65–72% yields (Scheme 2, 3ha–3ja). The reaction also worked with a cyclobutanol with an additional substituent group at the 3-position providing the expected product 3ka with 40% yield (Scheme 2).
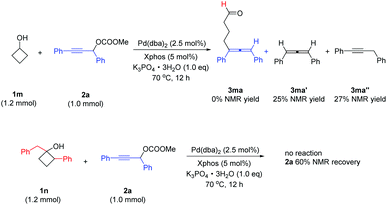
Notably, when benzocyclobutanol 1l was used in the reaction, product 3la with the cleavage of the proximal bond was selectively generated in 51% yield, and the distal bond cleavage product 3la′ was not observed (Scheme 3).
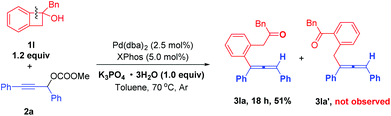 | ||
| Scheme 3 The reaction of benzocyclobutanol. Reaction conditions: 1l (1.2 mmol), 2a (1.0 mmol), Pd(dba)2 (2.5 mol%), XPhos (5.0 mol%), and K3PO4·3H2O (1.0 equiv.) in toluene (5.0 mL) at 70 °C. | ||
However, when R1 = H, the reaction did not provide the expected product 3ma, instead, by-products 1,3-diphenylpropadiene 3ma′ and 1,3-dipenylpropyne 3ma′′ were formed in 25% and 27% NMR yields, respectively.4 The reaction of the cyclobutanol 1n, which has an additional substituent at the 2-position, gave no allene product with 60% recovery of the starting material 2a (Scheme 4).
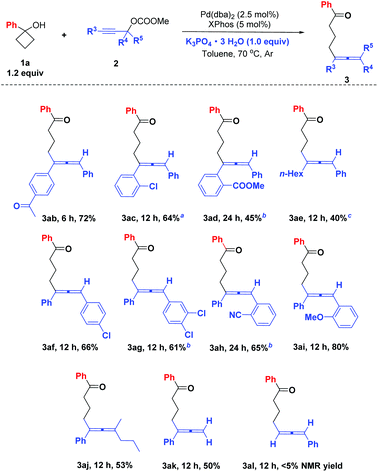
Next, the scope of propargylic carbonates 2 was also tested. When R5 is H, R3 and R4 substituents can be different aryl or alkyl groups. For example, 4-acetyl, 2-chloro, and 2-methoxycarbonyl substituted phenyls at the R3 position were tolerated providing the allene products 3ab–3ad in 45–72% yields. When R3 was n-hexyl, it could result in 40% yield of 3ae by decreasing the amount of 1a to 1.0 mmol and increasing the amount of propargylic carbonate 2e to 1.5 mmol. And 4-chloro, 3,4-dichloro, 2-cyano and 2-methoxy substituted phenyls could be introduced successfully to the R4 position to give the final products 3af–3ai in 61–80% yields. It should be noted that the catalyst loading should be improved to 5 mol% for 3ac, 3ad, 3ag and 3ah to make sure the complete consumption of the starting carbonates. Notably, besides the secondary propargylic carbonates, tertiary propargylic carbonates (R2 = Ph, R3 = nPr, R4 = Me) could also be used to afford the fully substituted allene product 3aj in a moderate yield. Besides, when R4 = R5 = H, the reaction afforded 3ak with 50% yield. However, when R3 = H, the corresponding allene product 3al was formed in a very low yield (<5% by 1H NMR) (Scheme 5).
In order to demonstrate the synthetic utility and practicality of the method, the allene product 3ab was synthesized on a gram scale. A 5.0 mmol scale reaction was carried out providing 1.2305 g of the desired product 3ab in 66% isolated yield. And the allene product 3ab can be subjected to some transformations. For example, it can be reduced to the secondary alcohol 4 with NaBH4, and reacted with the Wittig reagent producing product 5 (Scheme 6).
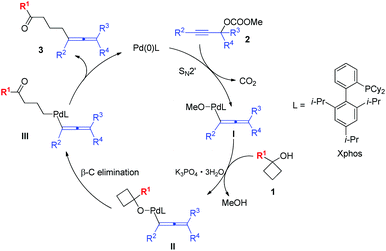
Based on the previous experimental results and knowledge,4,9 a possible mechanism was proposed as shown in Scheme 7. Firstly, the propargylic carbonate 2 reacted with Pd(0) via SN2′-type oxidative addition together with the elimination of a molecule of CO2, which leads to the allenyl palladium methoxide intermediate I. Then intermediate I underwent ligand exchange with cyclobutanol 1 generating intermediate II, which would provide intermediate III by β-C elimination. Intermediate III delivered the final product 3via reductive elimination and regenerated the Pd(0) species completing the catalytic cycle.
Conclusions
In summary, an efficient method to synthesize different multi-substituted δ-allenyl ketones via palladium-catalyzed ring opening of cyclobutanols and oxidative addition of propargylic carbonates was developed. This reaction had a wide substrate scope with tolerance to different functional groups, and this method may be easily expanded to gram scale synthesis with potential synthetic utility.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Basic Research Program of China (2015CB856600) and the National Natural Science Foundation of China (21502020) is greatly appreciated. We thank Yifan Cui of our group for reproducing the results of 3ca, 3ga, and 3ab represented in this study.Notes and references
- For a review on allenes in natural products and bioactive molecules, see: A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef PubMed.
- For reviews on the synthesis of different substituted allenes, see: (a) A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2002, 41, 2933 CrossRef; (b) N. Krause and A. Hoffmann-Röder, Tetrahedron, 2004, 60, 11671 CrossRef CAS; (c) G. B. Hammond, ACS Symp. Ser., 2005, 911, 204 CrossRef CAS; (d) K. M. Brummond and J. E. DeForrest, Synthesis, 2007, 795 CrossRef CAS; (e) M. Ogasawara, Tetrahedron: Asymmetry, 2009, 20, 259 CrossRef CAS; (f) S. Yu and S. Ma, Chem. Commun., 2011, 47, 5384 RSC; (g) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519 CrossRef CAS; (h) J. Ye and S. Ma, Org. Chem. Front., 2014, 1, 1210 RSC; (i) R. K. Neff and D. E. Frantz, Tetrahedron, 2015, 71, 7 CrossRef CAS.
- Selected examples, see: (a) E. Keinan and E. Bosch, J. Org. Chem., 1986, 51, 4006 CrossRef CAS; (b) P. Dixneuf, T. Guyot, M. Ness and S. Roberts, Chem. Commun., 1997, 2083 RSC; (c) R. Riveiros, D. Rodriguez, J. Sestelo and L. Sarandeses, Org. Lett., 2006, 8, 1403 CrossRef CAS PubMed; (d) H. Ito, Y. Sasaki and M. Sawamura, J. Am. Chem. Soc., 2008, 130, 15774 CrossRef CAS PubMed; (e) H. Ohmiya, H. Ito and M. Sawamura, Org. Lett., 2009, 11, 5618 CrossRef CAS PubMed; (f) Q. Li, J. Jeng and H. Gau, Eur. J. Org. Chem., 2014, 7916 CrossRef CAS; (g) T. Zhao, Y. Yang, T. Lessing and K. Szabó, J. Am. Chem. Soc., 2014, 136, 7563 CrossRef CAS PubMed; (h) H. Luo, Y. Yu and S. Ma, Org. Chem. Front., 2016, 3, 1705 RSC; (i) S. Kessler and J. Bäckvall, Angew. Chem., Int. Ed., 2016, 55, 3734 CrossRef CAS PubMed; (j) Q. Lu, S. Greßies, F. J. R. Klauck and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 6660 CrossRef CAS PubMed.
- P. Wu, M. Jia, W. Lin and S. Ma, Org. Lett., 2018, 20, 554 CrossRef CAS PubMed.
- (a) K. Jia, F. Zhang, H. Huang and Y. Chen, J. Am. Chem. Soc., 2016, 138, 1514 CrossRef CAS PubMed; (b) B. Casey, C. Eakin and R. Flowers II, Tetrahedron Lett., 2009, 50, 1264 CrossRef CAS PubMed; (c) H. Zeng, P. Pan, J. Chen, H. Gong and C. Li, Eur. J. Org. Chem., 2017, 1070 CrossRef CAS.
- For examples on the coupling reaction of the ring-opening of cyclobutanols via Pd catalysis, see: (a) T. Nishimura, K. Ohe and S. Uemura, J. Am. Chem. Soc., 1999, 121, 2645 CrossRef CAS; (b) T. Nishimura and S. Uemura, J. Am. Chem. Soc., 1999, 121, 11010 CrossRef CAS; (c) T. Nishimura, K. Ohe and S. Uemura, J. Org. Chem., 2001, 66, 1455 CrossRef CAS PubMed; (d) T. Nishimura, S. Matsumura, Y. Maeda and S. Uemura, Chem. Commun., 2002, 50 RSC; (e) T. Nishimura, S. Matsumura, Y. Maeda and S. Uemura, Tetrahedron Lett., 2002, 43, 3037 CrossRef CAS; (f) M. Ethirajan, H. Oh and J. Cha, Org. Lett., 2007, 9, 2693 CrossRef CAS PubMed; (g) A. Chtchemelinine, D. Rosa and A. Orellana, J. Org. Chem., 2011, 76, 9157 CrossRef CAS PubMed; (h) A. Ziadi and R. Martin, Org. Lett., 2012, 14, 1266 CrossRef CAS PubMed; (i) A. Ziadi, A. Correa and R. Martin, Chem. Commun., 2013, 49, 4286 RSC; (j) L. Chen, F. Sun, Y. Sun, Z. Xu, Z. Zheng, Y. Cui, J. Cao and L. Xu, Adv. Synth. Catal., 2018, 360, 411 CrossRef CAS.
- S. Matsumura, Y. Maeda, T. Nishimura and S. Uemura, J. Am. Chem. Soc., 2003, 125, 8862 CrossRef CAS PubMed.
- P. Khoury, W. Tam and J. Goddard, Tetrahedron, 2004, 60, 8103 CrossRef CAS.
- For a review, see: (a) J. Tsuji and T. Mandai, Angew. Chem., Int. Ed. Engl., 1995, 34, 2589 CrossRef CAS. See also: (b) S. Ogoshi, K. Tsutsumi, M. Ooi and H. Kurosawa, J. Am. Chem. Soc., 1995, 117, 10415 CrossRef CAS; (c) T. Konno, M. Tanikawa, T. Ishihara and H. Yamanaka, Chem. Lett., 2000, 1360 CrossRef CAS; (d) B. M. Trost and L. Debien, Chem. Sci., 2016, 7, 4985 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qo00192a |
| This journal is © the Partner Organisations 2019 |