Open Access Article
Open Access ArticleZinc-diffused silver indium selenide quantum dot sensitized solar cells with enhanced photoconversion efficiency†
Ganga
Halder
and
Sayan
Bhattacharyya
 *
*
Department of Chemical Sciences and Centre for Advanced Functional Materials, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur-741246, India. E-mail: sayanb@iiserkol.ac.in
First published on 20th February 2017
Abstract
Alloying of quantum dots (QDs) with suitable metal ions is one of the efficient tools to enhance the power conversion efficiency (PCE) of QD sensitized solar cells (QDSSCs). Ternary AgInX2 (X = S, Se) based liquid-junction QDSSCs have low PCEs due to the presence of defect states at internal atom vacancies. However, Zn2+ diffusion at these vacant sites can improve the carrier mobility of the QDs. The QDSSCs with Zn2+-diffused AgInSe2 (ZAISE) QDs have a broad light harvesting range up to the near-infrared (NIR) range. PCE was enhanced from a merely 1.67% for pristine AgInSe2 QDs (AISE) to 3.07% for ZAISE with a Zn/(Ag + In) ratio of 48.2%, aided by an optimized ZnS passivation layer on the photoanode. The device efficiency was further improved to 3.57% by applying dual passivation of amorphous TiO2 and SiO2 layers. This approach could effectively suppress the recombination pathways at the QD surface and thereby minimize back electron transfer from photoexcited electrons of QDs and from the conduction band of TiO2 to the polysulfide electrolyte. The combined strategy of alloying with Zn2+ and inorganic passivation could achieve the highest ever PCE of environmentally friendly AgInX2 based QDSSCs.
1. Introduction
Semiconductor QDs have size-controllable photophysical properties such as the tunability of their band gap, a high absorption coefficient, an ability to generate multiple excitons by a single photon and a large carrier lifetime which offers the possibility of boosting the PCE of photovoltaic devices beyond the Shockley–Queisser limit.1–5 In QDSSCs, narrow band gap cadmium and lead based chalcogenide semiconductors like CdS, CdSe, PbS, and PbSe are popularly employed as sensitizers and wider band gap metal oxide semiconductors like TiO2, ZnO and SnO2 are used as electron acceptors.6 However, the low to moderate PCE of these binary QDs lingers due to their limited light harvesting range and/or poor charge injection efficiency. In comparison, alloyed QDs are superior substitutes since their band gap can be tuned by changing their composition to obtain a broad light harvesting range and suitable band gap alignment for effective charge injection.7 Recently, copper and silver based ternary QDs have received attention for the fabrication of third generation photovoltaic devices due to their broad light harvesting range from the UV-visible to the NIR region. This broad range is very crucial for capturing a large number of solar photons. More importantly, these ternary alloyed QDs are free from toxic elements such as cadmium and lead which will open a new window for the commercial application of “non-toxic” QDs in QDSSCs for future environmental prospects.8–10Although ternary alloyed QDs are good light harvesters, the efficiency of the photovoltaic devices is limited due to their high density of trap states which act as recombination centers in QDSSCs.10–12 AgInSe2 (AISE) is a I-III-VI2 semiconductor having a direct bulk band gap of 1.19 eV, which is ideal for solar cell applications attributed to its high absorption coefficient. Although it has a broad light harvesting range, several vacancies at Ag and Se lattice sites along with internal atom vacancies lead to defect states.13,14 In order to improve the PCE, silver chalcogenides were manipulated through creation of Ag2S–AgInS2 based p–n junction heterostructures15,16 and AgInS2 QDs/In2S3 based photoelectrodes,17 or introducing a buffer layer between AgInS2 QD sensitizers and ZnO nanorod arrays.18 In spite of several efforts, the device efficiency is still low. The presence of defect states reduces the crystallinity, electrical conductivity and carrier mobility in ternary QDs. These trap states with a high density also act as recombination centers, thereby decreasing the photovoltage and photovoltaic performance.19,20 One of the ways to reduce the density of surface trap states is surface modification of ternary QDs where the passivation layers act as a barrier to minimize the electron and hole transfer rates.8,9,21
The diffusion of foreign metal ions into the vacant sites of ternary QD lattices is another suitable approach to improve the conductivity as well as PCE by minimizing the prevalent defect states. For example, Cu-diffused AISE QDs in organic/inorganic bulk-heterojunction devices decrease the internal resistance and increase the short-circuit current density (JSC) under white light illumination.22 These alloying strategies alter the energy difference (ΔECB) between the conduction energy levels of the QDs and metal oxide, henceforth increasing the injection efficiency of the photogenerated electrons. If ΔECB is large enough, then photogenerated electrons can be rapidly injected into the metal oxide to enhance the charge collection efficiency and PCE.23 Although QDSSCs with ternary alloyed Zn–CuInSe2 QD sensitizers have already achieved 11.6% PCE through manipulation of the conduction band energy level,24 the breakthrough in AISE QD sensitized QDSSCs is much in demand to achieve improved PCE by increasing the crystallinity and carrier mobility of the QDs as well as by applying appropriate surface passivation.
In this work, Zn2+-diffused AgInSe2 (ZAISE) QDs with a broad light harvesting range from visible to NIR were synthesized by a hot injection method. The chemical composition of the QDs was varied by altering the ratio of Zn/(Ag + In). Higher Zn diffusion increases the ZAISE band gap. Through different optimization steps, the best PCE obtained is 3.57% when the Zn/(Ag + In) at% is 48.2. Our strategy could enhance the PCE of these “non-toxic” QDSSCs by ∼23% as compared to previous attempts.
2. Results and discussion
2.1 Characterization of the QDs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
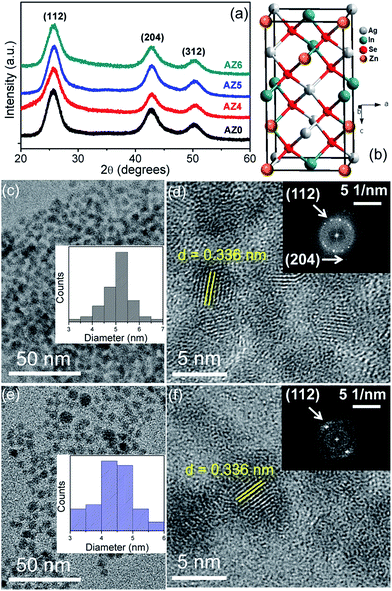
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of oleylamine and dodecanethiol. The Ag2Se QDs crystallize in the orthorhombic crystal structure according to JCPDS pattern 24-1041 (Fig. S1†). Thereafter the reaction mixture was further heated to 180 °C for the diffusion of In3+ and Zn2+ into the Ag2Se crystal lattice to form ZAISE QDs. Since the ionic radii of In3+ and Zn2+ are 1.44 and 0.68 Å, respectively, these ions can easily diffuse into the vacant sites of Ag2Se where the ionic radius of Ag+ is 1.54 Å.25,26 The QD samples denoted as AZ0, AZ4, AZ5 and AZ6 have a Zn:(Ag + In) at% of 0, 36, 48 and 58, respectively, as obtained by inductively coupled plasma mass spectrometry (ICP-MS) analyses. The powder X-ray diffraction (XRD) patterns in Fig. 1a show that all the QDs crystallize in the tetragonal crystal structure (Fig. 1b) with space group I
1 mixture of oleylamine and dodecanethiol. The Ag2Se QDs crystallize in the orthorhombic crystal structure according to JCPDS pattern 24-1041 (Fig. S1†). Thereafter the reaction mixture was further heated to 180 °C for the diffusion of In3+ and Zn2+ into the Ag2Se crystal lattice to form ZAISE QDs. Since the ionic radii of In3+ and Zn2+ are 1.44 and 0.68 Å, respectively, these ions can easily diffuse into the vacant sites of Ag2Se where the ionic radius of Ag+ is 1.54 Å.25,26 The QD samples denoted as AZ0, AZ4, AZ5 and AZ6 have a Zn:(Ag + In) at% of 0, 36, 48 and 58, respectively, as obtained by inductively coupled plasma mass spectrometry (ICP-MS) analyses. The powder X-ray diffraction (XRD) patterns in Fig. 1a show that all the QDs crystallize in the tetragonal crystal structure (Fig. 1b) with space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d according to the JCPDS pattern 75-0118. ICP-MS data show that on moving from AZ0 to AZ5, the drop in Ag:(Ag + In + Zn) is 30% whereas that of In:(Ag + In + Zn) is 70%. Therefore it is evident that a majority of Zn2+ sits in the In3+ sites than at Ag+ sites, and the relatively closer ionic radii of In3+ and Zn2+ are another validation to this argument. Also, the simultaneous diffusion of In3+ and Zn2+ in the Ag2Se lattice provides a competition between them to occupy the lattice vacancies. The crystallite size of the QDs is 3.2–3.5 nm which is calculated from the powder XRD pattern of the QDs using the Debye–Scherrer equation after subtracting the instrumental broadening. The representative transmission electron micrographs (TEMs) show that the particle size of the monodisperse QDs is around 4–6 nm with a moderately narrow size distribution (Fig. 1c and e). The AISE and ZAISE QDs were ligand-exchanged by replacing the initial hydrophobic oleylamine surfactant with mercaptopropionic acid (MPA) in order to obtain a maximum loading of the QDs on the TiO2 substrate and improve the film conductivity of QDSSCs.27 The interplanar spacing shows the high crystallinity and the indexed fast Fourier transform (FFT) patterns (Fig. 1d and f) confirm the purity of the as-synthesized QDs.
2d according to the JCPDS pattern 75-0118. ICP-MS data show that on moving from AZ0 to AZ5, the drop in Ag:(Ag + In + Zn) is 30% whereas that of In:(Ag + In + Zn) is 70%. Therefore it is evident that a majority of Zn2+ sits in the In3+ sites than at Ag+ sites, and the relatively closer ionic radii of In3+ and Zn2+ are another validation to this argument. Also, the simultaneous diffusion of In3+ and Zn2+ in the Ag2Se lattice provides a competition between them to occupy the lattice vacancies. The crystallite size of the QDs is 3.2–3.5 nm which is calculated from the powder XRD pattern of the QDs using the Debye–Scherrer equation after subtracting the instrumental broadening. The representative transmission electron micrographs (TEMs) show that the particle size of the monodisperse QDs is around 4–6 nm with a moderately narrow size distribution (Fig. 1c and e). The AISE and ZAISE QDs were ligand-exchanged by replacing the initial hydrophobic oleylamine surfactant with mercaptopropionic acid (MPA) in order to obtain a maximum loading of the QDs on the TiO2 substrate and improve the film conductivity of QDSSCs.27 The interplanar spacing shows the high crystallinity and the indexed fast Fourier transform (FFT) patterns (Fig. 1d and f) confirm the purity of the as-synthesized QDs.
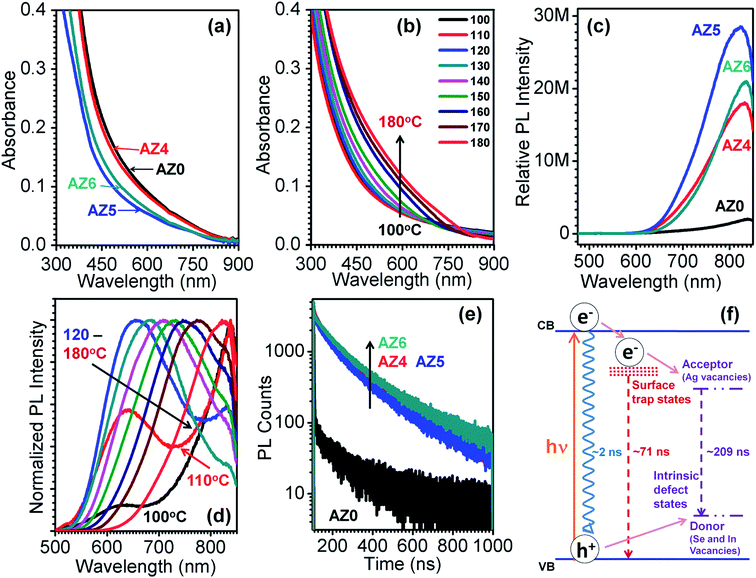
Fig. 2e shows the fluorescence decay curves of all the QDs at an excitation wavelength of 402 nm. The PL decay was recorded at 840, 834, 832 and 830 nm for AZ0, AZ4, AZ5 and AZ6 QDs, respectively. The PL decay profiles are found to be tri-exponential with an average lifetime of 232.7, 180.1, 184.8 and 203.9 ns for AZ0, AZ4, AZ5 and AZ6 QDs, respectively (Table 1). Fig. 2f provides the schematic energy level diagram showing the three recombination pathways with different lifetimes (τ) and their amplitude (A). The faster decay component (τ3 ∼ 2 ns) arises due to non-radiative processes e.g. Auger recombination and its contribution (A3) is the highest in AZ0. In AZ4 to AZ6, the contribution of this component is dominated by surface trap state emission (τ1 ∼ 66.6–75.6 ns) and donor–acceptor recombination (τ3 ∼ 196.4–222.1 ns) between the Ag+ vacancies as donor sites and In3+ and Se2− vacancies as acceptor sites.31 In the absence of Zn2+ (AZ0) the contributions from surface trap state emission and donor–acceptor recombination to the total average lifetime are lower as compared to those of AZ4–AZ6. Since τ2 increases from 196.4 ns to 222.1 ns for AZ4 to AZ6 QDs, respectively, it is evident that Zn2+ diffusion in the In3+ and Ag+ sites allows lesser donor–acceptor recombination. However, the contribution of surface trap state emission (A1) increases from 12.7 for AZ0 to 29.1–30.7 for AZ4–AZ6. The variation of τ1 and A1 implies that Zn2+ ions occupy surface sites till saturation before diffusing to the vacancy sites inside the AgInSe2 lattice. This is manifested from similar band gaps of AZ0 and AZ4, before increasing for AZ5 and AZ6 by Zn2+ lattice diffusion. In this case, segregation of a separate ZnSe phase at the QD surface is possible; however no such evidence is obtained from the XRD patterns in Fig. 1a.
| QD code | A 1 | τ 1 (ns) | A 2 | τ 2 (ns) | A 3 | τ 3 (ns) | χ 2 | 〈τ〉 (ns) |
|---|---|---|---|---|---|---|---|---|
| AZ0 | 12.68 | 37.7 | 53.15 | 240.8 | 34.17 | 1.33 | 1.13 | 232.7 |
| AZ4 | 29.49 | 66.6 | 69.65 | 196.4 | 0.85 | 2.67 | 1.04 | 180.1 |
| AZ5 | 30.68 | 67.9 | 68.23 | 202.5 | 1.09 | 1.69 | 1.09 | 184.8 |
| AZ6 | 29.08 | 75.6 | 70.07 | 222.1 | 0.85 | 1.94 | 1.06 | 203.9 |
2.2 Photovoltaic performance of the QDs
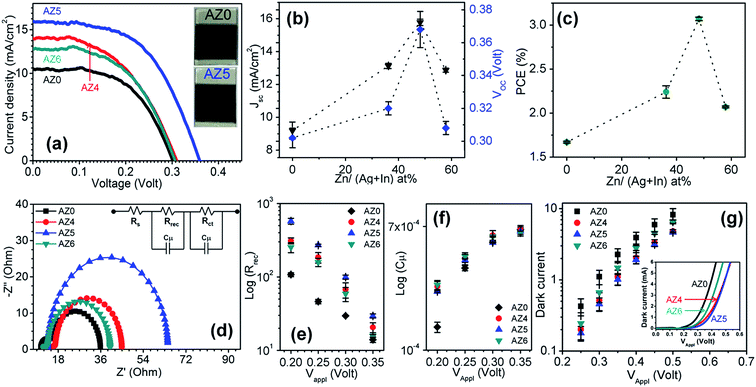
The working electrodes were prepared by dip coating of the as-prepared ∼9 μm thick TiO2 films on fluorine-doped tin oxide (FTO) coated glass substrates in aqueous dispersions of MPA-capped QDs. With the counter electrode made of a paste of microwave synthesized CuS powder32 and the sandwiched droplet of the electrolyte between the working and counter electrodes, at first the performance of AZ0–AZ6 based QDSSCs was tested. Here 8 cycles of ZnS passivation layers were applied on the working electrode by successive ionic layer adsorption and reaction (SILAR). Second, the number of ZnS SILAR cycles was optimized with the best performing QDs. In the third step, a double inorganic passivation of amorphous TiO2 and SiO2 was applied to further enhance the photovoltaic performance. Electrochemical impedance spectroscopy (EIS) was used to explain the trends and the role of passivation.| QDSSCs | Zn/(Ag + In) at% | J SC (mA cm−2) | V OC (V) | FF | η (%) |
|---|---|---|---|---|---|
| a Average of three cells in parallel. | |||||
| AZ0 | 0 | 9.91 ± 0.54 | 0.302 ± 0.004 | 0.555 ± 0.006 | 1.67 ± 0.10 |
| AZ4 | 36.2 | 13.13 ± 0.16 | 0.320 ± 0.004 | 0.526 ± 0.013 | 2.24 ± 0.07 |
| AZ5 | 48.2 | 15.78 ± 0.19 | 0.368 ± 0.011 | 0.529 ± 0.013 | 3.07 ± 0.02 |
| AZ6 | 57.9 | 12.87 ± 0.13 | 0.308 ± 0.004 | 0.520 ± 0.007 | 2.07 ± 0.01 |
The EIS measurements were carried out in the dark to highlight the significant improvement of the device efficiency upon zinc incorporation into the QDs.33Fig. 3d shows the Nyquist plots recorded in the frequency range from 0.1 Hz to 1 MHz at a forward bias of −0.35 V, which is very close to the VOC of the QDSSCs. The Nyquist plots are fitted with an equivalent circuit as shown in the Fig. 3d inset. All the EIS parameters e.g. series resistance (Rs), charge recombination resistance at photoanode and electrolyte interfaces (Rrec), and chemical capacitance (Cμ) are tabulated in Table 3. No significant change of Rs is observed for different QDSSCs since it depends upon the transport resistance of FTO and the connection setup which is equivalent for all the measurements. However, a considerably higher Rrec is observed in ZAISE than AISE QDSSCs (Fig. 3e). Since Rrec is inversely proportional to the charge recombination rate, the latter becomes sluggish at the interface of the ZAISE photoanode and the electrolyte than with the AISE photoanode. The photovoltaic data shown in Table 2 can be directly correlated with Rrec data which shows the significant improvement of the efficiency of ZAISE based devices. The Cμ of all the QDSSCs is similar at different applied voltages (Fig. 3f), due to the unchanged TiO2 conduction band position with different QD sensitizers. As a cross-check on the recombination rate in QDSSCs, their I–V characteristics were also measured in the dark (Fig. 3g inset) and the dark or recombination current is plotted as a function of applied voltage (Fig. 3g). The dark current in ZAISE QDSSCs is less than that in AISE QDSSCs at the same applied voltage which further proves the slower charge recombination at the photoanode/electrolyte interfaces in ZAISE QDSSCs.
| QDSSCSs | R s (Ω cm−2) | R rec (Ω cm−2) | C μ (mF cm−2) | f max (Hz) | τ n (ms) |
|---|---|---|---|---|---|
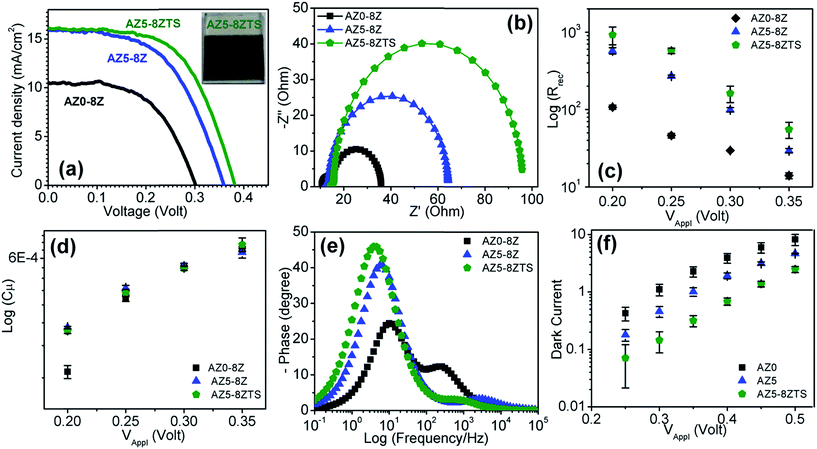
| AZ0-8Z | 10.65 | 12.58 | 0.70 | 12.12 | 13.11 |
| AZ4-8Z | 15.34 | 17.06 | 0.64 | 8.25 | 19.26 |
| AZ5-8Z | 12.25 | 30.78 | 0.68 | 5.62 | 28.27 |
| AZ6-8Z | 11.88 | 15.98 | 0.62 | 10.00 | 15.90 |
| AZ5-4Z | 12.1 | 21.58 | 0.67 | 8.25 | 19.26 |
| AZ5-10Z | 14.8 | 27.61 | 0.63 | 6.81 | 23.34 |
| AZ5-8ZTS | 12.75 | 46.87 | 0.65 | 3.83 | 41.50 |
The relatively higher efficiency of AZ5 QDSSCs even though the average lifetime of AZ5 colloidal QDs is lower than those of AZ0 and AZ6 is quite intriguing (Table 1). When the QD surface layer is passivated by 8 ZnS SILAR cycles, the surface trap state recombination is reduced. Since AZ5 has a relatively higher contribution of τ1, the QDSSCs with AZ5 QDs are mostly benefitted. Interestingly in this work, a total of 8 ZnS SILAR cycles are required to make a well passivated layer on the QD surface whereas there are reports where 4 ZnS SILAR cycles are employed to achieve the highest device efficiency.2,34,35 This clearly highlights the importance of reducing the surface trap state emission. The surface passivation however cannot alter the τ2 and τ3 components i.e. non-radiative and donor–acceptor recombination processes which are inherent to the QDs with a specific Zn2+ concentration. Due to the reduction in surface trap states, slower charge recombination occurs at the TiO2/QD/electrolyte interface which increases the PCE of AZ5-8Z QDSSCs. On the other hand, due to a large amount of non-radiative loss, PCE is the least for AZ0-8Z QDSSCs (Table 2).
| τn = 1/(2πfmax) | (1) |
| QDSSCs | J SC (mA cm−2) | V OC (V) | FF | η (%) |
|---|---|---|---|---|
| a Average of three cells in parallel. b Champion cell. | ||||
| AZ0-8Z | 9.91 ± 0.54 | 0.302 ± 0.004 | 0.555 ± 0.006 | 1.67 ± 0.10 |
| AZ5-8Z | 15.78 ± 0.19 | 0.368 ± 0.011 | 0.529 ± 0.013 | 3.07 ± 0.02 |
| AZ5-8ZTSa | 15.78 ± 0.23 | 0.378 ± 0.002 | 0.576 ± 0.016 | 3.41 ± 0.16 |
| AZ5-8ZTSb | 16.03 | 0.381 | 0.585 | 3.57 |
Several charge recombination processes which are responsible for the low PCE of QDSSCs include band edge recombination between the valence band holes and the conduction band electrons in QDs, back electron transfer to the electrolyte from photoexcited electrons of QDs and from the conduction band of TiO2, recombination of conduction band electrons of TiO2 with the valence band hole of QDs, and surface trapping of photoexcited electrons of QDs and conduction band electrons of TiO2.37 The modification of the photoanode surface with suitable inorganic and organic passivation layers is a key step to suppress these charge recombination pathways and improve the device efficiency.38–40 Inorganic passivation can suppress a majority of these recombination pathways which indeed is the key step to enhance the PCE of QDSSCs.34,41,42 In this work, when ZnS is deposited by SILAR onto the QD-coated TiO2 substrate, it likely passivates the dangling bonds present on the TiO2 surface through the formation of well-ordered Zn–O and S–Ti bonds. The amorphous TiO2 coating, on the QD-deposited TiO2 photoanode, acts as a buffer layer at the interface between TiO2/QDs and ZnS/SiO2 thus reducing the interfacial trap states to enhance the device efficiency. Further, the deposition of SiO2 on ZnS can passivate all the exposed Zn and S surface atoms through the formation of Si–S and O–Zn bonds at the interface of ZnS and SiO2.35 The coating of these different inorganic passivation layers is evident in the TEM image of the photoanode (Fig. S6†). However, the separate layers could not be distinguished in the ∼1.5 nm thick amorphous coating. The energy dispersive analysis of X-rays (EDAX) and elemental mapping in fact confirm the presence of Zn, S, O and Si elements.
To understand the role of passivation layers, QDs were deposited onto an insulating mesoporous SiO2 layer on FTO glass. Since there will be no charge injection from the QDs into the SiO2 layer, the changes in PL spectra (Fig. S7†) with different passivation layers can verify the significance of passivation. The PL intensity decreases by coating of an amorphous TiO2 layer onto the QDs due to injection of charge from QDs into TiO2. The PL intensity increases again by applying ZnS and SiO2 coatings over the film due to surface passivation of the QDs. ZnS and TiO2/SiO2 passivation however will be able to control neither the band edge recombination in QDs, nor the recombination pathways between QDs and the TiO2 photoanode which remain in contact without any interfacial layer. In fact these passivation layers at the interface of the photoanode and electrolyte potentially reduce or stop the back electron transfer from photoexcited electrons of QDs and from the conduction band of TiO2 to the electrolyte. A schematic is shown in Fig. 5 where the desired electron transfer pathways are shown by thicker curved arrows and the undesired recombination pathways are shown with dotted arrows. Overall, this strategic combination of alloying with Zn in AISE QDs, optimized ZnS layers and TiO2/SiO2 double passivation could significantly enhance the PCE of AISE based QDSSCs to 3.57%, which is ∼23% higher than the best of previous reports (Table 5).10,15–18,22,43–45
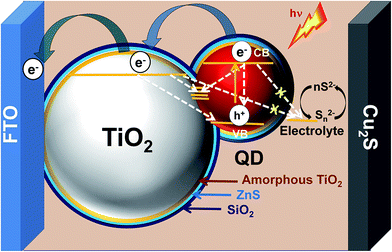 | ||
| Fig. 5 Schematic of the AZ5-8ZTS based QDSSC showing the electron transfer (solid curved arrows) and recombination pathways (dotted straight arrows). | ||
| QD | Synthesis method | Deposition methodb | Nature of devices | PCE (%) | Ref. |
|---|---|---|---|---|---|
| a HI = hot-injection method; HU = heating-up method. b SA = self-assembly; EPD = electrophoretic deposition; NA = not available. | |||||
| Cu diffused AgInS2 | HI + HUa | Spin coating | Bulk heterojunction | 1.13 | 22 |
| ZnO/ZnS/AgInS2 nanotube arrays | NA | Chemical etching + anion exchange + autoclave | Inorganic–organic hybrid solar cell | 2.11 | 18 |
| AgInS2 | Ligand-free synthesis | Direct deposition | Liquid junction | 0.80 | 10 |
| ZnSe-AgInSe2/CdS | HU | SA + SILARb | Liquid junction | 1.90 | 43 |
| Ag2S/AgInS2 heterostructures | HI | NA | Bulk heterojunction | 0.52 | 15 |
| Ag2S/AgInS2 nanoheterodimers | Thermolysis | Direct deposition | Liquid junction | 1.30 | 16 |
| AgInS2/In2S3 | NA | SILAR | Liquid junction | 0.70 | 17 |
| AgInS2-ZnS | HI | EPD | Liquid junction | 2.25 | 44 |
| AgInS2 | HU | SA via ligand exchange | Liquid junction | 2.9 | 45 |
| Zn-AgInSe2 | HI + HU | SA via ligand exchange | Liquid junction | 3.57 | This work |
3. Conclusions
Zn2+-diffused AgInSe2 QDs with a Zn:(Ag + In) at% of 36, 48 and 58 were synthesized by a hot-injection method and applied as light harvesters in QDSSCs. A majority of Zn2+ ions occupy the In3+ sites over Ag+ sites. The QDs had a broad light harvesting range from the visible to the NIR region and the PL lifetime decreases with Zn2+-diffusion up to a Zn:(Ag + In) at% of 48.2 (AZ5). The AISE and ZAISE QDs were ligand-exchanged with MPA to achieve a maximum loading of the QDs on the TiO2 photoanode. Apart from improving the crystallinity and carrier mobility of the QDs, diffusion of Zn2+ also tunes the intrinsic band gap. With 8 SILAR cycles of ZnS on top of the QD-deposited TiO2 photoanode the PCE was improved from 1.67% for AZ0 to 3.07% for AZ5. The passivation by ZnS reduces the surface trap state recombination whereas the non-radiative and donor–acceptor recombination processes likely remain unchanged. The PCE was further enhanced to 3.57% by dual inorganic passivation according to the TiO2/QD/amorphous TiO2/ZnS/SiO2 configuration. In addition to the suppression of surface trap states, the surface passivation reduces the recombination pathways of back electron transfer from photoexcited electrons of QDs and also from the conduction band of TiO2 to the polysulfide redox electrolyte. The record PCE of 3.57% is so far the highest achieved for ternary AgInX2 (X = S, Se) based QDSSCs. A similar strategy coupled with a surface passivation approach is effective in enhancing the device efficiency of other low performing QD systems in photovoltaic applications.4. Experimental section
4.1 Materials
Silver nitrate (AgNO3, Sigma Aldrich, 98%), indium chloride tetrahydrate (InCl3·4H2O, Sigma Aldrich, 99.999%), oleylamine (Sigma Aldrich, ≥98%), 1-octadecene (Sigma Aldrich, technical grade, 90%), 1-dodecanethiol (Sigma Aldrich, ≥98%), zinc acetate dihydrate Zn(OAc)2·2H2O (Merck India, 98%), zinc nitrate hexahydrate Zn(NO3)2·6H2O (reagent grade, 98%), sulfur powder (purified, Merck India), sodium sulfide flakes (Na2S·9H2O, purified, Merck India), potassium chloride (purified, Merck India), chloroform (Emplura, Merck India), ethanol (Absolute, Changshu Yangyuan, China) and methanol (Sigma Aldrich, ACS spectrophotometric grade, 98%) were used without further purification.4.2 Synthesis methodologies
The synthesis of AISE QDs was performed by following a literature reported method with slight modifications.304.3 Fabrication of QDSSCs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) in N-methyl 2-pyrrolidone. The paste was doctor bladed on pre-cleaned FTO, dried and annealed at 80 °C for 1.5 h.
1 w/w) in N-methyl 2-pyrrolidone. The paste was doctor bladed on pre-cleaned FTO, dried and annealed at 80 °C for 1.5 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) was employed as the electrolyte.
7 v/v) was employed as the electrolyte.
4.4 Characterization
The XRD measurements were carried out with a Rigaku powder X-ray diffractometer having Cu Kα = 1.54059 Å radiation. The ICP-MS measurements were performed by using a Thermo Scientific X-series using Plasma lab software. TEM images were obtained with a UHR-FEG TEM system (JEOL, Model JEM 2100 F) using a 200 kV electron source equipped with an energy dispersive X-ray spectroscope. To record the TEM images, EDAX and elemental mapping, the QD-sensitized photoanode with different inorganic coatings was crushed to powder and dispersed in isopropanol. UV-Vis absorbance spectra of the QD dispersion and the TiO2 photoanode were recorded using a Jasco V-670 spectrophotometer. To record the absorbance spectra of the TiO2 photoanode, the spectrophotometer was equipped with an integrating sphere. Room temperature PL spectra were measured with a Horiba Jobin Yvon Fluorolog using a Xe lamp as the excitation source with an excitation wavelength of 480 nm. The fluorescence lifetimes were recorded by using a time correlated single-photon counting spectrofluorimeter from HORIBA Jobin Yvon IBH. The fluorescence life time was calculated by fitting the decay curves by using an iterative fitting program provided by IBH. Photovoltaic measurements were carried out by using a solar simulator provided by Newport using a 300 W xenon lamp as a light source. The intensity of the light was adjusted to 100 mW cm−2 by using a reference silicon solar cell in the presence of a 1.5 G filter. The photovoltaic and the impedance measurements were carried out by using a CHI Electrochemical workstation. The plots were analyzed with inbuilt software in the electrochemical workstation.Acknowledgements
GH thanks the University Grants Commission (UGC), New Delhi for her fellowship. GH also thanks Dr M. Venkataramanan for his help with the PL measurements and Mr Arjun Halder for the XRD measurements. The financial support from the Department of Science & Technology – Science & Engineering Research Board (DST-SERB) under Sanction No EMR/2016/001703 is duly acknowledged. SB thanks IISER Kolkata for the academic and research fund.Notes and references
- M. C. Beard, J. Phys. Chem. Lett., 2011, 2, 1282–1288 CrossRef CAS PubMed.
- A. Sahasrabudhe and S. Bhattacharyya, Chem. Mater., 2015, 27, 4848–4859 CrossRef CAS.
- O. E. Semonin, J. M. Luther, S. Choi, H.-Y. Chen, J. Gao, A. J. Nozik and M. C. Beard, Science, 2011, 334, 1530–1533 CrossRef CAS PubMed.
- A. J. Nozik, Chem. Phys. Lett., 2008, 457, 3–11 CrossRef CAS.
- P. V. Kamat, Acc. Chem. Res., 2012, 45, 1906–1915 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834–2860 CAS.
- Z. Pan, K. Zhao, J. Wang, H. Zhang, Y. Feng and X. Zhong, ACS Nano, 2013, 7, 5215–5222 CrossRef CAS PubMed.
- Z. Pan, I. Mora-Seró, Q. Shen, H. Zhang, Y. Li, K. Zhao, J. Wang, X. Zhong and J. Bisquert, J. Am. Chem. Soc., 2014, 136, 9203–9210 CrossRef CAS PubMed.
- W. Li, Z. Pan and X. Zhong, J. Mater. Chem. A, 2015, 3, 1649–1655 CAS.
- K. P. Kadlag, P. Patil, M. J. Rao, S. Datta and A. Nag, CrystEngComm, 2014, 16, 3605–3612 RSC.
- M. G. Panthani, C. J. Stolle, D. K. Reid, D. J. Rhee, T. B. Harvey, V. A. Akhavan, Y. Yu and B. A. Korgel, J. Phys. Chem. Lett., 2013, 4, 2030–2034 CrossRef CAS PubMed.
- G. Halder and S. Bhattacharyya, J. Phys. Chem. C, 2015, 119, 13404–13412 CAS.
- J. Krustok, J. Raudoja, M. Krunks, H. Mändar and H. Collan, J. Appl. Phys., 2000, 88, 205–209 CrossRef CAS.
- B. Mao, C.-H. Chuang, F. Lu, L. Sang, J. Zhu and C. Burda, J. Phys. Chem. C, 2013, 117, 648–656 CAS.
- R. Bose, G. Manna, S. Jana and N. Pradhan, Chem. Commun., 2014, 50, 3074–3077 RSC.
- M. Jagadeeswararao, A. Swarnkar, G. B. Markad and A. Nag, J. Phys. Chem. C, 2016, 120, 19461–19469 CAS.
- Y. Wang, Q. Zhang, Y. Li and H. Wang, Nanoscale, 2015, 7, 6185–6192 RSC.
- J. Han, Z. Liu, K. Guo, J. Ya, Y. Zhao, X. Zhang, T. Hong and J. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 17119–17125 CAS.
- D. Aldakov, A. Lefrançois and P. Reiss, J. Mater. Chem. C, 2013, 1, 3756–3776 RSC.
- G. Hodes, J. Phys. Chem. C, 2008, 112, 17778–17787 CAS.
- H. McDaniel, N. Fuke, N. S. Makarov, J. M. Pietryga and V. I. Klimov, Nat. Commun., 2013, 4, 2887 Search PubMed.
- A. Guchhait and A. J. Pal, ACS Appl. Mater. Interfaces, 2013, 5, 4181–4189 CAS.
- A. G. Pattantyus-Abraham, I. J. Kramer, A. R. Barkhouse, X. Wang, G. Konstantatos, R. Debnath, L. Levina, I. Raabe, M. K. Nazeeruddin, M. Grätzel and E. H. Sargent, ACS Nano, 2010, 4, 3374–3380 CrossRef CAS PubMed.
- J. Du, Z. Du, J.-S. Hu, Z. Pan, Q. Shen, J. Sun, D. Long, H. Dong, L. Sun, X. Zhong and L.-J. Wan, J. Am. Chem. Soc., 2016, 138, 4201–4209 CrossRef CAS PubMed.
- M. A. Ahmed, K. E.-S. Rady, K. M. El-Shokrofy, A. A. Arais and M. S. Shams, Mater. Sci. Appl., 2014, 5, 932–942 CAS.
- J. E. Jaffe and A. Zunger, Phys. Rev. B: Condens. Matter, 1984, 29, 1882 CrossRef CAS.
- A. R. Kirmani, A. Kiani, M. M. Said, O. Voznyy, N. Wehbe, G. Walters, S. Barlow, E. H. Sargent, S. R. Marder and A. Amassian, ACS Energy Lett., 2016, 1, 922–930 CrossRef CAS.
- X. Tang, W. B. A. Ho and J. M. Xue, J. Phys. Chem. C, 2012, 116, 9769–9773 CAS.
- B. Chen, H. Zhong, W. Zhang, Z. Tan, Y. Li, C. Yu, T. Zhai, Y. Bando, S. Yang and B. Zou, Adv. Funct. Mater., 2012, 22, 2081–2088 CrossRef CAS.
- D. Yao, H. Liu, Y. Liu, C. Dong, K. Zhang, Y. Sheng, J. Cui, H. Zhang and B. Yang, Nanoscale, 2015, 7, 18570–18578 RSC.
- B. Mao, C.-H. Chuang, J. Wang and C. Burda, J. Phys. Chem. C, 2011, 115, 8945–8954 CAS.
- D. Ghosh, G. Halder, A. Sahasrabudhe and S. Bhattacharyya, Nanoscale, 2016, 8, 10632–10641 RSC.
- A. Sahasrabudhe, S. Kapri and S. Bhattacharyya, Carbon, 2016, 107, 395–404 CrossRef CAS.
- Z. Ren, J. Wang, Z. Pan, K. Zhao, H. Zhang, Y. Li, Y. Zhao, I. Mora-Sero, J. Bisquert and X. Zhong, Chem. Mater., 2015, 27, 8398–8405 CrossRef CAS.
- K. Zhao, Z. Pan, I. Mora-Seró, E. Cánovas, H. Wang, Y. Song, X. Gong, J. Wang, M. Bonn, J. Bisquert and X. Zhong, J. Am. Chem. Soc., 2015, 137, 5602–5609 CrossRef CAS PubMed.
- J.-Y. Kim, J. Yang, J. H. Yu, W. Baek, C.-H. Lee, H. J. Son, T. Hyeon and M. J. Ko, ACS Nano, 2015, 9, 11286–11295 CrossRef CAS PubMed.
- J. Tian and G. Cao, J. Phys. Chem. Lett., 2015, 6, 1859–1869 CrossRef CAS PubMed.
- K. Zhao, Z. Pan and X. Zhong, J. Phys. Chem. Lett., 2016, 7, 406–417 CrossRef CAS PubMed.
- N. Guijarro, J. M. Campiña, Q. Shen, T. Toyoda, T. Lana-Villarreal and R. Gómez, Phys. Chem. Chem. Phys., 2011, 13, 12024–12032 RSC.
- Z. Liu, M. Miyauchi, Y. Uemura, Y. Cui, K. Hara, Z. Zhao, K. Sunahara and A. Furube, Appl. Phys. Lett., 2010, 96, 233107 CrossRef.
- Z. Ren, Z. Wang, R. Wang, Z. Pan, X. Gong and X. Zhong, Chem. Mater., 2016, 28, 2323–2330 CrossRef CAS.
- Z. Pan and X. Zhong, J. Mater. Chem. A, 2016, 4, 18976–18982 CAS.
- T. Kameyama, Y. Douke, H. Shibakawa, M. Kawaraya, H. Segawa, S. Kuwabata and T. Torimoto, J. Phys. Chem. C, 2014, 118, 29517–29524 CAS.
- S. M. Kobosko, D. H. Jara and P. V. Kamat, ACS Appl. Mater. Interfaces DOI:10.1021/acsami.6b14604.
- C. Cai, L. Zhai, Y. Ma, C. Zou, L. Zhang, Y. Yang and S. Huang, J. Power Sources, 2017, 341, 11–18 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Powder XRD pattern of Ag2Se; Tauc plots; PL spectra of AZ4 and AZ6 QDs synthesized at different temperatures; J–V characteristics and variation of Nyquist plots, Rrec and Cμ as a function of the bias voltage of AZ5 QDSSCs with different ZnS SILAR cycles. See DOI: 10.1039/c7ta00268h |
| This journal is © The Royal Society of Chemistry 2017 |