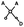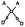Dual excitation wavelength system for combined fingerprint and high wavenumber Raman spectroscopy†
Laura E.
Masson
ab,
Christine M.
O'Brien
 abc,
Isaac J.
Pence
abd,
Jennifer L.
Herington
eg,
Jeff
Reese
e,
Ton G.
van Leeuwen
f and
Anita
Mahadevan-Jansen
*ab
abc,
Isaac J.
Pence
abd,
Jennifer L.
Herington
eg,
Jeff
Reese
e,
Ton G.
van Leeuwen
f and
Anita
Mahadevan-Jansen
*ab
aDepartment of Biomedical Engineering, Vanderbilt University, Nashville, USA. E-mail: anita.mahadevan-jansen@vanderbilt.edu
bBiophotonics Center, Vanderbilt University, Nashville, USA
cDepartment of Radiology, Washington University in St. Louis, St. Louis, USA
dInstitute of Biomedical Engineering, Imperial College London, London, UK
eDivision of Neonatology, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, USA
fBiomedical Engineering & Physics Department, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
gDepartment of Pharmacology, Vanderbilt University, Nashville, USA
First published on 31st October 2018
Abstract
A fiber optic probe-based Raman spectroscopy system using a single laser module with two excitation wavelengths, at 680 and 785 nm, has been developed for measuring the fingerprint and high wavenumber regions using a single detector. This system is simpler and less expensive than previously reported configurations of combined fingerprint and high wavenumber Raman systems, and its probe-based implementation facilitates numerous in vivo applications. The high wavenumber region of the Raman spectrum ranges from 2800–3800 cm−1 and contains valuable information corresponding to the molecular vibrations of proteins, lipids, and water, which is complimentary to the biochemical signatures found in the fingerprint region (800–1800 cm−1), which probes DNA, lipids, and proteins. The efficacy of the system is demonstrated by tracking changes in water content in tissue-mimicking phantoms, where Voigtian decomposition of the high wavenumber water peak revealed a correlation between the water content and type of water-tissue interactions in the samples. This dual wavelength system was then used for in vivo assessment of cervical remodeling during mouse pregnancy, a physiologic process with known changes in tissue hydration. The system shows that Raman spectroscopy is sensitive to changes in collagen content in the fingerprint region and hydration state in the high wavenumber region, which was verified using an ex vivo comparison of wet and dry weight. Simultaneous fingerprint and high wavenumber Raman spectroscopy will allow precise in vivo quantification of tissue water content in the high wavenumber region, paired with the high biochemical specificity of the fingerprint region.
1. Introduction
Raman spectroscopy is a sensitive and non-destructive optical method for obtaining molecular information from a material or tissue. The Raman spectrum is classically divided into two regions: the fingerprint or low wavenumber region, which ranges from 800–1800 cm−1, and the high wavenumber region, which ranges from 2800–3800 cm−1. The vast majority of Raman spectroscopic studies for biomedical applications have focused on the fingerprint region, which is rich in biochemical information including signatures associated with proteins, lipids, DNA, and blood.1–5 However, the high wavenumber region also contains valuable information corresponding to molecular vibrations of lipids, proteins, and especially water. The fingerprint and high wavenumber regions together provide a more complete biochemical picture of the sample composition.The region of the Raman spectrum which is accessible with a given system depends upon the excitation wavelength, grating, and type of detector used. Near infrared excitation wavelengths are optimal for many biomedical Raman applications because they result in greater penetration depths in tissue compared to UV or visible light.6 However, the Raman cross section is proportional to 1/λ4, dropping off as the wavelength increases.7 As Raman scattering and fluorescence are detected simultaneously, it is advantageous to minimize fluorescence and avoid saturating the detector while maximizing the Raman signal using longer excitation wavelengths. Excitation with a 785 or 830 nm laser diode balances these considerations and is commonly used to study the fingerprint region of the Raman spectrum. Silicon charge coupled devices (CCDs), which are used in many Raman spectroscopy systems, achieve their maximum quantum efficiency (≥50%) between 400 and 900 nm.8 With 785 or 830 nm excitation, Raman shifts corresponding to the fingerprint region therefore fall within the silicon detector's range of optimal sensitivity. However, depending upon the grating used, wavelengths corresponding to Raman shifts within the high wavenumber region may or may not be transmitted onto the detector. In either case, the detector exhibits low sensitivity, with quantum efficiency values of 20% or lower,9 to wavelengths corresponding to the high wavenumber region. This makes it infeasible to capture both the fingerprint and high wavenumber region on a silicon detector at 785 nm excitation.
One alternative is an InGaAs detector, which exhibits optimal performance in the range of 0.9 to 1.7 microns,8 and therefore may allow visualization of both the fingerprint region and a portion of the high wavenumber region.10 A dual transmission grating can also be used to transmit the fingerprint and high wavenumber regions of the Raman spectrum onto different vertical segments of either a silicon or InGaAs detector, allowing visualization of both regions simultaneously.10 Other specialized gratings have been developed to diffract both the fingerprint and high wavenumber region onto the detector.11 Two separate laser sources of different wavelengths-one to acquire fingerprint spectra and the other to acquire high wavenumber-can be combined with a dichroic beamsplitter to switch between the two regions in a single-detector system.12 Limitations of existing fingerprint and high wavenumber systems include high cost, reduced spectral resolution, low signal to noise ratio, and complexity, making it difficult to implement such systems in a clinical setting.
A Raman spectroscopy system capable of acquiring both fingerprint and high wavenumber Raman spectra has been developed using 680 and 785 nm excitation wavelengths produced by two laser diodes packaged in a single unit. To our knowledge, this is the first report of a system which uses a dual-wavelength laser unit coupled to a fiber optic probe to enable sequential acquisition of the fingerprint and high wavenumber regions in vivo. This system eliminates the need for a specialized detector or grating, and does not require any manual manipulation of internal optics to switch between fingerprint and high wavenumber spectral acquisition. Depending on the excitation wavelength selected, backscattered wavelengths corresponding to either the fingerprint or high wavenumber region will be captured by a conventional silicon CCD. Both excitation wavelengths can be transmitted to the sample, and scattered light from both spectral regions collected, using a single fiber optic probe. This system is capable of acquiring both fingerprint and high wavenumber Raman spectra with a flip of a switch. Unlike existing systems, such as those that use 785 nm excitation with a dual transmission grating, this system is more sensitive to the high wavenumber region due to the use of a shorter (680 nm) excitation wavelength. This configuration allows both the fingerprint (at 785 nm excitation) and the high wavenumber region (at 680 nm excitation) to fall within the CCD's wavelength range of optimal sensitivity. While combined fingerprint and high wavenumber Raman spectroscopy has previously been reported using a confocal Raman mircospectroscopy system,13 our fiber-optic probe-based setup facilitates in vivo application and eventual clinical translation.
The addition of the high wavenumber region facilitates analysis of water-tissue interactions within a sample. Water molecules are known to produce a weak Raman band due to O–H bending at approximately 1645 cm−1 in the fingerprint region.14 However, in biological samples, it is often difficult to distinguish between this O–H bending peak and the much stronger Amide I peak at approximately the same location. Water molecules also produce a strong, broad peak corresponding to O–H stretch centered at approximately 3350 cm−1 in the high wavenumber region.15 The Raman signal associated with water is much higher in intensity and easier to distinguish within the high wavenumber region than in the fingerprint region. This high wavenumber water peak represents a convolution of five distinct vibrational modes which reflect the hydrogen bonding state of the water molecules being probed. The five vibrational modes of water molecules represented by the high wavenumber water peak are DAA, DDAA, DA, DDA, and free OH,15 where D denotes a hydrogen bond donor, and A denotes a hydrogen bond acceptor. The high wavenumber water band also overlaps with the N–H stretch of protein13 at 3329 cm−1. Based on the broad O–H peak in the high wavenumber region, Raman spectroscopy facilitates a quantitative approach to studying hydration in tissue.
Previous studies have used high wavenumber Raman spectroscopy to study changes in water content and water-tissue interactions in tissues such as bone and skin.13,16–18 The ability to precisely quantify water content in the skin has been shown to be relevant for cosmetic purposes as well as for evaluating skin conditions such as atopic dermatitis.19 Differences in water content as measured using high wavenumber Raman spectroscopy have also been used to discriminate between oral cancer and the surrounding tissue.20 The combination of high wavenumber and fingerprint RS has been shown to enhance the performance of Raman spectroscopy in gastric,21 nasopharyngeal,22 colon,23 and cervical cancer detection.24 However, the instrumentation used for these combined studies relied on multiple detectors and/or multiple sources, making for complicated and expensive instrumentation.
An important biological process that involves changes in tissue hydration is cervical remodeling during pregnancy. It has been shown that the collagen matrix comprising the cervix becomes less dense and more disorganized as the cervix undergoes a softening and ripening process to prepare for delivery.25–28 The cervix has also been shown to increase in hydration during the course of pregnancy2,26,29,30 due to the upregulation of certain classes of aquaporins and increased expression of the hydrophilic molecule hyaluronic acid in the cervix.31–35 However, there is little data available regarding this hydration change and what role it may play in the process of preterm labor. Previous studies have used Raman spectroscopy in the fingerprint region to study changes to the pregnant cervix during parturition in a mouse model as well as in human patients.36–38 High wavenumber Raman spectroscopy using a fiber optic probe facilitates noninvasive assessment of in vivo cervical hydration state which, when paired with the biochemical specificity of the fingerprint region, is a powerful tool for studying cervical remodeling in the context of pregnancy and labor.
Combined fingerprint and high wavenumber Raman spectroscopy using dual excitation wavelengths facilitates a quantitative approach to studying tissue hydration while providing the rich biochemical information found in the fingerprint region. Here, the ability of this system to quantitatively assess water content is validated in a gelatin-based tissue mimicking phantom. The utility of this system for in vivo measurement is demonstrated using a mouse model of pregnancy, in which the addition of the high wavenumber region facilitates analysis of water-tissue interactions in the cervix. Accessing both the fingerprint and high wavenumber regions in a simplified probe-based system makes it possible to obtain a full biochemical picture of the cervical remodeling process during pregnancy.
2. Materials and methods
2.1 Raman spectroscopy system
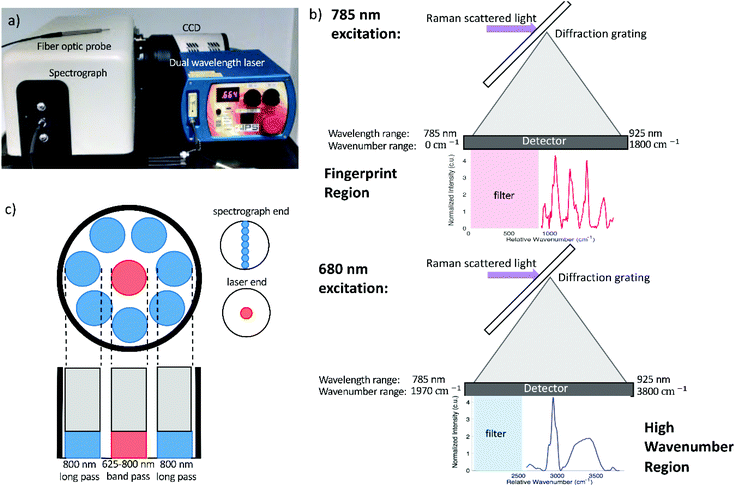
Raman spectra were acquired using a fiber optic probe-based Raman system. The Raman probe (developed in collaboration with EmVision, LLC) includes inline filtering to allow acquisition of both fingerprint and high wavenumber spectra and consists of seven 300-micron diameter collection fibers (0.22 NA) surrounding a single 300-micron excitation fiber (0.22 NA) (Fig. 1c).The excitation fiber includes a broad bandpass filter, allowing both the 680 and 785 nm excitation to reach the sample while preventing extraneous wavelengths of light from being transmitted. A ring-shaped 800 nm long pass filter is located at the distal tip of the collection fibers, which prevents the majority of backscattered laser light from being collected (Fig. 1d). The excitation fiber delivers 680 nm or 785 nm excitation light to the sample, with a spot size of approximately 2.05 μm diameter at the surface of the sample. The collection fibers then deliver the backscattered light to an imaging spectrograph (Holospec f/1.8i, Kaiser Optical Systems) which disperses the light onto a thermoelectrically cooled, deep depleted CCD camera (Pixis 256BR, Princeton Instruments). The system, which has a spectral resolution of approximately 7 cm−1, is controlled with a laptop and custom LabView program. The entire system is portable and fits on top of a cart (Fig. 1a) for easy transport.
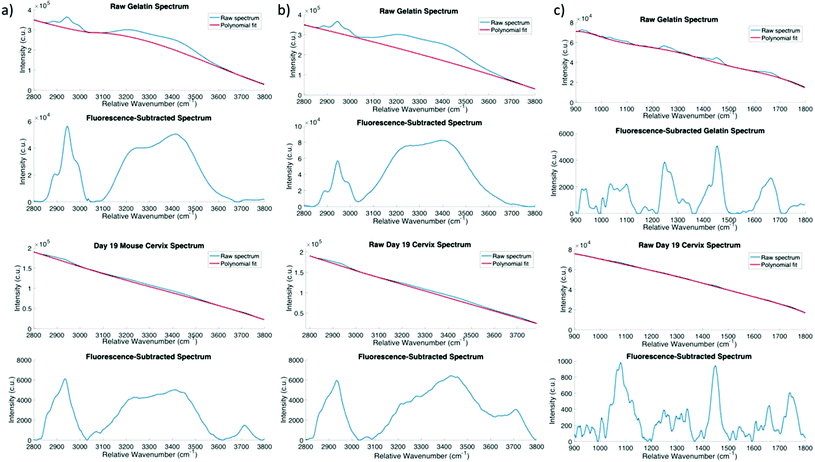
The laser (Innovative Photonics Solutions) consists of a 680 nm and 785 nm laser diode packaged in a single unit, and is able to switch between these wavelengths by flipping a switch on the front panel of the unit. Each diode is a Hybrid External Cavity Laser (HECL), which is based is based upon a semiconductor diode, and a Volume Brag Grating that are configured in a coupled cavity configuration to provide high levels of power and wavelength stability. A dichroic beam combiner is used to geometrically combine the 680 and 785 nm wavelengths. The maximum power output is 233 mW at 680 nm and 466 mW at 785 nm, and the maximum wavelength shift at these powers is 0.05 nm (1 cm−1). The HECL lasers shift with temperature at a rate of 0.007 nm °C−1. The spectral linewidth of both wavelengths is 0.1 nm or less. The spectrograph's diffraction grating disperses wavelengths from 785 nm to 925 nm onto the CCD. When 785 nm excitation is used, this wavelength range corresponds to Raman shifts of 0 to 1800 cm−1, allowing the fingerprint region of the Raman spectrum to be captured by the detector. When the sample is excited using 680 nm light, the wavelengths captured by the detector correspond to Raman shifts ranging from 1970 to 3800 cm−1, enabling visualization of the high wavenumber region. This detection scheme is depicted in Fig. 1b.
High wavenumber spectra were calibrated using the spectral peaks of a neon argon lamp, 4-acetamidophenol, and cyclohexane. Fingerprint region spectra were calibrated using a neon argon lamp, 4-acetamidophenol, and naphthalene. The spectral response of the system was initially determined using a NIST calibrated tungsten lamp, and subsequent daily calibration was performed using a piece of green glass which also has a broadband spectrum.
2.2 Phantom validation
Gelatin phantoms were prepared by adding gelatin powder to deionized water and stirring over low heat until all gelatin was dissolved. Gels were allowed to cool at room temperature for approximately two hours prior to measurement. Gelatin phantoms with known concentrations of 33.33%, 30.77%, 28.57%, 26.67%, 25%, 23.53%, 22.22%, 21.05%, 20%, 19.05%, 18.18%, 17.39%, 16.67%, 15.38%, 14.29%, 13.33%, 13%, 11.76%, 10.53%, 9.52% and 9.09% gelatin were prepared. This produced a range of 66.67%–90.91% water, which is representative of the percent water in most human tissues, with the exception of bone and adipose tissue.39 For spectral measurements, the fiber optic probe was placed in gentle contact with the surface of the gel, and spectra were acquired using a 1 second acquisition time with 10 accumulations and 80 mW incident power for both the high wavenumber region (680 nm excitation) and fingerprint region (785 nm excitation). Though these excitation parameters exceed the maximum permissible exposure for skin, a maximum temperature rise of 0.17 °C was calculated40 for the given excitation conditions in gelatin phantoms. The temperature rise was calculated as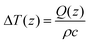 where Q(z) = μaHoe−μaz = μaH(z) where ρ is the density in kg m−3 and c is the specific heat (J (kg K)−1) of the irradiated material.40 Further, the phantom composition is not expected to change significantly during the time required to acquire sequential fingerprint and high wavenumber spectra. Measurements were taken from 4 different locations on the surface of the gels to account for any heterogeneity, and were then averaged.
where Q(z) = μaHoe−μaz = μaH(z) where ρ is the density in kg m−3 and c is the specific heat (J (kg K)−1) of the irradiated material.40 Further, the phantom composition is not expected to change significantly during the time required to acquire sequential fingerprint and high wavenumber spectra. Measurements were taken from 4 different locations on the surface of the gels to account for any heterogeneity, and were then averaged.
2.3 In vivo mouse cervix
In vivo Raman spectra were acquired from the cervices of five virgin nongravid (not pregnant) CD-1 wild type mice (Charles River Labs) as well as five wild type mice on day 19 of pregnancy. Timed matings were performed by placing 2–3 females with stud males overnight in a facility with 0700–1900 light–dark cycles. Day 1 of pregnancy was designated as the presence of a copulatory plug. Full-term gestational length is 19.5 days in our colony. All mouse experiments were conducted using protocols approved by Vanderbilt University Medical Center's Institutional Animal Care and Use Committee (IACUC) and in accordance with the regulations set forth in the NIH Guide for the Care and Use of Laboratory Animals. Cell smears obtained from vaginal lavage of nongravid mice were examined under a microscope to determine the stage of the estrous cycle; only mice in the diestrous phase were used for Raman spectral acquisition. All room lights were turned off during measurements, and mice were anesthetized with isofluorane during this procedure. A small plastic speculum tube was used to visualize the mouse cervix and place the fiber optic probe in gentle contact with the cervix. Spectra were acquired using a 0.5 second acquisition time and 10 accumulations at 80 mW incident power. Though these excitation parameters exceed the maximum permissible exposure for skin, a maximum temperature rise of 0.08 °C was calculated40 for the given excitation conditions in mouse cervix. The temperature rise was calculated as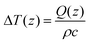 where Q(z) = μaHoe−μaz = μaH(z) where ρ is the density in kg m−3 and c is the specific heat (J (kg K)−1) of the irradiated material.40 Further, the tissue composition is not expected to change significantly during the time required to acquire sequential fingerprint and high wavenumber spectra, and any spectra which were affected by the movement of the mouse were discarded. Measurements were taken from 3–5 locations on the ectocervix for both the fingerprint and high wavenumber regions. The mice were then euthanized, and the cervix excised and weighed immediately to determine the wet weight. After acquiring ex vivo spectra using the same acquisition parameters, the cervix was lyophilized overnight and weighed to determine the dry weight. The percent water of each cervix was calculated as wet weight minus dry weight divided by wet weight. A nonnegative least squares biochemical model was used to determine the relative contributions of fat, water, and collagen to the measured cervix spectra. Cervix spectra with a fat coefficient greater than 1 were discarded. The remaining spectra from various locations in each mouse cervix were then averaged for both the in vivo and ex vivo spectra.
where Q(z) = μaHoe−μaz = μaH(z) where ρ is the density in kg m−3 and c is the specific heat (J (kg K)−1) of the irradiated material.40 Further, the tissue composition is not expected to change significantly during the time required to acquire sequential fingerprint and high wavenumber spectra, and any spectra which were affected by the movement of the mouse were discarded. Measurements were taken from 3–5 locations on the ectocervix for both the fingerprint and high wavenumber regions. The mice were then euthanized, and the cervix excised and weighed immediately to determine the wet weight. After acquiring ex vivo spectra using the same acquisition parameters, the cervix was lyophilized overnight and weighed to determine the dry weight. The percent water of each cervix was calculated as wet weight minus dry weight divided by wet weight. A nonnegative least squares biochemical model was used to determine the relative contributions of fat, water, and collagen to the measured cervix spectra. Cervix spectra with a fat coefficient greater than 1 were discarded. The remaining spectra from various locations in each mouse cervix were then averaged for both the in vivo and ex vivo spectra.
2.4 Data processing and analysis
The general approach used to remove fluorescence background in the measured spectra was first described by Lieber et al.,41 and has since been widely used to subtract fluorescence from Raman spectra.42–47 Briefly, this technique fits a polynomial of a specified degree to the raw spectrum consisting of both Raman features and fluorescence background. After the initial fit, any points for which the polynomial fit value is greater than the intensity of the measured spectrum are reassigned to their initial measured value. The fitting procedure is then repeated, this time fitting to the modified spectrum, until the number of data points being reassigned to their original value converges. The fluorescence background within high wavenumber spectra was removed using a segmented version of the automated polynomial fit procedure described above.41 The lower (2600–3035 cm−1) region was fit using a third order polynomial. For the upper region of the spectrum, a third order polynomial was fit to the regions directly above and below the water peak (3020–3080 cm−1 and 3600–3800 cm−1) and this fit was extrapolated over the entire upper region of the spectrum (3035–3850 cm−1). In this manner, the fluorescence background was sufficiently subtracted from the Raman spectra without overfitting to the broad water peak, as shown in Fig. 2a. While previous reports of high wavenumber Raman spectroscopy have used a linear fit to subtract fluorescence from high wavenumber spectra, as shown in Fig. 2b, this method of fluorescence subtraction does a poor job of fitting to the in vivo cervix spectra. Recent work characterizing the fluorescence background in high wavenumber Raman spectra of biological tissue demonstrated that 3rd order polynomial baseline fitting resulted in the lowest error in estimated water content compared to higher or lower degree polynomial fits.48 Fingerprint spectra were fit using the modified polynomial method with a 7th order polynomial to remove fluorescence background in gelatin phantoms and a 7th order polynomial in cervix spectra. Savitsky–Golay smoothing (3rd order, frame length of 11 pixels) was applied to all spectra to reduce noise, as described previously.41Fingerprint spectra for gelatin phantoms were not normalized, whereas cervix fingerprint spectra were mean normalized to account for variability among different mice. High wavenumber spectra were normalized for visualization purposes in order to remove sample-to-sample variability and to show changes in water content relative to other molecules present in the sample. High wavenumber spectra of gelatin were normalized to the –C–H stretch of protein at approximately 2940 cm−1, while high wavenumber cervix spectra were mean normalized in order to visualize the fingerprint and high wavenumber regions on the same set of axes. However, all subsequent analysis was performed using un-normalized, fluorescence subtracted spectra.
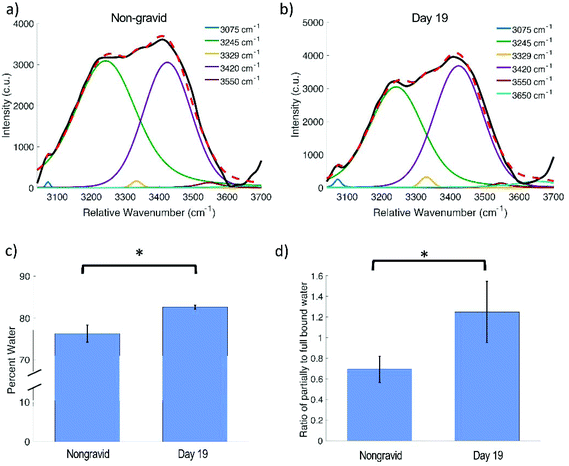
For the gelatin phantoms, data were analyzed by taking the ratio of the area under the curve of the water peak (3035 to 3680 cm−1) to the area under the curve of the entire high wavenumber spectrum (2850 to 3680 cm−1) for the average spectrum at each concentration, and then plotted as a function of the known percent water. All ratios were scaled such that the phantom comprised of 67% water had a ratio of exactly 0.67, corresponding to the 67.85% water that comprises the human body.39 A first order polynomial was then fit to the data. In a secondary analysis, five Voigtian curves with specified center wavenumbers were fit to the high wavenumber water peak from 3025 to 3680 cm−1 using nonlinear least squares fitting. Raman spectral peaks are best represented by Voigtian curves, which are a convolution of a Gaussian and a Lorentzian profile. The model adjusts the widths of the Gaussian and Lorentzian curves being convolved to obtain the Voigtians that best fit the data. The peak center locations were set to 3075 cm−1, 3245 cm−1, 3420 cm−1, 3550 cm−1, and 3650 cm−1, corresponding to DAA, DDAA, DA, DDA, and free water vibrational modes, respectively.15 The NH stretch at 3329 cm−1 was also incorporated into the model to account for any protein contribution in this region (Table 1).13 The same Voigtian fitting method was used for the high wavenumber spectra acquired from the in vivo mouse cervix. Statistical significance was determined using a two-sided two sample t-test (p < 0.05 significance level).
3. Results
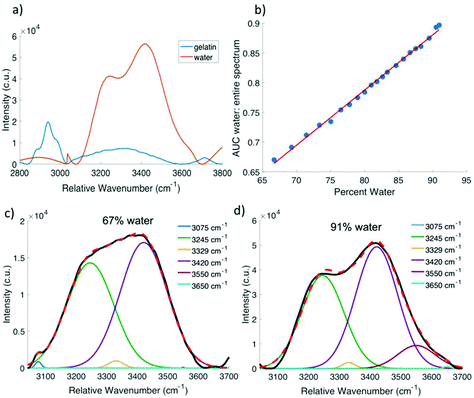
The developed fingerprint and high wavenumber system has a signal to noise ratio of 5.02 in the fingerprint region based on the measured peak of phenyalanine at 1006 cm−1 in cervix spectra, and 2.57 in the high wavenumber region based on the 3400 cm−1 peak of water. Peak ratios calculated based on repeated measurements of acetaminophen acquired on different days were used to assess the reproducibility and stability of the system. The variability in these measurements was 1.98% in the fingerprint region, and 0.54% in the high wavenumber region. The dual wavelength fiber optic probe has an approximately 94% collection efficiency, with an approximate excitation spot size of 205.40 μm at the surface of the sample. While the optical properties at the exact excitation wavelengths reported here were not available in the literature, measured optical properties of normal cervix at 674 nm and 811 nm have been previously reported.49 Monte Carlo modeling to show the propagation of excitation light from a 300 μm diameter fiber given the optical properties of cervical tissue yielded a 4.6 mm penetration depth of the excitation light at 811 nm compared to the penetration depth of 4.1 mm at 674 nm. Therefore, these two wavelengths differ in their penetration depths by 500 μm, and we expect this difference to be even lower for the wavelengths used in this system. This probe achieves approximately a 16.24 mm3 sampling volume at 811 nm excitation and a 13.30 mm3 at 641 nm excitation. As the Raman probe obtains a bulk, volumetric measurement, the lateral and spatial resolution are governed by this sampling volume.Gelatin-based tissue-mimicking phantoms were measured to demonstrate the utility of combined fingerprint and high wavenumber Raman spectroscopy for determining the biochemical composition of a sample, including quantitative hydration information. Gelatin phantoms of varying concentrations were measured to establish a correlation between the high wavenumber water peak and known water content. Spectral peaks in the fingerprint region (Fig. 3a) decreased in amplitude as the gel concentration decreased and water content increased. As gelatin is comprised primarily of denatured collagen, the peaks observed in the fingerprint region were primarily associated with protein and collagen, such as phenylalanine50 at 1003 cm−1, amide III51 at approximately 1247 cm−1, CH3 bending and CH2 scissoring52 at 1454 cm−1, and amide I51 at approximately 1665 cm−1. The high wavenumber spectra of gelatin phantoms were normalized to the C–H stretch of protein53 at approximately 2940 cm−1 in order to show changes in water content relative to gelatin.
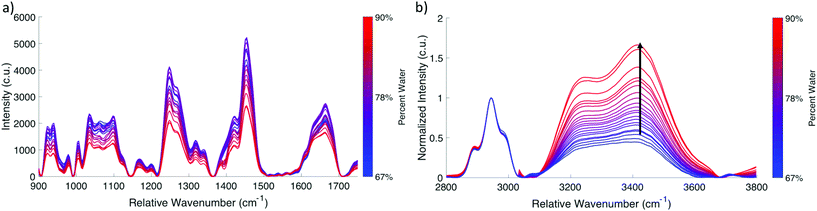 | ||
| Fig. 3 Gelatin phantom validation. (a) Fingerprint spectra. (b) High wavenumber spectra (normalized to 2940 cm−1 CH stretch, n = 4). | ||
Fig. 3b shows that as the water content of the gelatin phantom increased, the water peak increased in amplitude, indicating that high wavenumber Raman spectroscopy is sensitive to changes in water content of a gel-based phantom. In an effort to derive quantitative information regarding water content from the Raman spectrum, the ratio of the area under the water peak from 3035 cm−1 to 3680 cm−1 to the area under the entire high wavenumber spectrum from 2850 cm−1 to 3680 cm−1 was calculated and plotted as a function of the known percent water in each gel. This ratio was scaled such that the 67% water phantom corresponded to a ratio value of 0.67. Fig. 4b shows that this relationship is approximately linear with an R2 value of 0.9963. Voigtian fitting of the high wavenumber water peak for a phantom that is 67% water (Fig. 4c) and a phantom that is 91% water (Fig. 4d) shows a shift in the distribution of free and bound water molecules. The 91% water phantom is dominated by partially hydrogen bound water molecules, whereas the 67% water phantom shows a more equal distribution of fully hydrogen bound and partially hydrogen bound water molecules.
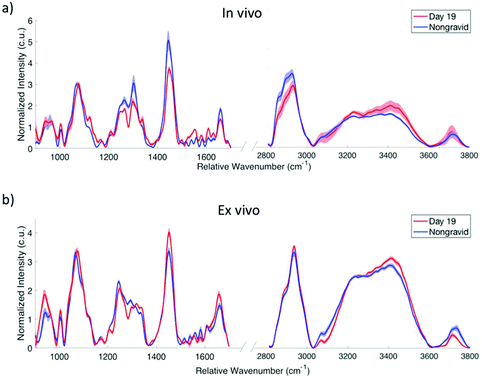
The combination of fingerprint and high wavenumber Raman spectroscopy has the ability to provide a full biochemical picture of the changes which occur as the cervix remodels during pregnancy. In the fingerprint spectra acquired from in vivo mouse cervix (Fig. 5a), the 1445 cm−1 peak associated with CH2/CH3 deformation in collagen is observed to decrease in day 19 mice compared to nongravid. The amide III peak at 1267 cm−1 as well as the 1303 cm−1 peak associated with CH2/CH3 twisting54 are lower in intensity in the day 19 mice, while the 1079 cm−1 peak associated with C–C stretching51 is higher in intensity in day 19 cervices. These results are consistent with previously published fingerprint Raman spectroscopic studies of biochemical changes in the mouse cervix during pregnancy.2Ex vivo results in the fingerprint region (Fig. 5b) demonstrate a similar change in the 1079 cm−1 peak, while the 1445 cm−1 peak appears to decrease in nongravid mice compared to day 19. In the high wavenumber region, the shoulders at approximately 2860 cm−1 and 2890 cm−1 are associated with CH stretch vibrations and are more pronounced in the in vivo nongravid spectra compared to the day 19.
An increase in the amplitude of the water peak is observed in day 19 mice in both the in vivo and ex vivo spectra. Voigtian decomposition of the in vivo high wavenumber water peak shows a shift in the distribution of water molecules between nongravid (Fig. 6a) and day 19 (Fig. 6b) mouse cervices. The percent water in the cervix as determined based on the wet and dry weights (found in ESI Table 4†) was found to be significantly lower in nongravid mice compared to day 19 (p = 0.0129), as shown in Fig. 6c. Nongravid mouse cervices had a mean percent water of 76.34%, while day 19 cervices had a mean of 82.65%. These values are in agreement with previous measurements of cervical hydration demonstrating increased water content in the pregnant cervix.2,29,32 Further, there is a statistically significant difference between the ratio of the area under the Voigtian peaks associated with fully hydrogen bound water molecules (centered at approximately 3075 and 3245 cm−1) and the area under the Voigtian peaks associated with partially hydrogen bound water molecules (centered at approximately 3433 and 3562 cm−1) in nongravid and day 19 mouse cervices (p = 0.0496) as shown in Fig. 6d. The average ratio of fully hydrogen bound water to partially hydrogen bound water was 0.70 ± 0.12 for nongravid mouse cervix, and 1.24 ± 0.29 for day 19 mouse cervix.
4. Discussion
A system has been developed which can use 680 and 785 nm excitation light to map the high wavenumber and fingerprint region of the Raman spectrum, respectively, onto a silicon detector. Many Raman spectroscopy systems for biomedical applications utilize a silicon CCD for detection paired with excitation at approximately 785 nm to acquire spectra in the fingerprint region. However, this configuration is not conducive to high wavenumber Raman spectroscopy due to the limited sensitivity of the silicon detector at wavelengths exceeding 900 nm. The dual excitation wavelength approach is simpler and more cost effective than previously developed combined fingerprint and high wavenumber Raman spectroscopy systems, which typically utilized specialized detectors or gratings. Further, the use of dual excitation wavelengths results in an improvement in spectral resolution compared to previously reported systems which rely on a customized grating11 while circumventing the falloff in sensitivity associated with longer wavelengths. One limitation of the dual wavelength approach lies in the slightly different penetration depths and sampling volumes interrogated in each region. While this difference is not believed to significantly impact our results, future work to develop a correction factor or adjust the laser output to compensate for this effect may allow direct comparison between the fingerprint and high wavenumber regions. Furthermore, the Raman scattered light which is detected by our system falls within the same wavelength range (approximately 785–925 nm) regardless of the excitation wavelength used. Although the penetration depth achieved by the two excitation wavelengths differ, the resulting Raman scattered photons will propagate in the same manner.With the addition of a diode laser capable of producing two different wavelengths of light, Raman systems developed for fingerprint application can be easily adapted to capture both the fingerprint and high wavenumber regions. Though this is not the first report of a combined fingerprint and high wavenumber system utilizing dual excitation wavelengths, to our knowledge it is the first report of such a system using a single laser module coupled to a fiber optic probe. While the system developed by Puppels et al. has been demonstrated for water quantification based on high wavenumber spectra in a variety of tissues, and in fact reports superior spectral resolution (<5 cm−1), this confocal microspectroscopy configuration is limited to skin and ex vivo applications.13 The ability to implement this approach in a probe-based configuration is critical to enable in vivo applications, such as the investigation of cervical remodeling. This work validates the use of combined fingerprint and high wavenumber Raman spectroscopy to quantitatively probe molecular interactions of water as well as other biochemical changes in a gelatin phantom, then demonstrates the utility of using both spectral regions to understand cervical change during pregnancy.
The results obtained from gelatin phantoms demonstrate the feasibility of using high wavenumber Raman spectroscopy to assess water content in tissues and materials. It has previously been shown that the ratio of the area under the water peak from 3350 to 3550 cm−1 to the area under the protein peak correlates with known water content in skin,13 and that the water content calculated using this method enables discrimination of oral cancers from normal tissue.20 High wavenumber Raman spectroscopy has also shown increased water content in dysplastic cervical tissue compared to normal cervix.55 Such results motivate the use of a spectral metric to determine water content noninvasively. As tissue composition varies widely from one organ to another, the spectral ratio which is best suited to quantifying water content may also vary depending upon the application. The results of this work indicate that the ratio of the area under the water peak (3035 cm−1 to 3680 cm−1) to the area under the entire high wavenumber spectrum (from 2850 cm−1 to 3680 cm−1) correlates linearly with known sample water content in gelatin phantoms (R2 = 0.9963) (Fig. 4b). In its current form, this model assumes the same Raman scattering cross section for the O–H bonds that comprise the water band and the C–H bonds that comprise the majority of the remainder of the high wavenumber spectrum. The accuracy of this metric would likely be improved by accounting for differences in the Raman scattering cross sections. Analysis of gelatin phantom spectra using the previously reported ratio13 of water at 3350–3550 cm−1 to protein at 2910–2965 cm−1 displayed a lower correlation with known water content (R2 = 0.8397) (ESI Fig. 2†). However, this ratio accounts only for the portion of the water peak corresponding to partially bound water, whereas the higher correlation of the ratio we report here may result from accounting for the changing spectral contributions of partially bound, fully bound, and free water. As the water content of the phantoms increases, the high wavenumber water peak increases in amplitude and protein-associated signatures in the fingerprint region decrease in amplitude (Fig. 3a and b). The ratio of the area under the water peak to the area under the entire high wavenumber spectrum is able to capture these associated changes in water and protein concentration to accurately quantify water content.
Analysis of the spectral shape of the water peak and decomposition into its component peaks reveals the distribution of free, partially bound, and fully bound water molecules. Previous work has explored the impact of temperature and pressure on the location, intensity, and width of the individual peaks that comprise the water band in pure water samples.15,56,57 This approach has also been used to tease out the interactions between water molecules and lipids and proteins as a function of depth in the skin,58 suggesting that it may be possible to learn more about the molecular interactions of water in the pregnant cervix using this technique. Most previous studies have used Gaussian fitting to determine the contribution of individual peaks to the overall high wavenumber water band. However, a Voigtian profile, which is a convolution of a Gaussian and Lorentzian function, has been shown to accurately reconstruct Raman spectral features,59 and as such was used in our model. A comparison of the spectrum of pure water to that of pure gelatin revealed a small but noticeable contribution from NH2 in gelatin within the same region as the high wavenumber water peak (Fig. 4a), leading to the incorporation of the NH2 peak into the Voigtian fitting model to improve its accuracy. In gelatin phantoms, the ratio of the area under the Voigtian peaks associated with fully hydrogen bound water molecules to the area under the peaks associated with partially hydrogen bound water molecules decreased as the water content increased. As the ratio of water to gelatin in the phantom increases, there may be fewer sites available on the gelatin molecules for water to interact and bind, thus contributing to the observed shift toward partially bound and free water. This phenomenon has also been observed in the skin, where unbound water increased when all protein sites for water binding were occupied.60
Previous Raman spectroscopy studies have shown that the fingerprint region is sensitive to changes in collagen and lipid content as well as vascularity of the cervix during pregnancy.2,36 Though decreased amplitude of fingerprint peaks associated with collagen and protein content were observed in day 19 mice compared to nongravid, prior work has indicated that total collagen content of the cervix remains constant during the remodeling process.29,61 This observed decrease is likely due to the increasing hydration of the cervix, which results in a net decrease in collagen concentration.29 All studies of cervical hydration to date have used ex vivo approaches, such as a comparison of dry versus wet weight, to determine changes in cervix water content during pregnancy. Such work has indicated that the cervix undergoes an increase in hydration, mediated by hydrophilic molecules such as hyaluronic acid and glycosaminoglycans, as well as an upregulation of aquaporins, during pregnancy.32 Combined fingerprint and high wavenumber Raman spectroscopy is capable of quantitatively assessing cervical hydration in vivo while simultaneously monitoring other biochemical changes. In this study, day 19 mice were measured on the morning of their nineteenth day of gestation. As the mice were sacrificed following in vivo Raman measurements, the exact time until delivery is unknown. The cervix changes rapidly during the ripening and dilation phases, which occur in the hours leading up to delivery in mice.27 Therefore, differences in the stage of the ripening or dilation process for each mouse may be responsible for the variability observed in the distribution of free and bound water for day 19 cervices, as shown in Fig. 6d. Nonetheless, a clear difference in the water peak intensity and a significant change in distribution between free and bound water is observed. The addition of the high wavenumber region facilitates in vivo assessment of cervical hydration change during pregnancy, indicating a clear increase in water content using mean normalized spectra (Fig. 5a). Additionally, a significantly higher contribution of partially hydrogen bound water molecules is observed compared to fully bound water molecules in the cervices of day 19 mice (Fig. 6d). This suggests that as the water content of the cervix increases during pregnancy and the amount of collagen remains constant, the number of available hydrogen binding sites on collagen and other extracellular matrix molecules decreases, similar to the trend that is observed in the gelatin phantoms. This shift in the interaction of water molecules with cervical tissue may serve as a biomarker of healthy cervical remodeling.
High wavenumber peaks associated with lipid molecules are more pronounced in the in vivo spectra of nongravid mice compared to in vivo day 19, which may be indicative of increased phospholipids in the nongravid cervix. Lipid signal from fat pads surrounding the cervix may also contribute to changes in these signatures. The nongravid cervix, at approximately 3 mm in diameter, is smaller than the pregnant cervix. The fiber optic probe, which is 2.1 mm in diameter, is more likely to collect Raman scattered photons from the surrounding tissue in nongravid mice, especially when taking measurements from the outer circumference of the cervix. We hypothesize that the primary differences between the in vivo and ex vivo spectra shown in Fig. 5a and b are the result of lipid contribution from the surrounding fat pads in vivo. The use of a fiber optic probe configuration capable of collecting information from superficial depths within the mouse cervix while minimizing signal contamination from tissue behind and surrounding the cervix should eliminate the majority of these differences between in vivo and ex vivo spectra. The peak observed at approximately 3712 cm−1 in both the in vivo and ex vivo cervix spectra does not appear to be an artifact of fluorescence subtraction in preprocessing, although the origin of this peak is yet unclear. Some have reported the existence of a dangling OH bond responsible for a peak observed beyond the primary OH band in the high wavenumber region, though the reported position of this peak varies and further investigation is needed to confirm its source.62,63
The ability to measure hydration of tissues in vivo is essential to obtain an accurate measurement, as tissue water content will begin to change as soon as the tissue is removed from the body. High wavenumber Raman spectroscopy provides a comprehensive understanding of how hydration impacts the structure and function of biological tissues such as the cervix,64 and fingerprint Raman spectroscopy assesses the effects of other biochemical components. By probing the various vibrational hydrogen bonds associated with water, we can tease out more specific information about the dynamics of hydration in addition to the amount of hydration. This simplified fingerprint and high wavenumber system shows great utility for rapidly and noninvasively providing a full biochemical picture of any sample of interest, including tissues such as cervix, bone, skin, and others. This simplified dual wavelength, probe-based system for fingerprint and high wavenumber Raman spectroscopy will facilitate wider application of Raman spectroscopy by combining the rich biochemical signatures, such as proteins, lipids, and nucleic acids, found in the fingerprint region with information regarding water-tissue interactions from the high wavenumber region.
5. Conclusions
A system using two different excitation wavelengths and a single fiber optic probe has been developed for simultaneous fingerprint and high wavenumber Raman spectroscopy. This configuration is cost effective, user-friendly, and ideally suited for in vivo biomedical applications. The addition of the high wavenumber region facilitates quantitative analysis of tissue water content in addition to the biochemical signatures found in the fingerprint region. The efficacy of this approach has been demonstrated to quantify water content and evaluate molecular interactions of water in tissue-mimicking phantoms. This system was then used to identify changes in the distribution of water molecules in the mouse cervix between nongravid mice and mice at the end of pregnancy. This approach has the potential to provide precise in vivo tissue water quantification to be used in a wide range of applications. Combined fingerprint and high wavenumber Raman spectroscopy in a dual excitation system can improve the accuracy of Raman spectroscopy for diagnostic applications and will facilitate a better understanding of water-tissue interactions in physiological processes.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Bibhash Paria and Naoko Brown for their help in executing mouse experiments, and Scott Rudder of Innovative Photonics Solutions for his helpful information regarding the laser design. This work was supported by the National Institutes of Health in part by the National Institute of Biomedical Imaging and Bioengineering training grant T32EB021937 (LEM), and NIH R01 HD081121 (JR and AMJ). This work was also supported in part by the Netherlands Organisation for Scientific Research (NWO)-SPORT 12864 (TVL).References
- A. S. Haka, Z. Volynskaya, J. A. Gardecki, J. Nazemi, R. Shenk, N. Wang, R. R. Dasari, M. Fitzmaurice and M. S. Feld, J. Biomed. Opt., 2009, 14, 54023 CrossRef PubMed.
- C. M. O'Brien, J. L. Herington, N. Brown, I. J. Pence, B. C. Paria, J. C. Slaughter, J. Reese and A. Mahadevan-Jansen, Sci. Rep., 2017, 7, 6835 CrossRef PubMed.
- R. E. Kast, G. K. Serhatkulu, A. Cao, A. K. Pandya, H. Dai, J. S. Thakur, V. M. Naik, R. Naik, M. D. Klein, G. W. Auner and R. Rabah, Biopolymers, 2008, 89, 235–241 CrossRef CAS PubMed.
- C. A. Lieber, S. K. Majumder, D. L. Ellis, D. D. Billheimer and A. Mahadevan-Jansen, Lasers Surg. Med., 2008, 40, 461–467 CrossRef PubMed.
- L. Franzen, Adv. Drug Delivery Rev., 2015, 89, 91–104 CrossRef CAS PubMed.
- F. LaPlant, Lasers, Spectrographs, and Detectors, Springer, Berlin, Heidelberg, 2010, pp. 1–24 Search PubMed.
- C. M. Penney, L. M. Goldman and M. Lapp, Nat. Phys. Sci., 1972, 235, 110–112 CrossRef CAS.
- F. Adar, S. Atzeni, J. R. Gilchrist, L. Goldstone and J. Noonan, Optoelectron. World.
- Y. Kamata, S. Miyazaki, M. Muramatsu, H. Suzuki, K. Miyaguchi, T. G. Tsuru, S. Takagi and E. Miyata, International Society for Optics and Photonics, ed. J. D. Garnett and J. W. Beletic, 2004, vol. 5499, p. 210 Search PubMed.
- I. P. Santos, P. J. Caspers, T. Bakker Schut, R. van Doorn, S. Koljenović and G. J. Puppels, J. Raman Spectrosc., 2015, 46, 652–660 CrossRef CAS.
- J. Wang, K. Lin, W. Zheng, K. Yu Ho, M. Teh, K. Guan Yeoh and Z. Huang, Sci. Rep., 2015, 5, 12957 CrossRef CAS PubMed.
- A. H. Chau, J. T. Motz, J. A. Gardecki, S. Waxman, B. E. Bouma and G. J. Tearney, J. Biomed. Opt., 2008, 13, 40501 CrossRef PubMed.
- P. J. Caspers, H. A. Bruining, G. J. Puppels, G. W. Lucassen and E. A. Carter, J. Invest. Dermatol., 2001, 116, 434–442 CrossRef CAS PubMed.
- P. C. Cross, J. Burnham and P. A. Leighton, J. Am. Chem. Soc., 1937, 59, 1134–1147 CrossRef CAS.
- Q. Hu, S. Ouyang, J. Li and Z. Cao, J. Raman Spectrosc., 2017, 48, 610–617 CrossRef CAS.
- M. Unal, S. Yang and O. Akkus, DOI:10.1016/j.bone.2014.07.021.
- M. Unal and O. Akkus, Bone, 2015, 81, 315–326 CrossRef CAS PubMed.
- P. J. Caspers, G. W. Lucassen and G. J. Puppels, Biophys. J., 2003, 85, 572–580 CrossRef CAS PubMed.
- M. Janssens, J. van Smeden, G. J. Puppels, A. P. M. Lavrijsen, P. J. Caspers and J. A. Bouwstra, Br. J. Dermatol., 2014, 170, 1248–1255 CrossRef CAS PubMed.
- E. M. Barroso, R. W. H. Smits, T. C. Bakker Schut, I. ten Hove, J. A. Hardillo, E. B. Wolvius, R. J. Baatenburg de Jong, S. Koljenović and G. J. Puppels, Anal. Chem., 2015, 87, 2419–2426 CrossRef CAS PubMed.
- K. Lin, J. Wang, W. Zheng, K. Y. Ho, M. Teh, K. G. Yeoh and Z. Huang, Cancer Prev. Res., 2016, 9, 476–483 CrossRef CAS PubMed.
- W. Huang, S. Wu, M. Chen, L. Sun, Y. Li, M. Huang, S. Huang, Z. Xu, R. Chen and H. Zeng, J. Raman Spectrosc., 2015, 46, 537–544 CrossRef CAS.
- M. S. Bergholt, K. Lin, J. Wang, W. Zheng, H. Xu, Q. Huang, J. Ren, K. Y. Ho, M. Teh, S. Srivastava, B. Wong, K. G. Yeoh and Z. Huang, J. Biophotonics, 2016, 9, 333–342 CrossRef CAS PubMed.
- S. Duraipandian, W. Zheng, J. Ng, J. J. H. Low, A. Ilancheran and Z. Huang, Anal. Chem., 2012, 84, 5913–5919 CrossRef CAS PubMed.
- R. Word, X.-H. Li, M. Hnat and K. Carrick, Semin. Reprod. Med., 2007, 25, 069–079 CrossRef CAS PubMed.
- J. Vink and K. Myers, Best Pract. Res. Clin. Obstet. Gynaecol. DOI:10.1016/J.BPOBGYN.2018.03.007.
- B. Timmons, M. Akins and M. Mahendroo, Trends Endocrinol. Metab., 2010, 21, 353–361 CrossRef CAS PubMed.
- D. M. Rimmer, J. Endocrinol., 1973, 57, 413–418 CAS.
- C. P. Read, R. A. Word, M. A. Ruscheinsky, B. C. Timmons and M. S. Mahendroo, Reproduction, 2007, 134, 327–340 CAS.
- N. Uldbjerg, G. Ekman, A. Malmström, K. Olsson and U. Ulmsten, Am. J. Obstet. Gynecol., 1983, 147, 662–666 CrossRef CAS PubMed.
- Y. M. Soh, A. Tiwari, M. Mahendroo, K. P. Conrad and L. J. Parry, Endocrinology, 2012, 153, 6054–6064 CrossRef CAS PubMed.
- J. Anderson, N. Brown, M. S. Mahendroo and J. Reese, Endocrinology, 2006, 147, 130–140 CrossRef CAS PubMed.
- K. J. Straach, J. M. Shelton, J. A. Richardson, V. C. Hascall and M. S. Mahendroo, Glycobiology, 2005, 15, 55–65 CrossRef CAS PubMed.
- E. Ducza, A. Csányi and R. Gáspár, Int. J. Mol. Sci., 2017, 18, 2593 CrossRef PubMed.
- E. El Maradny, N. Kanayama, H. Kobayashi, B. Hossain, S. Khatun, S. Liping, T. Kobayashi and T. Terao, Hum. Reprod., 1997, 12, 1080–1088 CrossRef CAS PubMed.
- C. M. O'Brien, E. Vargis, A. Rudin, J. C. Slaughter, G. Thomas, J. M. Newton, J. Reese, K. A. Bennett and A. Mahadevan-Jansen, Am. J. Obstet. Gynecol., 2018, 218, 528.e1–528.e18 CrossRef PubMed.
- C. M. O'Brien, E. Vargis, B. C. Paria, K. A. Bennett, A. Mahadevan-Jansen and J. Reese, Acta Paediatr., 2014, 103, 715–721 Search PubMed.
- E. Vargis, N. Brown, K. Williams, A. Al-Hendy, B. C. Paria, J. Reese and A. Mahadevan-Jansen, Ann. Biomed. Eng., 2012, 40, 1814–1824 CrossRef PubMed.
- H. H. Mitchell, T. S. Hamilton, F. R. Steggerda and H. W. Bean, J. Biol. Chem., 1945, 158, 625–637 CAS.
- J. Wells, P. Konrad, C. Kao, E. D. Jansen and A. Mahadevan-Jansen, J. Neurosci. Methods, 2007, 163, 326–337 CrossRef PubMed.
- C. A. Lieber and A. Mahadevan-Jansen, Appl. Spectrosc., 2003, 57, 1363–1367 CrossRef CAS PubMed.
- H. Daniel, A. M. Gholami, D. Berry, C. Desmarchelier, H. Hahne, G. Loh, S. Mondot, P. Lepage, M. Rothballer, A. Walker, C. Böhm, M. Wenning, M. Wagner, M. Blaut, P. Schmitt-Kopplin, B. Kuster, D. Haller and T. Clavel, ISME J., 2014, 8, 295–308 CrossRef CAS PubMed.
- K. Hamada, K. Fujita, N. I. Smith, M. Kobayashi, Y. Inouye and S. Kawata, J. Biomed. Opt., 2008, 13, 44027 CrossRef PubMed.
- M. V. Schulmerich, J. H. Cole, J. M. Kreider, F. Esmonde-White, K. A. Dooley, S. A. Goldstein and M. D. Morris, Appl. Spectrosc., 2009, 63, 286–295 CrossRef CAS PubMed.
- R. O. P. Draga, M. C. M. Grimbergen, P. L. M. Vijverberg, C. F. P. van Swol, T. G. N. Jonges, J. A. Kummer and J. L. H. Ruud Bosch, Anal. Chem., 2010, 82, 5993–5999 CrossRef CAS PubMed.
- A. Robichaux-Viehoever, E. Kanter, H. Shappell, D. Billheimer, H. Jones and A. Mahadevan-Jansen, Appl. Spectrosc., 2007, 61, 986–993 CrossRef CAS PubMed.
- C. Ricci, C. Eliasson, N. A. Macleod, P. N. Newton, P. Matousek and S. G. Kazarian, Anal. Bioanal. Chem., 2007, 389, 1525–1532 CrossRef CAS PubMed.
- E. M. Barroso, T. C. Bakker Schut, P. J. Caspers, I. P. Santos, E. B. Wolvius, S. Koljenović and G. J. Puppels, J. Raman Spectrosc., 2018, 49, 699–709 CrossRef CAS.
- R. Hornung, T. H. Pham, K. A. Keefe, M. W. Berns, Y. Tadir and B. J. Tromberg, Hum. Reprod., 1999, 14, 2908–2916 CrossRef CAS PubMed.
- A. Mizuno, H. Kitajima, K. Kawauchi, S. Muraishi and Y. Ozaki, J. Raman Spectrosc., 1994, 25, 25–29 CrossRef CAS.
- C. J. Frank, R. L. McCreery and D. C. B. Redd, Anal. Chem., 1995, 67, 777–783 CrossRef CAS PubMed.
- A. Mahadevan-Jansen, M. F. Mitchell, N. Ramanujam, A. Malpica, S. Thomsen, U. Utzinger and R. Richards-Kortum, Photochem. Photobiol., 1998, 68, 123–132 CrossRef CAS PubMed.
- S. Leikin, V. A. Parsegian, W.-H. Yang and G. E. Walrafen, Proc. Natl. Acad. Sci. U. S. A., 1997, 11312–11317 CrossRef CAS.
- C. J. Frank, D. C. B. Redd, T. S. Gansler and R. L. McCreery, Anal. Chem., 1994, 66, 319–326 CrossRef CAS PubMed.
- J. Mo, W. Zheng, J. J. H. Low, J. Ng, A. Ilancheran and Z. Huang, Anal. Chem., 2009, 81, 8908–8915 CrossRef CAS PubMed.
- Q. Sun, Vib. Spectrosc., 2009, 51, 213–217 CrossRef CAS.
- G. E. Walrafen, M. R. Fisher, M. S. Hokmabadi and W.-H. Yang, J. Chem. Phys., 1986, 85, 6970–6982 CrossRef CAS.
- C. Choe, J. Lademann and M. E. Darvin, Analyst, 2016, 141, 6329–6337 RSC.
- T. Sundius, J. Raman Spectrosc., 1973, 1, 471–488 CrossRef CAS.
- R. Vyumvuhore, A. Tfayli, H. Duplan, A. Delalleau, M. Manfait and A. Baillet-Guffroy, Analyst, 2013, 138, 4103 RSC.
- M. L. Akins, K. Luby-Phelps and M. Mahendroo, J. Biomed. Opt., 2010, 15, 26020 CrossRef PubMed.
- H. Abramczyk, B. Brozek-Pluska, M. Krzesniak, M. Kopec and A. Morawiec-Sztandera, Spectrochim. Acta, Part A, 2014, 129, 609–623 CrossRef CAS PubMed.
- P. N. Perera, K. R. Fega, C. Lawrence, E. J. Sundstrom, J. Tomlinson-Phillips and D. Ben-Amotz, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 12230–12234 CrossRef CAS PubMed.
- C. A. Patil, N. Bosschaart, M. D. Keller, T. G. van Leeuwen and A. Mahadevan-Jansen, Opt. Lett., 2008, 33, 1135 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8an01989d |
| This journal is © The Royal Society of Chemistry 2018 |