Open Access Article
Open Access ArticleFast selective trapping and release of picoliter droplets in a 3D microfluidic PDMS multi-trap system with bubbles
Richard W.
Rambach
a,
Preetika
Biswas
ab,
Ashutosh
Yadav
ab,
Piotr
Garstecki
 c and
Thomas
Franke
c and
Thomas
Franke
 *ab
*ab
aSoft Matter and Biological Physics Group, Universität Augsburg, Universitätsstr. 1, D-86159 Augsburg, Germany
bDivision of Biomedical Engineering, School of Engineering, University of Glasgow, Oakfield Avenue, G12 8LT Glasgow, UK. E-mail: Thomas.Franke@glasgow.ac.uk
cInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: garst@ichf.edu.pl
First published on 17th November 2017
Abstract
The selective manipulation and incubation of individual picoliter drops in high-throughput droplet based microfluidic devices still remains challenging. We used a surface acoustic wave (SAW) to induce a bubble in a 3D designed multi-trap polydimethylsiloxane (PDMS) device to manipulate multiple droplets and demonstrate the selection, incubation and on-demand release of aqueous droplets from a continuous oil flow. By controlling the position of the acoustic actuation, individual droplets are addressed and selectively released from a droplet stream of 460 drops per s. A complete trapping and releasing cycle can be as short as 70 ms and has no upper limit for incubation time. We characterize the fluidic function of the hybrid device in terms of electric power, pulse duration and acoustic path.
Introduction
Droplets are a powerful tool in microfluidics due to their ability to act as small sample containers.1 They provide closed entities and are used to encapsulate bacteria, cells, biochemical solutes and other reactants of interest. They are extremely versatile and have been used in a myriad of different applications from cell sorting, drug discovery2 and delivery,3 high-throughput screening,4 and directed evolution of cells and enzymes5 to protein crystallization.6 Droplets provide several advantages over conventional techniques. It is possible to generate and work with a large number of droplets,7 they can hold ultra-small volumes and it is possible to operate complex fluidic handling protocols automatically.8,9 The metering, splitting, merging and moderating of the speed of a single droplet has been impressively demonstrated.10–12Yet, most of the microfluidic devices developed have focussed on the analysis of droplets in arrays (i.e. in batches),13–22 while others operate on single drops one by one in series.23–28 One important feature of droplet manipulation in series is the capture and immobilization of individual droplets, which remains challenging. Existing approaches have been able to capture single droplets13–19,29 and cells30 but the selection of individual droplets/particles in these studies is random. In contrast, the controlled trapping of individual droplets has been shown using small groves in the channel but the system involves only a very small flow or droplet rate10,31 (e.g. 10 μl h−1 for the dispersed phase resulting in about 30 drops per s). The release of trapped droplets has been achieved by flowing an excess continuous phase of oil or by reversing the flow of the trapped droplets.10,14–16,29,32 In a trapping system for giant unilamellar vesicles (GUVs) the continuous phase has to be increased over a critical value for releasing the trapped GUVs,33 which is quite similar to another publication.34 To overcome these limitations, other studies have been made. A system has been shown that is able to trap and release single cancer cells and particles with a hydrodynamic and pressure driven actuation system (one valve per trap), yet with a rather slow performance (e.g. several seconds for loading)35 Very recently, two devices, using surface acoustic waves, have also been demonstrated, yet they similarly suffer from a slow performance and low droplet rate36,37 (about 2–3 drops per s). While controlled trapping and fusion of droplets have been demonstrated using high-voltage electric fields,38,39 these approaches require charged interfaces, a condition that limits their range of applications and is potentially harmful for biological samples. A general method for the controlled trapping and release of multiple picoliter droplets at the microfluidic scale and at high speed has not been shown.
Here we demonstrate the use of surface acoustic waves (SAWs) for the controlled fast trapping, incubation and release of individually selected droplets from a continuously flowing stream with a high rate of 460 drops per s. The system allows us to manipulate individual droplets on demand without disturbing the flow of other droplets or altering the density, temperature or size of the droplets. Each drop can be individually addressed, manipulated and released by selecting the appropriate operation frequency of the SAW and can be incubated for variable incubation times as required.
Fabrication and experimental setup
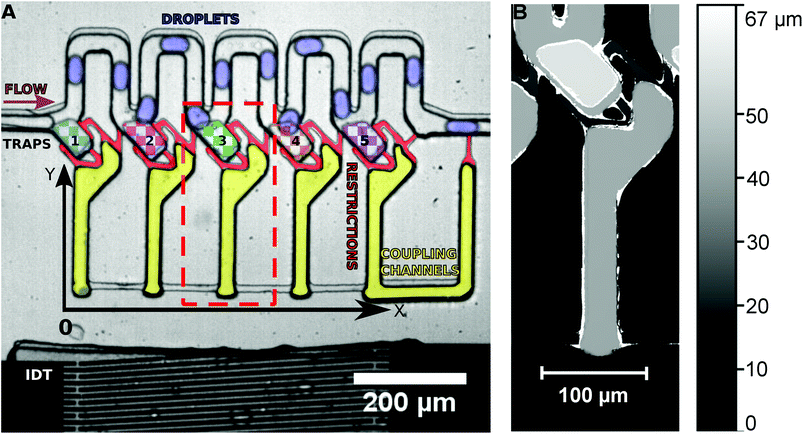
An SU-8 (negative photoresist) 3D structure on a silicon wafer is produced by photolithography to act as a mask for the fabrication of a multilayer PDMS microchannel by soft lithography. A first SU-8 layer of 15 μm thickness is spin coated on a silicon wafer, soft baked, exposed to the mask of the first layer and hard baked. It is then coated with a second layer of SU-8 (15 μm) and soft baked. The mask of the second layer is then aligned with the exposed structures of the first layer and exposure is done followed by hard baking and spin coating of SU-8 (15 μm) for the third layer. The third layer is produced aligning the mask for the third layer with the structures of the second layer as described above. After the hard baking, the SU-8 structure is developed using MR-Dev 600 (Micro Resist Technology GmbH). The final structure fabricated is shown in Fig. 1.The PDMS microchannel produced was bonded with a thin PDMS foil coated on a SU-8 coated Si wafer using O2 plasma, to seal the open channels.
A tapered interdigital transducer (TIDT) with an aperture of 500 μm, a wavelength from 23 to 24.3 μm and 60 finger pairs was used to generate surface acoustic waves (SAWs). It was produced by depositing 100 nm of aluminium on a LiNbO3 (128° y-cut) substrate (17.5 mm × 17.5 mm) with an adhesive layer in between LiNbO3 and aluminium. A frequency generator (SML-01, Rhode & Schwartz) was connected to the IDT along with an amplifier (ZHL-1-2W, Mini Circuits) in between.
The PDMS microchannel was mounted onto the IDT chip and connected to two micro-syringe pumps (PHD2000, Harvard apparatus). The two pumps injected deionised water (dispersive phase) and oil (3M Novec 7500 Engineered Fluid, continuous phase), respectively. The water was stained with Patent Blue (1 mg ml−1), and a surfactant (fluorosurfactant ammonium carboxylate, DuPoint Krytox 157) was added to the oil at a concentration of 0.5 wt%. The setup was observed under an optical microscope (CKX41, Olympus, Germany), and a high speed camera (FASTCAM 1024 PCI, Photron) was used to record the trapping of droplets. For all the results, a flow rate of 350 μl h−1 (continuous phase) and 75 μl h−1 (dispersive phase) was used. The drop rate of the averaged 45 pl droplets was determined to be 460 drops per s and can be calculated using:
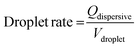 | (1) |
Experiment and results
The multilayer PDMS device is placed on a piezoelectric substrate (lithium niobate) with the produced IDT. The water and the oil flow intersect at the T-junction, where droplets of water are created in the continuous phase of oil. The droplet emulsion flows into the microchannel system from the left side (see Fig. 1), where they pass by the traps without entering them. The traps are loaded on demand by a single acoustic pulse. The droplets in the channels are on average 30 μm in width, 50 μm in length and 30 μm in height. They are in a squeezed shape in the channels as the channel walls prevent a spherical shape. The microchannel system consists of the main channel (30 μm height) where the droplets are travelling, the traps (45 μm height) and the coupling channels (30 μm height) connected by small restriction shunts (15 μm height). As the restrictions have only a height of 15 μm, any trapped droplet would have to considerably deform in order to escape the trap though the restrictions. Thus, the high Laplace pressure required for deformation prevents droplets from squeezing through the restrictions.The frequency generator applies a desired RF signal to the LiNbO3 crystal using the aluminium fingers. The LiNbO3 crystal will vibrate due to the inverse piezoelectric effect and if the spacing between the fingers is half of the wavelength of the RF signal, constructive interference occurs generating a SAW which propagates in the y-direction. The orientation of the LiNbO3 (128° y-cut) guarantees that mainly Rayleigh waves are excited.
The transducer is a tapered IDT (also named slanted IDT), i.e. the spacing between the fingers changes linearly over the aperture. This allows the IDT to be actuated at different spots along the x-direction using different frequencies.
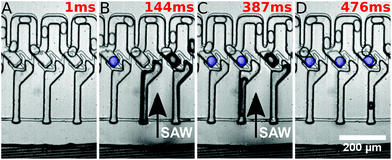
In the initial state, droplets pass by the traps and all traps are empty. By the application of a long SAW pulse (350 ms) in the middle of the PDMS channels (between traps 3 and 4) a bubble is generated, as shown in Fig. 2. When the SAW is turned off at a user defined time (point in time is independent of the positions of the drops), the bubble retreats and droplets enter the traps. All traps are loaded with a single droplet each. The traps being 45 μm in height (in comparison with the 30 μm height of the main channel) provide a room for the droplets to expand and the trapped droplets become spherical in shape. The droplets can be stored in the traps for several minutes.
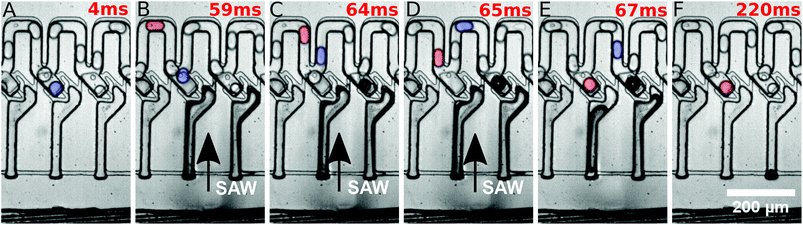
They were then released by on-demand actuation as shown in Fig. 3. With different frequencies the position of the acoustic path can be controlled (compare Fig. 4). Depending on where the SAW hits the coupling channel, the bubble is generated at different locations. It actuates the appropriate trap pushing out the droplet inside the trap. It is then immediately refilled by a subsequent droplet (see Fig. 3).
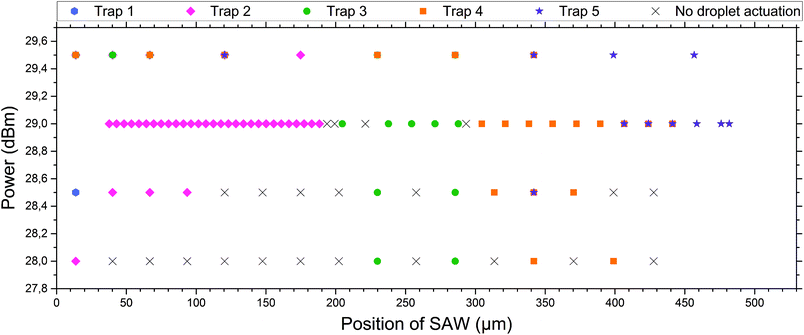 | ||
| Fig. 4 Droplet release in dependence of power and position of the SAW. For different positions (the result of different applied frequencies) the SAW hits the coupling channels of the microfluidic system at different spots and consequently different traps (marked by colours, see the inserted label and compare with Fig. 1) are actuated. The actuation was performed using different powers of SAW. At powers below 29 dBm, not all droplets can be pushed out of the appropriate traps and there are positions where no droplet can be actuated (black crosses). Above 29 dBm multiple traps are actuated by a single SAW pulse and a selective release of single droplets is not possible. At 29 dBm selective release of individual droplets is possible. The first trap cannot be actuated properly during this specific experimental setup, as the IDT was slightly shifted to the right of the microfluidic system, and therefore an actuation of the very left trap was not possible. The errors in the measurements, corresponding to the error of the frequency generator for power and frequency, were very small and thus neglected in the graph. A pulse length of 350 ms was used. | ||
Different powers are used to actuate the droplets in the traps. For powers below 28 dBm, actuation was not possible. For powers of 28 dBm and 28.5 dBm different traps can be actuated and individual droplets can be released, but actuating trap 5 was not possible. For a power of 29 dBm, all traps can be selectively activated and the system is very robust. For a power of 29.5 dBm the traps could be actuated, but not each individually. A single SAW pulse at a distinct position releases two droplets, each of a different trap. The result has been summarised in Fig. 4. It shows the power vs. the position of the traps where the SAW is applied.
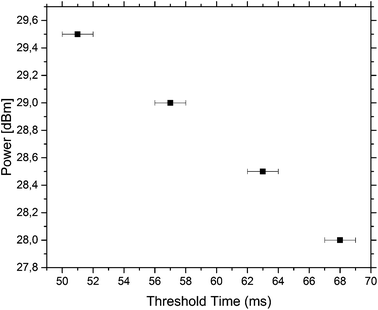
The minimum time needed to actuate the traps was measured for different powers. The results are summarised in Fig. 5. The data were measured for the third trap and it was found that the trap could be actuated with a small pulse length of 57 ms with a power of 29 dBm. At lower powers, the pulse length needed for pushing a droplet out of the third trap increases.
Discussion and conclusions
The applied technique of SAWs is an established practice in the field of microfluidics, and several applications have already been demonstrated40–42 like fluid mixing,43,44 fluid translation,45–48 jetting and atomization,49,50 particle, droplet and cell sorting,51–54 reorientation of nano-objects like carbon nanotubes55 and liquid crystals,56 pumping of fluids57 and microcentrifugation.58,59Here, the generated water droplets in oil, using a T-junction, are directed to the trapping system. The excited SAW propagating on the piezoelectric substrate hits the coupling channel. The position is controlled by the applied frequency. At this location it generates a bubble,60 possibly by heating up the oil.61–68 The bubble grows, while acoustic energy is applied, over time and pushes the oil out of the coupling channels through the restrictions. After the SAW is switched off, the bubble collapses within several milliseconds, inducing a spontaneous high pressure gradient. Droplets are sucked into the traps and each trap is filled with one droplet each.
The traps have a height of 45 μm in contrast to the 30 μm main channel. The channels are 30 μm in width and height. The droplets generated pass through the channels in a squeezed state and when a droplet is sucked into a trap, it expands to a spherical shape and gets trapped. The Laplace pressure generated by deforming into a non-spherical shape hinders droplets from escaping the trap and makes the system more robust. The restrictions in the system have a height of 15 μm, increasing this pressure effect by deforming and guarantee that no droplet passes through a restriction.
The SAW is used to actuate droplets in individual traps. The tapered IDT is actuated at different positions, controlled by the applied frequency, to hit individual traps. The application of the SAW generates a bubble in the coupling channel at the specific location, which pushes the oil into the traps through the restrictions. This in turn pushes the droplet out of the trap. When the SAW is switched off, the bubble collapses and creates a pressure gradient that sucks another droplet into the same trap.
To achieve the actuation of individual droplets, the SAW is switched on in small pulses of 350 ms and a constant power of 29 dBm is used for all measurements. These values are chosen as this combination of power and pulse length works for the setup and all traps can be individually actuated. It should be noted that the third trap can be actuated individually with a much smaller pulse length of 57 ms at a power of 29 dBm but this pulse length cannot actuate all the traps individually. When the power is lowered, the pulse length needed to actuate a droplet increases. As the SAW, generating the bubble by the induced energy, couples into the channel far away from the droplets, potential contents in the drops, like cells, are not directly exposed to the acoustic field and the actuation is non-invasive. This makes this approach ideal for sensitive samples.
It is observed that a higher concentration of the surfactant, leading to a lower surface tension, decreases the robustness of the system. The trapped droplets are then able to squeeze through the restrictions as the Laplace pressure induced by deformation is less. A stable trapping is not possible at much higher surfactant concentrations. This observation is similar to the findings from earlier experiments with simpler channel designs using single-layer PDMS fabrication where a robust and reliable capture was not feasible either.
We have shown a robust system for the fast trapping and selective release of picoliter droplets on demand. This system with a rate of 460 drops per s demonstrates a fast trapping and release of individual droplets in 57 ms. It is for the first time that the controlled release and trapping of individual traps has been demonstrated in a continuous high-speed flow. Most of the previous studies either relied on the surface charged trapping of droplets or trapped droplets randomly based on a probabilistic distribution. None of the existing systems work at high speed flow rates with high drop rates of over 400 drops per s. For other systems, the release of trapped droplets often relies on disrupting the continuous flow and reversing the flow of the continuous phase.
Some of the applications of the present system are obvious, as droplets could be loaded with various contents, including cells, then observed in the traps for a defined time and finally pushed out into the constant flow again. This is particularly useful for examining random samples in a running system. Importantly, the system offered robust and stable operation over a long time (half an hour or more), allowing the holding of the droplets in the observation field over user-defined intervals, even without switching off or altering the continuous flow. During observation we could not measure the shrinkage of drops due to water dissolving in the continuous oil phase or PDMS. However, for extremely long incubation times of several days as necessary for some cell assays, evaporation could be further reduced by applying a water saturated atmosphere as has been demonstrated before in droplet based fluidic systems.69 The ability to manipulate the droplets in multiple traps makes it possible to compare the chemical processes taking place in two or more droplets containing different analytes. This feature could be used e.g. in a quality check at different times for a running experiment. Our approach is non-invasive and biocompatible since the drops are not exposed to acoustic fields directly but are stirred only by the flow and pressure differences caused by an acoustically formed bubble, far away from the droplet sample. Another major advantage of this system is that it is independent of the cargo of the droplets. The device we presented here is a versatile platform for droplet incubation and measurement for continuous fast flowing droplet systems that can simply be used in combination with other operations on the fluidic chip and can be integrated as an additional module of a fluidic chip (μTAS-chip) without interacting with other components on the chip nor interacting with the flow conditions. The acoustic trapping mechanism could be further developed for on-demand trapping of single droplets. This would provide a more controlled way for observing and measuring during a running experiment. If the selective readout of traps is combined with a more complex microfluidic channel design, the sorting of the observed droplets into another outlet is feasible. After trapping, the contents in droplets could be mixed via acoustic streaming induced by a SAW of a lower power and pulse length.70 Also the acoustic merging of two droplets in a modified trap seems feasible in the near future.25 The droplet trap has the potential for further multiplexing. Based on the design we have demonstrated here, the footprint of a single trap is about 0.15 mm2. On a standard microscopic slide of 26 mm × 76 mm, therefore, about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 traps could be integrated. These examples show the broad range of potential applications of this method, which we plan to explore next.
000 traps could be integrated. These examples show the broad range of potential applications of this method, which we plan to explore next.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. W. R. and T. F. acknowledge the support by the “Bayerisches Staatsministerium für Umwelt und Verbraucherschutz”, the German Academic Exchange Service (DAAD) and the Center for NanoScience (CeNS). T. F. particularly thanks the DFG for continuous financial support and the cluster of excellence Nanosystems Initiative Munich (NIM). R. W. R. thanks Michael Heyman and Lloyd Ung for very helpful discussions and hints.References
- S.-Y. Teh, R. Lin, L.-H. Hung and A. P. Lee, Lab Chip, 2008, 8, 198–220 RSC.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS PubMed.
- B. Ziaie, Adv. Drug Delivery Rev., 2004, 56, 145–172 CrossRef CAS PubMed.
- J. J. Agresti, E. Antipov, A. R. Abate, K. Ahn, A. C. Rowat, J.-C. Baret, M. Marquez, A. M. Klibanov, A. D. Griffiths and D. A. Weitz, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4004–4009 CrossRef CAS PubMed.
- M. T. Guo, A. Rotem, J. A. Heyman and D. A. Weitz, Lab Chip, 2012, 12, 2146 RSC.
- B. Zheng, L. S. Roach and R. F. Ismagilov, J. Am. Chem. Soc., 2003, 125, 11170–11171 CrossRef CAS PubMed.
- F. Dutka, A. S. Opalski and P. Garstecki, Lab Chip, 2016, 16, 2044–2049 RSC.
- T. S. Kaminski, O. Scheler and P. Garstecki, Lab Chip, 2016, 16, 2168–2187 RSC.
- M. A. Czekalska, T. S. Kaminski, S. Jakiela, K. Tanuj Sapra, H. Bayley and P. Garstecki, Lab Chip, 2015, 15, 541–548 RSC.
- P. M. Korczyk, L. Derzsi, S. Jakieła and P. Garstecki, Lab Chip, 2013, 13, 4096 RSC.
- V. van Steijn, P. M. Korczyk, L. Derzsi, A. R. Abate, D. A. Weitz and P. Garstecki, Biomicrofluidics, 2013, 7, 24108 CrossRef PubMed.
- L. Derzsi, T. S. Kaminski and P. Garstecki, Lab Chip, 2016, 16, 893–901 RSC.
- S. S. Bithi and S. A. Vanapalli, Biomicrofluidics, 2010, 4, 44110 Search PubMed.
- H. Boukellal, S. Selimović, Y. Jia, G. Cristobal and S. Fraden, Lab Chip, 2009, 9, 331–338 RSC.
- A. Huebner, D. Bratton, G. Whyte, M. Yang, A. J. DeMello, C. Abell and F. Hollfelder, Lab Chip, 2009, 9, 692–698 RSC.
- H. Nuss, C. Chevallard, P. Guenoun and F. Malloggi, Lab Chip, 2012, 12, 5257–5261 RSC.
- C. H. J. Schmitz, A. C. Rowat, S. Köster and D. A. Weitz, Lab Chip, 2009, 9, 44–49 RSC.
- W. Shi, J. Qin, N. Ye and B. Lin, Lab Chip, 2008, 8, 1432–1435 RSC.
- M. Sun, S. S. Bithi and S. A. Vanapalli, Lab Chip, 2011, 11, 3949–3952 RSC.
- Q. Zhang, S. Zeng, J. Qin and B. Lin, Electrophoresis, 2009, 30, 3181–3188 CrossRef CAS PubMed.
- S. S. Bithi and S. A. Vanapalli, Soft Matter, 2015, 11, 5122–5132 RSC.
- Y. Bai, X. He, D. Liu, S. N. Patil, D. Bratton, A. Huebner, F. Hollfelder, C. Abell and W. T. S. Huck, Lab Chip, 2010, 10, 1281 RSC.
- L. Schmid, D. A. Weitz and T. Franke, Lab Chip, 2014, 14, 3710–3718 RSC.
- D. J. Collins, T. Alan, K. Helmerson and A. Neild, Lab Chip, 2013, 13, 3225–3231 RSC.
- M. Sesen, T. Alan and A. Neild, Lab Chip, 2014, 14, 3325–3333 RSC.
- M. Sesen, T. Alan and A. Neild, Lab Chip, 2015, 15, 3030–3038 RSC.
- S. Li, X. Ding, F. Guo, Y. Chen, M. I. Lapsley, S.-C. S. Lin, L. Wang, J. P. McCoy, C. E. Cameron and T. J. Huang, Anal. Chem., 2013, 85, 5468–5474 CrossRef CAS PubMed.
- J. Nam, H. Lim, C. Kim, J. Yoon Kang and S. Shin, Biomicrofluidics, 2012, 6, 24120–2412010 CrossRef PubMed.
- C. Dammann and S. Köster, Lab Chip, 2014, 14, 2681–2687 RSC.
- L. Lin, Y.-S. Chu, J. P. Thiery, C. T. Lim and I. Rodriguez, Lab Chip, 2013, 13, 714 RSC.
- B. Ahn, K. Lee, H. Lee, R. Panchapakesan, L. Xu, J. Xu and K. W. Oh, Lab Chip, 2011, 11, 3915 RSC.
- X. Chen, S. Shojaei-Zadeh, M. L. Gilchrist and C. Maldarelli, Lab Chip, 2013, 13, 3041 RSC.
- A. Yamada, S. Lee, P. Bassereau and C. N. Baroud, Soft Matter, 2014, 10, 5878 RSC.
- P. Abbyad, R. Dangla, A. Alexandrou and C. N. Baroud, Lab Chip, 2011, 11, 813–821 RSC.
- T. Yeo, S. J. Tan, C. L. Lim, D. P. X. Lau, Y. W. Chua, S. S. Krisna, G. Iyer, G. S. Tan, T. K. H. Lim, D. S. W. Tan, W.-T. Lim and C. T. Lim, Sci. Rep., 2016, 6, 22076 CrossRef CAS PubMed.
- M. Sesen, C. Devendran, S. Malikides, T. Alan and A. Neild, Lab Chip, 2017, 17, 438–447 RSC.
- J. H. Jung, G. Destgeer, J. Park, H. Ahmed, K. Park and H. J. Sung, Anal. Chem., 2017, 89, 2211–2215 CrossRef CAS PubMed.
- W. Wang, C. Yang and C. M. Li, Lab Chip, 2009, 9, 1504 RSC.
- W. Wang, C. Yang, Y. Liu and C. M. Li, Lab Chip, 2010, 10, 559 RSC.
- G. Destgeer and H. J. Sung, Lab Chip, 2015, 15, 2722–2738 RSC.
- X. Ding, P. Li, S.-C. S. Lin, Z. S. Stratton, N. Nama, F. Guo, D. Slotcavage, X. Mao, J. Shi, F. Costanzo and T. J. Huang, Lab Chip, 2013, 13, 3626–3649 RSC.
- T. Dung Luong and N. Trung Nguyen, Micro Nanosyst., 2010, 2, 217–225 CrossRef.
- T. Frommelt, M. Kostur, M. Wenzel-Schäfer, P. Talkner, P. Hänggi and A. Wixforth, Phys. Rev. Lett., 2008, 100, 1–4 CrossRef PubMed.
- C. Y. Lee, C. L. Chang, Y. N. Wang and L. M. Fu, Int. J. Mol. Sci., 2011, 12, 3263–3287 CrossRef CAS PubMed.
- A. Wixforth, C. Strobl, C. Gauer, A. Toegl, J. Scriba and Z. v. Guttenberg, Anal. Bioanal. Chem., 2004, 379, 982–991 CrossRef CAS PubMed.
- T. Franke and A. Wixforth, ChemPhysChem, 2008, 9, 2140–2156 CrossRef CAS PubMed.
- A. Renaudin, P. Tabourier, J.-C. Camart and C. Druon, J. Appl. Phys., 2006, 100, 116101 CrossRef.
- A. Renaudin, P. Tabourier, V. Zhang, J. C. Camart and C. Druon, Sens. Actuators, B, 2006, 113, 389–397 CrossRef CAS.
- M. Tan, J. Friend and L. Yeo, Phys. Rev. Lett., 2009, 103, 24501 CrossRef PubMed.
- A. Winkler, S. M. Harazim, S. B. Menzel and H. Schmidt, Lab Chip, 2015, 15, 3793–3799 RSC.
- T. Franke, S. Braunmüller, L. Schmid, A. Wixforth and D. A. Weitz, Lab Chip, 2010, 10, 789 RSC.
- V. Skowronek, R. W. Rambach, L. Schmid, K. Haase and T. Franke, Anal. Chem., 2013, 85, 9955–9959 CrossRef CAS PubMed.
- R. W. Rambach, V. Skowronek and T. Franke, RSC Adv., 2014, 4, 60534–60542 RSC.
- V. Skowronek, R. W. Rambach and T. Franke, Microfluid. Nanofluid., 2015, 19, 335–341 CrossRef CAS.
- C. J. Strobl, C. Schaeflein, U. Beierlein, J. Ebbecke and A. Wixforth, Appl. Phys. Lett., 2004, 85, 1427 CrossRef CAS.
- Y. J. Liu, X. Ding, S. C. S. Lin, J. Shi, I. K. Chiang and T. J. Huang, Adv. Mater., 2011, 23, 1656–1659 CrossRef CAS PubMed.
- X. Y. Du, Y. Q. Fu, J. K. Luo, A. J. Flewitt and W. I. Milne, J. Appl. Phys., 2009, 105, 24508 CrossRef.
- R. V. Raghavan, J. R. Friend and L. Y. Yeo, Microfluid. Nanofluid., 2010, 8, 73–84 CrossRef CAS.
- H. Li, J. R. Friend and L. Y. Yeo, Biomed. Microdevices, 2007, 9, 647–656 CrossRef PubMed.
- T. Lee, J. G. Ok, H. S. Youn and L. J. Guo, in IEEE Int. Ultrason. Symp. Proc., 2014, pp. 1041–1044.
- D. Beyssen, L. Le Brizoual, O. Elmazria, P. Alnot, I. Perry and D. Maillet, in 2006 IEEE Ultrasonics Symposium, IEEE, 2006, vol. 1, pp. 949–952.
- J. Kondoh, N. Shimizu, Y. Matsui, M. Sugimoto and S. Shiokawa, in IEEE Ultrasonics Symposium, 2005, IEEE, 2005, vol. 2, pp. 1023–1027.
- W. Tseng, J. Lin, W. Sung, S.-H. Chen and G.-B. Lee, J. Micromech. Microeng., 2006, 16, 539–548 CrossRef CAS.
- J. Kondoh, N. Shimizu, Y. Matsui, M. Sugimoto and S. Shiokawa, Sens. Actuators, A, 2009, 149, 292–297 CrossRef CAS.
- J. K. Luo, Y. Q. Fu and W. I. Milne, in Modeling and Measurement Methods for Acoustic Waves and for Acoustic Microdevices, ed. M. G. Beghi, InTech, 2013, pp. 515–556 Search PubMed.
- Z. Yang, S. Matsumoto, H. Goto, M. Matsumoto and R. Maeda, Sens. Actuators, A, 2001, 93, 266–272 CrossRef CAS.
- T. Luong, V. Phan and N.-T. Nguyen, Microfluid. Nanofluid., 2011, 10, 619–625 CrossRef CAS.
- B. H. Ha, K. S. Lee, G. Destgeer, J. Park, J. S. Choung, J. H. Jung, J. H. Shin and H. J. Sung, Sci. Rep., 2015, 5, 11851 CrossRef PubMed.
- S. Köster, F. E. Angilè, H. Duan, J. J. Agresti, A. Wintner, C. Schmitz, A. C. Rowat, C. A. Merten, D. Pisignano, A. D. Griffiths and D. A. Weitz, Lab Chip, 2008, 8, 1110 RSC.
- J. Reboud, Y. Bourquin, R. Wilson, G. S. Pall, M. Jiwaji, A. R. Pitt, A. Graham, A. P. Waters and J. M. Cooper, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15162–15167 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |